Abstract
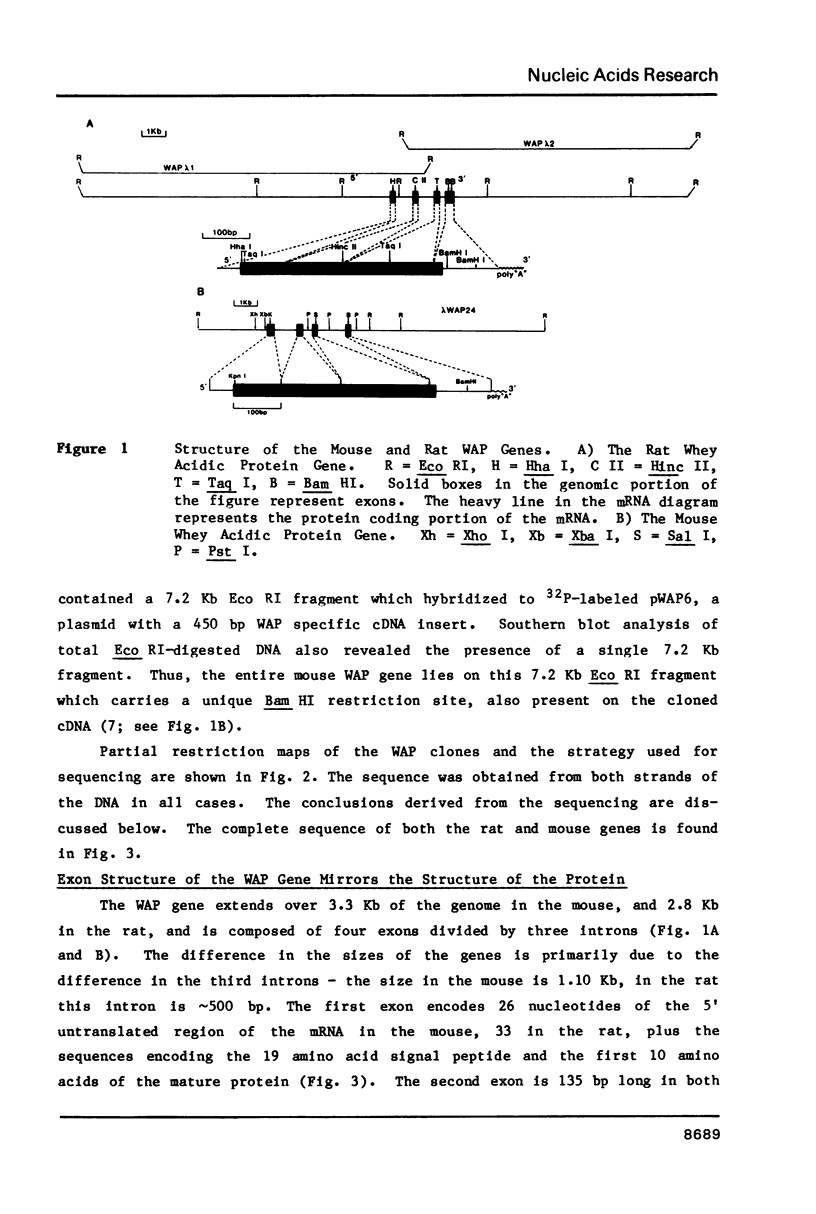
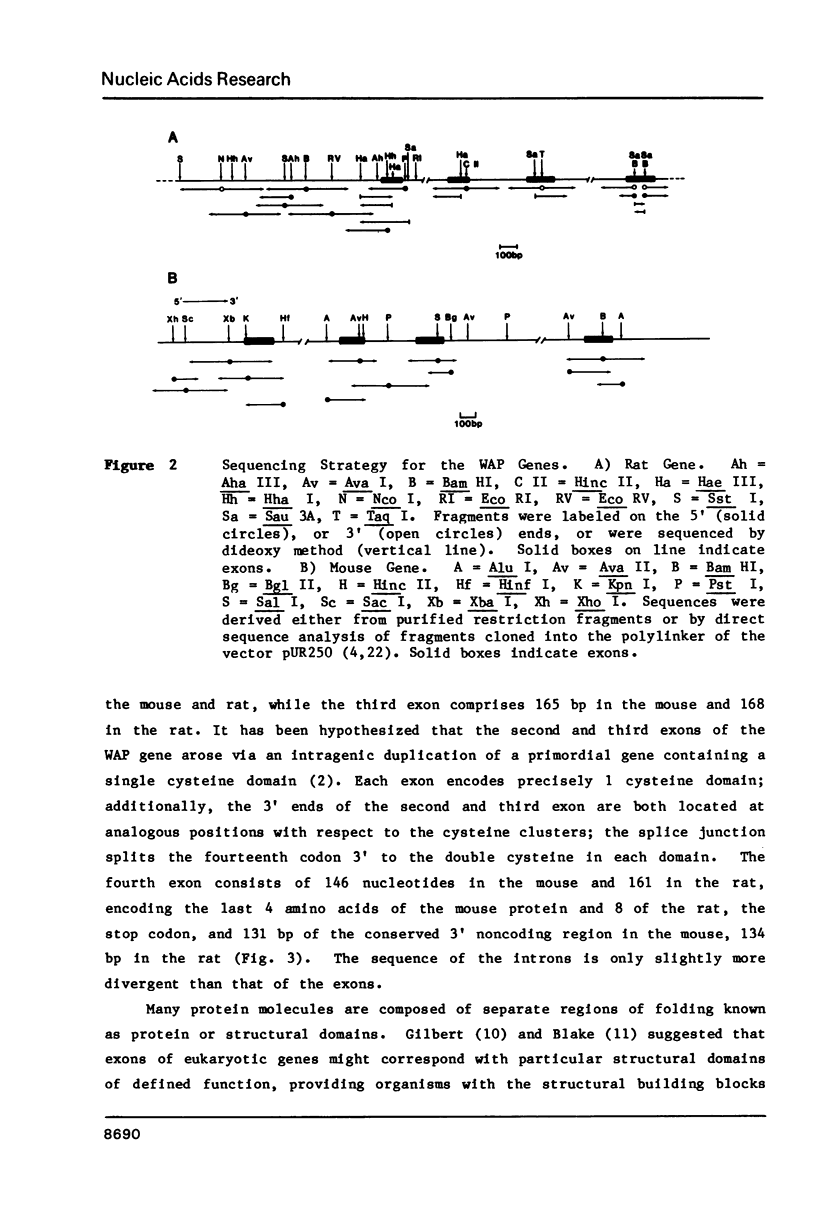
Whey acidic protein (WAP), a hormonally-regulated 14,000 dalton cysteine-rich protein, is the principal whey protein found in rodent milk. Genomic clones encompassing both the 2.8 Kb rat and 3.3 Kb mouse WAP genes have been characterized. The genes consist of four exons and three introns. The middle two exons encode the two cysteine-rich regions which probably form separate protein domains. Homology in the 5' flanking DNA of the mouse and rat extends at least 325 bp upstream of the putative CAP site, including a precisely conserved stretch of 50 bp around the unusual TATA and CAAT sites. The homology previously observed between the 3' noncoding sequences of the rat and mouse mRNAs extends at least 20 bp into the 3' flanking region. Several potential glucocorticoid receptor binding sites have been found in the 5' flanking region of the WAP gene. The conservation of the 5' flanking region of the WAP genes may be related to regulation of expression of WAP by peptide and/or steroid hormones.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Berget S. M. Are U4 small nuclear ribonucleoproteins involved in polyadenylation? Nature. 1984 May 10;309(5964):179–182. doi: 10.1038/309179a0. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Dandekar A. M., Robinson E. A., Appella E., Qasba P. K. Complete sequence analysis of cDNA clones encoding rat whey phosphoprotein: homology to a protease inhibitor. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3987–3991. doi: 10.1073/pnas.79.13.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenth J., Low B. W., Richardson J. S., Wright C. S. The toxin-agglutinin fold. A new group of small protein structures organized around a four-disulfide core. J Biol Chem. 1980 Apr 10;255(7):2652–2655. [PubMed] [Google Scholar]
- Drenth J. The structure of neurophysin. J Biol Chem. 1981 Mar 25;256(6):2601–2602. [PubMed] [Google Scholar]
- Eiferman F. A., Young P. R., Scott R. W., Tilghman S. M. Intragenic amplification and divergence in the mouse alpha-fetoprotein gene. Nature. 1981 Dec 24;294(5843):713–718. doi: 10.1038/294713a0. [DOI] [PubMed] [Google Scholar]
- Gilbert W. Why genes in pieces? Nature. 1978 Feb 9;271(5645):501–501. doi: 10.1038/271501a0. [DOI] [PubMed] [Google Scholar]
- Hennighausen L. G., Sippel A. E. Characterization and cloning of the mRNAs specific for the lactating mouse mammary gland. Eur J Biochem. 1982 Jun 15;125(1):131–141. doi: 10.1111/j.1432-1033.1982.tb06660.x. [DOI] [PubMed] [Google Scholar]
- Hennighausen L. G., Sippel A. E., Hobbs A. A., Rosen J. M. Comparative sequence analysis of the mRNAs coding for mouse and rat whey protein. Nucleic Acids Res. 1982 Jun 25;10(12):3733–3744. doi: 10.1093/nar/10.12.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennighausen L. G., Sippel A. E. Mouse whey acidic protein is a novel member of the family of 'four-disulfide core' proteins. Nucleic Acids Res. 1982 Apr 24;10(8):2677–2684. doi: 10.1093/nar/10.8.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrlich P., Hynes N. E., Ponta H., Rahmsdorf U., Kennedy N., Groner B. The endogenous proviral mouse mammary tumor virus genes of the GR mouse are not identical and only one corresponds to the exogenous virus. Nucleic Acids Res. 1981 Oct 10;9(19):4981–4995. doi: 10.1093/nar/9.19.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs A. A., Richards D. A., Kessler D. J., Rosen J. M. Complex hormonal regulation of rat casein gene expression. J Biol Chem. 1982 Apr 10;257(7):3598–3605. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miyata T., Hayashida H., Kikuno R., Hasegawa M., Kobayashi M., Koike K. Molecular clock of silent substitution: at least six-fold preponderance of silent changes in mitochondrial genes over those in nuclear genes. J Mol Evol. 1982;19(1):28–35. doi: 10.1007/BF02100221. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Goodman R. H., Horovitch S. J., Habener J. F. Primary structure of the gene encoding rat preprosomatostatin. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3337–3340. doi: 10.1073/pnas.81.11.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motojima K., Oka T. 5'-Terminal sequence of the mRNA of mouse whey acidic protein contains three possible sites of interaction with 18S rRNA. Biochem Biophys Res Commun. 1983 Oct 14;116(1):167–172. doi: 10.1016/0006-291x(83)90396-0. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvihill E. R., LePennec J. P., Chambon P. Chicken oviduct progesterone receptor: location of specific regions of high-affinity binding in cloned DNA fragments of hormone-responsive genes. Cell. 1982 Mar;28(3):621–632. doi: 10.1016/0092-8674(82)90217-3. [DOI] [PubMed] [Google Scholar]
- Nathans J., Hogness D. S. Isolation, sequence analysis, and intron-exon arrangement of the gene encoding bovine rhodopsin. Cell. 1983 Oct;34(3):807–814. doi: 10.1016/0092-8674(83)90537-8. [DOI] [PubMed] [Google Scholar]
- Payvar F., DeFranco D., Firestone G. L., Edgar B., Wrange O., Okret S., Gustafsson J. A., Yamamoto K. R. Sequence-specific binding of glucocorticoid receptor to MTV DNA at sites within and upstream of the transcribed region. Cell. 1983 Dec;35(2 Pt 1):381–392. doi: 10.1016/0092-8674(83)90171-x. [DOI] [PubMed] [Google Scholar]
- Pfahl M. Specific binding of the glucocorticoid-receptor complex to the mouse mammary tumor proviral promoter region. Cell. 1982 Dec;31(2 Pt 1):475–482. doi: 10.1016/0092-8674(82)90140-4. [DOI] [PubMed] [Google Scholar]
- Qasba P. K., Safaya S. K. Similarity of the nucleotide sequences of rat alpha-lactalbumin and chicken lysozyme genes. Nature. 1984 Mar 22;308(5957):377–380. doi: 10.1038/308377a0. [DOI] [PubMed] [Google Scholar]
- Quinto C., Quiroga M., Swain W. F., Nikovits W. C., Jr, Standring D. N., Pictet R. L., Valenzuela P., Rutter W. J. Rat preprocarboxypeptidase A: cDNA sequence and preliminary characterization of the gene. Proc Natl Acad Sci U S A. 1982 Jan;79(1):31–35. doi: 10.1073/pnas.79.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards D. A., Rodgers J. R., Supowit S. C., Rosen J. M. Construction and preliminary characterization of the rat casein and alpha-lactalbumin cDNA clones. J Biol Chem. 1981 Jan 10;256(1):526–532. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rüther U. pUR 250 allows rapid chemical sequencing of both DNA strands of its inserts. Nucleic Acids Res. 1982 Oct 11;10(19):5765–5772. doi: 10.1093/nar/10.19.5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. J. DNA sequence analysis by primed synthesis. Methods Enzymol. 1980;65(1):560–580. doi: 10.1016/s0076-6879(80)65060-5. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stein J. P., Catterall J. F., Kristo P., Means A. R., O'Malley B. W. Ovomucoid intervening sequences specify functional domains and generate protein polymorphism. Cell. 1980 Oct;21(3):681–687. doi: 10.1016/0092-8674(80)90431-6. [DOI] [PubMed] [Google Scholar]
- Yu-Lee L. Y., Rosen J. M. The rat casein multigene family. I. Fine structure of the gamma-casein gene. J Biol Chem. 1983 Sep 10;258(17):10794–10804. [PubMed] [Google Scholar]
- Zamierowski M. M., Ebner K. E. A radioimmunoassay for mouse alpha-lactalbumin. J Immunol Methods. 1980;36(3-4):211–220. doi: 10.1016/0022-1759(80)90126-x. [DOI] [PubMed] [Google Scholar]



