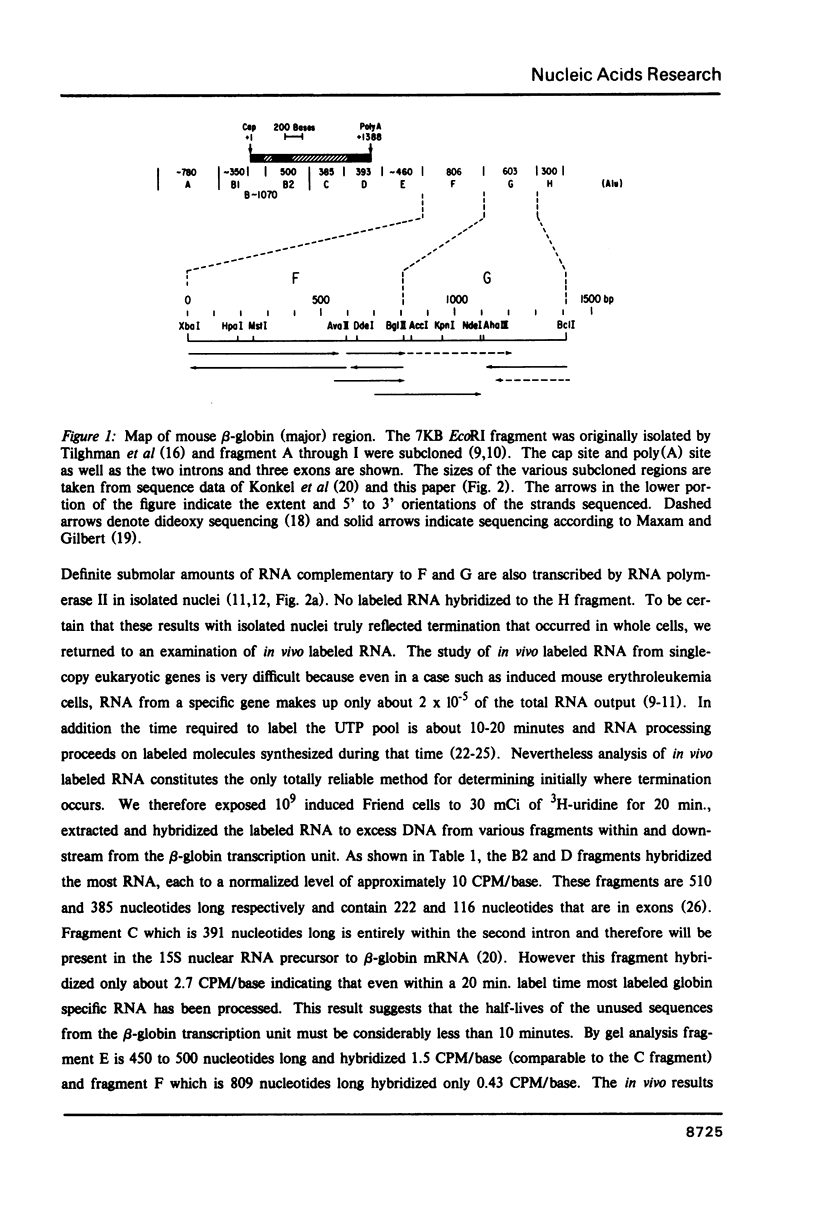
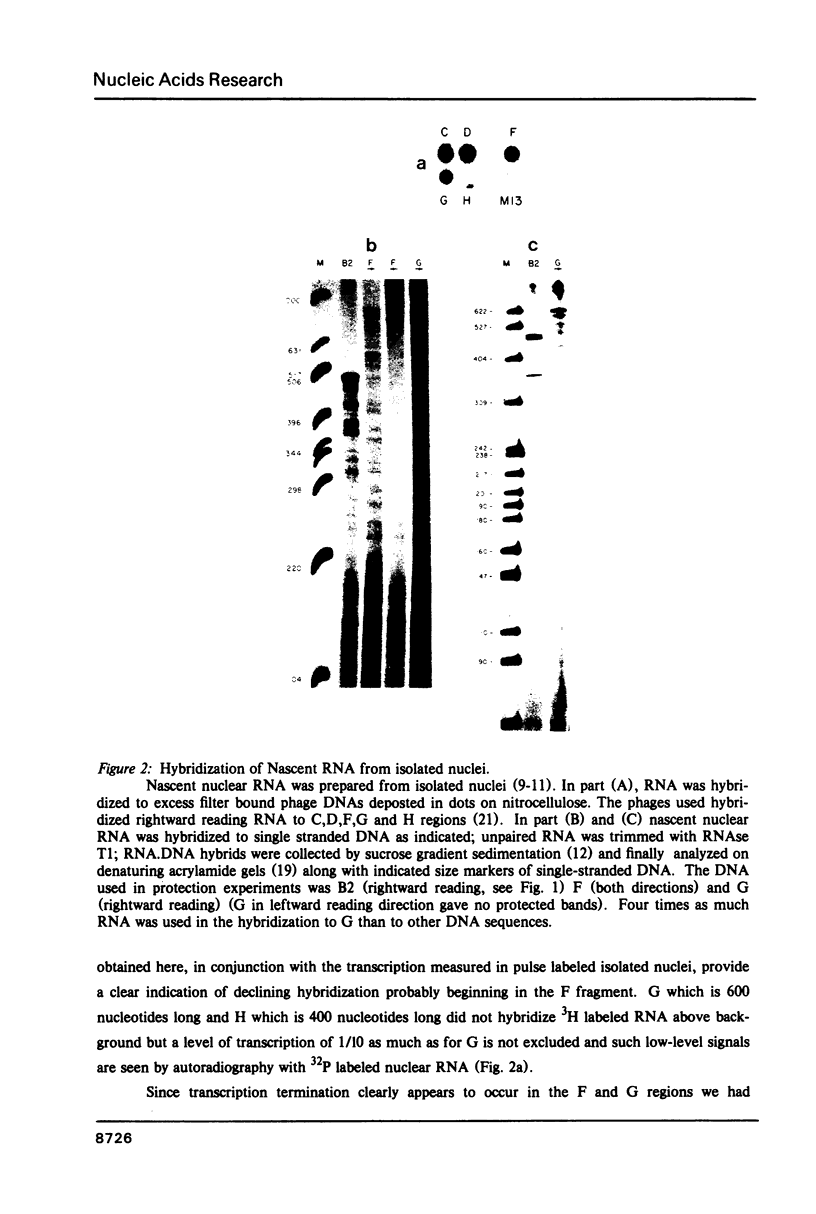
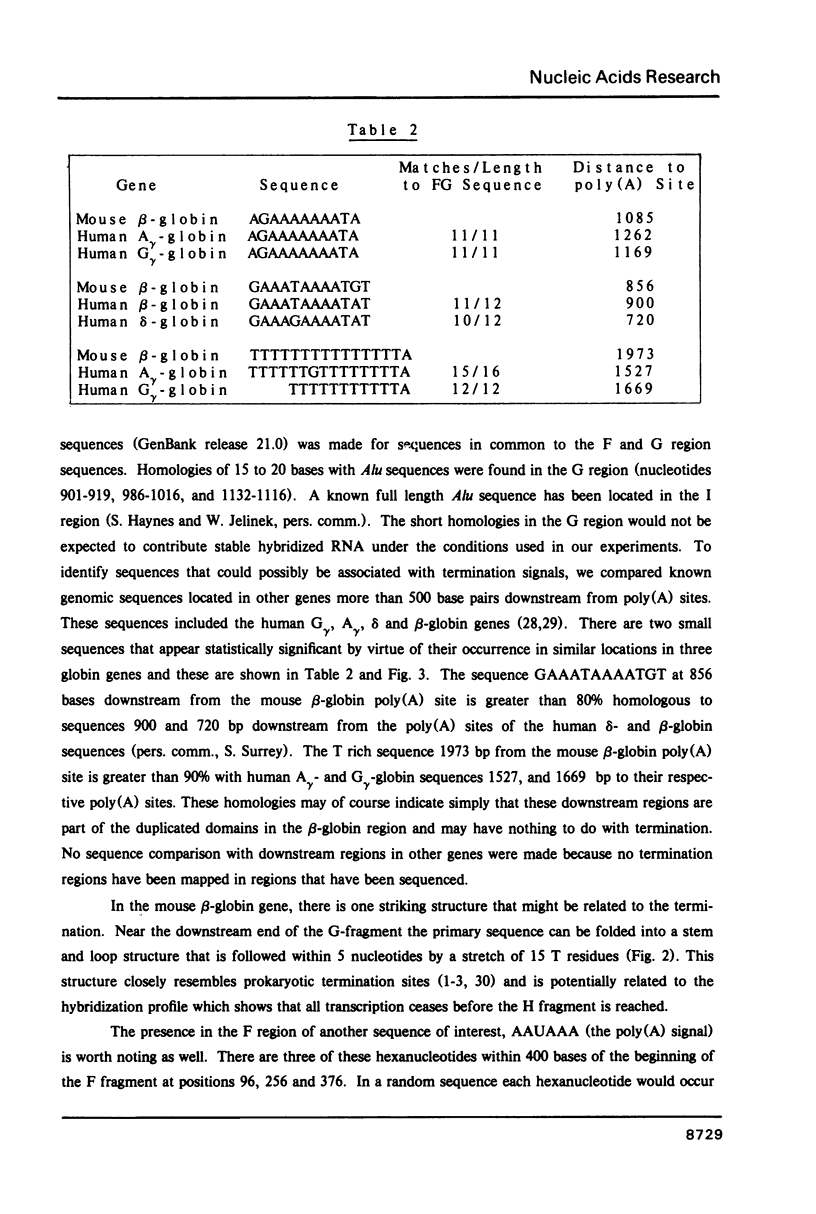
Abstract
Transcription of RNA from the beta-globin (major) gene in nuclei from induced mouse erythroleukemia cells terminates within the region between 700 to 2000 bases downstream from the poly(A) addition site, but not at particularly favored sites. In addition we present the first analysis of in vivo labeled RNA from a cell transcription unit that shows RNA termination in the same region as diagnosed by analysis of in vitro labeled RNA. The region in which termination occurs is contained in 1414 bp that were sequenced beginning 600 bases downstream from the poly(A) site. There is an increased frequency of the sequence AATAAA at the beginning and a stem and loop structure followed by a string of Ts near the end of this region.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Gottesman M. Promoter occlusion: transcription through a promoter may inhibit its activity. Cell. 1982 Jul;29(3):939–944. doi: 10.1016/0092-8674(82)90456-1. [DOI] [PubMed] [Google Scholar]
- Bogenhagen D. F., Brown D. D. Nucleotide sequences in Xenopus 5S DNA required for transcription termination. Cell. 1981 Apr;24(1):261–270. doi: 10.1016/0092-8674(81)90522-5. [DOI] [PubMed] [Google Scholar]
- Burton Z. F., Gross C. A., Watanabe K. K., Burgess R. R. The operon that encodes the sigma subunit of RNA polymerase also encodes ribosomal protein S21 and DNA primase in E. coli K12. Cell. 1983 Feb;32(2):335–349. doi: 10.1016/0092-8674(83)90453-1. [DOI] [PubMed] [Google Scholar]
- Curtis P. J., Mantei N., Weissmann C. Characterization and kinetics of synthesis of 15S beta-globin RNA, a putative precursor of beta-globin mRNA. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):971–984. doi: 10.1101/sqb.1978.042.01.098. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Harpold M. M., Wilson M. C., Darnell J. E., Jr Chinese hamster polyadenylated messenger ribonucleic acid: relationship to non-polyadenylated sequences and relative conservation during messenger ribonucleic acid processing. Mol Cell Biol. 1981 Feb;1(2):188–198. doi: 10.1128/mcb.1.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing P., Shenk T. The adenovirus type 5 E1A transcriptional control region contains a duplicated enhancer element. Cell. 1983 Jul;33(3):695–703. doi: 10.1016/0092-8674(83)90012-0. [DOI] [PubMed] [Google Scholar]
- Hofer E., Darnell J. E., Jr The primary transcription unit of the mouse beta-major globin gene. Cell. 1981 Feb;23(2):585–593. doi: 10.1016/0092-8674(81)90154-9. [DOI] [PubMed] [Google Scholar]
- Hofer E., Hofer-Warbinek R., Darnell J. E., Jr Globin RNA transcription: a possible termination site and demonstration of transcriptional control correlated with altered chromatin structure. Cell. 1982 Jul;29(3):887–893. doi: 10.1016/0092-8674(82)90450-0. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel D. A., Tilghman S. M., Leder P. The sequence of the chromosomal mouse beta-globin major gene: homologies in capping, splicing and poly(A) sites. Cell. 1978 Dec;15(4):1125–1132. doi: 10.1016/0092-8674(78)90040-5. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills D. R., Dobkin C., Kramer F. R. Template-determined, variable rate of RNA chain elongation. Cell. 1978 Oct;15(2):541–550. doi: 10.1016/0092-8674(78)90022-3. [DOI] [PubMed] [Google Scholar]
- Montell C., Fisher E. F., Caruthers M. H., Berk A. J. Inhibition of RNA cleavage but not polyadenylation by a point mutation in mRNA 3' consensus sequence AAUAAA. Nature. 1983 Oct 13;305(5935):600–605. doi: 10.1038/305600a0. [DOI] [PubMed] [Google Scholar]
- Poncz M., Schwartz E., Ballantine M., Surrey S. Nucleotide sequence analysis of the delta beta-globin gene region in humans. J Biol Chem. 1983 Oct 10;258(19):11599–11609. [PubMed] [Google Scholar]
- Puckett L., Darnell J. E. Essential factors in the kinetic analysis of RNA synthesis in HeLa cells. J Cell Physiol. 1977 Mar;90(3):521–534. doi: 10.1002/jcp.1040900315. [DOI] [PubMed] [Google Scholar]
- Roberts J. W. Termination factor for RNA synthesis. Nature. 1969 Dec 20;224(5225):1168–1174. doi: 10.1038/2241168a0. [DOI] [PubMed] [Google Scholar]
- Roberts J. W. Transcription termination and late control in phage lambda. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3300–3304. doi: 10.1073/pnas.72.9.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Salditt-Georgieff M., Darnell J. E., Jr A precise termination site in the mouse beta major-globin transcription unit. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4694–4698. doi: 10.1073/pnas.80.15.4694. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Salditt-Georgieff M., Darnell J. E., Jr A precise termination site in the mouse beta-major globin transcription unit. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2274–2274. doi: 10.1073/pnas.81.7.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salditt-Georgieff M., Harpold M., Sawicki S., Nevins J., Darnell J. E., Jr Addition of poly(A) to nuclear RNA occurs soon after RNA synthesis. J Cell Biol. 1980 Sep;86(3):844–848. doi: 10.1083/jcb.86.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salditt-Georgieff M., Sheffery M., Krauter K., Darnell J. E., Jr, Rifkind R., Marks P. A. Induced transcription of the mouse beta-globin transcription unit in erythroleukemia cells. Time-course of induction and of changes in chromatin structure. J Mol Biol. 1984 Feb 5;172(4):437–450. doi: 10.1016/s0022-2836(84)80016-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S. H., Slightom J. L., Smithies O. A history of the human fetal globin gene duplication. Cell. 1981 Oct;26(2 Pt 2):191–203. doi: 10.1016/0092-8674(81)90302-0. [DOI] [PubMed] [Google Scholar]
- Stow N. D. Cloning of a DNA fragment from the left-hand terminus of the adenovirus type 2 genome and its use in site-directed mutagenesis. J Virol. 1981 Jan;37(1):171–180. doi: 10.1128/jvi.37.1.171-180.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman S. M., Tiemeier D. C., Polsky F., Edgell M. H., Seidman J. G., Leder A., Enquist L. W., Norman B., Leder P. Cloning specific segments of the mammalian genome: bacteriophage lambda containing mouse globin and surrounding gene sequences. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4406–4410. doi: 10.1073/pnas.74.10.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981 Feb 26;289(5800):751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]