Abstract
Ophiocordyceps unilateralis (Ascomycota: Hypocreales) is a specialized parasite that infects, manipulates and kills formicine ants, predominantly in tropical forest ecosystems. We have reported previously, based on a preliminary study in remnant Atlantic Forest in Minas Gerais (Brazil), that O. unilateralis represents a species complex. On each of the four species of infected carpenter ant (Camponotus) collected, the fungus—characterized macroscopically by a single stalk arising from the dorsal neck region on which the sexual structures (stromatal plates) are borne laterally—can readily be distinguished both microscopically and functionally. Here, we describe and discuss the biology, life cycle and infection strategies of O. unilateralis s.l. and hypothesize that there may be hundreds of species within the complex parasitizing formicine ants worldwide. We then address the diversity within related hypocrealean fungi, with particular reference to symbionts (mutualists through to parasites), and argue that the widely-quoted total of extant fungi (1.5 million species) may be grossly underestimated.
Key words: zombie ants, Ophiocordyceps, Hypocreales, tropical forests, fungal diversity, symbionts
“How many species?” has been the rallying cry for systematists and ecologists, championing the interests of their particular organismic group, since the question was posed over 30 years ago.1,2 Although it was answered soon after for the Kingdom Fungi3—with an estimated total in the region of 1.5 million species, of which, less than 100,000 (∼7%) have been described, thus far4—this group of organisms still receives relatively little press in terms of its biodiversity and the pivotal role it plays in ecosystem functioning. Recently, however, the subject has been revisited within the context of microbes associated with beetles.5 Of the near one million species of insects that have been described, beetles are by far the most numerous. However, in terms of overall biodiversity, “beetles may represent just the tip of the iceberg,”5 since “A single beetle species itself can house an entire community of associated [predominantly fungal] species.”5 The premise put forward is that microbial diversity and ecology have been neglected in favor of other disciplines, especially in the area of biological mutualisms and antagonisms between macro- and micro-organisms.1,5 An illustration of Ophiocordyceps unilateralis on its ant host was chosen by the latter authors to emphasize their point, that—for most of the insect-microbial interactions—the ecology and, more pragmatically, the pharmacological properties of the microbes involved remain unknown.
We have now shown6 that the interactions between O. unilateralis and its Camponotus hosts are much more complex than has previously been supposed.7–10 It had been posited that the morphological variation noted in global collections of this fungus from formicine ants “is undoubtedly a consequence of its wide geographic range,”8 and that this would best be resolved at the varietal level, as with the Ophiocordyceps species complexes associated with myrmicine and ponerine ants.11,12 However, working in remnant fragments of Atlantic Forest (Mata Atlántica) in Minas Gerais, Brazil,6 we discovered a far more complex scenario: one that will necessitate a re-evaluation of the diversity of species within Ophiocordyceps unilateralis s.l., as well as in the genus Ophiocordyceps and related genera of Hypocreales—and, eventually, within the kingdom fungi—with particular reference to tropical forests. These ecosystems harbor a hyperdiversity of fungi, especially when we consider they are found as symbionts (ranging from mutualists to parasites) of the extremely diverse flora and entomofauna, and even of other fungi. Based on such studies, it may also be possible to better interpret their role in arthropod population dynamics and, holistically, in ecosystem functioning.
The Host-Pathogen System: A Diversity of Interactions
As has been emphasized previously in reference 2 and 5, insect-microbial associations have been a source of useful drugs: although, “The pharmacological properties of the ant-infesting fungus [O. unilateralis s.l.] have yet to be investigated.”5 What can we expect from such specialized fungi, and from related entomopathogenic Hypocreales, in general? Throughout the life cycle, there are unique challenges that must be met by equally unique metabolic activities. The fungal pathogen must attach securely to the arthropod exoskeleton and penetrate it—avoiding or suppressing host defences—then, control the behavior of the host before killing it; and finally, it must protect the cadaver from microbial and scavenger attack. What is not generally realized—certainly not by drug companies—is that such coevolved fungi are pleiomorphic, with well-defined parasitic, necrotrophic and saprophytic phases: each one morphologically, genetically and physiologically distinct. Only now, are we beginning to try to understand the mechanisms and the biochemical pathways that drive these systems.
Our initial studies are focused on investigating the form and function of the weaponry deployed by the pathogen (O. unilateralis s.l.) to breach the ant host's formidable defenses. We suppose that the heavy armament “of choice” is the stromatal plate (the sexual stage or teleomorph) produced laterally on a stalk or clava emerging from behind the head of the ant—the emblematic feature of the zombie- ant fungus—buried within the stroma are the flask-shaped, thick-walled ascomata producing sac-like structures called asci. Each of these is fitted with a specialized firing cap containing the missiles: large (from 80 > 200 µm long) multiseptate, sexual spores (ascospores). These are released under pressure from the launch pad—the ant cadaver—typically, affixed to the underside of a leaf on an understorey forest shrub. We also assume that the system is designed to operate when ant activity is at its peak and the potential targets are in range and, therefore, that microclimatic factors (light, temperature, humidity) will play a crucial role. What we do know, from our preliminary study in reference 6, is that there is a species complex of macroscopically-similar pathogens (O. unilateralis s.l.) producing not only sexual stages (teleomorphs), but also asexual stages (anamorphs), that differ widely in form and function, each equipped with in-built insurance mechanisms to increase the chances of success if the primary missiles fail to hit their targets.
Currently, we are working on documenting the diverse strategies employed by this newly-delimited complex of Ophiocordyceps species to reach new hosts. Thus, we will be in a better position to re-interpret the taxonomic, as well as the ecological significance of the worldwide collections of the zombie-ant fungus, especially of the somewhat bizarre, multiple asexual stages (synanamorphs) described on Polyrhachis ants infected with O. unilateralis s.l. from West Africa.8
From studies of other entomopathogenic fungi,13 we know that there are also critical host-recognition factors to trigger the formation of the limpet-like appressorium: the “beach-head” from which the infective hypha drills through the complex multi-layered exoskeleton using a combination of mechanical pressure and enzymes (chitinase, lipase and protease). We now have circumstantial evidence from our work with freshly-released ascospores of Ophiocordyceps from different Camponotus hosts, which suggests that there are also sophisticated signals between pathogen and host in this species complex since recognizable infection structures (appressoria) are never produced in vitro, only the secondary (“insurance”) spores mentioned above: sticky-capped conidia on hair-like extensions (capilliconidiophores) for species with delicate, filiform ascospores; thick-walled, resting spores (chlamydospores) for the larger, more robust ascospores. Once the infective hypha breaches the exoskeleton, avoiding the cellular and humoral defences of the ant, the fungus grows in the hemocoel as free-living yeast cells. However, time periods are not clear cut, but we suppose that in a matter of days the yeast phase colonizes the hemocoel and then produces the nerve toxins to alter the behavior of the ant causing it to climb and bite onto vegetation, often choosing the adaxial leaf midrib and a specific orientation—hence, the zombie-ant epithet6—where it soon dies. During this phase, we expect—based on work from other systems—that there is a complex interplay of protein expression between fungal parasite and manipulated host. It would come as no surprise to discover that much of the host-pathogen interaction occurs prior to protein expression, via RNA silencing or suppression of silencing. Hyphal outgrowths from the body orifices and joints further secure the ant to the substrate whilst, internally, the saprophytic mycelium, consisting of lipidrich storage bodies, colonizes and mummifies the cadaver. The logistics governing production of the fruiting stalk appear to be controlled to some extent by external conditions, but, probably in a matter of weeks, rather than days for the synanamorphs, the stromata are fertile and the cycle is completed.
Diversity and Importance of Fungi in Tropical Forest Ecosystems
The zombie-ant fungus has a pantropical forest distribution on formicine hosts, and may also be found in warm-temperate forest systems. However, like the ants, it is far more common in tropical forests. Our study,6 in an outlying fragment of the fast-disappearing Atlantic rainforest, has revealed unexpected diversity within O. unilateralis s.l., which, if repeated on a global scale, could mean that there are tens if not hundreds of species still to be delimited within the complex. Moreover, there are similar Ophiocordyceps complexes within tropical ants, in general, yet to be investigated.10 So, we will be hoping to address the question: to what degree does this diversity scale up when we step back and look at a fragment of forest or at a biome, such as the Atlantic Forest, and imagine all of the host-symbiont interactions, focusing on the hypocrealean fungi? But how does this sit with the oft-repeated mantra that arthropods are the true “kings of the jungle,” especially when species diversity is the parameter of interest? It has been proposed that there is an intermediate body size at which organisms are globally most species diverse,14 effectively a humped distribution of species diversity that is concentrated somewhere between small and large insects. According to this view, smaller organisms (i.e., microbes) have cosmopolitan distributions. However, much of the data presented concern predominantly free-living organisms and even for these, the view has been challenged.15 It is becoming increasingly apparent that microbes such as fungi may be more influenced by biogeography than previously thought, and the symbiotic lifestyle can be an important factor in speciation.16
Not only does this make the question of “how many species” appear imponderable— and, thereby, makes the original estimated figure of 1.5 million fungal species redundant, even though this was later still held to be a viable working hypothesis17— it also raises basic questions about the role of these pathogens in ecosystem functioning, particularly in tropical forests where ants are the drivers.18 We have focused here on Ophiocordyceps as the flagship genus for a re-examination of fungal diversity. However, there are many similar fungal: macro-organism associations in tropical forest systems that we are only beginning to uncover, let alone investigate in any depth. Another example, also involving hypocrealean fungi, should help to emphasize the biodiversity, food webs and multi-trophic interactions involved in such systems.
Theobroma gileri, the only species of the chocolate-tree genus found west of the Andean Cordillera, occurs in restricted pockets in fragile cloud forest systems on the western border of Ecuador and Colombia. Searches to find the centre of origin of a devastating disease of cocoa (Theobroma cacao) revealed a complex associated with its rare indigenous forest host (T. gileri) and the causal fungal pathogen (Moniliophthora roreri).19 Not only is there a guild of novel mycoparasites colonizing the pathogen, but also a suite of unusual invertebrate natural enemies. One of these, a bizarre dipteran larva—equipped with a hooked abdomen to cling onto the substrate as it feeds on fungal spores covering the pods high in the canopy—proved to be difficult to identify. Attempts to follow the life cycle, in order to obtain adult flies for taxonomic studies, were thwarted since all the pupae were hyperparasitized by a wasp belonging to a genus described over a century ago but not collected since (A. Polaszek, Natural History Museum, London, pers. comm.). Moreover, tissue samples taken from healthy pods and stems of T. gileri revealed a rich diversity of endophytic fungi, with many representatives in the Hypocreales,20 including a new clade of Trichoderma spp.21 Molecular examination of this novel endophytic clade in the commercially-important genus Trichoderma, is revealing only now the presence of seven new species within the T. harzianum complex alone (G.J. Samuels, ARS-USDA, Washington DC, pers. comm.).
Perspectives
The reciprocal question to that posed in the introduction to this paper, is: “Why should we care how many species there are?”2 As highlighted before,2,5 microbial ecology has been neglected, and we need to know how these systems function and their microbial species composition: the pragmatic incentive is that there could be “products of great use to humans.”22 The latter authors have recently made an important contribution in addressing this largely conjectural analysis, and provided evidence that “novel biology [of tropical forest endophytes] will indeed yield novel chemistry of potential value.”22 Since tropical forest systems are fast disappearing, there is an added urgency if we are “to understand the structure and functioning of ecosystems, particularly tropical ecosystems, much better than we do. And we cannot hope to do this if we do not even know what there is there, and why tropical diversity is what it is?”2 Fungi underpin tropical forest systems. If we only know a small fraction of the extant fungal species,4 we have a lot of catching up to do. The task has suddenly become even more daunting with the very latest estimate of the number of fungi being put at 5.1 million species, based on molecular data.23 Prospects are not bright, however, as we are rapidly losing the tropical forest ecosystems, as well as the mycologists who can contribute to understanding them.
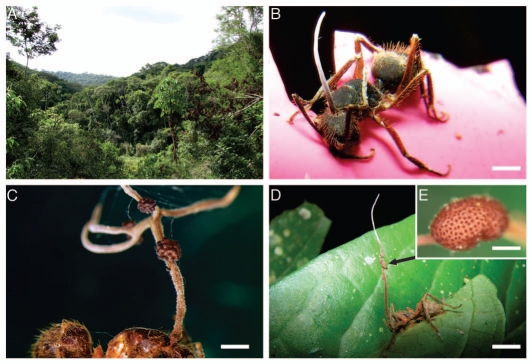
Figure 1.
Most common carpenter ant species infected with Ophiocordyceps s.l. in Atlantic rain forest in Minas Gerais, Brazil. (A) Sample area, Mata do Paraíso, near Viçosa, a small (300 ha) reserve of remnant forest; (B) Camponotus rufipes showing early stage in development of Ophiocordyceps camponoti-rufipedis with a single asexual synnema arising from the dorsal pronotum, the ant had died biting into a plastic label identifying a disease-outbreak site, (bar = 1.5 mm); (C) Camponotus balzani with mature Ophiocordyceps camponoti-balzani, showing the elaborate, branching, antler-like synnema/clava bearing scattered ascostromata with prominent semi-erumpent ascomata (compare E, bar = 1.0 mm); (D) Camponotus rufipes in more typical death position biting into leaf margin, with unbranched synnema/clava producing groups of stromata (arrow, bar = 4 mm); (E) inset, shows the ascomatal necks with the ascomata buried within the stroma (bar = 0.4 mm).
Figure 2.
Variations on a theme. (A) Ascospore of Ophiocordyceps camponoti-rufipedis germinating to produce secondary spores (capilliconidia) on needle-like capilliconidiophores (bar = 30 µ); (B) Ascospore of O. camponoti-melanotici producing true Hirsutella phialide with swollen base tapering to a needle-like neck (bar = 15 µm); (C) Ascospore of O. camponoti-balzani becoming swollen with bead-like cells and the formation centrally of an appressorial-like structure (bar = 30 µm); (D) Ascospore of an un-named Ophiocordyceps sp. from Camponotus atriceps producing numerous swollen side branches of unknown function (bar = 30 µm); (E and F) Two undescribed, specialized mycoparasites on Ophiocordyceps illustrating the biodiversity and complexity associated with the system (E, bar = 2 mm; F, bar = 2 mm).
Acknowledgments
H.C.E. is a visiting scientist in the Postgraduate Program in Entomology, funded by the Brazilian National Council for Research (CNPq, grant no. 401610/2009-8). S.L.E. is in receipt of a CNPq scholarship (300920/2010-5).
References
- 1.May RM. How many species are there on earth? Science. 1988;241:1441–1449. doi: 10.1126/science.241.4872.1441. [DOI] [PubMed] [Google Scholar]
- 2.May RM. How many species? Phil Trans R Soc Lond B. 1990;330:293–304. [Google Scholar]
- 3.Hawksworth DLH. The fungal dimension of biodiversity: magnitude, significance and conservation. Mycol Res. 1991;95:641–655. [Google Scholar]
- 4.Kirk PM, Cannon PF, Minter DM, Stalpers JA, editors. Dictionary of the Fungi. 10th edition. Wallingford, UK: CAB International; 2008. [Google Scholar]
- 5.Berenbaum MR, Elsner T. Bugs' bugs. Science. 2008;322:52–53. doi: 10.1126/science.1164873. [DOI] [PubMed] [Google Scholar]
- 6.Evans HC, Elliot SL, Hughes DP. Hidden diversity behind the zombie-ant fungus Ophiocordyceps unilateralis: four new species described from carpenter ants in Minas Gerais, Brazil. PLoS ONE. 2011;6:17024. doi: 10.1371/journal.pone.0017024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans HC. Natural control of arthropods, with special reference to ants (Formicidae), by fungi in the tropical high forest of Ghana. J Appl Ecol. 1974;11:37–49. [Google Scholar]
- 8.Evans HC, Samson RA. Cordyceps species and their anamorphs pathogenic on ants (Formicidae) in tropical forest ecosystems. II. The Camponotus (Formicinae) complex. Trans Br mycol Soc. 1984;82:127–150. [Google Scholar]
- 9.Samson RA, Evans HC, Hoekstra ES. Notes on entomogenous fungi from Ghana. VI The genus Cordyceps. Proc K Nederl Akad Wetensch, Ser C. 1982;85:589–605. [Google Scholar]
- 10.Evans HC. Entomopathogenic fungi associated with ants (Formicidae): a review. In: Misra JK, Horn BW, editors. Trichomycetes and Other Fungal Groups. Enfield, USA: Science Publishers; 2002. pp. 119–144. [Google Scholar]
- 11.Evans HC, Groden E, Bischoff JF. New fungal pathogens of the red ant, Myrmica rubra, from the UK and implications for ant invasions in the USA. Fungal Biol. 2010;114:451–456. doi: 10.1016/j.funbio.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Evans HC, Samson RA. Cordyceps species and their anamorphs pathogenic on ants (Formicidae) in tropical forest ecosystems. I. The Cephalotes (Myrmicinae) complex. Trans Br Mycol Soc. 1982;79:431–453. [Google Scholar]
- 13.Samson RA, Evans HC, Latge JP. Atlas of Entomopathogenic Fungi. Berlin: Springer-Verlag; 1988. [Google Scholar]
- 14.Finlay BJ. Global dispersal of free-living microbial eukaryote species. Science. 2002;296:1061–1063. doi: 10.1126/science.1070710. [DOI] [PubMed] [Google Scholar]
- 15.Green J, Bohannan JM. Spatial scaling of microbial diversity. Trends Ecol Evol. 2006;21:501–507. doi: 10.1016/j.tree.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Giraud T, Refrégier G, Le Gac M, de Vienne DM, Hood ME. Speciation in fungi. Fungal Genet Biol. 2008;45:791–802. doi: 10.1016/j.fgb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Hawksworth DL. The magnitude of fungal diversity: the 1.5 million species estimate revisited. Mycol Res. 2001;105:1422–1432. [Google Scholar]
- 18.Hölldobler B, Wilson EO. The Ants. Cambridge, MA: Harvard University Press; 1990. [Google Scholar]
- 19.Evans HC, Holmes KA, Reid AP. Phylogeny of the frosty pod pathogen of cocoa. Pl Pathol. 2003;52:149–160. [Google Scholar]
- 20.Evans HC, Holmes KA, Thomas SE. Endophytes and mycoparasites associated with an indigenous forest tree, Theobroma gileri, in Ecuador and a preliminary assessment of their potential as biocontrol agents of cocoa diseases. Mycol Progress. 2003;2:149–160. [Google Scholar]
- 21.Samuels GJ. Trichoderma: Systematics, the sexual state and ecology. Phytopath. 2006;96:195–206. doi: 10.1094/PHYTO-96-0195. [DOI] [PubMed] [Google Scholar]
- 22.Smith SA, Tank DC, Boulanger LA, Bascom-Slack CA, Eisenman K, et al. Bioactive endophytes warrant intensified exploration and conservation. PLoS One. 2008;3:3052. doi: 10.1371/journal.pone.0003052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blackwell M. The fungi: 1, 2, 3… 5.1 million species? Am J Bot. 2011;98:426–438. doi: 10.3732/ajb.1000298. [DOI] [PubMed] [Google Scholar]