Abstract
Purpose
Patients with malignancy sometimes develop painful mucositis and require patient-controlled analgesia (PCA) to treat their pain. Pain disrupts sleep and there is some evidence that analgesic medications also disrupt sleep. This study examined whether treatment with the sedative hypnotic eszopiclone could improve self-reports of sleep, fatigue, and pain as well as decrease opioid self-administered via PCA.
Methods
Inpatients who developed mucositis severe enough to require PCA treatment were randomized double-blind to a 2-day trial on eszopiclone or placebo-administered at bedtime. Patients completed questionnaires which assessed sleep, pain, and fatigue. PCA medication was calculated in terms of morphine equivalents. Data were analyzed with unpaired t tests and repeated measures analysis of variance.
Results
Twenty-two patients were randomized to placebo and 23 to eszopiclone. Groups were comparable in age and treatment characteristics. Mean pain scores were lower in the eszopiclone group at all time points (morning p = 0.01, afternoon p = 0.04, evening p = 0.04). The eszopiclone group reported increased sleep time (p < 0.05), fewer nighttime awakenings (p < 0.001), better self-reported sleep quality (p = 0.01), and depth (p = 0.04). There were no significant differences between eszopiclone and placebo in terms of self-reports of fatigue or opioid usage.
Conclusion
Sedative hypnotic agents improve sleep and analgesia even in the setting of considerable pain and discomfort.
Keywords: Pain, Sleep, Fatigue, Opioids, Eszopiclone, Sedative hypnotics, Patient-controlled analgesia, Mucositis, Cancer
Introduction
Pain, insomnia, and fatigue are common symptom complaints of cancer patients. Although dramatic improvements have come about in recognizing and treating cancer related pain, less progress has been made in treating fatigue or sleep disorders.
Patients with malignancy who develop moderate to severe pain are commonly treated with opioids. One of the less commonly recognized side effects of opiate use is sleep disruption [1]. Although the human literature is small, it is clear that opiates, while sedating, are also profoundly sleep disruptive. We previously reported that opiate use significantly increased light sleep and decreased deep sleep [2]. Sleep disruption lowers pain threshold [3], and opioid medications themselves disrupt deep sleep [4], thereby putting in place a potential vicious cycle of pain, insomnia, more pain, and more insomnia. The quality of sleep influences daytime pain which in turn negatively influences nighttime sleep [5–7]. Animal and human studies demonstrate that experimentally induced sleep disruption lowers the threshold for detection of painful stimuli [8–10]. Although opiates are obviously helpful for pain, they do so at certain “costs”: They increase next day fatigue, constipation, and other side effects; they disrupt sleep which further increases next day fatigue; and finally, by virtue of their sleep disruptive properties, they lower the threshold for pain stimuli, thereby insuring that continuing or even higher doses of opiates are required.
Oral mucositis is a particularly painful side effect of certain types of chemotherapy [11–13]. High-dose melphalan used in autologous stem cell transplantation for multiple myeloma and malignant lymphoma is a frequent cause of mucositis. Total body irradiation (TBI) is also commonly associated with mucositis when patients receive TBI in conjunction with myeloablative allogeneic stem cell transplantation. Mucositis is commonly treated with opiates and, if severe, with patient-controlled analgesia (PCA) devices. These devices are advantageous because they allow smooth safe parenteral dosing of opiates to patients in moderate to severe pain. A baseline continuing infusion of opiate is programmed, and the device allows a certain amount of prn self-medication administration for additional pain relief. With PCAs, opiate usage can be quantitated in terms of morphine equivalents per 24 h or in smaller time intervals (e.g., nighttime vs daytime).
Eszopiclone (Lunesta™) is a non-benzodiazepine hypnotic agent used for treatment of insomnia. Roth et al. recently reported that eszopiclone improved pain reports in insomniac patients who also suffered from rheumatoid arthritis [14]. Because patients with extensive mucositis commonly experience severe pain, we wondered if a hypnotic agent might improve their pain. This study examined the effect of eszopiclone on sleep, pain, and fatigue in patients with hematologic malignancy undergoing chemotherapy and/or hematopoietic stem cell transplantation. The study also examined if eszopiclone treatment would lead to decreased opioid requirements via PCA in these patients.
Methods
The study was approved by the UCSD IRB and is a registered clinical trial (ClinicalTrials.gov # NCT00365261).
Patient eligibility
Patients were eligible for participation if they required inpatient hospitalization for hematologic malignancy, if they were between 20 and 75 years old, were able to tolerate oral medication, and were able to provide informed consent. Patients were excluded if they had a current history of substance abuse, had a history of allergic response to eszopiclone, required additional oral or parenteral opioids after starting PCA opioid treatment, or were regularly taking a prescribed sleeping pill more often than four times per week.
These patients were variously diagnosed with acute and chronic myeloid leukemia, multiple myeloma, Waldenstrom’s macroglobulinemia, Pre-B acute lymphoblastic leukemia, diffuse large B cell lymphoma, T cell lymphoma, and T cell pro-lymphocytic lymphoma. Patients were treated with (1) high-dose melphalan, (2) cytosine arabinoside (total body irradiation), and (3) the BEAM regimen comprising of busulfan, etoposide, cytosine arabinoside, and melphalan.
Procedures
Inpatients at UCSD’s Thornton Hospital who were felt to be at risk for developing mucositis were approached upon admission and invited to participate in the study. Written informed consent was obtained.
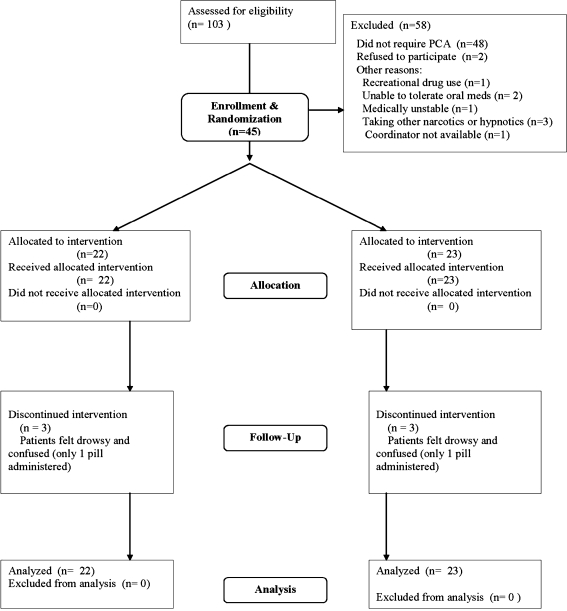
One hundred three inpatients were approached before they developed painful mucositis. Two declined to participate, 48 never developed mucositis, seven were ineligible, and one patient was missed (see flow chart, Fig. 1). Forty-five patients were enrolled and randomized to treatment.
Fig. 1.
Patient flow diagram
Dosing
Medications were administered double-blind for two successive days on this parallel group design study. Patients received eszopiclone 3 mg (or placebo) at 9 p.m. Patients who were >64 years or individuals receiving potent 3A4 inhibitors such as ketoconazole received 2 mg eszopiclone. These doses are commonly employed and the dose adjustment for older patients and for patients receiving 3A4 inhibitors is suggested in the prescribing information. Active and placebo medications were obtained from Sepracor and randomization, and blinding was provided by the UCSD Research Pharmacy.
Self-report data
Upon enrolling in the study, patients were familiarized with the brief subjective report instruments. Patients were asked to rate their pain and fatigue every 6 h after dosing, while awake. Pain was assessed with a 10-cm visual analog scale (0 = “no pain at all”; 10 = “severe, uncontrolled pain”). Patients completed the five-item Profile of Mood States Scale, Short Form (POMS-SF) Fatigue–Inertia Scale [15] to rate their fatigue complaints (higher scores denote more fatigue). Sleep quality was assessed every morning by self-report. Sleep latency was defined as the number of minutes taken to fall asleep after bedtime. Patient were also asked how long they slept as well as how many times they woke during the night. In addition, they rated their depth and quality of sleep (on a scale of 0 (poor) to 10 (excellent)), as well as their current level of sleepiness (0 = very sleepy to 10 = not sleepy at all).
PCA dosing
PCAs were programmed to deliver either morphine or dilaudid at fixed rate with optional self-administered prn boluses. Medication was summarized per nursing shift, and dilaudid doses were converted into morphine equivalents by multiplying the dose by 5.
Statistical analysis
Data were analyzed with two-sample tests and repeated measures analysis of variance.
Results
Forty-five patients were randomized: 22 to group placebo and 23 to group eszopiclone. Groups were comparable in age (mean (SD): eszopiclone arm 45.52 (2.72) years vs placebo 46.00 (3.38) years; p = 0.91) and treatment (e.g., allotransplantation vs autotransplantation p = 0.92).
Side effects
Three patients in each group received only one dose of medication because of drowsiness or confusion. There were no other side effects. Table 1 provides summary data from the study.
Table 1.
Effect of eszopiclone vs placebo on pain, fatigue, and sleep
| Variable | Eszopiclone | Placebo | p valued | |
|---|---|---|---|---|
| Mean (SEM) | Mean (SEM) | |||
| Pain scorea | Morning | 3.72 (0.38) | 5.41 (0.47) | 0.01 |
| Range 1–10 | Afternoon | 3.65 (0.45) | 5.05 (0.49) | 0.04 |
| Evening | 3.42 (0.49) | 4.79 (0.43) | 0.04 | |
| Fatigue scorea | Morning | 2.41 (0.20) | 2.77 (0.17) | 0.17 |
| POMS 5-item scale | Afternoon | 2.35 (0.21) | 2.79 (0.17) | 0.11 |
| Evening | 2.75 (0.35) | 2.81 (0.19) | 0.89 | |
| Sleep | Total night sleep time (min) | 426.20 (30.40) | 342.13 (27.64) | 0.05 |
| Latencyc (min) | 70.39 (25.85) | 51.90 (12.44) | 0.52 | |
| # of awakenings at night | 2.96 (0.35) | 5.78 (0.59) | <0.001 | |
| Qualityb (range 0–10) | 6.74 (0.57) | 4.65 (0.59) | 0.01 | |
| Depthb (range 0–10) | 6.50 (0.50) | 4.93 (0.57) | 0.04 | |
| Current sleepinessb (range 0–10) | 4.33 (0.63) | 5.13 (0.64) | 0.38 | |
aHigh scores indicate worse symptoms (e.g., 1 = no pain, 10 = worst imaginable pain)
bHigh scores indicate better sleep (e.g., 0 = poor sleep quality, 10 = excellent sleep quality)
cAfter removing one outlier with value >400 min, the mean (SEM) were eszopiclone 50.88 (17.78) min vs placebo 51.90 (12.44) min; p = 0.96
d p values based on t test (non-parametric two-sample tests gave similar results)
Sleep and fatigue effects
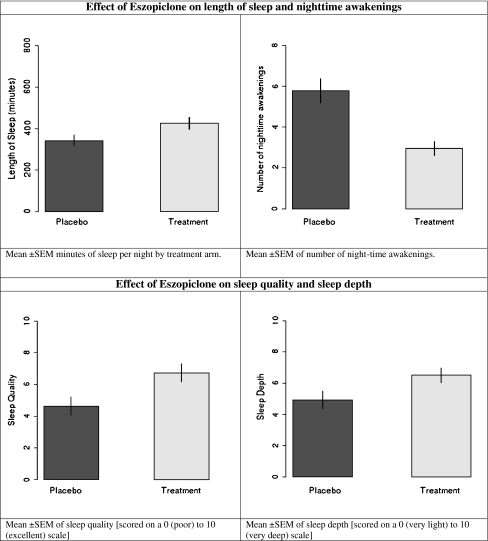
The eszopiclone group reported increased sleep time (p < 0.05), fewer nighttime awakenings (p < 0.001), better self-reported sleep quality (p = 0.01), and depth (p = 0.04; see Table 1 and Fig. 2). There were no significant differences between the groups in terms of sleep latency (p = 0.52) or current level of sleepiness (p = 0.38). There was no significant difference between the groups in terms of fatigue reports (see Table 1).
Fig. 2.
Sleep effects
Pain effects
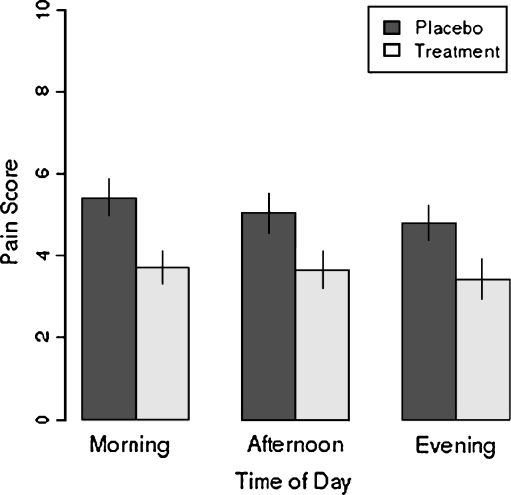
Mean pain scores over the course of 2 days’ treatment were significantly lower in the eszopiclone group than the placebo group (see Fig. 3 and Table 1) in the morning (p = 0.01), in the afternoon (p = 0.04), as well as in the evening (p = 0.04).
Fig. 3.
Effects of eszopiclone on pain. Mean ± SEM pain score by time of day and treatment arm; pain score for each patient at each time point is the average score across 2 days
Effects on PCA usage
There were no significant differences in terms of opiate usage: Median levels (25%, 75%) were 40.94 (23.37, 66.19) mg morphine in the placebo arm vs 36.35 mg (17.57, 58.46) in the eszopiclone arm (p = 0.73 using Wilcoxon rank sum test).
Discussion
The central finding of this study was that eszopiclone led to significant reductions in self-reported pain throughout the day. However, no effect of eszopiclone was observed on dose of opiates self-administered via PCA. If the analgesic effect of eszopiclone were due just to drug carry-over and sedation, one might expect the effect to be apparent only on the morning ratings of pain. However, Fig. 3 reveals that, after eszopiclone, pain ratings were consistently lower on the next morning, afternoon, and evening. Furthermore, as Table 1 reveals, fatigue scores were not significantly different between the eszopiclone and placebo groups at any of the three time points and, if anything, the eszopiclone group had lower fatigue complaints. Thus, the effects of eszopiclone on pain does not appear to reflect sedative effects of the compound.
The study also confirmed that eszopiclone improved subjective estimates of sleep, even in a group of patients who were very ill and in pain. While it is not surprising to detect an effect of a hypnotic agent on sleep [16], it is important to keep in mind that insomnia is common and demoralizing to patients and that insomnia in the context of serious medical illness is all the more burdensome.
In future studies, it may be desirable to assess sleep via polysomnography to determine which aspects of objectively measured sleep are most associated with the beneficial effects of eszopiclone on pain. Such data may help inform future research because sedative hypnotic agents act via diverse mechanisms, and it is thus possible that some agents may be more effective in relieving pain. Insomnia (lack of refreshing sleep) is defined on the basis of subjective estimates of sleep. It is an interesting theoretical question whether the beneficial effects of a sedative hypnotic agent on pain and self-reported sleep stem from specific actions on sleep physiology or on patient recall. Certainly, an extensive basic science literature demonstrates that sleep disruption increases pain sensitivity (see for instance [3]).
The study is limited by its small sample size. It is possible that comparisons of borderline significance (i.e., p < 0.15) might reach conventional criteria of significance with a larger sample size, but, in our study, with the exception of afternoon fatigue levels (p = 0.11), at no other time points was eszopiclone associated with markedly lower levels of fatigue. Sleep and fatigue are overlapping but distinct constructs [17]. In this study, their dissociation was beneficial to patients, i.e., patients reported improved sleep without reporting increasing levels of daytime fatigue.
The PCA contrasts revealed no differences between the groups. There was a large variance in PCA usage which is an expected observation because the minimum effective analgesic dose for the same procedure is highly variable [18]. It is possible that an effect on self-administered PCA dosing might be observed with a longer observation period or a different PCA dosing regimen.
This study is limited by its short-term period of observation. The pre-specified design contrasted active treatment with placebo over a 2-day interval. We have no data on our patients’ fatigue, pain, or sleep after they completed the trial. It would be important to know if these observations could be replicated and extended in a longer-term clinical trial. While this short clinical trial is a limitation, it is important to note that this double-blind study found that the sedative hypnotic drug was associated with consistently decreased reports of pain in the morning, the afternoon, and the evening—an average reduction of pain reports by ∼30%. In addition, the active treatment did not increase patients’ daytime fatigue—always a concern in cancer patients who already have high fatigue levels. Furthermore, the active drug had clear beneficial effects on patients’ sleep. On the one hand, it is not surprising that a hypnotic drug would improve sleep reports; on the other hand, the fact that one can demonstrate benefits in this context—painful oral mucositis in patients who are hospitalized—is a promising observation. We feel our observations are an important first step in improving symptom control. Natural next steps would entail replication and extension to longer duration clinical trials and perhaps other settings of pain.
The literature has generally focused on effects of sedative hypnotic agents on sleep in insomniac patients or on the side effect and safety profile. Our findings suggest that such agents may be helpful in patients who are quite medically ill and in pain. Even in such patients, sleep reports are improved, and interestingly, pain reports are diminished. Replication of this sort of study in other inpatients, perhaps no longer restricted to those requiring PCA devices, would be important. Similarly, future study might well examine the effects of sedative hypnotic medications on quality of life in patients with other major medical illnesses and pain.
Acknowledgments
This investigator-initiated study was funded by Sepracor Pharmaceuticals (#Esrc054). The authors appreciate the assistance of Peter Curtin, M.D. and Januario Castro, M.D.
Conflict of interest
This research was supported on an investigator-initiated grant to Dr. Dimsdale by Sepracor Pharmaceuticals.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Moore P, Dimsdale J. Opioids, sleep, and cancer-related fatigue. Med Hypotheses. 2002;58:77–82. doi: 10.1054/mehy.2001.1461. [DOI] [PubMed] [Google Scholar]
- 2.Dimsdale J, Norman D, Dejardin D, Wallace M. The effects of opioids on sleep architecture. J Clin Sleep Med. 2007;15:33–36. [PubMed] [Google Scholar]
- 3.Roehrs T, Hyde M, Blaisdell B, Greenwald M, Roth T. Sleep loss and REM sleep loss are hyperalgesic. Sleep. 2006;29:137–139. doi: 10.1093/sleep/29.2.145. [DOI] [PubMed] [Google Scholar]
- 4.Moore J, Kelz M. Opiates, sleep, and pain: the adenosinergic link. Anesthesiology. 2009;111:1175–1176. doi: 10.1097/ALN.0b013e3181bdfa2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raymond I, Nielsen TA, Lavigne G, Manzini C, Choiniere M. Quality of sleep and its daily relationship to pain intensity in hospitalized adult burn patients. Pain. 2001;92:381–388. doi: 10.1016/S0304-3959(01)00282-2. [DOI] [PubMed] [Google Scholar]
- 6.Affleck G, Urrows S, Tennen H, Higgins P, Abeles M. Sequential daily relations of sleep, pain intensity, and attention to pain among fibromyalgia patients. Pain. 1996;68:363–368. doi: 10.1016/S0304-3959(96)03226-5. [DOI] [PubMed] [Google Scholar]
- 7.Haack M, Sanchez E, Mullington J. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30(9):1145–1152. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kundermann B, Krieg J, Schreiber W, Lautenbacher S. The effect of sleep deprivation on pain. Pain Res Manag. 2004;9:25–32. doi: 10.1155/2004/949187. [DOI] [PubMed] [Google Scholar]
- 9.Moldofsky H, Scarisbrick P. Induction of neurasthenic musculoskeletal pain syndrome by selective sleep stage deprivation. Psychosom Med. 1976;38:35–44. doi: 10.1097/00006842-197601000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Onen S, Alloui A, Gross A, Eschallier A, Dubray C. The effects of sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. J Sleep Res. 2001;10:35–42. doi: 10.1046/j.1365-2869.2001.00240.x. [DOI] [PubMed] [Google Scholar]
- 11.Strobel ES, Bauchmuller K, Ihorst G, Engelhardt M. Frequency, severity and risk factors for oral mucositis after BEAM conditioning and autologous peripheral blood stem cell transplantation: a single center analysis and review of the literature. Leuk Lymphoma. 2007;48:2255–2260. doi: 10.1080/10428190701636492. [DOI] [PubMed] [Google Scholar]
- 12.Bensinger W, Schubert M, Ang KK, Brizel D, Brown E, Eilers JG, Elting L, Mittal BB, Schattner MA, Spielberger R, Treister NS, Trotti AM., 3rd NCCN Task Force report: prevention and management of mucositis in cancer care. J Natl Compr Canc Netw. 2008;6(Suppl 1):S1–S21. [PubMed] [Google Scholar]
- 13.Niscola P, Romani C, Cupelli L, Scaramucci L, Tendas A, Dentamaro T, Amadori S, de Fabritiis P. Mucositis in patients with hematologic malignancies: an overview. Haematologica. 2007;92(2):222–231. doi: 10.3324/haematol.10232. [DOI] [PubMed] [Google Scholar]
- 14.Roth T, Price JM, Amato DA, Rubens RP, Roach JM, Schnitzer TJ. The effect of eszopiclone in patients with insomnia and coexisting rheumatoid arthritis. Prim Care Companion J Clin Psychiatry. 2009;11(6):292–301. doi: 10.4088/PCC.08m00749bro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNair D, Lorr M, Droppleman L. Manual for the profile of mood states. San Diego: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 16.Ancoli-Israel S, Krystal AD, McCall WV, Schaefer K, Wilson A, Claus R, Rubens R, Roth T. A 12-week, randomized, double-blind, placebo-controlled study evaluating the effect of eszopiclone 2 mg on sleep/wake function in older adults with primary and comorbid insomnia. Sleep. 2010;33:225–234. doi: 10.1093/sleep/33.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailes S, Libman E, Baltzan M, Amsel R, Schondorf R, Fichten C. Brief and distinct empirical sleepiness and fatigue scales. J Psychosom Res. 2006;60:605–613. doi: 10.1016/j.jpsychores.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Portenoy R. Clinical application of opioid analgesics. In: Sinatra RS, Hord AH, Ginsberg B, Preble LM, editors. Acute pain: mechanisms and management. St. Louis: Mosby Year Book; 1992. pp. 93–101. [Google Scholar]