Abstract
The presence of macrophages in dental pulp is well known. However, whether these macrophages proliferate and differentiate in the dental pulp in situ, or whether they constantly migrate from the blood stream into the dental pulp remains unknown. We have examined and compared the development of dental pulp macrophages in an organ culture system with in vivo tooth organs to clarify the developmental mechanism of these macrophages. The first mandibular molar tooth organs from ICR mice aged between 16 days of gestation (E16) to 5 days postnatally were used for in vivo experiments. Those from E16 were cultured for up to 14 days with or without 10% fetal bovine serum. Dental pulp tissues were analyzed with immunohistochemistry to detect the macrophages and with reverse transcription and the polymerase chain reaction (RT-PCR) for the detection of factors related to macrophage development. The growth curves for the in vivo and in vitro cultured cells revealed similar numbers of F4/80-positive macrophages in the dental pulp. RT-PCR analysis indicated the constant expression of myeloid colony-stimulating factor (M-CSF) in both in-vivo- and in-vitro-cultured dental pulp tissues. Anti-M-CSF antibodies significantly inhibited the increase in the number of macrophages in the dental pulp. These results suggest that (1) most of the dental pulp macrophages proliferate and differentiate in the dental pulp without a supply of precursor cells from the blood stream, (2) M-CSF might be a candidate molecule for dental pulp macrophage development, and (3) serum factors might not directly affect the development of macrophages.
Keywords: Dental pulp, Immunohistochemistry, Macrophage, M-CSF, Organ culture, Mouse (ICR)
Introduction
Macrophages are a heterogenous population of cells, characterized by their morphology, function, and metabolism. At least two types of macrophages are distributed in the body: resident and exudative macrophages (Daems and Brederoo 1972). Whereas exudative macrophages migrate into sites of inflammation in response to several chemokines, resident macrophages are ubiquitously distributed around the body under normal healthy conditions (Daems and Brederoo 1973; Daems et al. 1976, 1979; Soranzo et al. 1978; Daems and van der Rhee 1980). According to the concept of the mononuclear phagocyte system (van Furth et al. 1972), both these types of macrophages are derived from blood monocytes, which originate in the bone marrow. Furthermore, macrophages are generally accepted to be relatively short-lived cells and do not show any proliferative activity (van Furth 1975, 1980, 1989, 1992). However, several recent studies have demonstrated the in situ proliferation of resident macrophages in various tissues in response to several cytokines, such as myeloid colony-stimulating factors (GM-CSF and M-CSF), secreted into the proliferation microenvironment (Naito et al. 1996; Sawa et al. 2003; Douglass et al. 2008).
Large numbers of macrophages exist in the dental pulp and are considered to be the major immunocompetent cells that fight against bacterial infections caused by dental caries (Nakakura-Ohshima et al. 2003; Zhang et al. 2006). In reality, macrophages migrate toward the infection site in pulpitis. However, whether dental pulp macrophages proliferate and differentiate in situ, or whether monocytes derived from the circulation differentiate into macrophages remains uncertain.
The tissue organ culture system is commonly used to examine the development of the tooth organ (Fujiwara 1997; Fujiwara et al. 2005). Our previous studies have indicated that 2 days of development in culture is almost equal to 1 day of in vivo development, and that the calcification of dentin and enamel can be detected following culture under either serum-supplemented or serumless conditions (Evans et al. 1988; Nakamura et al. 1994). In the system presented here, the migration of blood monocytes from the circulation can be excluded, because of the isolation of tooth organs from the mandibles.
In this study, we have used immunohistochemistry to examine the number and phenotype of macrophages in in-vivo- and in-vitro-cultured dental pulp as a product of time in order to determine whether these cells proliferate and differentiate in situ. We have also used reverse transcription with the polymerase chain reaction (RT-PCR) to analyze the possible factors responsible for the proliferation and differentiation of these macrophages.
Materials and methods
The experimental protocol used in this study was reviewed and approved by the Animal Care Committee of Showa University.
Mice
Pregnant ICR mice were purchased from Sankyo Laboratory Service (Tokyo, Japan) and maintained under routine conditions at the Laboratory Animal Center of Showa University. Mandibular first molars taken from mice aged from 16 days of gestation (E16) to 5 days postnatally (5dPN) were examined.
Organ culture
E16 mandibular first molar tooth organs were dissected and maintained for up to 14 days in a Tronwell culture system with serum-supplemented or a serumless, chemically defined medium as previously described (Evans et al. 1988; Nakamura et al. 1994). The explants were placed on 0.2-μm pore-size Millipore filter discs (Bedford, Mass., USA) and supported by stainless steel mesh triangles. The explants were cultured in Grobstein Falcon dishes under optimal humidity conditions in an atmosphere containing 5% CO2 and 95% air. The medium used was Fitton-Jackson modified BGJb medium (Sigma, St. Louis, Mo., USA), with or without 10% FBS (fetal bovine serum), and supplemented with 100 mM ascorbic acid, 100 U/ml penicillin, and 100 mg/ml streptomycin. The medium (pH 7.4 at the start of each culture) was changed every other day.
To examine the inhibitory effect of M-CSF on the development of dental pulp resident macrophages, a neutralizing antibody against M-CSF (R & D Systems, Abingdon, Berkshire, UK) was added to the culture medium at a final concentration of 16 μg/ml. For control experiments, isotype-matched rat IgG2b (eBioscience, Burlingame, Calif., USA) was added to the medium at the same concentration.
Tissue preparation
Mandibles from E16 to 5dPN mice and first molar tooth organs cultured for 2–14 days were fixed with 4% paraformaldehyde in 0.01 M phosphate-buffered saline (PBS) at pH 7.4. After decalcification with 10% EDTA, specimens were immersed in 5%, 15%, and 30% sucrose, embedded in Tissue–Tek O.C.T Compound (Sakura Finetek USA, Torrance, USA), and snap-frozen in a mixture of acetone and dry ice for analysis by immunohistochemistry. Sections (10 μm thick) were processed for hematoxylin and eosin staining and immunohistochemistry.
Antibodies
Four markers were applied in this study for the identification of macrophage-lineage cells in the dental pulp: F4/80, CD68, ER-MP20, and ER-MP58. F4/80 is usually used as the marker for resident macrophages, whereas CD68 is a marker of macrophage activation (Leenen et al. 1994; Dambach et al. 2002; Lloyd et al. 2008). ER-MP20 and ER-MP58 are both used as markers for monocytic progenitor cells (Leene et al. 1990, 1994; Chan et al. 1998; Kennedy and Abkowitz 1998). Proliferating cell nuclear antigen (PCNA) was used as a proliferation marker (Hillmeister et al. 2008; Vassiliou et al. 2010).
Anti-F4/80, CD68, ER-MP20 (Ly-6C), ER-MP58, and PCNA monoclonal antibodies were purchased from SEROTEC (Oxfordshire, UK). Goat anti-rat IgG antibodies, conjugated with either Alexa Fluor 488 or Alexa Fluor 594, were purchased from Molecular Probes (Eugene, Ore., USA). Biotinylated goat anti-rat IgG antibody was purchased from VECTOR (Burlingame, Calif., USA).
Immunohistochemical procedures
Frozen sections (10 μm thick) were cut from the embedded tissues, placed on poly-L-lysine-coated glass slides, and air-dried. After incubation in 0.3% H2O2-methanol for 30 min, the sections were incubated with 5% normal goat serum in PBS containing 5% bovine serum albumin and 0.025% Triton X-100, followed by incubation with each of the monoclonal antibodies. After several rinses, the sections were incubated with biotinylated goat anti-rat IgG antibody and then with an avidin-biotin-bound horseradish peroxidase complex. After being washed, the sections were incubated with a mixture of 3,3′-diaminobenzidine tetrahydrochloride (0.5 mg/ml; WAKO, Osaka, Japan) and H2O2 at a final concentration of 0.03% in 0.1 M TRIS-HCl buffer at pH 7.6. Counterstaining was achieved with methyl green. As controls, sections were incubated with either normal rat serum or PBS instead of the primary antibody. After the immunolabeling step, the number of F4/80-, CD68-, ER-MP20-, and ER-MP58-positive cells in the dental pulp was counted (100×100 μm).
For the double-labeling of F4/80- and CD68- or ER-MP20-positive macrophages, sections were first incubated with the F4/80 antibody and then with the Alexa-Fluor-488-conjugated goat anti-rat IgG antibody. After being washed, sections were incubated with anti-CD68 or anti-ER-MP20 antibody and then with Alexa-Fluor-594-conjugated goat anti-rat IgG antibody.
For the double-labeling of PCNA- and F4/80-positive macrophages, sections were first boiled with antigen retrieval buffer (10 mM sodium citrate, pH 6.0) for 10 min before the sections were incubated with anti-mouse-PCNA mouse monoclonal antibody (BD Biosciences Pharmingen, San Diego, Calif., USA) and then with Alexa-Fluor-488-conjugated goat anti-mouse IgG antibody. After being washed, sections were incubated with anti-F4/80 antibody and then with Alexa-Fluor-594-conjugated goat anti-rat IgG antibody. After several washes with PBS, sections were mounted with AquaMount (Polysciences, Warrington, Pa., USA) and observed with a Nikon ECLIPSE 50i fluorescent microscope (Tokyo, Japan).
RT-PCR analysis
The in vivo and in vitro cultured mandibular first molar tooth organs were homogenized after the careful removal of enamel organs under a dissection microscopy. Total RNA was extracted from each sample by using an RNeasy Mini kit (QIAGEN, Tokyo, Japan). A total of 4 mg RNA served as a template for RT-PCR, which was performed by means of an Gene Amp Gold RNA PCR (Applied Biosystems, Tokyo, Japan) with mG-CSF, mGM-CSF, and mM-CSF sequence-aligned primers. The primers used for PCR analysis were as follows: GM-CSF forward, 5′-GCG TGA CAT TAA AGA AGC TG-3′; reverse, 5′-CTC AGG AGG AGC AAT GAT CTT G-3′; G-CSF forward, 5′-GGG ACA AGA CAT CCC TGT TT-3′; reverse, 5′-CTG TGA GGA CAG GAA ACC CT-3′; M-CSF forward, 5′-CTA GGG GCC AGC ATT AGA CC-3′; reverse, 5′-GAC ACA TAC TAC ACC CCA GAG G-3′; and beta-actin forward, 5′-GCG TGA CAT TAA AGA GAA GCT G-3′; reverse, 5′-CTC AGG AGG AGC AAT GAT CTT G-3′. Amplification conditions were as follows: the RT reaction was performed at 25°C for 10 min and at 42°C for 12 min; the PCR started at 95°C for 10 min, followed by 30 cycles consisting of a denaturing step at 95°C for 20 s, an annealing step at 60°C for 1 min, and an extension step at 72°C for 2 min. Final extension took place at 72°C for 7 min. PCR products were mixed with 10× loading buffer (TAKARA BIOTECHNOLOGY, Shiga, Japan) at the ratio 10:1 (v/v) and were separated by electrophoresis at 100 V for 30 min on a 1.0% agarose gel.
Statistical analyses
Statistical significance was evaluated by using the Student’s unpaired t-test or Mann-Whitney U test, for which P values of <0.01 were considered significant.
Results
Immunohistochemical detection of resident macrophages in dental pulp during in vivo development
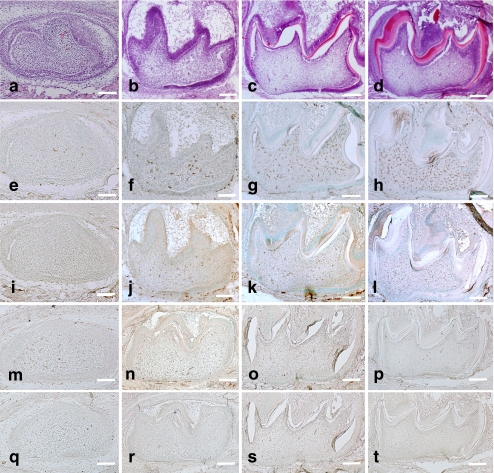
In the dental pulp of the first mandibular molar tooth organ at age E16, dental pulp cells adjacent to the basement membrane became polarized, whereas no dentin matrix was secreted at this time (Fig. 1a). A small number of F4/80-, ER-MP20- (Ly- 6C), and ER-MP58-positive cells were detected, whereas CD68-positive cells were not detected at this stage (Fig. 1e, i, m, q). At 0dPN, dental pulp cells beneath the basement membrane differentiated into odontoblasts and secreted dentin matrix (Fig. 1b). F4/80- and ER-MP20-positive cells were observed throughout the dental pulp (Fig. 1f, n), and CD68- and ER-MP58-positive cells were also detected at this stage (Fig. 1j, r). During this development, the odontoblasts secreted more dentin matrix and formed calcified dentin (Fig. 1c, d). Inner enamel epithelial cells differentiated into secretory ameloblasts and formed enamel (Fig. 1c, d).
Fig. 1.
In vivo development of mouse mandibular first molars at E16 (a, e, i, m, q), 0dPN (b, f, j, n, r), 3dPN (c, g, k, o, s), and 5dPN (d, h, l, p, t). Hematoxylin and eosin (H-E) staining indicated the development of tooth organs (a–d). Immunohistochemical detection of F4/80-positive cells (e–h), CD68-positive cells (i–l), ER-MP20-positive cells (m–p), and ER-MP58-positive cells (q–t) indicated an increase in the number of macrophages with tooth organ development. Bars 100 μm
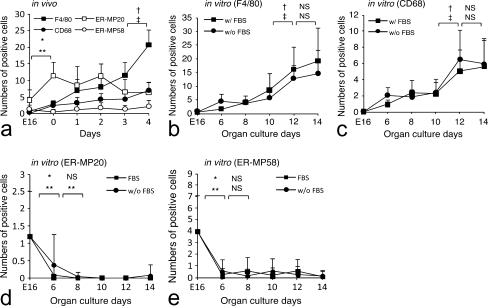
The number of F4/80- and CD68-positive cells increased significantly with development (Fig. 1g, h, k, l; see also quantitation below). In contrast, the number of ER-MP20- and ER-MP58-positive cells decreased or were constant at a low level (Fig. 1o, p, s, t; see also quantitation below). At all of the developmental stages, the number of CD68-positive cells was lower than that of the F4/80-positive cells (see quantitation below).
These results suggest that the macrophages actively proliferate within the dental pulp, even if a relatively low number of monocytes might penetrate from the bloodstream.
Immunohistochemical detection of resident macrophages in in-vitro-cultured dental pulp
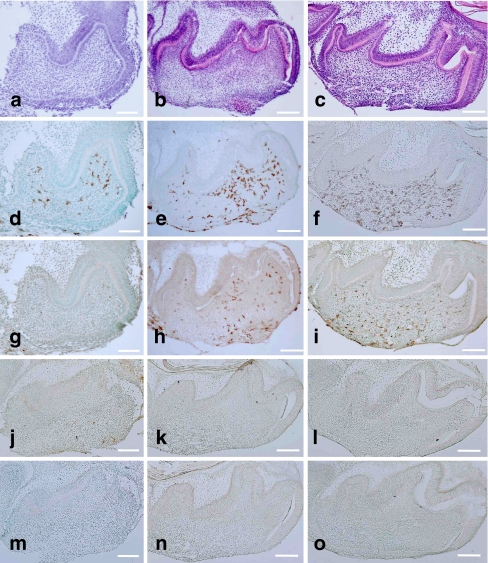
First mandibular molar tooth organs taken from mice aged E16 were used for organ culture. When cultured in serum-supplemented media, tooth organs developed with time. By 6 days, odontoblasts secreted dentin matrix (Fig. 2a), and the formation of dentin and enamel proceeded at 10 and 14 days (Fig. 2b, c). The number of F4/80-positive cells increased significantly during development (Figs. 2d–f, 3b). CD68-positive cells were detected from 6 days of culture, and the number of these cells increased significantly during development (Figs. 2g-i, 3c). ER-MP20- and ER-MP58-positive cells decreased significantly during development (Figs. 2j-o, 3d, e). The number of F4/80-positive cells was always higher than that of the CD68-positive cells (Fig. 3b, c). The same results were obtained from the organ culture experiments in the serumless, chemically defined media condition (Fig. 3b, c). No significant differences were detected between the numbers of F4/80- and CD68-positive cells under either of the two culture conditions (Fig. 3b, c). The number of these cells in vivo was higher than the organ culture tooth organ cells (Fig. 3a-c).
Fig. 2.
Development of E16 mouse mandibular first molars in organ culture under serum-supplemented conditions for 6 days (a, d, g, j, m), 10 days (b, e, h, k, n), and 14 days (c, f, i, l, o). H-E staining indicated the development of tooth organs (a–c). Immunohistochemical detection of F4/80-positive cells (d–f), CD68-positive cells (g–i), ER-MP20-positive cells (j–l), and ER-MP58-positive cells (m–o) indicated an increase in the number of macrophages with tooth organ development. Bar 100 μm
Fig. 3.
Numbers of F4/80- , CD68- , ER-MP20-, and ER-MP58-positive cells in dental pulp with development. Data are representative of means±SD; P values of <0.01 were considered to be significant. a In vivo development of F4/80-, CD68-, ER-MP20-, and ER-MP58-positive cells. b Development of F4/80-positive cells in organ culture with or without fetal bovine serum (w/FBS, w/o FBS). c Development of CD68-positive cells in organ culture with or without fetal bovine serum (w/FBS, w/o FBS). d Development of ER-MP20-positive cells in organ culture with or without fetal bovine serum (w/FBS, w/o FBS). e Development of ER-MP58-positive cells in organ culture with or without fetal bovine serum (w/FBS, w/o FBS). *P<0.01, †: in vivo (F4/80), in vitro (w/FBS), ‡: in vivo (CD68), in vitro (w/o FBS), *: in vivo (ER-MP20), in vitro (w/FBS), **: in vivo (ER-MP58), in vitro (w/o FBS); NS not significant
The organ culture results strongly support the possibility of the direct proliferation of macrophages within the dental pulp and also indicate the lack of contribution from serum factors for macrophage development.
Double-immunostaining of macrophages
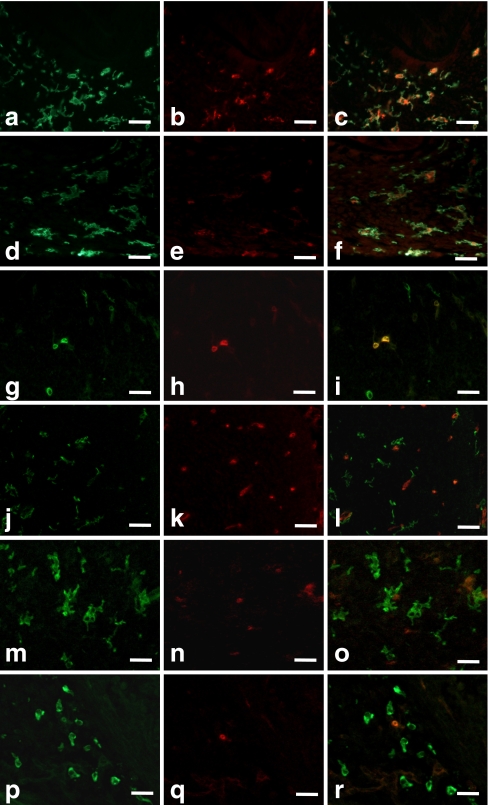
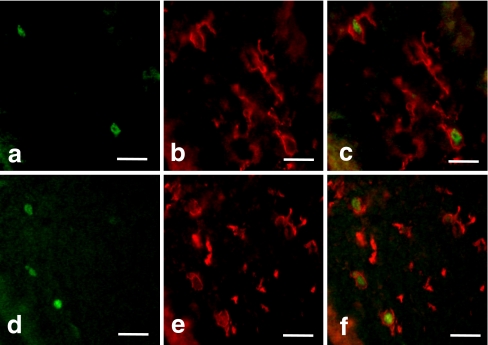
Double-staining of the macrophages with the anti-F4/80 and anti-CD68 antibodies in vivo and in vitro showed that all CD68-positive cells were also F4/80-positive, whereas the F4/80 single-positive cells were distributed throughout the dental pulp (Fig. 4c, f). Most of the macrophages were relatively large and had well-developed cell processes (Fig. 4a-f).
Fig. 4.
Double-immunostaining of dental pulp macrophages with anti-F4/80 (a, d) and anti-CD68 (b, e) antibodies in 4dPN (a–c) and 14-day-cultured tooth organs (d–f). All CD68-positive cells were F4/80-positive (c, f). Double-immunostaining of dental pulp macrophages with anti-F4/80 (g, j, m, p) and anti-ER-MP20 (h, k, n, q) antibodies in E16 (g–i), 3dPN (j–l), 8-day (m–o), and 14-day-cultured tooth organs (p–r). Bar 25 μm
Double-staining of the macrophages with anti-F4/80 and anti-ER-MP20 antibodies in vivo and in vitro showed that all F4/80-positive cells were also ER-MP20-positive at age E16 (Fig. 4g-i). During development, the number of F4/80 single-positive cells increased, whereas the number of ER-MP20 single-positive cells decreased. As a result, the number of double-positive cells decreased (Figs. 3a, d, 4j-r).
These results suggest that immature ER-MP20-positive cells present in the interstitial area within the dental pulp differentiate into F4/80-positive cells via the ER-MP20 and F4/80 double-positive developmental stage.
Double-immunostaining of macrophages with anti-F4/80 and anti-PCNA antibodies
As the results from the in vivo and in vitro immunostaining strongly suggested the direct proliferation of F4/80-positive cells within the dental pulp, we next examined the expression of PCNA in F4/80-positive cells. Double-staining of macrophages with anti-F4/80 and anti-PCNA antibodies in vivo and in vitro revealed that some of the F4/80-positive cells were also PCNA-positive (Fig. 5), which indicated that the F4/80-positive cells were proliferating within the peripheral dental pulp.
Fig. 5.
Double-immunostaining of dental pulp macrophages with anti-PCNA (a, d) and anti-F4/80 (b, e) antibodies in 4dPN (a–c) and 8-day-cultured tooth organs (d–f). All PCNA-positive cells were F4/80-positive (c, f). Bar 25 μm
RT-PCR analysis of dental pulp
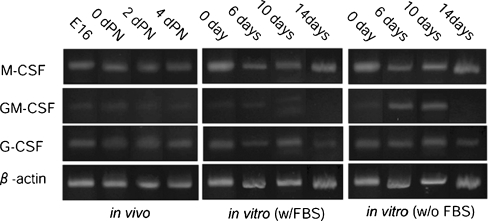
Next, we examined the factors relating to the development of macrophages in the dental pulp. The expression of three cytokines important for leukocyte development (G-CSF, GM-CSF, and M-CSF) was examined by RT-PCR (Fig. 6). Among these three cytokines, the high expression of M-CSF was detected constitutively during the examined period in both in vivo and in vitro dental pulp cells. The expression of GM-CSF was detected at all of the examined times, although this expression was not stable. G-CSF was also expressed constantly during the developmental period.
Fig. 6.
RT-PCR analysis of the expression of M-CSF, GM-CSF, G-CSF, and β-actin in dental pulp of in vivo and organ culture with or without fetal bovine serum (w/FBS, w/oFBS)
Effects of M-CSF-neutralizing antibody on macrophage development in dental pulp
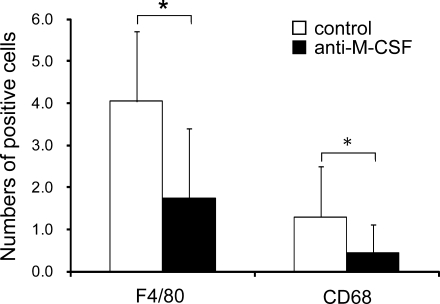
Finally, we examined the effect of M-CSF on macrophage development in dental pulp by means of the organ culture system. The addition of anti-M-CSF in the culture medium resulted in a significant reduction of F4/80-positive and CD68-positive cells in the dental pulp compared with the isotype matched control at day 12 of culture (P<0.01; Fig. 7). No obvious developmental defects could be detected in the tooth organs following the addition of the antibody (data not shown).
Fig. 7.
Effects of the M-CSF neutralizing antibody on the development of macrophages in dental pulp. The number of F4/80- and CD68-positive cells was significantly decreased by the addition of the antibody to the medium. Data are representative of means±SD. *P values of <0.01 were considered to be significant
Discussion
In this study, we used the tooth organ culture system to examine the proliferation of macrophages within the dental pulp. For cultivation, tooth organs were isolated from the mandibles, a procedure that resulted in the exclusion of a blood monocyte invasion from the circulation. In the tooth organ culture system, the number of macrophages in the dental pulp increased during organ development. At age E16, the dental pulp contained a few F4/80-positive macrophages. Even if these F4/80-positive cells had migrated into the dental pulp from the bloodstream as monocytes, our results provide direct evidence that the macrophages proliferate in the dental pulp in situ.
The number of macrophages was always higher in vivo than in vitro. As blood vessels were developed in the dental pulp of in vivo tooth organs, the monocytes must have migrated from the blood stream into the dental pulp. This environmental difference might explain the difference between the number of macrophages observed under the two conditions. However, the number of ER-MP20-positive cells gradually decreased during development. In contrast, the number of F4/80-positive macrophages increased during development. Furthermore, under the in vivo and in vitro culture conditions, F4/80-positive macrophages were also PCNA-positive, and the number of F4/80-positive cells was always higher than the CD68-positive cells. These results strongly imply that, even if a small number of macrophages is supplied from the blood stream, most of the F4/80-positive macrophages in the dental pulp represent a self-proliferating population that is distinct from the monocyte population and that develops into functionally activated CD68-positive cells in situ.
Several studies have suggested that macrophages proliferate in various organs. During ontogeny, primitive macrophages develop, proliferate, and differentiate into fetal macrophages before the production of monocytes in the fetal hematopoietic tissues (Naito and Wisse 1977; Naito et al. 1986, 1989, 1990, 1991; Takahashi et al. 1989; Higashi et at. 1992). Yamamoto et al. (2008) have identified macrophages in osteopetrotic (op/op) mice. These animals contain approximately half the number of macrophages found in healthy animals. Resident macrophages are capable of proliferating under various experimental conditions (Wisse 1974; Widmann and Fahimi 1975; Hibbs et al. 2007). In mice that have been rendered monocytopenic following the administration of strontium-89 (Yamada et al. 1990; Naito and Takahashi 1991), the number of Kupffer cells is maintained by the proliferation of resident Kupffer cells.
The production of monocytes and macrophages is controlled by various cytokines, such as interleukin-6 (IL-6), IL-3, GM-CSF, and M-CSF. Among these, M-CSF is the most important key molecule for mediating the development and differentiation of a restricted macrophage lineage belonging to the mononuclear phagocyte system and for the production of heterogeneous macrophage populations (Rutherford et al. 1993; Naito et al. 1996; Douglass et al. 2008). Moreover, M-CSF has been reported to stimulate the growth of macrophages, and resident macrophages exist in the local tissues, such as the Kupffer cells in the liver, microglial cells in the brain, mesangial cells in the kidney, and osteoclasts in bone (Takashima et al. 1995; Douglass et al. 2008). M-CSF-derived macrophages are larger in size, develop more abundant intracellular organelles, and extend more well-developed cell processes than GM-CSF-derived and IL-3-derived macrophages (Morioka et al. 1994).
The macrophages detected in the dental pulp in this study were also large and extended several cell processes. RT-PCR analysis also revealed the constitutive expression of M-CSF mRNA in the dental pulp, as previously indicated by Sawa et al. (2003). The lack of F4/80-positive resident macrophages in the dental pulp was furthermore indicated in osteopetrotic (op/op) mice, which carried an M-CSF mutation (Nagahama et al. 1998). Neutralizing antibodies against M-CSF significantly inhibited the proliferation of resident macrophages in the dental pulp in our study. Tooth organs developed in an organ culture system in both serum-supplemented and serum-less conditions. Under both conditions, the number of F4/80-positive cells increased similarly. These results indicate that M-CSF is the main cytokine responsible for the development of resident macrophages in the dental pulp.
Macrophages in the dental pulp are considered to be one of the major types of immunocompetent cells that fight dental pulp infections. Several studies have mentioned the possibility of resident macrophages being involved in the regulatory function and differentiation of odontoblasts (Ohshima et al. 1995; Tsuruga et al. 1999; Nakakura-Ohshima et al. 2003). However, the precise functions of the macrophages in the dental pulp have not yet been clarified. According to Zhang et al. (2006), two types of cells with well-developed cell processes exist in the dental pulp: the CD11c-positive sentinel and F4/80-positive interstitial cells. The CD11c-positive cells constitutively express toll-like receptors 2 and 4 and displayed high migration activities. They rapidly move to the infected regions in the dental pulp and then migrate into the local lymph nodes. In contrast, F4/80-positive cells exhibit low migration activity. Therefore, CD11c-positive cells and F4/80-positive cells are considered to be sentinel and interstitial cells, respectively. In our study, we have not examined the further phenotypic characterization of resident macrophages. Whether these cells show the same phenotypic and functional characterizations in vivo and in vitro remains undetermined and should be considered in future investigations.
In conclusion, our in vivo and in vitro studies indicate that dental pulp macrophages proliferate and differentiate in situ. The main factor involved in the development of these macrophages might be M-CSF.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Part of this study was supported by Grant-in-Aid for Scientific Research (21592342, 20592148) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. This study was also supported in part by the High-Tech Research Center Project for Private Universities from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
References
- Chan J, Leenen PJ, Bertoncello I, Nishikawa SI, Hamilton JA. Macrophage lineage cells in inflammation: characterization by colony-stimulating factor-1 (CSF-1) receptor (c-Fms), ER-MP58, and ER-MP20 (Ly-6C) expression. Blood. 1998;92:1423–1431. [PubMed] [Google Scholar]
- Daems WT, Brederoo P. The fine structure and peroxidase activity of resident and exudate peritoneal macrophages in the guinea pig. Adv Exp Med Biol. 1972;15:19–31. [Google Scholar]
- Daems WT, Brederoo P. Electron microscopical studies on the structure, phagocytic properties, and peroxidatic activity of resident and exudate peritoneal macrophages in guinea pigs. Z Zellforsch Mikrosk Anat. 1973;144:247–297. doi: 10.1007/BF00307305. [DOI] [PubMed] [Google Scholar]
- Daems WT, van der Rhee HJ. Peroxidase and catalase in monocytes, macrophages, epithelioid cells and giant cells of the rat. In: van Furth R, editor. Mononuclear phagocytes, functional aspects. The Hague: Nijhoff; 1980. pp. 43–60. [Google Scholar]
- Daems WT, Koerten HK, Soranzo MR. Differences between monocyte-derived and tissue macrophages. Adv Exp Med Biol. 1976;73:27–40. doi: 10.1007/978-1-4684-3297-8_3. [DOI] [PubMed] [Google Scholar]
- Daems WT, Roos D, van Berkel TJC, van der Rhee HJ. The subcellular distribution and biochemical properties of peroxidase in monocytes and macrophages. In: Dingle JT, Jacques PJ, editors. Lysosomes in biology and pathology. Amsterdam: North-Holland; 1979. pp. 463–516. [PubMed] [Google Scholar]
- Dambach DM, Watson LM, Gray KR, Durham SK, Laskin DL. Role of CCR2 in macrophage migration into the liver during acetaminophen-induced hepatotoxicity in the mouse. Hepatology. 2002;35:1093–1103. doi: 10.1053/jhep.2002.33162. [DOI] [PubMed] [Google Scholar]
- Douglass TG, Driggers L, Zhang JG, Hoa N, Delgado C, Williams CC, Dan Q, Sanchez R, Jeffes EWB, Wepsic HT, Myers MP, Koths K, Martin R, Jadus MR. Macrophage colony stimulating factor: not just for macrophages anymore! A gateway into complex biologies. Int Immunopharmacol. 2008;10:1354–1376. doi: 10.1016/j.intimp.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Evans J, Bringas P, Jr, Nakamura M, Nakamura E, Santos V, Slavkin HC. Metabolic expression of intrinsic developmental programs for dentine and enamel biomineralization in serumless, chemically-defined, organotypic culture. Calcif Tissue Int. 1988;42:220–230. doi: 10.1007/BF02553747. [DOI] [PubMed] [Google Scholar]
- Fujiwara N. In vitro formation of cementum in mouse molar germs cultured in newly developed organ culture system. Jpn J Oral Biol. 1997;39:143–154. doi: 10.2330/joralbiosci1965.39.143. [DOI] [Google Scholar]
- Fujiwara N, Tabata MJ, Endoh M, Ishizeki K, Nawa T. Insulin-like growth factor-I stimulates cell proliferation in the outer layer of Hertwig’s epithelial root sheath and elongation of the tooth root in mouse molars in vitro. Cell Tissue Res. 2005;320:69–75. doi: 10.1007/s00441-004-1065-5. [DOI] [PubMed] [Google Scholar]
- van Furth R. Modulation of monocyte production. In: van Furth R, editor. Mononuclear phagocytes in immunity, infection, and pathology. Oxford: Blackwell Scientific; 1975. pp. 161–172. [Google Scholar]
- van Furth R. Cells of the mononuclear phagocyte system. Nomenclature in terms of sites and conditions. In: van Furth R, editor. Mononuclear phagocytes, functional aspects. The Hague: Nijhoff; 1980. pp. 1–30. [Google Scholar]
- van Furth R. Origin and turnover of monocytes and macrophages. Curr Top Pathol. 1989;79:125–147. [PubMed] [Google Scholar]
- van Furth R. Production and migration of monocytes and kinetics of macrophages. In: van Furth R, editor. Mononuclear phagocytes. Biology of monocytes and macrophages. Dordrecht: Kluwer Academic; 1992. pp. 3–12. [Google Scholar]
- van Furth R, Cohn ZA, Hirsch JG, Humphrey JH, Spector WG, Langevoort HL. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ. 1972;46:845–852. [PMC free article] [PubMed] [Google Scholar]
- Hibbs ML, Quilici C, Kountouri N, Seymour JF, Armes JE, Burgess AW, Dunn AR. Mice lacking three myeloid colony-stimulating factors (G-CSF, GM-CSF, and M-CSF) still produce macrophages and granulocytes and mount an inflammatory response in a sterile model of peritonitis. J Immunol. 2007;178:6435–6443. doi: 10.4049/jimmunol.178.10.6435. [DOI] [PubMed] [Google Scholar]
- Higashi K, Naito M, Takeya M, Ando M, Araki S, Takahashi K. Ontogenetic development, differentiation, and phenotypic expression of macrophages in fetal rat lungs. J Leukoc Biol. 1992;51:444–454. doi: 10.1002/jlb.51.5.444. [DOI] [PubMed] [Google Scholar]
- Hillmeister P, Lehmann KE, Bondke A, Witt H, Duelsner A, Gruber C, Busch HJ, Jankowski J, Ruiz-Noppinger P, Hossmann KA, Ivo R, Buschmann IR. Induction of cerebral arteriogenesis leads to early-phase expression of protease inhibitors in growing collaterals of the brain. J Cereb Blood Flow Metab. 2008;28:1811–1823. doi: 10.1038/jcbfm.2008.69. [DOI] [PubMed] [Google Scholar]
- Kennedy DW, Abkowitz JL. Mature monocytic cells enter tissues and engraft. Proc Natl Acad Sci USA. 1998;95:14944–14949. doi: 10.1073/pnas.95.25.14944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenen PJ, Melis M, Slieker WA, van Ewijk W. Murine macrophage precursor characterization. II. Monoclonal antibodies against macrophage precursor antigens. Eur J Immunol. 1990;20:27–34. doi: 10.1002/eji.1830200105. [DOI] [PubMed] [Google Scholar]
- Leenen PJ, de Bruijn MF, Voerman JS, Campbell PA, van Ewijk W. Markers of mouse macrophage development detected by monoclonal antibodies. J Immunol Methods. 1994;174:5–19. doi: 10.1016/0022-1759(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Lloyd CM, Phillips AR, Cooper GJ, Dunbar PR. Three-colour fluorescence immunohistochemistry reveals the diversity of cells staining for macrophage markers in murine spleen and liver. J Immunol Methods. 2008;334:70–81. doi: 10.1016/j.jim.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Morioka Y, Naito M, Sato T, Takahashi K. Immunophenotypic and ultrastructural heterogeneity of macrophage differentiation in bone marrow and fetal hematopoiesis of mouse in vitro and in vivo. J Leukoc Biol. 1994;55:642–651. doi: 10.1002/jlb.55.5.642. [DOI] [PubMed] [Google Scholar]
- Nagahama SI, Cunningham ML, Lee MY, Byers MR. Normal development of dental innervation and nerve/tissue interactions in the colony-stimulating factor-1 deficient osteopetrotic mouse. Dev Dyn. 1998;211:52–59. doi: 10.1002/(SICI)1097-0177(199801)211:1<52::AID-AJA5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Naito M, Takahashi K. The role of Kupffer cells in glucan-induced granuloma formation in the liver of mice depleted of blood monocytes by administration of strontium-89. Lab Invest. 1991;64:664–674. [PubMed] [Google Scholar]
- Naito M, Wisse E. Observations on the fine structure and cytochemistry of sinusoidal cells in fetal and neonatal rat liver. In: Knook DL, Wisse E, editors. Kupffer cells and other sinusoidal liver cells. Amsterdam: Elsevier North Holland Biomedical; 1977. pp. 497–505. [Google Scholar]
- Naito M, Yamamura F, Takeya M, Takahashi K. Ultrastructural analysis of Kupffer cell progenitors. In: Kirn A, Knook DL, Wisse E, editors. Cells of the hepatic sinusoid. Rijswijk: Kupffer Cell Foundation; 1986. pp. 13–20. [Google Scholar]
- Naito M, Yamamura F, Nishikawa SI, Takahashi K. Development, differentiation and maturation of fetal mouse yolk sac macrophages in cultures. J Leukoc Biol. 1989;46:1–10. doi: 10.1002/jlb.46.1.1. [DOI] [PubMed] [Google Scholar]
- Naito M, Takahashi K, Nishikawa SI. Development, differentiation and maturation of macrophages in the fetal mouse liver. J Leukoc Biol. 1990;48:27–37. doi: 10.1002/jlb.48.1.27. [DOI] [PubMed] [Google Scholar]
- Naito M, Hayashi SI, Yoshida H, Nishikawa SI, Shultz LD, Takahashi K. Abnormal differentiation of tissue macrophage populations in “osteopetrosis” (op) mice defective in the production of macrophage colony stimulating factor. Am J Pathol. 1991;139:657–667. [PMC free article] [PubMed] [Google Scholar]
- Naito M, Umeda S, Yamamoto T, Moriyama H, Umezu H, Hasegawa G, Usuda H, Shultz LD, Takahashi K. Development, differentiation, and phenotypic heterogeneity of murine tissue macrophages. J Leukoc Biol. 1996;59:133–138. doi: 10.1002/jlb.59.2.133. [DOI] [PubMed] [Google Scholar]
- Nakakura-Ohshima K, Watanabe J, Kenmotsu S, Ohshima H. Possible role of immunocompetent cells and the expression of heat shock protein-25 in the process of pulpal regeneration after tooth injury in rat molars. J Electron Microsc. 2003;52:581–591. doi: 10.1093/jmicro/52.6.581. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Bringas P, Jr, Nanci A, Zeichner-David M, Ashdown B, Slavkin HC. Translocation of enamel proteins from inner enamel epithelia to odontoblasts during mouse tooth development. Anat Rec. 1994;238:383–396. doi: 10.1002/ar.1092380313. [DOI] [PubMed] [Google Scholar]
- Ohshima H, Sato O, Kawahara I, Maeda T, Takano Y. Responses of immunocompetent cells to cavity preparation in rat molars: an immunohistochemical study using OX6-monoclonal antibody. Connect Tissue Res. 1995;32:303–311. doi: 10.3109/03008209509013738. [DOI] [PubMed] [Google Scholar]
- Rutherford MS, Witsell A, Schook LB. Mechanisms generating functionally heterogeneous macrophages: chaos revisited. J Leukoc Biol. 1993;53:602–618. doi: 10.1002/jlb.53.5.602. [DOI] [PubMed] [Google Scholar]
- Sawa Y, Horie Y, Yamaoka Y, Ebata N, Kim T, Yoshida S. Production of colony-stimulating factor in human dental pulp fibroblasts. J Dent Res. 2003;82:96–100. doi: 10.1177/154405910308200204. [DOI] [PubMed] [Google Scholar]
- Soranzo MR, Koerten HK, Daems WT. Peroxidase activity and morphometric analysis of alveolar macrophages in guinea-pigs. J Reticuloendothel Soc. 1978;23:343–359. [PubMed] [Google Scholar]
- Takahashi K, Yamamura F, Naito M. Differentiation, maturation and proliferation of macrophages in the mouse yolk sac: a light-microscopic, enzyme-cytochemical, immunohistochemical, and ultrastructural study. J Leukoc Biol. 1989;45:87–96. doi: 10.1002/jlb.45.2.87. [DOI] [PubMed] [Google Scholar]
- Takashima A, Edelbaum D, Kitajima T, Shadduck RK, Gilmore GL, Xu S, Taylor RS, Bergstresser PR, Ariizumi K. Colony-stimulating factor-1 secreted by fibroblasts promotes the growth of dendritic cell lines (XS series) derived from murine epidermis. J Immunol. 1995;154:5128–5135. [PubMed] [Google Scholar]
- Tsuruga E, Sakakura Y, Yajima T, Shide N. Appearance and distribution of dendritic cells and macrophages in dental pulp during early postnatal morphogenesis of mouse mandibular first molars. Histochem Cell Biol. 1999;112:193–204. doi: 10.1007/s004180050407. [DOI] [PubMed] [Google Scholar]
- Vassiliou I, Lolis E, Nastos C, Tympa A, Theodosopoulos T, Dafnios N, Fragulidis G, Frangou M, Kondi-Pafiti A, Smyrniotis V. The combined effect of erythropoietin and granulocyte macrophage colony stimulating factor on liver regeneration after major hepatectomy in rats. World J Surg Oncol. 2010;8:57–62. doi: 10.1186/1477-7819-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse E. Kupffer cell reactions in rat liver under various conditions as observed in the electron microscope. J Ultrastruct Res. 1974;46:499–520. doi: 10.1016/S0022-5320(74)90070-7. [DOI] [PubMed] [Google Scholar]
- Widmann JJ, Fahimi HD. Proliferation of mononuclear phagocytes (Kupffer cells) and endothelial cells in regenerating rat liver. Am J Pathol. 1975;80:349–366. [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Naito M, Takahashi K. Kupffer cell proliferation and glucan-induced granuloma formation in mice depleted of blood monocytes by strontium-89. J Leukoc Biol. 1990;47:195–205. [PubMed] [Google Scholar]
- Yamamoto T, Kaizu C, Kawasaki T, Hasegawa G, Umezu H, Ohashi R, Sakurada J, Jiang S, Shultz L, Naito M. Macrophage colony-stimulating factor is indispensable for repopulation and differentiation of Kupffer cells but not for splenic red pulp macrophages in osteopetrotic (op/op) mice after macrophage depletion. Cell Tissue Res. 2008;332:245–256. doi: 10.1007/s00441-008-0586-8. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kawashima N, Suda H, Nakano Y, Takano Y, Azuma M. The existence of CD11c+ sentinel and F4/80+ interstitial dendritic cells in dental pulp and their dynamics and functional properties. Int Immunol. 2006;18:1375–1384. doi: 10.1093/intimm/dxl070. [DOI] [PubMed] [Google Scholar]