Abstract
Formins are an important and evolutionarily well conserved class of actin binding proteins with essential biological functions. Although their molecular roles in actin regulation have been clearly demonstrated in vitro, their functions at the cellular or organism levels are still poorly understood. To illustrate this problem, but also to demonstrate potential ways forward, we focus here on the DAAM group of formins. In vertebrates, DAAM group members have been demonstrated to be important regulators of cellular and tissue morphogenesis but, as for all formins, the molecular mechanisms underlying these morphogenetic functions remain to be uncovered. The genome of the fruitfly Drosophila encodes a single DAAM gene that is evolutionarily highly conserved. Recent work on dDAAM has already provided a unique combination of observations and experimental opportunities unrivalled by any other Drosophila formin. These comprise in vitro actin polymerisation assays, subcellular studies in culture and in vivo, and a range of developmental phenotypes revealing a role in tracheal morphogenesis, axonal growth and muscle organization. At all these levels, future work on dDAAM will capitalize on the power of fly genetics, raising unique opportunities to advance our understanding of dDAAM at the systems level, with obvious implications for other formins.
Key words: formin, actin cytoskeleton, DAAM, drosophila, axon growth, filopodia formation
Formins are evolutionarily highly conserved actin assembly factors with essential biological functions such as the regulation of cell division, cell motility and cell adhesion. Metazoan formins can be subdivided into seven clades: Diaphanous (DIA), formins (FMN), formin homology domain containing proteins (FHOD), delphilins, inverted formins (INF), formin-related genes in leukocytes (FRL) and Dishevelled-associated activators of morphogenesis (DAAM). All these metazoan formins share at least two functional domains, the formin homology domains 1 and 2 (FH1, FH2).1,2 Of these, the FH2 domain is both necessary and sufficient to nucleate actin in vitro. Although some formins have been implicated in the regulation of microtubules,3 the best understood molecular function of formins is to promote the nucleation and elongation of unbranched actin filaments.4,5 Accordingly, they play pivotal roles in the formation of various actin-based cellular structures, such as stress fibers, filopodia, focal adhesions and contractile rings.6,7 Their importance is further illustrated by loss-of-function analysis in vivo, for example, knockout mice for mDia1, FMN1, FMN2 and Delphilin exhibit myelo-proliferative defects, mild limb deformities, meiotic cell division defects and enhanced synaptic plasticity, respectively.8 However, although the molecular functions of formins in actin regulation are well established and their importance has been clearly demonstrated at the cellular and organism level, it is largely unclear through which molecular mechanisms and in which subcellular contexts they contribute to cytoskeletal regulation in a particular cell type or tissue.4,8 This is mainly due to the fact that much of our present knowledge about formins is based on in vitro assays and cellular studies relying on the overexpression of truncated protein isoforms, but an in depth in vivo analysis at the subcellular level in multicellular organisms is missing for most formins. Furthermore, formins display a high degree of redundancy (15 formin genes in mouse or human, 18–21 in fish), but our current understanding of the functional differences between different formin members is insufficient, not to mention the potential functional overlap with other protein classes also involved in the regulation of actin assembly. Hence, it follows that the lack of understanding at the cellular level is even more pronounced at the complex level of whole tissues, organs or organisms.
To gain a better understanding of formin functions, interesting new opportunities have arisen during the last five years through work in the fruitfly on the single Drosophila DAAM ortholog, dDAAM. Only a few studies have been published on dDAAM so far, but these investigations revealed a unique combination of observations and experimental opportunities, with a high potential for advancing our principal understanding of formin functions. To illustrate this point, we will review our current knowledge on DAAM formins, focussing first on the vertebrate Daam genes, then on the Drosophila gene, dDAAM.
Vertebrate DAAM Family Members: An Important Class of Formins
Members of the DAAM group of formins contain a GTPase binding (GBD), a diaphanous inhibitory (DID), an N-terminal dimerization (DD), a coiled-coil (CC), an FH1, an FH2 and a diaphanous autoregulatory domain (DAD) (Fig. 1A), which makes them closest relatives of the DIA and the FRL groups of formins.4,8 Despite of their overall similarity, comparative studies between hDaam1, mDia1 and the yeast formin Bni1p revealed certain differences in the crystal structure of their FH2 domains, and a difference in their catalytic efficiencies has also been reported.9,10 Furthermore, FH2 domains of some formins are better actin nucleation factors when combined with a DAD domain, and hDaam1 displayed the largest difference in this assay among the four formins tested.11 These specific structural and kinetic properties strongly suggest that even closely related formins may divert in their catalytic and regulatory functions. DAAM family members might therefore play unique cell biological roles which are not or only partly redundant with other formins.
Figure 1.
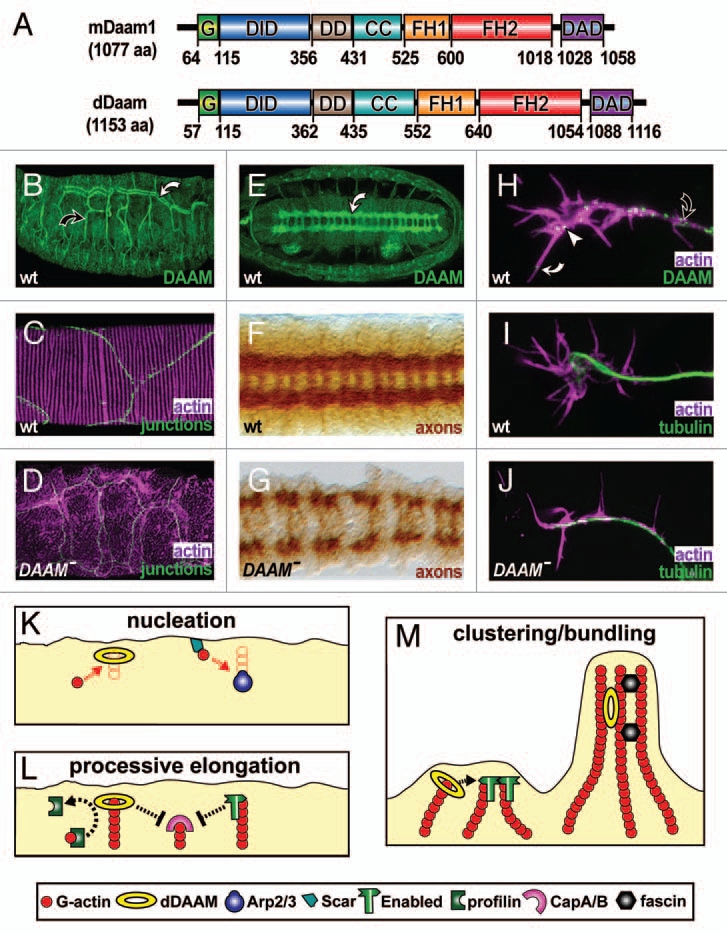
Properties and functions of Drosophila DAAM. (A) Mouse and Drosophila DAAM are of similar length and display the same functional domains: GTPase binding (G), diaphanous inhibitory (DID), N-terminal dimerisation (DD), coiled-coil (CC), formin homology 1 and 2 (FH1, FH2), diaphanous autoregulatory domain (DAD); residues demarcating the functional domains are shown below. (B) In embryos, dDAAM is strongly expressed in main (white curved arrow) and side branches (black curved arrow) of tracheal trees. (C) At high magnification, tracheae show parallel lines of F-actin enrichment (magenta) that run across cellular junctions (green, stained for De-Cadherin) in the main airways. (D) In tracheae of dDAAM loss-of-function mutant embryos, ordered F-actin patterns are abolished. (E) dDAAM is strongly expressed in the ladder-shaped neuropile within the embryonic CNS (white curved arrow). (F) In wild-type embryos, the axonal marker BP102 labels the ladder-like arrangement of the neuropile. (G) In dDAAM loss-of-function mutant embryos, the neuropile is severely disrupted indicating strong axonal growth defects. (H) In cultured primary embryonic neurons, dDAAM displays a punctate pattern along the axon (black curved arrow), but also at the growth cone (white arrow head) and in filopodia (white curved arrow). (I) In wild-type neurons, growth cones frequently display a hand-shaped broad appearance. (J) In dDAAM loss-of-function mutant neurons, growth cones tend to be narrow and display significantly reduced numbers of filopodia. (K–M) Potential functions of dDAAM during filopodia formation in Drosophila neurons. (K) dDAAM acts as an actin nucleator in parallel to Scar complex/Arp2/3 complex activity. (L) Like enabled, dDAAM promotes processive elongation of actin filaments, expected to collaborate with profilin and potentially antagonising the capping activities of the CapA/B complex. (M) The ability to bind and bundle actin filaments in vitro25 suggests potential roles for dDAAM in clustering of actin filament barbed ends (together with enabled; left) or the stabilisation of F-actin bundles (together with other bundlers, such as fascin; right).
The genomes of the common vertebrate model organisms, such as mouse, frog and chick, encode two DAAM family members which are broadly expressed in highly dynamic spatiotemporal patterns throughout embryogenesis.12,13 Prominent tissues of expression are the developing central nervous system (CNS), somites, dermomyotomes and heart. Within the embryonic CNS, the Daam1 and Daam2 genes typically display complementary expression patterns including the brain, spinal cord and retina,12,13 suggesting that they carry out cell-specific tasks instead of acting redundantly in these contexts.
Work on DAAM in vertebrate tissue culture models revealed a whole range of cellular functions that are consistent with the typical roles of formins in cytoskeletal regulation. Such roles of Daam1 include regulation of the shape and branching of porcine aortic endothelial cells,14 contribution to RhoA-dependent actin assembly in platelets,15 inhibition of cell proliferation and migration in endothelial cells,16 regulation of stress fiber formation and centrosome re-orientation in COS-7 and U2OS cells17 and microtubule stabilisation in endothelial cells.16 Interestingly, some of these cellular functions of DAAMs display context-dependent variations. For example, Daam1 promotes stress fiber formation in COS-7 and U2OS cells,17 but destabilizes stress fibers in endothelial cells.16 In addition, Xenopus data argue for Daam being the activator of RhoA,18 whereas studies in mammalian COS-7 cells and platelets suggest that Daam1 is a direct effector of Rho GTPases.14,15 In spite of these apparent controversies, the roles in stress fiber formation, centrosome orientation and microtubule stabilisation are novel functions for this formin family, providing exciting new opportunities for understanding DAAM function at the cellular level.
In vivo analysis of the vertebrate DAAM proteins began with the identification of the founding member of the DAAM subclass as a Dishevelled (Dsh) binding protein that has been implicated in non-canonical Wnt/Fz signalling during Xenopus gastrulation.18 Injection of morpholinos or deletion constructs confirmed such morphogenetic roles in convergent extension movements both in frog19–21 and zebrafish embryos.22 Protein localization studies with a GFP-fused Daam1 protein revealed a dynamic subcellular pattern in zebrafish notochord cells including the plasma membrane, cytoplasmic vesicles and F-actin rich cytoskeletal structures.22 Reported analyses in mouse are currently restricted to a hypomorphic Daam1 mutant allele, which showed important roles in heart morphogenesis.23 However, these mammalian studies have failed so far to support roles for DAAMs in Fz/Dsh signalling, or as an upstream regulator of RhoA. Complete loss-of-function analyses in mice are therefore needed to clarify these potential roles.
In conclusion, an extensive body of work has been published on vertebrate DAAM group members, clearly identifying them as important regulators of cell and tissue morhogenesis. However, as for all other formins, the mechanism by which vertebrate DAAMs perform these functions at cellular or organism levels are poorly understood.
Drosophila DAAM: A Typical Formin with Important in vivo Functions
The Drosophila genome encodes six formin genes, each representing one of the metazoan subclasses, except for the delphilin family which is missing in flies.2 While some of them (i.e., diaphanous and the FMN family member cappuccino) have been heavily studied in reference 8 and 24, dDAAM is so far the only invertebrate formin (with the exception of a D. discoideum formin) that has been investigated by detailed biophysical assays in vitro.25 These studies established that the FH2 domain of dDAAM behaves as a “bona fide” formin in actin nucelation and polymerization assays. Additionally, these data showed that the FH1 domain of dDAAM interacts with profilin-actin during processive actin assembly.25 The functional importance of this DAAM-profilin interaction has been further confirmed by in vivo studies and in primary neuron cultures.26,27 Interestingly, dDAAM was also shown to bind to the sides of actin filaments in vitro, which might suggest potential roles in actin filament bundling in cellular contexts.25
In Drosophila embryos, diaphanous and dDAAM are the most broadly expressed formins28 (Kalmár T and Mihály J, unpublished results). Accordingly, dDAAM has been shown to contribute to a range of developmental processes, none of which seems to relate to non-canonical Wnt/Fz signalling so far. At the cellular blastoderm stage the dDAAM protein displays a strong plasma membrane accumulation, particularly in the apical zone (Kalmár T and Mihály J, unpublished data). In agreement with this finding, embryos lacking both maternally and zygotically derived dDAAM fail to cellularise,29 a phenotype that still awaits further investigation. During later stages, dDAAM is highly expressed in the cardioblasts and the dorsal vessel (Drosophila heart) and, accordingly, heart tube morphogenesis is impaired in dDAAM mutant embryos (Molnár I and Mihály J, unpublished results). The latter observation is consistent with findings in mDaam1 mutant mice,23 suggesting that DAAM family formins play evolutionarily conserved roles in this context.
Another prominent embryonic dDAAM expression domain is the tracheal system (Fig. 1B), a well patterned network of tubular epidermal invaginations that serves as the respiratory organ.29 In agreement with this expression pattern, dDAAM mutant larvae display severe tracheal cuticle defects with collapsed and flattened tracheal tubes. In the tracheal system of wild type embryos, actin cables run in parallel beneath the apical surfaces of tracheal cells, perpendicular to the tube axis (Fig. 1C).29 In contrast, in dDAAM mutant tracheal cells, apical actin levels are lower than in wild-type, actin bundles are much shorter and thinner and patterned actin organization is almost completely lost29 (Fig. 1D). These studies established that the parallel running actin cables define the taenidial fold pattern of the cuticle, an important architectural element composed of extracellular matrix that maintains the tubular structure of tracheae. The loss of their organization is therefore the likely cause for tracheal collapse in DAAM mutant larvae.29 Moreover, this analyses presented genetic evidences that dDAAM is regulated by the RhoA small GTPase in the tracheal system and cooperates with Src family kinases during patterning of the tracheal cuticle. Given that RhoD and human DIA2C regulate endosome dynamics through Src activation,30 and RhoB induced Dia1 activation has been implicated in the regulation of endosome trafficking,31 it seems possible that the RhoA/dDAAM/Src module is not simply required to organize apical actin bundles. For example, it could also regulate exocytosis, thus orchestrating spatially patterned cuticle deposition during taenidial fold formation. Another intriguing aspect of apical actin organization in the tracheal system concerns the case of the main tracheal airways (white curved arrow in Fig. 1B) where actin bundles are perfectly aligned across cell boundaries (Fig. 1C). Since the dDAAM protein co-localizes with apical actin cables at cell adherens junctions, beyond its subcellular actin organizer function, dDAAM might play an important role in a transcellular patterning phenomenon as well.29
Finally, DAAM is strongly expressed in the developing nervous system and DAAM loss-of-function mutant embryos display severe disruptions of axonal compartments in the CNS (called neuropile; Fig. 1E–G). These defects include errors in midline crossing and breaks in longitudinal and commissural tracts27 (Fig. 1G), but also axonal stall phenotypes of motoraxons.32 Axonal growth is a key event of nervous system development and regeneration, depending on both the actin and microtubule cytoskeleton. 33,34 Consistently, a large number of actin regulators have been shown to be essential for axonal growth and guidance in many organisms including Drosophila.33–36 However, the findings for dDAAM were the first to demonstrate a role for any formin in axonal growth by true loss-of-function analysis.27 In addition, dDAAM is described to be the only formin expressed at detectable levels in embryonic Drosophila neurons.26,35 Unhindered by redundant functions of different formins, these observations offered new opportunities to address the function of formins in the nervous system. Genetic interaction studies using the CNS phenotypes as readouts have revealed so far functional links between dDAAM and Rac GTPases (Rac1, Rac2, Mtl), Enabled (Ena), Drosophila profilin (Chickadee, Chic) and two components of the Arp2/3 complex (Sop2, Arp66B),26,27 providing a promising starting point to unravel the genetic networks within which dDAAM operates in the nervous system. Interestingly, RhoA failed to genetically interact with dDAAM in the nervous system, tempting to speculate that dDAAM in neurons is activated through Rac GTPases rather than RhoA.
Refined Analyses of Drosophila DAAM in Primary Neurons
Important progress has been made by extending the study of dDAAM function to cultured embryonic primary neurons (Fig. 1H–J). Unlike in vivo studies, primary neurons provide refined subcellular readouts for cytoskeletal dynamics fully amenable to Drosophila genetics.37,38 So far, loss of dDAAM function in primary neurons was found to cause strong phenotypes, including reduction in filopodia numbers and filopodia length, an increase in the rates of filopodial elongation and retraction and a significant tendency to have longer axons.26,27,32 These findings delivered potential subcellular explanations for the observed in vivo functions of dDAAM in the CNS, as explained in greater detail elsewhere in reference 27 and 32. Studies in these primary neurons have also shown that Arp2/3 is the only other obvious nucleator besides dDAAM: the combined loss of dDAAM and Arp2/3 functions caused a complete absence of lamellipodia and filopodia and a severe general depletion of F-actin.26 Consequently, F-actin networks in Arp2/3 deficient neurons are primarily formin-derived, whereas dDAAM mutant neurons contain Arp2/3-derived networks. This constellation provides unique opportunities to compare the functions of these two nucleators in isolation. So far, experiments using this strategy have led to a model in which formins and Arp2/3 contribute through one common mode to filopodia formation,26 rather than through distinct molecular mechanisms as suggested previously in reference 39. Such overlapping roles of distinct nucleators are further supported by data from Hela and mouse melanoma cells showing that Arp2/3 and the formin mDia2 both contribute filaments to lamellipodial F-actin networks.40
The process of filopodia formation and dynamics in primary Drosophila neurons provides a promising context in which to study molecular mechanisms of dDAAM at the cellular level. These mechanisms of dDAAM potentially involve nucleation, processive elongation and F-actin bundling activities, most of which are expected to operate in close coordination with other factors, such as Arp2/3, Enabled, profilin, fascin or capping proteins (summarised in Fig. 1K–M). As mentioned before, some of these factors have already been shown to form complexes with dDAAM in vitro and/or to functionally interact with dDAAM in the nervous system in vivo, providing a promising starting point for studies in filopodia. Beyond their roles in actin regulation, DAAMs, such as other formins, are also recognised for their direct roles in microtubule regulation and stabilization,16 but the underlying mechanisms are poorly understood.3,7 Since the microtubule cytoskeleton of Drosophila primary neurons is also highly amenable to investigations,32,41 this cellular system has the potential to develop into a powerful experimental platform to investigate the links between formins and microtubules.
Conclusions and Perspectives
Drosophila DAAM clearly has become a very promising paradigm for work on formins, amenable to genetic and experimental approaches in well defined cellular contexts in vivo and in culture. The data reported so far are promising and provide an impressive number of observations and experimental approaches that can now be integrated and used to advance our insights into dDAAM function at the systems level. Further work will unravel the complex genetic networks that govern dDAAM functions during axonal growth or other actin-based cellular processes. Such knowledge will advance our principal understanding of cytoskeletal dynamics regulation at the cellular level, which certainly is one of the major challenges of modern cell biology.42 As clearly shown for Drosophila growth cones, the cytoskeletal regulators are evolutionarily well conserved at the molecular and cellular levels.32,35 Therefore, work on dDAAM will have implications beyond Drosophila and provide explanations for the important roles that DAAM and related formins play in mammals in neuronal and non-neuronal contexts alike.
Acknowledgments
We are grateful to Tom Millard for comments and critical reading. Work in the laboratory of J.M. is supported by OTKA grant K82039 and a Pfizer Hungary Award. A.P. and N.S.S. are supported by grants from the Wellcome Trust (077748/Z/05/Z and 092403/Z/10/Z) and the BBSRC (BB/I002448/1), as well as a studentship of the Fundacao para a Ciencia e a Tecnologia to C.G.P.
References
- 1.Chalkia D, Nikolaidis N, Makalowski W, Klein J, Nei M. Origins and evolution of the formin multigene family that is involved in the formation of actin filaments. Mol Biol Evol. 2008;25:2717–2733. doi: 10.1093/molbev/msn215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Higgs HN, Peterson KJ. Phylogenetic analysis of the formin homology 2 domain. Mol Biol Cell. 2005;16:1–13. doi: 10.1091/mbc.E04-07-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartolini F, Gundersen GG. Formins and microtubules. Biochim Biophys Acta. 2010;1803:164–173. doi: 10.1016/j.bbamcr.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- 5.Paul AS, Pollard TD. Review of the mechanism of processive actin filament elongation by formins. Cell Motil Cytoskel. 2009;66:606–617. doi: 10.1002/cm.20379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mellor H. The role of formins in filopodia formation. Biochim Biophys Acta. 2009;1803:191–200. doi: 10.1016/j.bbamcr.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 7.Chesarone MA, DuPage AG, Goode BL. Unleashing formins to remodel the actin and microtubule cytoskeletons. Nat Rev Mol Cell Biol. 2010;11:62–74. doi: 10.1038/nrm2816. [DOI] [PubMed] [Google Scholar]
- 8.Campellone KG, Welch MD. A nucleator arms race: cellular control of actin assembly. Nat Rev Mol Cell Biol. 2010;11:237–251. doi: 10.1038/nrm2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu J, Meng W, Poy F, Maiti S, Goode BL, Eck MJ. Structure of the FH2 domain of Daam1: implications for formin regulation of actin assembly. J Mol Biol. 2007;369:1258–1269. doi: 10.1016/j.jmb.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamashita M, Higashi T, Suetsugu S, Sato Y, Ikeda T, Shirakawa R, et al. Crystal structure of human DAAM1 formin homology 2 domain. Genes Cells. 2007;12:1255–1265. doi: 10.1111/j.1365-2443.2007.01132.x. [DOI] [PubMed] [Google Scholar]
- 11.Gould CJ, Maiti S, Michelot A, Graziano BR, Blanchoin L, Goode BL. The formin DAD domain plays dual roles in autoinhibition and actin nucleation. Curr Biol. 2011;21:384–390. doi: 10.1016/j.cub.2011.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kida Y, Shiraishi T, Ogura T. Identification of chick and mouse Daam1 and Daam2 genes and their expression patterns in the central nervous system. Brain Res Dev Brain Res. 2004;153:143–150. doi: 10.1016/j.devbrainres.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Nakaya MA, Habas R, Biris K, Dunty WC, Jr, Kato Y, He X, et al. Identification and comparative expression analyses of Daam genes in mouse and Xenopus. Gene Expr Patterns. 2004;5:97–105. doi: 10.1016/j.modgep.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Aspenstrom P, Richnau N, Johansson AS. The diaphanous-related formin DAAM1 collaborates with the Rho GTPases RhoA and Cdc42, CIP4 and Src in regulating cell morphogenesis and actin dynamics. Exp Cell Res. 2006;312:2180–2194. doi: 10.1016/j.yexcr.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Higashi T, Ikeda T, Shirakawa R, Kondo H, Kawato M, Horiguchi M, et al. Biochemical characterization of the Rho GTPase-regulated actin assembly by diaphanousrelated formins, mDia1 and Daam1, in platelets. J Biol Chem. 2008;283:8746–8755. doi: 10.1074/jbc.M707839200. [DOI] [PubMed] [Google Scholar]
- 16.Ju R, Cirone P, Lin SD, Griesbach H, Slusarski DC, Crews CM. Activation of the planar cell polarity formin DAAM1 leads to inhibition of endothelial cell proliferation, migration and angiogenesis. Proc Natl Acad Sci USA. 2010;107:6906–6911. doi: 10.1073/pnas.1001075107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ang SF, Zhao ZS, Lim L, Manser E. DAAM1 is a formin required for centrosome re-orientation during cell migration. PLoS One. 2010;5:13064. doi: 10.1371/journal.pone.0013064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- 19.Kim GH, Han JK. Essential role for beta-arrestin 2 in the regulation of Xenopus convergent extension movements. EMBO J. 2007;26:2513–2526. doi: 10.1038/sj.emboj.7601688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu W, Sato A, Khadka D, Bharti R, Diaz H, Runnels LW, et al. Mechanism of activation of the Formin protein Daam1. Proc Natl Acad Sci USA. 2008;105:210–215. doi: 10.1073/pnas.0707277105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato A, Khadka DK, Liu W, Bharti R, Runnels LW, Dawid IB, et al. Profilin is an effector for Daam1 in non-canonical Wnt signaling and is required for vertebrate gastrulation. Development. 2006;133:4219–4231. doi: 10.1242/dev.02590. [DOI] [PubMed] [Google Scholar]
- 22.Kida YS, Sato T, Miyasaka KY, Suto A, Ogura T. Daam1 regulates the endocytosis of EphB during the convergent extension of the zebrafish notochord. Proc Natl Acad Sci USA. 2007;104:6708–6713. doi: 10.1073/pnas.0608946104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li DQ, Hallett MA, Zhu WQ, Rubart M, Liu Y, Yang ZY, et al. Dishevelled-associated activator of morphogenesis 1 (Daam1) is required for heart morphogenesis. Development. 2011;138:303–315. doi: 10.1242/dev.055566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wasserman S. FH proteins as cytoskeletal organizers. Trends Cell Biol. 1998;8:111–115. doi: 10.1016/s0962-8924(97)01217-8. [DOI] [PubMed] [Google Scholar]
- 25.Barko S, Bugyi B, Carlier MF, Gombos R, Matusek T, Mihaly J, et al. Characterization of the biochemical properties and biological function of the formin homology domains of Drosophila DAAM. J Biol Chem. 2010;285:13154–13169. doi: 10.1074/jbc.M109.093914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonçalves-Pimentel C, Gombos R, Mihály J, Sánchez-Soriano N, Prokop A. Dissecting regulatory networks of filopodia formation in a Drosophila growth cone model. PLoS One. 2011;6:18340. doi: 10.1371/journal.pone.0018340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matusek T, Gombos R, Szecsenyi A, Sánchez-Soriano N, Czibula A, Pataki C, et al. Formin proteins of the DAAM subfamily play a role during axon growth. J Neurosci. 2008;28:13310–13319. doi: 10.1523/JNEUROSCI.2727-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka H, Takasu E, Aigaki T, Kato K, Hayashi S, Nose A. Formin3 is required for assembly of the F-actin structure that mediates tracheal fusion in Drosophila. Dev Biol. 2004;274:413–425. doi: 10.1016/j.ydbio.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 29.Matusek T, Djiane A, Jankovics F, Brunner D, Mlodzik M, Mihaly J. The Drosophila formin DAAM regulates the tracheal cuticle pattern through organizing the actin cytoskeleton. Development. 2006;133:957–966. doi: 10.1242/dev.02266. [DOI] [PubMed] [Google Scholar]
- 30.Gasman S, Kalaidzidis Y, Zerial M. RhoD regulates endosome dynamics through Diaphanous-related Formin and Src tyrosine kinase. Nat Cell Biol. 2003;5:195–204. doi: 10.1038/ncb935. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez-Borja M, Janssen L, Verwoerd D, Hordijk P, Neefjes J. RhoB regulates endosome transport by promoting actin assembly on endosomal membranes through Dia1. J Cell Sci. 2005;118:2661–2670. doi: 10.1242/jcs.02384. [DOI] [PubMed] [Google Scholar]
- 32.Sánchez-Soriano N, Gonçalves-Pimentel C, Beaven R, Haessler U, Ofner L, Ballestrem C, et al. Drosophila growth cones: a genetically tractable platform for the analysis of axonal growth dynamics. Dev Neurobiol. 2010;70:58–71. doi: 10.1002/dneu.20762. [DOI] [PubMed] [Google Scholar]
- 33.Lowery LA, van Vactor D. The trip of the tip: understanding the growth cone machinery. Nat Rev Mol Cell Biol. 2009;10:332–343. doi: 10.1038/nrm2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dent EW, Gupton SL, Gertler FB. The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sánchez-Soriano N, Tear G, Whitington P, Prokop A. Drosophila as a genetic and cellular model for studies on axonal growth. Neural Develop. 2007;2:9. doi: 10.1186/1749-8104-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pak CW, Flynn KC, Bamburg JR. Actin-binding proteins take the reins in growth cones. Nat Rev Neurosci. 2008;9:136–147. doi: 10.1038/nrn2236. [DOI] [PubMed] [Google Scholar]
- 37.Prokop A, Küppers-Munther B, Sánchez-Soriano N. Using primary neuron cultures of Drosophila to analyse neuronal circuit formation and function. In: Hassan BA, editor. The making and un-making of neuronal circuits in Drosophila. New York: Springer Science + Business Media; 2011. In press. [Google Scholar]
- 38.Sánchez-Soriano N, Gonçalves-Pimentel C, Beaven R, Prokop A. Using Drosophila growth cones to dissect F-actin network regulation at the cellular level. In: Mellor H, editor. Actin 2009. Bristol: 2010. [Google Scholar]
- 39.Mattila PK, Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol. 2008;9:446–454. doi: 10.1038/nrm2406. [DOI] [PubMed] [Google Scholar]
- 40.Yang C, Czech L, Gerboth S, Kojima S, Scita G, Svitkina T. Novel roles of formin mDia2 in lamellipodia and filopodia formation in motile cells. PLoS Biol. 2007;5:317. doi: 10.1371/journal.pbio.0050317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sánchez-Soriano N, Pimentel C, Travis M, Haessler U, Ofner L, Dajas-Bailador F, et al. CSHL Meeting on Axon Guidance, Synaptic Plasticity and Regeneration. 2009. New insights into growth cone advance and filopodia formation in a Drosophila growth cone model. [Google Scholar]
- 42.Insall RH, Machesky LM. Actin dynamics at the leading edge: from simple machinery to complex networks. Dev Cell. 2009;17:310–322. doi: 10.1016/j.devcel.2009.08.012. [DOI] [PubMed] [Google Scholar]


