The Abelson interactor (Abi) has a conserved role in Arp2/3-dependent actin polymerization, regulating WASP and WAVE. In this study, the function of Abi was analyzed in the context of the developing fly visual system, and the steps in the molecular regulation of WAVE activity by its regulatory complex in vivo were identified.
Abstract
A tight spatial-temporal coordination of F-actin dynamics is crucial for a large variety of cellular processes that shape cells. The Abelson interactor (Abi) has a conserved role in Arp2/3-dependent actin polymerization, regulating Wiskott-Aldrich syndrome protein (WASP) and WASP family verprolin-homologous protein (WAVE). In this paper, we report that Abi exerts nonautonomous control of photoreceptor axon targeting in the Drosophila visual system through WAVE. In abi mutants, WAVE is unstable but restored by reexpression of Abi, confirming that Abi controls the integrity of the WAVE complex in vivo. Remarkably, expression of a membrane-tethered WAVE protein rescues the axonal projection defects of abi mutants in the absence of the other subunits of the WAVE complex, whereas cytoplasmic WAVE only slightly affects the abi mutant phenotype. Thus complex formation not only stabilizes WAVE, but also provides further membrane-recruiting signals, resulting in an activation of WAVE.
INTRODUCTION
Growth cone motility and axon guidance require a number of signaling pathways to transmit extracellular signals to intracellular signal transduction cascades converging on a dynamic cytoskeleton (Pak et al., 2008; Lowery and Van Vactor, 2009; O'Donnell et al., 2009). The dynamics of the actin cytoskeleton are controlled by a number of conserved proteins, such as formins, Spire-like proteins, and the Arp2/3 complex (Kerkhoff, 2006; Pollard, 2007). The Arp2/3 complex represents an efficient actin nucleation machine activated by members of the Wiskott-Aldrich syndrome protein/WASP family verprolin-homologous protein (WASP/WAVE) protein family (Goley and Welch, 2006). Given the low intrinsic nucleating activity of the Arp2/3 complex, WASP and WAVE proteins play a central role as nucleation-promoting factors (NPF) to drive actin polymerization in space and time (Stradal and Scita, 2006; Insall and Machesky, 2009; Pollitt and Insall, 2009). Despite their similar biochemical properties, WASP and WAVE proteins fulfill distinct cellular functions. WAVE function is essential for proper formation and protrusion of lamellipodia, whereas WASP is primarily required for membrane internalization through endocytosis and vesicle movement. Given the essential function for membrane protrusions in nonneuronal cells, it has been assumed that WAVE would play a similar role in protruding growth cones. However, the role of Arp2/3-dependent actin polymerization in neurons is still controversial. In hippocampal neurons, a dominant-negative approach shows that the Arp2/3 complex is dispensable for lamellipodia formation but acts as a negative regulator of growth cone translocation (Strasser et al., 2004). In contrast, knockdown of the Arp2/3 complex in hippocampal neurons, as well as in neuroblastoma cells, impairs lamellipodia and filopodia formation in growth cones (Korobova and Svitkina, 2008). Recent studies using primary Drosophila mutant neurons confirmed an essential role of the Arp2/3 complex in regulating growth cone motility (Goncalves-Pimentel et al., 2011). Likewise, loss-of-function studies in different model systems document an important role of WAVE proteins in regulating Arp2/3-dependent actin polymerization during nervous system development. In mice, all three WAVE isoforms differentially localize at the leading edges of growth cones and knockout of both WAVE1 and WAVE2 causes several neuroanatomical defects (Dahl et al., 2003; Nozumi et al., 2003; Soderling et al., 2003; Yan et al., 2003; Kim et al., 2006). In particular, disruption of the brain-enriched WAVE1 isoform results in abnormal growth cone morphology, decreased neurite outgrowth, and a reduced number of dendritic spines, which are important structures for the formation of excitatory synaptic connections (Dahl et al., 2003; Soderling et al., 2003, 2007; Kim et al., 2006;). Defects in nervous system development were also observed for the knockdown of wve-1 in Caenorhabditis elegans causing defects in both axon outgrowth and guidance (Shakir et al., 2008). In Drosophila, WAVE acts as the main Arp2/3 regulator during axonal growth, whereas WASP is required for cell fate decisions during sensory organ development (Ben-Yaacov et al., 2001; Zallen et al., 2002). Mutants lacking wave show severe CNS defects, including ectopic midline crossing and motor nerve branching (Zallen et al., 2002; Schenck et al., 2003). Thus the requirement for WAVE proteins in the nervous system is conserved.
Over the past few years, insight into molecular regulation of WAVE/WASP proteins has been achieved. WASP and WAVE proteins are regulated by similar molecular principles (Derivery and Gautreau, 2010; Padrick and Rosen, 2010). Both, WASP and WAVE exist in multi-protein complexes, are primarily inactive, and become activated by different signals. WASP proteins are predominantly found in an autoinhibited conformation within a stable complex with the WASP-interacting protein (Anton et al., 2007; Ramesh and Geha, 2009). This autoinhibition is released by the cooperative binding of the small GTPase Cdc42, the phospholipid phosphatidylinositol 4,5-biphosphate, and the F-BAR protein Cip4/Toca-1 (Ho et al., 2004). Pure WAVE proteins are basally active but in vivo, trans-inhibited in a pentameric protein complex with the Abelson interactor (Abi), Nap1/Kette, hematopoietic stem progenitor cell 300 (HSPC300), and, specifically, Rac-1 associated protein 1 (Sra-1; Eden et al., 2002; Gautreau et al., 2004; Derivery et al., 2009; Lebensohn and Kirschner, 2009).
WAVE complex stability depends on its integrity and coinciding signals, such as activated Rac, phosphorylation, and binding to phospholipids, lead to the full activation of WAVE in vitro (Kunda et al., 2003; Lebensohn and Kirschner, 2009). High-resolution crystal structure of a recombinant WAVE1 complex recently confirmed that the catalytic verprolin-cofilin-acidic (VCA) motif of WAVE is sequestered by a combination of intramolecular and intermolecular contacts within the WAVE complex (Chen et al., 2010). The crystal structure also provides a plausible mechanism for how Rac1 and phospholipids could cooperatively recruit the complex to membranes and how they might release the trans-inhibition of WAVE. Contrary to earlier, simplified models, the Rac1 effector Sra-1 is not a peripheral subunit but forms a heterodimer with Nap1/Kette, which creates an oppositely charged platform for the WAVE-HSPC300-Abi trimer. The catalytic VCA domain of WAVE is sequestered by Sra-1. On Rac1 binding to Sra-1, the VCA domain is released, and WAVE becomes active. This model also implies that acidic phospholipids cooperate with Rac1 to recruit the complex at the membrane by binding to the positively charged faces of the Sra-1/Nap1/Kette platform and the polybasic region of WAVE.
Despite this significant progress in our understanding of the WAVE complex biochemistry, less is known about how processes driven by actin dynamics are coordinated by WAVE and its regulatory complex, including Abi in vivo. Genetic studies have demonstrated that Abi family proteins orchestrate cell migration, cell adhesion, and cell differentiation during neuronal and cardiovascular development (Ring et al., 2011; Grove et al., 2004; Proepper et al., 2007; Pollitt and Insall, 2008; Stephan et al., 2008; Lin et al., 2009; Schmidt et al., 2009; Dubielecka et al., 2011). These differential functions are reflected by the modular domain structure of the Abi protein. Abi represents a multi-domain adaptor protein that is not only an integral component of the WAVE complex but also directly interacts with WASP, Diaphanous (Dia1), and the nonreceptor tyrosine kinase Abl (Dai and Pendergast, 1995; Shi et al., 1995; Juang and Hoffmann, 1999; Bogdan et al., 2005; Innocenti et al., 2005; Ryu et al., 2009; Liebau et al., 2011). Abi contains an amino-terminal, WAVE-binding domain (WAB), which is followed by the Kette/NAP1-interacting homeodomain homologous region (HHR), and a carboxy-terminal SRC homology 3 domain (SH3), which directly binds and activates Abl and WASP (Bogdan et al., 2005; Stephan et al., 2008; Ryu et al., 2009). Unlike vertebrates, Drosophila has only a single gene each for wave, wasp, and abi, and in vivo analyses are not complicated by redundancy (Ben-Yaacov et al., 2001; Zallen et al., 2002; Lin et al., 2009). Loss of both maternal and zygotic abi functions severely disrupts CNS development, resulting in embryonic lethality (Lin et al., 2009).
In this study, we analyzed the zygotic function of abi in the context of the developing fly visual system, and dissected the molecular regulation of WAVE activity by Abi in vivo. The developing Drosophila visual system has served as a good model to identify and study factors, including cytoskeletal regulators that control axonal growth and axonal targeting (Martin et al., 1995; Berger et al., 2008). The adult fly eye is a compound eye comprising ∼750 individual eye units called ommatidia (Ting and Lee, 2007). Each ommatidium harbors eight photoreceptor neurons (R-cells) that are specialized for light sensitivity (Choe and Clandinin, 2005; Ting and Lee, 2007). The so-called outer photoreceptor cells R1 to R6 terminate in the first optic ganglion, which is called the lamina. The inner photoreceptor cells (R7 and R8) in turn pass through the lamina and terminate in the next optic ganglion, the medulla (Clandinin and Zipursky, 2002; Tayler and Garrity, 2003; Yamaguchi et al., 2006; Morante and Desplan, 2008). The exact termination of the growth cones of the photoreceptor axons at the lamina also requires the presence of a set of specialized glial cells and neurons highlighting the importance of cellular interactions for proper axonal targeting (Poeck et al., 2001; Dearborn and Kunes, 2004; Chotard et al., 2005; Chotard and Salecker, 2007).
Genetic analysis and cell-specific rescue experiments demonstrate that Abi and WAVE, but not WASP, are required in neurons of the target area to regulate non-cell-autonomous photoreceptor axon targeting. We show that, in Drosophila eye, Abi is mostly found in 400- to 500-kDa protein complexes, cofractionating with WAVE and Kette, and that Abi is required for the integrity of the WAVE complex in vivo. Structure–function analysis shows that WAVE activity requires interaction with Abi but also membrane interactions mediated by the phosphatidylinositol 3,4,5-trisphosphate–binding domain. Moreover, we were able to suppress the abi mutant phenotype by reexpression of a membrane-tethered WAVE protein in the absence of the WAVE complex. Furthermore, we show that the rescue ability of membrane-recruited WAVE is due to an activation of Arp2/3 in the absence of Abi. This demonstrates that membrane recruitment of WAVE is sufficient for its activation in vivo.
RESULTS
Neuronal abi controls photoreceptor axon targeting
To analyze abi function during development, we generated an abi mutant by imprecise excision of the EY20423 transposon located in the abi gene (Figure 1A). In the allele abiΔ20, most of the protein-coding region, including the start codon, is removed, resulting in a complete loss of Abi protein expression (Figure 1B) but not affecting the expression of the neighboring twinfilin gene (Figure 1C). abiΔ20 mutants show early pupal lethality in homozygosity, as previously reported for an abi mutation generated by gene targeting (Lin et al., 2009). Ubiquitous reexpression (daGal4) of Abi in the mutant background fully rescues the lethality of the abiΔ20 allele (Supplemental Table S1), confirming this mutant is an abi loss-of-function mutation.
FIGURE 1:
abi is required for photoreceptor targeting. (A) Schematic overview of the abi locus with the neighboring twf gene. The position of the transposon used to generate the abi mutant is indicated. A dashed line indicates the extension of the genomic deletion in the abi mutant. (B and C) Western blot analysis of larval brain extracts. (B) The abiΔ20 mutation results in a loss of Abi protein, whereas (C) the expression of the adjacent Twf protein is unaffected. (D–P) Analysis of photoreceptor projection pattern in the fly larval visual system in abi mutants. (D) Schematic overview of the projection pattern of photoreceptor axons (adapted from Tayler and Garrity, 2003). (E) In wild type, Abi (gray) is expressed in photoreceptor axons, as well as in the lamina (la, arrows) and medulla (me, asterisk). (F–H) R-cell axons (green, R1–R8; antibody 24B10) show a stereotyped projection to the lamina and medulla with R2–R5 (red, marked by rough-τlacZ; α-β-galactosidase) terminating in the lamina (note a few axons overshooting the lamina, asterisk in G). (J–M) The loss of abi leads to a highly abnormal targeting of R-cell axons with axonal bundling and uneven appearance of the lamina with gaps and clumps (K). The vast majority of the R2–R5 axons fail to terminate in the lamina and terminate in deeper layer medulla (L). (I and N) Rescue of the abi-dependent projection defects of R-cell axons. Neural resupply (N) of Abi (elavGal4 > Abi) rescues abi-mediated targeting abnormalities of R-cell axon whereas (I) glial reexpression of Abi does not (repoGal4 > Abi). (O–P) Quantification of the photoreceptor targeting defects. (O) The number of axonal bundles in the medulla per optic lobe was quantified for the indicated genotypes. abi, elav > Abi: neural reexpression of Abi in abi mutant animals. abi, repo > Abi: glial reexpression of Abi in abi mutant animals. Error bars represent SEM. ***, p < 0.001 (analysis of variance [ANOVA]); n.s., not significant. (P) Severity of R2–R5 overshooting defects in the indicated genotypes. Animals used for the quantification of axonal bundles were grouped according to the numbers of R2–R5 axons overshooting the lamina. The numbers represent percent of optic lobes with R2–R5 axons in the medulla. abi, elav > Abi: neural reexpression of Abi in abi mutant animals.
Since the loss of the Abi interaction partner Kette results in axon targeting defects in the larval visual system (Hummel et al., 2000), we determined the projection pattern of retinal axons in abiΔ20 mutants. To monitor the projection pattern of R-cells in wild type and abi mutants, we visualized the axons of all photoreceptors R1–R8 and, concomitantly, the axons of the outer R-cell axons R2–R5, which terminate in the lamina (Figure 1D).
In wild type, Abi is expressed in R-cell axons (Figure 1E, arrowheads), as well as in the target area (Figure 1E, asterisk). The axons of all R-cells target in a highly stereotyped manner to the lamina and medulla (Figure 1F), whereas the R-cells R2–R5 project to the lamina with only few axons overshooting (Figure 1, G, asterisk, and P). In abi mutants, axonal targeting is strongly affected (Figure 1, J–M). R-cell axons terminate in the brain, but the projection pattern appears highly irregular. Individual axons fasciculate and form abnormal bundles and gaps in the lamina (Figure 1K, arrows). In the medulla, axons do not appear as individual axons anymore, but form bundles (7.7 axonal bundles per optic lobe vs. 0.1 in wild type; see Figure 1O). Furthermore, large numbers of R2–R5 axons overshoot the lamina and misproject into the medulla (in 94% of the brains >15 axons vs. 0% of the brains in wild type; Figure 1, L and P). The differentiation of abi mutant R-cells appears normal, as the numbers of different subsets of R-cell nuclei in abi mutant eye imaginal disks are indistinguishable from wild type (Supplemental Figure S1). We conclude that the abnormal projection pattern and lamina overshooting is not due to abnormal development of R-cells.
It has been reported that the correct projection pattern of R-cell axons not only depends on the R-cells themselves and neurons in the target area, but also on the presence of specialized glia cells in the lamina (Poeck et al., 2001; Ting and Lee, 2007). Therefore, the abi-dependent targeting defects might either be due to compromised neuronal or glial abi function. To differentiate between these possibilities we conducted differential rescue experiments and quantified the number of axonal bundles in medulla, as well as the overshooting of R2–R5 axons. Expression of Abi in all glia cells (repoGal4) in abi mutants does not modulate the targeting defects, whereas Abi expression in all neurons (elavGal4) completely restores the axonal projection pattern (Figure 1, I, N, O, and P). Taken together, the mutant analysis and the rescue experiments show that abi is required in neurons to ensure proper R-cell projections.
abi function is required in the target area
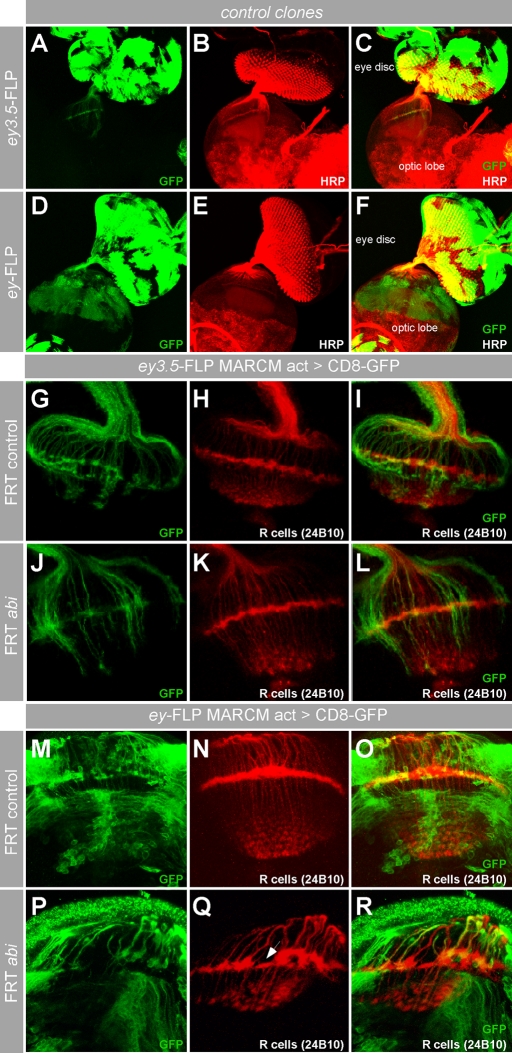
Arp2/3-mediated actin polymerization has an essential function for membrane protrusions in nonneuronal cells (Goley and Welch, 2006). Thus Abi might play a similar cell-autonomous role in protruding growth cones of photoreceptor axons. We also assumed that Abi plays a cell-autonomous role in axonal outgrowth and axon targeting of R-cells. To verify this assumption, we performed a mosaic analysis with a repressible cell marker (MARCM) analysis (Lee and Luo, 2001) that allowed us to induce and simultaneously visualize abi mutant cell clones in the visual system (Figure 2). We generated abi mutant photoreceptor cells using two different eyeless (ey) promotor-driven flippases (FLP), called ey3.5-FLP (Bazigou et al., 2007) and ey-FLP (Newsome et al., 2000). ey3.5-FLP is an eye disk–specific FLP excluding the brain target area (Figure 2, A–C), whereas ey-FLP drives FLP expression in the eye disk and in the optic lobe (Figure 2, D–F). Interestingly, the projections of ey3.5-FLP–induced abi mutant R-cells into a wild-type target were largely indistinguishable from control clones (Figure 2, J–L, vs. 2, G–I). This suggests that abi is not required in the projecting R-cell axons, but rather acts in neurons in the target area. To test this, we used the ey-FLP driver to induce large abi mutant cell clones in the target area, as well as in the eye disk (Figure 2, M–R). Compared with control clones (Figure 2, M–O), strong axonal targeting defects can be observed in brains within large abi mutant clones (Figure 2, P–R, arrow in Q).
FIGURE 2:
abi function is required in target area neurons. (A–F) Comparison of the spatial activity of different flippase sources used for clonal analysis. (A–C) Eye disk–specific flippase activity (ey3.5-FLP) induces dominantly CD8-GFP–labeled cell clones (green) only in the eye imaginal disk including R-cells and their axons. (D–F) Genetic mosaics induced by ey-FLP leads to CD8-GFP expression in the eye disk as well as in the brain target area. Anti-HRP (red) was used as general neuronal marker to visualize brain structures. (G–R) MARCM analysis of abi in the developing visual system. R-cell axons are visualized with the antibody 24B10 (red); cell clones are dominantly marked by CD8-GFP expression (green). (G–L) Analysis of abi mutant cell clones generated only in the eye disk by ey3.5-FLP. As in control clones (G–I), axons of abi mutant R-cells show a normal targeting (J–L). (M–R) Analysis of abi mutant cell clones generated in the eye disk and the brain target area by ey-FLP. In contrast to control clones (M–O), mutant cell clones lacking abi function (P–R) are associated with severe lamina targeting defects (arrow in Q).
Rescue experiments further support this nonautonomous function of Abi. Reexpression of Abi in mutant animals only in the eye disk, but including the R-cells (using the eye disk–specific GMRGal4 driver), did not rescue axonal targeting defects (Figure S2, A–C). The nonautonomous requirement of Abi was further confirmed by analyzing mutant clones induced by the heat shock–driven expression of FLP (Figure S2, D–F). These data show that neurons in the target area require abi function to regulate targeting of R-cell axons.
An axonal scaffold in the target area is disorganized in abi mutants
Analysis of the organization of the optic lobe revealed that certain neuron–neuron and neuron–glia interactions mediate the precise formation of the R-cell projection pattern (Dearborn and Kunes, 2004; Yoshida et al., 2005; Sugie et al., 2010). For example, optic lobe neurons provide an axonal scaffold controlling the correct migration of glia cells, which is essential for retinal axon targeting. R-cell axons misproject if the glia cells fail to migrate due to the absence or malformation of the axonal tracts of optic lobe neurons (Dearborn and Kunes, 2004; Yoshida et al., 2005).
To test whether the loss of abi influences the organization of the target area in the optic lobe, we visualized the axonal scaffold mediating correct glia migration, using cytoplasmic expression of β-galactosidase from a lacZ gene under the control of a wingless (wg) promoter (Dearborn and Kunes, 2004; Figure 3). In wild type, several wg-lacZ–labeled fascicles emerge from each of the dorsal and ventral Wg domains and extend to the lamina region (Figure 3, A, C, E, and G). By contrast, in abi mutant brains, scaffold axons project aberrantly and some glia cells line these abnormal trajectories and accumulate in abnormal destinations (Figure 3, B, D, F, and H). However, reexpression of Abi in abi mutants in the wg pattern does not rescue the photoreceptor projection defects (data not shown), suggesting an additional requirement of Abi in additional neurons of the target area. This indicates that the organization of the target area, including the establishment of an axon scaffold, is affected by the loss of abi.
FIGURE 3:
abi controls the organization of an axonal scaffold in the target area. (A–H) Analysis of scaffold axon fascicles in the brain target area. (A, C, E, and G) In wild-type scaffold, axon fascicles grow out from two Wingless (Wg)-expressing domains (green, marked by wg-lacZ; visualized with α-β-galactosidase). The axon fascicles extend to neuropile regions marked by intense horseradish peroxidase staining (red). Glia cell nuclei (blue, anti-Repo) are located around the neuropile regions. (B, D, F, and H) In abi mutant brains the organization of the brain target area in the visual system is disturbed. The Wg-expressing domains appear broadened and irregular (arrows in B). The trajectories of wg-lacZ–labeled axon fascicles are abnormal. The neuropile regions and the position of the surrounding glia cell nuclei also appear disturbed (compare D vs. C and F vs. E).
Abi acts through WAVE during R-cell axon targeting
Abi represents a multi-domain adaptor protein that is not only able to regulate WAVE but also WASP activity (Bogdan et al., 2005; Innocenti et al., 2005; Liebau et al., 2011). Distinct interaction domains within the Abi protein mediate the functional relationship between Abi and both Arp2/3 activators (Figure 4A). Abi binds through its N-terminal WAB domain to WAVE, whereas the C-terminal SH3 directly binds WASP (Bogdan et al., 2005; Innocenti et al., 2005). In the N-terminus next to the WAB domain, Abi contains an HHR that is necessary for Kette binding (Ryu et al., 2009). To analyze the domain requirement of Abi function in photoreceptor axon targeting, we performed a structure–function analysis using neuronal reexpression of distinct Abi deletion transgenes in the abi mutant background. Rescue activity of each transgene was quantified by measuring the numbers of abnormal axon bundles in the medulla and overshooting R2–R5 axons observed in abi mutants (Figure 4, B–G). Reexpression of wild-type Abi completely rescues the abi mutant phenotype (Figure 4B, quantification in Figure 4, F and G). Removal of the C-terminal SH3 domain (AbiΔC) mediating binding and activation of WASP does not affect the rescue activity of the truncated Abi protein (Figure 4, C, F, and G). In contrast, an Abi variant unable to bind to WAVE (AbiΔN) (Bogdan et al., 2005) does not exhibit any rescue activity, demonstrating that the WAB is essential for abi function (Figure 4, D, F, and G). Interestingly, expression of Abi-WAB-HHR (a truncated Abi protein containing only the WAVE- and the Kette-interacting domain but lacking all proline-rich regions, including the SH3 domain) rescues the phenotypic traits observed in abi mutants (Figure 4, E–G). The number of axonal bundles in the medulla is comparable to wild-type levels and to levels upon expression of a full-length Abi protein (0.5 vs. 0.1 and 0.1, respectively). The overshooting of R2–R5 axons is clearly rescued, but to a slightly weaker extend compared with the full-length Abi (11% of optic lobes with >15 and 32% with 10–15 R2–R5 axons overshooting vs. 0 and 3%, respectively). Since ubiquitous reexpression of Abi-WAB-HHR (using the daGal4 driver line) also completely rescues lethality of abi mutants (data not shown), the first N-terminal 179 amino acids most likely constitute the minimal region of Abi needed for essential functions of Abi during development.
FIGURE 4:
The N-terminus of Abi is required during photoreceptor targeting. (A) Schematic overview of the domain structure of Abi and Abi variants used in the analysis. Numbers indicate the amino acid position of an indicated domain. WAB (red); HHR (gray) required for Kette binding; P (gray): proline-rich regions; SH3 (magenta) required for WASP interaction. rescue: summary of ability of Abi variants to rescue R-cell targeting defect upon neural reexpression (+ indicates rescue; − indicates no rescue). (B–E′′) Representative images of projection patterns of all R-cell axons and R2–R5 axons of the indicated genotypes. R-cell axons (R1–R8, red) are visualized by anti-24B10, and R2–R5 axons (green) are marked by the ro-τlacZ reporter (α-β-galactosidase). Abi variants were expressed in all neurons using the elavGal4 driver line. Whereas full-length Abi and a C-terminal truncated Abi protein (AbiΔC) (B–C′′) are able to rescue the abi-dependent projection defects, an Abi protein lacking the N-terminal WAB domain (AbiΔN) fails to rescue the R-cell targeting phenotype (D–D′′). Expression of the Abi N-terminus alone (Abi-WAB-HHR) also restores the projection defects of R-cell axons. *RFP expression in the Bolwig's nerve marks the 68E landing site of the ΦC31 integrase system for generating site-specific transgenic fly lines (Bischof et al., 2007). (F–G) Quantification of the rescue experiments using Abi variants. (F) The number of axonal bundles in the medulla per optic lobe was quantified for the indicated Abi variants upon neural reexpression in the abi mutant background. Abi: full-length Abi; AbiΔC: Abi variant lacking C-terminal SH3 domain; AbiΔN: Abi variant lacking N-terminal WAB domain; Abi-WAB-HHR: Abi variant consisting only of N-terminal WAB- and HHR domain. Error bars represent SEM and data sets of rescue experiments refer to the abi mutant. ***, p < 0.001 (ANOVA); n.s., not significant. (G) Severity of R2–R5 overshooting defects in the indicated genotypes. Numbers represent percent of optic lobes with R2–R5 axons in the medulla.
wave and arp2/3 but not wasp function are required for R-cell axon targeting
Zygotic wave mutants mostly die at early larval stages, with some rare wandering third instar larvae escapers (Zallen et al., 2002). Thus we next analyzed those wave escapers to test the function of WAVE during targeting of R-cell axons. Loss of wave results in severe photoreceptor targeting defects, similar to those seen in abi mutants (Figure 5B). In contrast, wasp mutant brains show no axonal targeting phenotype in the visual system, confirming that the Abi-WASP interaction is not essential in photoreceptor targeting (Figures 4C and 5C,). Similarly, reexpression of WAVE in neurons, but not in glia cells, completely restores a wild-type axonal projection in wave-deficient brains (Figure 5, D and E). Further rescue experiments with distinct truncated WAVE variants revealed the domain requirements of WAVE activity in vivo. Expression of WAVE lacking the Abi-interacting domain (WHD; Echarri et al., 2004) does not restore the targeting deficits in wave mutants (WAVEΔN, Figure 5, F and H). WAVE variants lacking the basic region mediating membrane lipid binding (WAVEΔB) or both the basic and the proline-rich regions (WAVEΔB+ΔP), exhibit strongly reduced rescue activities (Figure 5, G and H). On the other hand, deletion of the Arp2/3-activating VCA domain completely abolishes WAVE activity, suggesting that WAVE-induced, Arp2/3-mediated actin polymerization is required in photoreceptor targeting. We consistently found similar targeting defects in mutant cell clones for the gene sop2, which encodes one of the Arp2/3 regulatory subunits p40/Arpc1(Figure S3).
FIGURE 5:
Abi acts through WAVE during photoreceptor axon targeting. (A–G) Representative images of R-cell projection patterns of the indicated genotypes. R-cell axons (green) are visualized with 24B10. (A–C) Compared with wild type, the loss of wave, but not wasp, leads to an axonal targeting phenotype similar to abi mutants with axonal bundling and uneven appearance of the lamina with gaps and clumps. (D–G) Rescue of the wave-dependent projection defect. Resupply of full-length Wave in glial cells (repoGal4 > WAVE) does not improve the R-cell targeting in the wave mutant background (D). Neural reexpression of full-length Wave (elavGal4 > WAVE) in wave mutants restores a wild-type appearance of the projection pattern of R-cell axons (E). In contrast, a Wave variant lacking the N-terminal Abi-binding region (WAVEΔN) is unable to rescue the targeting defects neurally expressed in wave mutants (F). Expression of a Wave protein missing the basic region (WAVEΔB) mediating membrane association only partially restores the wave-dependent targeting defects upon neural expression (G). (H) Schematic overview of the domain structure of WAVE and WAVE variants used in the rescue experiments. WHD (green): WAVE homology domain mediating Abi binding; B (gray): basic region required for lipid binding; PolyPro (blue): proline-rich regions; VCA (red) region required for Arp2/3 binding and activation. rescue: summary of ability of WAVE variants to rescue R-cell targeting defect upon neural reexpression (+ indicates full rescue; − indicates no rescue; −/+ indicates partial rescue).
We next analyzed whether WAVE has a similar nonautonomous function in regulating photoreceptor targeting. And indeed, we found that wave function is also required in the target area neurons and not in R-cells. Eye disk–specific reexpression of WAVE in mutant animals (using the eye disk–specific GMRGal4 driver) does not rescue axonal targeting defects (Figure S4A). Conversely, eye disk–specific suppression of wave function by RNA interference (RNAi) does not affect axonal targeting (Figure S4B), whereas pan-neural RNAi knockdown in the elav or scabrous (sca) pattern results in axonal targeting defects (Figure S4, C and D). Clonal analysis confirmed a nonautonomous function of WAVE in regulating photoreceptor axon targeting. ey-FLP–induced wave mutant cell clones in the target area, but not ey3.5-FLP–induced mutant R-cells, show strong axonal targeting defects (Figure S5, A–F). The nonautonomous requirement of WAVE was further shown by analyzing mutant clones induced by the heat shock–driven expression of FLP (Figure S5, G–L). Thus we propose that Abi acts through WAVE to regulate Arp2/3-mediated actin polymerization in the target area neurons.
Integrity of the WAVE complex in vivo
The dependence of proper R-cell axon targeting on the interaction of Abi and WAVE and the independence from wasp function suggests that WAVE is the major activator of Arp2/3 during photoreceptor targeting. Thus R-cell axon targeting seems to differ in this respect from Drosophila myoblast fusion and wing epithelium polarization, where both wave and wasp functions are required (Fricke et al., 2009; Gildor et al., 2009). The distribution of protein complexes containing Abi, WASP, and WAVE might be different in the nervous system compared with epithelia or muscle tissue. We have previously shown that Abi is present in wing disk epithelia in 200- to 300-kDa complexes with WASP, but also together with WAVE in complexes at 400–500 kDa (Fricke et al., 2009). Gel filtration analysis from adult heads containing mainly nervous system tissue revealed that a large fraction of Abi cofractionates with WAVE and the WAVE complex subunit Kette in 400- to 500-kDa protein complexes. A smaller fraction of Abi cofractionates with WASP in 200- to 300-kDa complexes that hardly contain WAVE and the WAVE complex member Kette (Figure 6A). This suggests that the composition of WAVE and WASP complexes might depend on the tissue or cell type, and that Abi mainly associates with WAVE in the nervous system. Consistent with previous cell culture studies Abi is required for the stability of WAVE in vivo (Kunda et al., 2003; Figure 6). In the eye disk, WAVE levels are clearly reduced in abi mutant cell clones (Figure 6, B and C). Additionally, WAVE levels in brain lysates are strongly down-regulated in abi and kette mutants, whereas WASP levels appear unaffected (Figure 6D, lanes 2 and 4; see also Figure 6E, quantifications for lanes 2 and 4). More importantly, neuronal reexpression (elavGal4) of Abi (Figure 6D, lane 5; Figure 6E, quantification for lane 5) or Kette (Figure 6D, lane 3; Figure 6E, quantification for lane 3) restores the level of endogenous WAVE, depending on the presence of the WAVE-binding domain in Abi (Figure 6, D and E, lane 6 vs. lane 1 and lane 4). Similarly, the reduced Kette and Sra-1 levels in abi mutants are restored by neuronal resupply of Abi (Figure 6, D and E). Further expression analysis suggests that increased reexpression improves complex stabilization. Fivefold overexpression of Kette in kette mutants (Figure 6D, lane 3; see also Figure 6E, quantifications for lane 5) more efficiently restores endogenous proteins above wild-type levels. It is worth mentioning at this point that we cannot fully exclude Abi also affecting WAVE protein levels due to an effect at the transcriptional or translational level, in addition to the effect on WAVE stability. Taken together, whenever Abi or other WAVE complex members, such as Kette, are missing, the stability of WAVE is compromised, resulting in similar axonal projection defects.
FIGURE 6:
Members of the WAVE complex are required for the integrity of WAVE in vivo. (A) Gel filtration profiles of WASP, WAVE, Abi, and Kette from lysates of heads of adult flies. The elution profile of proteins of known molecular weight is indicated at the bottom. Abi mainly cofractionates with WAVE and Kette (fractions 18–20). A portion of Abi cofractionates also with WASP (fractions 21–22) that hardly contain WAVE and Kette. (B–C) WAVE stability requires abi function in vivo. Cell clones lacking abi were generated in the eye imaginal disk and are marked by the absence of GFP (green, GMR-GFPMyr). Mutant cell clones (dashed lines in C) in the eye imaginal disk lacking abi function show a pronounced instability of endogenous WAVE protein (red, anti-WAVE). (D–E) Western blot analysis of larval brain extracts from wild type, kette, abi, and mutant larvae expressing indicated proteins. Quantification of protein levels (E) was done with the Image QuantTL software package (GE Healthcare, Waukesha, WI). Data were normalized with respect to tubulin levels. The loss of abi or kette leads to an instability of the members of the WAVE complex (WAVE, Abi, Kette, Sra-1) in vivo. Concomitantly, protein levels of WASP appear unchanged. Neural expression of Abi and Kette in abi and kette mutants, respectively, restores the integrity of the WAVE complex in vivo. Expression of an N-terminal truncated Abi protein does not lead to an increase of WAVE levels in the mutant background (blue in (E), quantification for lane 6 vs. lane 4). In contrast, an Abi variant lacking the WASP-binding domain (AbiΔC) elevates WAVE protein levels upon neural expression in the mutant background (blue in (E), quantification for lane 7 vs. lane 4). Please note that the used anti-Abi antibody does not recognize the AbiΔC protein variant (see Supplemental Information).
Membrane recruitment of WAVE regulates its biological activity
Previous in vitro studies suggested the WAVE complex is not only required for the stability of WAVE, but also directly or indirectly regulates WAVE activity (Innocenti et al., 2004; Steffen et al., 2004). To examine WAVE activity in the absence of the WAVE complex in vivo, we reexpressed WAVE in abi mutants and monitored R-cell axon targeting (Figure 7A). Since previous in vitro studies indicated Abi might activate WAVE by relocalizing WAVE to the leading edge, we also expressed a membrane-tethered WAVE (Myr-WAVE) in abi mutants (Figure 7B). A cytoplasmic WAVE exerts little biological activity upon neuronal expression. WAVE expression weakly rescues the abi-dependent deficits in axonal targeting (Figure 7A; quantification in Figure 7, E and F; 5.0 ± 2.0 axonal bundles in the medulla vs. 7.7 ± 2.1 in the abi mutant). A WAVE variant (WAVEΔB+ΔP) lacking the basic and the proline-rich regions (WAVEΔB+ΔP, Figure 7, E and F) also weakly restores axonal targeting to an extent similar to that of a cytosplasmic full-length WAVE protein. In contrast, expression of Myr-WAVE substantially rescues the frequency and severity of the abi mutant defects (Figure 7B; quantification in Figure 7, E and F). Membrane-recruited WAVE significantly suppresses the appearance of axonal bundles in the medulla (1.8 ± 1.8 vs. 7.7 ± 2.1 in the abi mutant; see Figure 7E). We found that 51% of abi mutant brains expressing Myr-WAVE show from zero to five R2–R5 axons overshooting the lamina compared with 0% of abi mutant brains and 68% of wild-type brains (Figure 7F). Thus we conclude membrane recruitment is important for the activity of WAVE in vivo.
FIGURE 7:
WAVE activity at the membrane in the absence of the WAVE complex (A–F) Analysis of ability of WAVE variants to rescue the abi-dependent projection defects. (A–D′′) Representative images of projection patterns of all R-cell axons and R2–R5 axons of the indicated genotypes. R-cell axons (R1–R8, red) are visualized by anti-24B10 and R2–R5 axons (green) are marked by the ro-τlacZ reporter (α-β-Galactosidase). WAVE variants were expressed in all neurons using the elavGal4 driver line. A full-length WAVE protein expressed in neurons in the abi mutant background weakly rescues the abi-dependent projection defects (A–A′′). A membrane-targeted WAVE variant expressed from an arbitrary genomic site (Myr-WAVE, B–B′′), as well as expressed from a specific site (Myr-WAVE68E, C–C′′) partially rescues the projection defects by abi. In contrast, a membrane-targeted Wave lacking the Arp2/3-activating VCA domain (Myr-WAVEΔVCA68E, D–D′′) expressed from the same genomic site fails completely to rescue the abi mutant phenotype. * RFP expression in the Bolwig's nerve marks the 68E landing site of the ΦC31 integrase system for generating site-specific transgenic fly lines (Bischof et al., 2007). (E–F) Quantification of the rescue experiments using WAVE variants in the abi mutant background. (E) The number of axonal bundles in the medulla per optic lobe was quantified for the indicated WAVE variants upon neural reexpression in the abi mutant background. WAVE: full-length WAVE; Myr-WAVE: membrane-tethered WAVE expressed from an arbitrary genomic site; Myr-WAVE68E: membrane-tethered WAVE expressed from a specific site (68E); Myr-WAVEΔVCA68E: membrane-tethered WAVE lacking the Arp2/3-activating domain expressed from a specific site (68E). Error bars represent SEM and data sets of rescue experiments were referred to the abi mutant. ***, p < 0.001; **, p < 0.01 (ANOVA); n.s., not significant. (F) Severity of R2–R5 overshooting defects in the indicated genotypes. Numbers represent percentage of optic lobes with R2–R5 axons in the medulla.
These findings prompted us to test whether membrane-tethered WAVE might promote actin polymerization. In contrast to cytoplasmic WAVE (Figure S6A), overexpression of Myr-WAVE in wing imaginal disks results in a significant elevation in the level of F-actin in hetero- and homozygous abi mutant background (Figure S6, B and E). Thus membrane recruitment results in an increased actin nucleation–promoting activity of WAVE. Conversely, the overexpression of a membrane-tethered WAVE variant lacking the VCA domain (Myr-WAVEΔVCA) does not induce actin polymerization, but results in a reduction of F-actin (Figure S6C). To finally test whether the increased rescue efficiency of the membrane-tethered WAVE corresponds to an enhanced actin-nucleating activity, we compared the rescue activities of Myr-WAVE and Myr-WAVEΔVCA in abi mutants. To ensure an equal expression rate, we integrated both transgenes into the same landing site (68E) using the ΦC31-mediated transgenesis strategy (Bischof et al., 2007). Expression of Myr-WAVE68E results in a similar rescue of axonal bundles in medulla in abi mutants as observed for Myr-WAVE (Figure 7, C, E, and F; 2.3 ± 1.6 for Myr-WAVE68E vs. 1.8 ± 1.8 for Myr-WAVE). The rescue activity of Myr-WAVE68E with respect to the overshooting of R2–R5 axons is weaker than Myr-WAVE (19% of optic lobes with from zero to five R2–R5 axons vs. 51%, respectively). However, expression of Myr-WAVEΔVCA68E fails to rescue the phenotypic defects in abi mutant brains (Figure 7, D–F). Expression of Myr-WAVEΔVCA68E in abi mutant brains does not significantly change the axonal bundling defect in the medulla compared with abi mutants (Figure 7E; 6.6 ± 1.5 vs. 7.7 ± 2.1, respectively). Furthermore, expression of Myr-WAVEΔVCA68E leads only to a very weak improvement of R2–R5 mistargeting compared with abi mutants (9% of brains with 5–10 R2–R5 axons overshooting the lamina vs. 0 and 60% of brains with >15 R2–R5 axons overshooting vs. 94%, respectively). As Myr-WAVE68E and the Myr-WAVEΔVCA68E are expressed at equal levels, we conclude that the rescue of activity of membrane-tethered WAVE is due to an activation of Arp2/3. These findings strongly indicate that WAVE activation in vivo can be achieved upon membrane targeting of WAVE in the absence of the WAVE complex.
DISCUSSION
In summary, we have shown that abi and wave functions are required for early targeting of R-cell axons but are not needed in the R-cells themselves. Our observations strongly suggest a nonautonomous role for the Arp2/3 activator WAVE and its regulator Abi in the brain target area, indicating that in their absence proper cellular communications between projecting R-cell axons and neurons in the target area might be disrupted. It is well established that WAVE and its regulatory complex are effectors of the activated GTPase Rac (Yan et al., 2003; Innocenti et al., 2004) and one might also assume a similar nonautonomous role for Rac, as for WAVE and Abi. Previous analysis of genetic mosaics in the Drosophila brain lacking rac function indeed revealed an unexpected degree of nonautonomous effects in axon guidance and branching (Ng et al., 2002).
How might Abi/WAVE control the targeting of retinal axons into the optic lobe? The formation of the photoreceptor projection pattern depends on complex bidirectional interactions between R-cell axons and different populations of glia, as well as neurons in the lamina target field (Mast et al., 2006). In wild type, incoming photoreceptor axons induce the outgrowth of scaffold axons, which in turn act as substrates for glia migration (Dearborn and Kunes, 2004). Conversely, lamina glia cells provide an essential stop signal for photoreceptor axons to terminate their outgrowth in the lamina (Poeck et al., 2001). These findings highlight the importance of the correct organization of the target area in the establishment of the R-cell projection pattern. The abnormal projections of the wg-positive scaffold axons indicate that neuronal Abi function might be required for the correct organization of the target area. The precise organization of the optic lobe by Abi could control axonal targeting directly (neuron–neuron; Sugie et al., 2010) or indirectly (neuron–glia; Dearborn and Kunes, 2004; Yoshida et al., 2005). The failure of the Abi reexpression in the wingless domain to rescue suggests that Abi/WAVE function is needed in additional neurons in the target area.
Loss-of-function studies in different model organisms clearly revealed a conserved function of Abi/WAVE in regulating axon guidance and axonal outgrowth in developing nervous systems. However, the precise role of WAVE-induced, Arp2/3-mediated actin polymerization in neuronal development is still controversial. Inhibition of Arp2/3 activity in cultured hippocampal neurons resulted in increased axon length but no significant effects on growth cone morphology (Strasser et al., 2004; Pinyol et al., 2007), whereas it has been recently reported that the knockdown of the Arp2/3 complex impairs lamellipodia and filopodia formation in growth cones of hippocampal neurons and neuroblastoma cells (Korobova and Svitkina, 2008). Recent studies using primary Drosophila mutant neurons confirmed an essential role of the Arp2/3 complex in regulating growth cone motility (Goncalves-Pimentel et al., 2011).
Analyzing early retinal axon targeting in abi mutants represents a good experimental paradigm to measure WAVE activity in vivo. The targeting process does not require wasp function, but only wave function, in contrast to other developmental processes (Fricke et al., 2009; Gildor et al., 2009). The functional rescue assay in abi mutants allows examination of the activity of WAVE and WAVE variants in the absence of other WAVE complex subunits. Membrane recruitment of WAVE in abi mutants results in a partial but clear rescue of R-cell projection defects. Several conclusions can be made based on these data. Membrane localization is sufficient to confer partial activity to WAVE without regulation by the WAVE complex. Members of the WAVE complex are not only required to control the integrity of WAVE but also provide means for the membrane recruitment of WAVE. We conclude from our rescue experiments that WAVE activated by artificial membrane targeting induces activation of the Arp2/3 complex. It would be interesting to see whether and to what extent an artificial activation of Arp2/3 will rescue the phenotypic traits associated with a loss of wave. As cytoplasmic, full-length WAVE exerts only slight rescue activity, we propose that the Abi/WAVE complex might not only control membrane relocalization but also might be required for full activation of WAVE. The finding that membrane recruitment of WAVE leads to a partial activation might also be true for mammalian neurons. It has recently been shown that artificial membrane recruitment of WAVE partially rescues axonal growth defects in rac-deficient cerebellar granule neurons (Tahirovic et al., 2010).
Taken together, recruitment of WAVE to the membrane leads to activation of the Arp2/3 complex and is an important step during its activation but not the only one. Other important signals might include a specific state of phosphorylation and interaction with activated Rac (Lebensohn and Kirschner, 2009). We propose that the analysis of Drosophila photoreceptor axon targeting in abi mutants will facilitate investigation of WAVE activity and regulation by the WAVE complex, as well as other signals independent of the WAVE complex in the context of a developmental process in vivo.
MATERIALS AND METHODS
Drosophila genetics
w1118, Df(3R)Exel8159, FRT40A scarΔ37, elavC155Gal4, tubGal4, FRT82B, FRT82B GMR-GFPMyr, FRT82B tubP-Gal80, FRT40A tubP-Gal80, repoGal4, daGal4, scaGal4, gcmGal4, GMRGal4, UAS-mCD8-GFP, hs-FLP, elavC155Gal4, UAS-mCD8-GFP, twfEP3701 (Bloomington Stock Center, Bloomington, IN), P{EPgy2}EY20423 (Drosophila Gene Disruption Project at Berkeley); wasp1, wasp3 (Ben-Yaacov et al., 2001); rough-tlacZ (Garrity et al., 1999); Δ2-3, Ki (Robertson et al., 1988); LGMRGal4 (Wernet and Desplan, 2004); lamaGal4 (Chotard et al., 2005); c855aGal4 (Hrdlicka et al., 2002); ey-FLP (Newsome et al., 2000); ey3.5-FLP (Bazigou et al., 2007); UAS-WaveRNAi (NIG-FLY), UAS-Wave (Zallen et al., 2002). The transgenes UAS-Abi, UAS-AbiΔN, UAS-AbiΔC, UAS-WaveΔN, UAS-WaveΔB, UAS-WaveMyr were generated as described previously (Bogdan et al., 2005). All crosses were performed at 25°C. See specific references and Supplemental Information for details regarding all mutant alleles and constructs described throughout this study.
Western blot analysis and immunohistochemistry
For Western blot analysis, brains from third instar wandering larvae of the different genotypes were collected and homogenized in lysis buffer (2 μl/brain; 50 mM Tris-HCl, pH 7.5, 150 mM KCl, 5 nM MgCl2, 0.25M sucrose, 0.5% Triton100 + protease inhibitor cocktail [Roche, Indianapolis, IN]). Lysates were centrifuged at 13,000 rpm for 20 min at 4°C. The lipid phase was removed, and the centrifugation step was repeated. Equal amounts of protein lysates were separated by SDS–PAGE (10%) and analyzed by Western blot. Antibodies were used as follows: rabbit α-Abi (1:1000, peptide antibody raised against the C-terminal part of Abi: (C)SHIGMHTLGRNINRN; affinity-purified, gift from A. Gautreau; recognizes strongly endogenous Abi but not AbiΔC); α-Kette (1:1000); α-Sra-1 (1:1000); α-WAVE (1:5000); mouse α-tubulin (1:400 E7; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA); and rabbit α-twinfilin (1:2000 [ Wahlstrom et al., 2001]).
Third instar wandering larvae were dissected and stained as previously described (Hummel et al., 2000). Primary antibodies were used at the following dilutions: mouse 24B10: 1:40 (α-Chaoptin); rat α-Elav: 1:10 (7E810); mouse α-Prospero: 1:10 (MR1A); mouse α-Rough: 1:100 (ro-62C2A8; Developmental Studies Hybridoma Bank); guinea pig α-Abi (1:700, this work); rabbit α-β-galactosidase: 1:1000 (Cappel); rabbit α-GFP: 1:1000 (MP Biomedicals, Solon, OH); goat α-HRP-Cy3: 1:300 (Dianova, Hamburg, Germany); goat α-HRP-Cy5 1:200 (Dianova); guinea pig α-Senseless: 1:1000 (Nolo et al., 2000); mouse anti-Seven up: 1:300 and guinea pig α-Wave: 1:5000 (Bogdan et al., 2005).
Fluorescent images were collected on a Zeiss (Jena, Germany) LSM510 confocal system. Images were processed with Adobe Photoshop (San Jose, CA).
Gel filtration
Gel filtration experiments were carried out as described previously (Fricke et al., 2009).
Supplementary Material
Acknowledgments
We thank A. Gautreau for sharing the unpublished Abi antibody. We thank M. Petrovic, R. Fricke, and T. Hummel for helpful discussions; I. Bunse for sequencing the abi mutant; all members of the Klämbt and Bogdan lab for help throughout the project; and A. Püschel for critical reading of the manuscript. This work was supported by grants to S.B. from the Deutsche Forschungsgemeinschaft and by a predoctoral fellowship of the Boehringer Ingelheim foundation to R.S.
Abbreviations used:
- Abi
Abelson interactor
- ANOVA
analysis of variance
- FLP
flippases
- GFP
green fluorescent protein
- HHR
homeodomain homologous region
- HSPC300
hematopoietic stem progenitor cell 300
- MARCM
mosaic analysis with a repressible cell marker
- NPF
nucleation-promoting factors
- R-cells
photoreceptor neurons
- RNAi
RNA interference
- SH3
SRC homology 3 domain
- VCA
verprolin-cofilin-acidic
- WAB
WAVE-binding domain
- WASP
Wiskott-Aldrich syndrome protein
- WAVE
WASP family verprolin-homologous protein
- WHD
WAVE lacking the Abi-interacting domain
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-02-0121) on September 7, 2011.
REFERENCES
- Anton IM, Jones GE, Wandosell F, Geha R, Ramesh N. WASP-interacting protein (WIP): working in polymerisation and much more. Trends Cell Biol. 2007;17:555–562. doi: 10.1016/j.tcb.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Bazigou E, Apitz H, Johansson J, Loren CE, Hirst EMA, Chen PL, Palmer RH, Salecker I. Anterograde jelly belly and Alk receptor tyrosine kinase signaling mediates retinal axon targeting in Drosophila. Cell. 2007;128:961–975. doi: 10.1016/j.cell.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Ben-Yaacov S, Le Borgne R, Abramson I, Schweisguth F, Schejter ED. Wasp, the Drosophila Wiskott-Aldrich syndrome gene homologue, is required for cell fate decisions mediated by Notch signaling. J Cell Biol. 2001;152:1–13. doi: 10.1083/jcb.152.1.1-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J, Senti KA, Senti G, Newsome TP, Asling B, Dickson BJ, Suzuki T. Systematic identification of genes that regulate neuronal wiring in the Drosophila visual system. PLoS Genet. 2008;4:e1000085. doi: 10.1371/journal.pgen.1000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof J, Maeda RK, Hediger M, Karch F, Basler K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci USA. 2007;104:3312–3317. doi: 10.1073/pnas.0611511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan S, Stephan R, Lobke C, Mertens A, Klambt C. Abi activates WASP to promote sensory organ development. Nat Cell Biol. 2005;7:977–984. doi: 10.1038/ncb1305. [DOI] [PubMed] [Google Scholar]
- Chen Z, Borek D, Padrick SB, Gomez TS, Metlagel Z, Ismail AM, Umetani J, Billadeau DD, Otwinowski Z, Rosen MK. Structure and control of the actin regulatory WAVE complex. Nature. 2010;468:533–538. doi: 10.1038/nature09623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe KM, Clandinin TR. Thinking about visual behavior; Learning about photoreceptor function. Curr Topics Dev Biol. 2005;69:187–213. doi: 10.1016/S0070-2153(05)69007-2. [DOI] [PubMed] [Google Scholar]
- Chotard C, Leung W, Salecker I. glial cells missing and gcm2 cell autonomously regulate both glial and neuronal development in the visual system of Drosophila. Neuron. 2005;48:237–251. doi: 10.1016/j.neuron.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Chotard C, Salecker I. Glial cell development and function in the Drosophila visual system. Neuron Glia Biol. 2007;3:17–25. doi: 10.1017/S1740925X07000592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clandinin TR, Zipursky SL. Making connections in the fly visual system. Neuron. 2002;35:827–841. doi: 10.1016/s0896-6273(02)00876-0. [DOI] [PubMed] [Google Scholar]
- Dahl JP, Wang-Dunlop J, Gonzales C, Goad ME, Mark RJ, Kwak SP. Characterization of the WAVE1 knock-out mouse: implications for CNS development. J Neurosci. 2003;23:3343–3352. doi: 10.1523/JNEUROSCI.23-08-03343.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z, Pendergast AM. Abi-2, a novel SH3-containing protein interacts with the c-Abl tyrosine kinase and modulates c-Abl transforming activity. Genes Dev. 1995;9:2569–2582. doi: 10.1101/gad.9.21.2569. [DOI] [PubMed] [Google Scholar]
- Dearborn R Jr, Kunes S. An axon scaffold induced by retinal axons directs glia to destinations in the Drosophila optic lobe. Development. 2004;131:2291–2303. doi: 10.1242/dev.01111. [DOI] [PubMed] [Google Scholar]
- Derivery E, Gautreau A. Generation of branched actin networks: assembly and regulation of the N-WASP and WAVE molecular machines. Bioessays. 2010;32:119–131. doi: 10.1002/bies.200900123. [DOI] [PubMed] [Google Scholar]
- Derivery E, Lombard B, Loew D, Gautreau A. The Wave complex is intrinsically inactive. Cell Motil Cytoskeleton. 2009;66:777–790. doi: 10.1002/cm.20342. [DOI] [PubMed] [Google Scholar]
- Dubielecka PM, Ladwein KI, Xiong X, Migeotte I, Chorzalska A, Anderson KV, Sawicki JA, Rottner K, Stradal TE, Kotula L. Essential role for Abi1 in embryonic survival and WAVE2 complex integrity. Proc Natl Acad Sci USA. 2011;108:7022–7027. doi: 10.1073/pnas.1016811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echarri A, Lai MJ, Robinson MR, Pendergast AM. Abl interactor 1 (Abi-1) wave-binding and SNARE domains regulate its nucleocytoplasmic shuttling, lamellipodium localization, and wave-1 levels. Mol Cell Biol. 2004;24:4979–4993. doi: 10.1128/MCB.24.11.4979-4993.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden S, Rohatgi R, Podtelejnikov AV, Mann M, Kirschner MW. Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature. 2002;418:790–793. doi: 10.1038/nature00859. [DOI] [PubMed] [Google Scholar]
- Fricke R, Gohl C, Dharmalingam E, Grevelhorster A, Zahedi B, Harden N, Kessels M, Qualmann B, Bogdan S. Drosophila Cip4/Toca-1 integrates membrane trafficking and actin dynamics through WASP and SCAR/WAVE. Curr Biol. 2009;19:1429–1437. doi: 10.1016/j.cub.2009.07.058. [DOI] [PubMed] [Google Scholar]
- Garrity PA, Lee CH, Salecker I, Robertson HC, Desai CJ, Zinn K, Zipursky SL. Retinal axon target selection in Drosophila is regulated by a receptor protein tyrosine phosphatase. Neuron. 1999;22:707–717. doi: 10.1016/s0896-6273(00)80730-8. [DOI] [PubMed] [Google Scholar]
- Gautreau A, Ho HY, Li J, Steen H, Gygi SP, Kirschner MW. Purification and architecture of the ubiquitous Wave complex. Proc Natl Acad Sci USA. 2004;101:4379–4383. doi: 10.1073/pnas.0400628101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gildor B, Massarwa R, Shilo BZ, Schejter ED. The SCAR and WASp nucleation-promoting factors act sequentially to mediate Drosophila myoblast fusion. EMBO Rep. 2009;10:1043–1050. doi: 10.1038/embor.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goley ED, Welch MD. The ARP2/3 complex: an actin nucleator comes of age. Nat Rev Mol Cell Biol. 2006;7:713–726. doi: 10.1038/nrm2026. [DOI] [PubMed] [Google Scholar]
- Goncalves-Pimentel C, Gombos R, Mihaly J, Sanchez-Soriano N, Prokop A. Dissecting regulatory networks of filopodia formation in a Drosophila growth cone model. PLoS One. 2011;6:e18340. doi: 10.1371/journal.pone.0018340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove M, et al. ABI2-deficient mice exhibit defective cell migration, aberrant dendritic spine morphogenesis, and deficits in learning and memory. Mol Cell Biol. 2004;24:10905–10922. doi: 10.1128/MCB.24.24.10905-10922.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho HY, Rohatgi R, Lebensohn AM, Le M, Li J, Gygi SP, Kirschner MW. Toca-1 mediates Cdc42-dependent actin nucleation by activating the N-WASP-WIP complex. Cell. 2004;118:203–216. doi: 10.1016/j.cell.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Hrdlicka L, Gibson M, Kiger A, Micchelli C, Schober M, Schock F, Perrimon N. Analysis of twenty-four Gal4 lines in Drosophila melanogaster. Genesis. 2002;34:51–57. doi: 10.1002/gene.10125. [DOI] [PubMed] [Google Scholar]
- Hummel T, Leifker K, Klämbt C. The Drosophila HEM-2/NAP1 homolog KETTE controls axonal pathfinding and cytoskeletal organization. Genes Dev. 2000;14:863–873. [PMC free article] [PubMed] [Google Scholar]
- Innocenti M, et al. Abi1 regulates the activity of N-WASP and WAVE in distinct actin-based processes. Nat Cell Biol. 2005;7:969–976. doi: 10.1038/ncb1304. [DOI] [PubMed] [Google Scholar]
- Innocenti M, Zucconi A, Disanza A, Frittoli E, Areces LB, Steffen A, Stradal TE, Di Fiore PP, Carlier MF, Scita G. Abi1 is essential for the formation and activation of a WAVE2 signalling complex. Nat Cell Biol. 2004;6:319–327. doi: 10.1038/ncb1105. [DOI] [PubMed] [Google Scholar]
- Insall RH, Machesky LM. Actin dynamics at the leading edge: from simple machinery to complex networks. Dev Cell. 2009;17:310–322. doi: 10.1016/j.devcel.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Juang JL, Hoffmann FM. Drosophila abelson interacting protein (dAbi) is a positive regulator of abelson tyrosine kinase activity. Oncogene. 1999;18:5138–5147. doi: 10.1038/sj.onc.1202911. [DOI] [PubMed] [Google Scholar]
- Kerkhoff E. Cellular functions of the Spir actin-nucleation factors. Trends Cell Biol. 2006;16:477–483. doi: 10.1016/j.tcb.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Kim HJ, DiBernardo AB, Sloane JA, Rasband MN, Solomon D, Kosaras B, Kwak SP, Vartanian TK. WAVE1 is required for oligodendrocyte morphogenesis and normal CNS myelination. J Neurosci. 2006;26:5849–5859. doi: 10.1523/JNEUROSCI.4921-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korobova F, Svitkina T. Arp2/3 complex is important for filopodia formation, growth cone motility, and neuritogenesis in neuronal cells. Mol Biol Cell. 2008;19:1561–1574. doi: 10.1091/mbc.E07-09-0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunda P, Craig G, Dominguez V, Baum B. Abi, Sra1, and Kette control the stability and localization of SCAR/WAVE to regulate the formation of actin-based protrusions. Curr Biol. 2003;13:1867–1875. doi: 10.1016/j.cub.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Lebensohn AM, Kirschner MW. Activation of the WAVE complex by coincident signals controls actin assembly. Mol Cell. 2009;36:512–524. doi: 10.1016/j.molcel.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 2001;24:251–254. doi: 10.1016/s0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
- Liebau S, et al. An SK3 Channel/nWASP/Abi-1 complex is involved in early neurogenesis. Plos One. 2011;6:e18148. doi: 10.1371/journal.pone.0018148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TY, Huang CH, Kao HH, Liou GG, Yeh SR, Cheng CM, Chen MH, Pan RL, Juang JL. Abi plays an opposing role to Abl in Drosophila axonogenesis and synaptogenesis. Development. 2009;136:3099–3107. doi: 10.1242/dev.033324. [DOI] [PubMed] [Google Scholar]
- Lowery LA, Van Vactor D. The trip of the tip: understanding the growth cone machinery. Nat Rev Mol Cell Biol. 2009;10:332–343. doi: 10.1038/nrm2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KA, Poeck B, Roth H, Ebens AJ, Ballard LC, Zipursky SL. Mutations disrupting neuronal connectivity in the Drosophila visual system. Neuron. 1995;14:229–240. doi: 10.1016/0896-6273(95)90281-3. [DOI] [PubMed] [Google Scholar]
- Mast JD, Prakash S, Chen PL, Clandinin TR. The mechanisms and molecules that connect photoreceptor axons to their targets in Drosophila. Seminars Cell Dev Biol. 2006;17:42–49. doi: 10.1016/j.semcdb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Morante J, Desplan C. The color-vision circuit in the medulla of Drosophila. Curr Biol. 2008;18:553–565. doi: 10.1016/j.cub.2008.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome TP, Asling B, Dickson BJ. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development. 2000;127:851–860. doi: 10.1242/dev.127.4.851. [DOI] [PubMed] [Google Scholar]
- Ng J, Nardine T, Harms M, Tzu J, Goldstein A, Sun Y, Dietzl G, Dickson BJ, Luo L. Rac GTPases control axon growth, guidance and branching. Nature. 2002;416:442–447. doi: 10.1038/416442a. [DOI] [PubMed] [Google Scholar]
- Nolo R, Abbott LA, Bellen HJ. Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell. 2000;102:349–362. doi: 10.1016/s0092-8674(00)00040-4. [DOI] [PubMed] [Google Scholar]
- Nozumi M, Nakagawa H, Miki H, Takenawa T, Miyamoto S. Differential localization of WAVE isoforms in filopodia and lamellipodia of the neuronal growth cone. J Cell Sci. 2003;116:239–246. doi: 10.1242/jcs.00233. [DOI] [PubMed] [Google Scholar]
- O'Donnell M, Chance RK, Bashaw GJ. Axon growth and guidance: receptor regulation and signal transduction. Annu Rev Neurosci. 2009;32:383–412. doi: 10.1146/annurev.neuro.051508.135614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padrick SB, Rosen MK. Physical mechanisms of signal integration by WASP family proteins. Annu Rev Biochem. 2010;79:707–735. doi: 10.1146/annurev.biochem.77.060407.135452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak CW, Flynn KC, Bamburg JR. Actin-binding proteins take the reins in growth cones. Nat Rev Neurosci. 2008;9:136–147. doi: 10.1038/nrn2236. [DOI] [PubMed] [Google Scholar]
- Pinyol R, Haeckel A, Ritter A, Qualmann B, Kessels MM. Regulation of N-WASP and the Arp2/3 complex by Abp1 controls neuronal morphology. PLoS One. 2007;2:e400. doi: 10.1371/journal.pone.0000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeck B, Fischer S, Gunning D, Zipursky SL, Salecker I. Glial cells mediate target layer selection of retinal axons in the developing visual system of Drosophila. Neuron. 2001;29:99–113. doi: 10.1016/s0896-6273(01)00183-0. [DOI] [PubMed] [Google Scholar]
- Pollard TD. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol Struct. 2007;36:451–477. doi: 10.1146/annurev.biophys.35.040405.101936. [DOI] [PubMed] [Google Scholar]
- Pollitt AY, Insall RH. Abi mutants in Dictyostelium reveal specific roles for the SCAR/WAVE complex in cytokinesis. Curr Biol. 2008;18:203–210. doi: 10.1016/j.cub.2008.01.026. [DOI] [PubMed] [Google Scholar]
- Pollitt AY, Insall RH. WASP and SCAR/WAVE proteins: the drivers of actin assembly. J Cell Sci. 2009;122:2575–2578. doi: 10.1242/jcs.023879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proepper C, Johannsen S, Liebau S, Dahl J, Vaida B, Bockmann J, Kreutz MR, Gundelfinger ED, Boeckers TM. Abelson interacting protein 1 (Abi-1) is essential for dendrite morphogenesis and synapse formation. EMBO J. 2007;26:1397–1409. doi: 10.1038/sj.emboj.7601569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh N, Geha R. Recent advances in the biology of WASP and WIP. Immunol Res. 2009;44:99–111. doi: 10.1007/s12026-008-8086-1. [DOI] [PubMed] [Google Scholar]
- Ring C, Ginsberg MH, Haling J, Pendergast AM. Abl-interactor-1 (Abi1) has a role in cardiovascular and placental development and is a binding partner of the alpha4 integrin. Proc Natl Acad Sci USA. 108:149–154. doi: 10.1073/pnas.1012316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson HM, Preston CR, Phillis RW, Johnsonschlitz DM, Benz WK, Engels WR. A stable genomic source of P element transposase in Drosophila melanogaster. Genetics. 1988;118:461–470. doi: 10.1093/genetics/118.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu JR, Echarri A, Li R, Pendergast AM. Regulation of cell-cell adhesion by Abi/Diaphanous complexes. Mol Cell Biol. 2009;29:1735–1748. doi: 10.1128/MCB.01483-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenck A, Bardoni B, Langmann C, Harden N, Mandel JL, Giangrande A. CYFIP/Sra-1 controls neuronal connectivity in Drosophila and links the Rac1 GTPase pathway to the fragile X protein. Neuron. 2003;38:887–898. doi: 10.1016/s0896-6273(03)00354-4. [DOI] [PubMed] [Google Scholar]
- Schmidt KL, Marcus-Gueret N, Adeleye A, Webber J, Baillie D, Stringham EG. The cell migration molecule UNC-53/NAV2 is linked to the ARP2/3 complex by ABI-1. Development. 2009;136:563–574. doi: 10.1242/dev.016816. [DOI] [PubMed] [Google Scholar]
- Shakir MA, Jiang K, Struckhoff EC, Demarco RS, Patel FB, Soto MC, Lundquist EA. The Arp2/3 activators WAVE and WASP have distinct genetic interactions with Rac GTPases in Caenorhabditis elegans axon guidance. Genetics. 2008;179:1957–1971. doi: 10.1534/genetics.108.088963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Alin K, Goff SP. Abl-interactor-1, a novel SH3 protein binding to the carboxy-terminal portion of the Abl protein, suppresses v-abl transforming activity. Genes Dev. 1995;9:2583–2597. doi: 10.1101/gad.9.21.2583. [DOI] [PubMed] [Google Scholar]
- Soderling SH, Guire ES, Kaech S, White J, Zhang F, Schutz K, Langeberg LK, Banker G, Raber J, Scott JD. A WAVE-1 and WRP signaling complex regulates spine density, synaptic plasticity, and memory. J Neurosci. 2007;27:355–365. doi: 10.1523/JNEUROSCI.3209-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderling SH, Langeberg LK, Soderling JA, Davee SM, Simerly R, Raber J, Scott JD. Loss of WAVE-1 causes sensorimotor retardation and reduced learning and memory in mice. Proc Natl Acad Sci USA. 2003;100:1723–1728. doi: 10.1073/pnas.0438033100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen A, Rottner K, Ehinger J, Innocenti M, Scita G, Wehland J, Stradal TE. Sra-1 and Nap1 link Rac to actin assembly driving lamellipodia formation. EMBO J. 2004;23:749–759. doi: 10.1038/sj.emboj.7600084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan R, Grevelhorster A, Wenderdel S, Klambt C, Bogdan S. Abi induces ectopic sensory organ formation by stimulating EGFR signaling. Mech Dev. 2008;125:183–195. doi: 10.1016/j.mod.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Stradal TE, Scita G. Protein complexes regulating Arp2/3-mediated actin assembly. Curr Opin Cell Biol. 2006;18:4–10. doi: 10.1016/j.ceb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Strasser GA, Rahim NA, VanderWaal KE, Gertler FB, Lanier LM. Arp2/3 is a negative regulator of growth cone translocation. Neuron. 2004;43:81–94. doi: 10.1016/j.neuron.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Sugie A, Umetsu D, Yasugi T, Fischbach KF, Tabata T. Recognition of pre- and postsynaptic neurons via nephrin/NEPH1 homologs is a basis for the formation of the Drosophila retinotopic map. Development. 2010;137:3303–3313. doi: 10.1242/dev.047332. [DOI] [PubMed] [Google Scholar]
- Tahirovic S, Hellal F, Neukirchen D, Hindges R, Garvalov BK, Flynn KC, Stradal TE, Chrostek-Grashoff A, Brakebusch C, Bradke F. Rac1 regulates neuronal polarization through the WAVE complex. J Neurosci. 2010;30:6930–6943. doi: 10.1523/JNEUROSCI.5395-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayler TD, Garrity PA. Axon targeting in the Drosophila visual system. Curr Opin Neurobiol. 2003;13:90–95. doi: 10.1016/s0959-4388(03)00004-7. [DOI] [PubMed] [Google Scholar]
- Ting CY, Lee CH. Visual circuit development in Drosophila. Curr Opin Neurobiol. 2007;17:65–72. doi: 10.1016/j.conb.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Wahlstrom G, Vartiainen M, Yamamoto L, Mattila PK, Lappalainen P, Heino TI. Twinfilin is required for actin-dependent developmental processes in Drosophila. J Cell Biol. 2001;155:787–796. doi: 10.1083/jcb.200108022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernet MF, Desplan C. Building a retinal mosaic: cell-fate decision in the fly eye. Trends Cell Biol. 2004;14:576–584. doi: 10.1016/j.tcb.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Wolf R, Heisenberg M, Desplan C. Motion vision is independent of color in Drosophila. Proc Natl Acad Sci USA. 2008;105:4910–4915. doi: 10.1073/pnas.0711484105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, et al. WAVE2 deficiency reveals distinct roles in embryogenesis and Rac-mediated actin-based motility. EMBO J. 2003;22:3602–3612. doi: 10.1093/emboj/cdg350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Soustelle L, Giangrande A, Umetsu D, Murakami S, Yasugi T, Awasaki T, Ito K, Sato M, Tabata T. DPP signaling controls development of the lamina glia required for retinal axon targeting in the visual system of Drosophila. Development. 2005;132:4587–4598. doi: 10.1242/dev.02040. [DOI] [PubMed] [Google Scholar]
- Zallen JA, Cohen Y, Hudson AM, Cooley L, Wieschaus E, Schejter ED. SCAR is a primary regulator of Arp2/3-dependent morphological events in Drosophila. J Cell Biol. 2002;156:689–701. doi: 10.1083/jcb.200109057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.