Abstract
Vitamin B12 deficiency is emerging as a growing public health problem. The most commonly used diagnostic tests are limited in accuracy, sensitivity, and are non-specific for B12 deficiency. The aim of this study was to develop a simple B12 Breath Test (BBT) to more accurately evaluate vitamin B12 status as an alternative to the most common diagnostic test, serum B12 levels. The breath test is based on the metabolism of sodium 1-13C-propionate to 13CO2 which requires B12 as a cofactor. We initially compared the BBT to current B12 diagnostic methods in 58 subjects. Subjects also received a second BBT 1–3 days after initial testing to evaluate reproducibility of results. Propionate dosage, fasting times, and collection periods were compared respectively. The dose of sodium 1-13C-propionate (10 to 50 mg) gave equivalent results while an 8 hour fast was essential. Statistical analysis revealed that breath collection times could be reduced to just a baseline and 10 and 20 minutes following propionate dosing. We also measured the incidence of B12 deficiency with the BBT in 119 patients with chronic pancreatitis, Crohn’s disease, small intestinal bacterial overgrowth, and subjects over 65 years of age. The BBT results agreed with previous publications showing a higher incidence of B12 deficiency in these patients. The BBT may provide clinicians with a non-invasive, accurate, reliable, and reproducible diagnostic test to detect vitamin B12 deficiency.
Introduction
Vitamin B12 (cobalamin) deficiency is emerging as a common clinically important problem. The 3,000 person Framingham Study indicated that almost 40% of these generally healthy adults had low serum B12 levels, <258 pmol/l (1). Cobalamin levels at 258 pmol/l or lower are at risk for neurologic signs and symptoms of B12 deficiency. Patients with clinically important B12 deficiency may not be aware of this disorder since many are asymptomatic. If untreated, vitamin B12 deficiency may cause significant morbidity largely related to hematological and neurological aberrations.
A serum B12 level is the most commonly used diagnostic test in cases of suspected deficiency and is typically measured by automated competitive displacement assays. The perception, based almost exclusively on semantics, tradition, and convenience, is that serum B12 provides an accurate indication of a patient’s vitamin B12 status. Although this test is inexpensive and readily available, many publications suggest serum B12 levels do not reflect vitamin deficiency and frequently shows false positives and negative results (2–6). Normal lab values range from 200–900 pg/ml and values at the lower range (anywhere from 100–400 pg/ml) are not considered diagnostic as serum vitamin B12 levels appear to be maintained at a certain level at the expense of long-term tissue stores.
Although it may be true that vitamin B12 status may affect circulating homocysteine by “backing up” the metabolism of methionine, it is also not a distinct measure of vitamin B12 status. Serum Homocysteine (HC) can become elevated by several confounding factors such as age, smoking, vitamin B6 status, genetic abnormalities, clinical folate deficiency, and certain classes of medications (i.e, carbazepine, methotrexate). The serum MMA levels are generally accepted as the most accurate available test for detecting B12 deficiency (7). However, serum MMA levels must be interpreted with caution in patients with chronic renal failure due to their tendency to accumulate MMA (8). The assay is not normalized to account for dilution or concentration caused by kidney function. Therefore, it can give falsely high values in patients with renal insufficiency. The typical method for measurement of serum MMA is stable isotope dilution analysis by liquid chromatograph-mass spectrometer/mass spectrometer (LC-MS/MS) (9). Thus, the analytical methods for this metabolite are expensive for routine use and not widely used in clinical practice.
In light of the shortcomings of these vitamin B12 deficiency screening procedures, we developed a simple, non-invasive, and low-cost diagnostic breath test to more detect B12 functional deficiency. The B12 Breath Test (BBT) quantitates the metabolism of 13C-labeled propionate to 13CO2 by the pathway shown in Fig. 1. 13C is a non-radioactive, naturally occurring isotope which is safe for human use. The pathway from propionate to CO2 requires B12 as a cofactor. We hypothesized that individuals with B12 deficiency would have lower 13CO2 recovery compared with individuals with normal vitamin B12 status.
Figure 1.
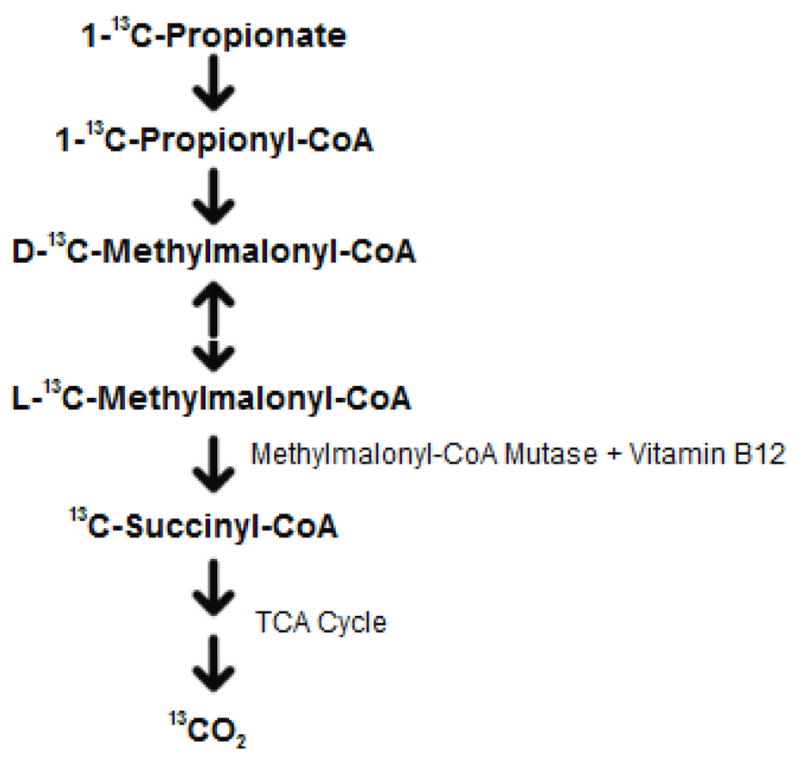
Principal of the vitamin B12 breath test using sodium 1-13C-bicarbonate and detecting breath 13CO2. Methylmalonyl-CoA mutase is a vitamin B12 dependant enzyme.
We prospectively evaluated how well the BBT identified vitamin B12 deficient individuals by measuring methylmalonic acid (MMA), homocysteine (HC) and cobalamin levels in the blood. We also evaluated the reproducibility of the test, conducted a sodium 1-13C-propionate dose ranging study, determined if the number of breath collections could be reduced, and assessed if the 8-hour fast could be shortened. After the initial test development studies, we measured the incidence of B12 deficiency with the BBT in 119 subjects with chronic pancreatitis, Crohn’s disease, small intestinal bacterial overgrowth, and subjects over 65 years of age. Previous reports have shown these subgroups have a higher incidence of B12 deficiency.
Methods
These studies were conducted under full review and approval by the University of Florida Institutional Review Board. All subjects were 18 years of age or older and gave informed consent prior to enrolling into these studies. No subjects were receiving supplemental doses of vitamin B12 for the past year (>100 mcg/day).
For Study group 1, we enrolled 26 adults at potential risk for B12 deficiency (25–97 yrs, mean=63) and 32 healthy controls (18–64 yrs, mean=39). The initial subject demographics are detailed in Table 1. Based on prior published research reports, we defined at-risk individuals for B12 deficiency as either ≥65 years of age or had at least one of the following gastrointestinal diseases: chronic pancreatitis, Crohn’s disease, or small intestinal bacterial overgrowth. Chronic pancreatitis was previously confirmed by abnormal CT scans showing pancreatic calcification, pancreatic atrophy or enlargement of the main pancreatic duct. Crohn’s disease was previously confirmed by endoscopy and/or histology, while small intestinal bacterial overgrowth individuals had a positive 14C-xylose breath test. The thirty-two healthy controls were used to determine the normal range of the BBT. Overall, at-risk subjects were significantly older than healthy controls. The genders of at-risk individuals were equally divided while the healthy controls had an abundance of female subjects. At-risk subjects were mostly Caucasian, while the control group was 50% minority (Hispanic). No appreciable differences were seen in the body mass index (BMI) between groups.
Table 1.
Participant Demographics for Study Group 1.
| At-risk Subjects | Healthy Subjects | |
|---|---|---|
| Number | 26 | 32 |
| Age (years) | Mean = 63 yrs (range 25–97 yrs) | Mean = 39 yrs (range 18–64 yrs) |
| Gender | 13 males, 13 females | 7 males, 25 females |
| Race | 23 Caucasians, 1 African American, 1 Hispanic, 1 Other | 14 Caucasians, 1 African American, 16 Hispanic, 1 Other |
| BMI (kg/m2) | Mean = 25.2 (range 16.4–35.7) | Mean = 26.1 (range 17.1–38.4) |
The demographics of study group 2 included 119 subjects as detailed in Table 2. These at-risk individuals were either ≥65 years of age or had at least one of the following gastrointestinal diseases: chronic pancreatitis, Crohn’s disease, or small intestinal bacterial overgrowth. Confirmation of these diseases as inclusion criteria was similar to study group 1 above.
Table 2.
Participant demographics of Study Group 2 for BBT and MMA Comparison Studies.
| Group | Number of Subjects | Gender | Mean Age ± SD |
|---|---|---|---|
| Crohn’s Disease | 33 | 9 M 24 F | 40 ± 15 |
| Chronic Pancreatitis | 9 | 5 M 4 F | 63 ± 14 |
| Sm. Intestinal Bacterial Overgrowth | 44 | 4 M 40 F | 55 ± 14 |
| Over 65 yrs old | 32 | 8 M 24 F | 77 ± 8 |
Blood samples were collected after an overnight fast (8 hr) to determine serum vitamin B12, homocysteine, and methylmalonic acid levels. All three B12 biomarkers were measured for group 1 but serum methylmalonic was only measured for group 2. The serum determinations were conducted by Quest Diagnostics using standard methods and reference intervals established by their laboratory.
The initial BBT utilized 120 minutes of breath collections for Group 1 subjects who had been fasting and not smoking for at least 8 hours prior to testing. Two baseline breath samples (−10 and −5 minutes) were collected into 10 ml Exetainer tubes (Labco, Ltd., High Wycombe, UK) just prior to dosing. Group 1 subjects were orally administered 50 mg sodium 1-13C-propionate (gift of Cambridge Isotope Laboratories, Andover, MA) dissolved in 30 ml water followed by another 170 ml of water. Sodium propionate is on the FDA Generally Regard as Safe List. Breath samples were collected every 10 minutes after dosing for the first hour and every 15 minutes for the second hour. Breath samples were sent to Metabolic Solutions (Nashua, NH) for assay.
The amount of 13CO2 in the Exetainer breath storage tubes was measured with a Europa Scientific 20/20 gas isotope ratio mass spectrometer (Europa Scientific, Crewe, United Kingdom). The ratio of 13CO2 to 12CO2 (mass 45 to 44) was measured in the sample and compared to a reference gas (5% CO2, balance 75% N2, 20% O2). The reference gas was calibrated with international standards. The units of measurement were atom % 13C and defined by:
Standards of carbon dioxide gas at 3 different levels of atom % 13C were run before and after each daily run to check instrument performance. The analytical precision of the instrument is 0.0001 atom % 13C.
The atom % 13C values of each breath sample were used to calculate the percent of the dose recovered in the breath during each time period. The area under the curve (AUC) for each time period was calculated by the linear trapezoid method, using the atom % 13C for the two points during the time period. The percent of the dose metabolized at each time point was calculated as:
Total 13C Excreted (mmol) = % 13C (AUC) × CO2 production (mmol/min) × Time (min) CO2 production was estimated from the basal metabolic rate (BMR) estimation for adults as described by Schofield (10). BMR was converted to CO2 production using the energy equivalent of a typical diet, 23.85 kJ/liter CO2 (11), and the gas constant for CO2 at 22.263 moles/liter. The percent dose metabolized at each time point was calculated as:
The BBT was reported as the cumulative area-under-the-curve for the total breath collection time period. Receiver operator curves were used to determine the normal range of the BBT using the healthy control subjects.
Several different components were assessed in Study Group 1 during the development of the BBT as follows:
1. Comparison of BBT to Serum B12 Tests
We determined the sensitivity and specificity of the BBT compared to current B12 diagnostic serology techniques. Subjects were classified as B12 deficient if MMA levels were greater than 243 nmol/l. The BBT (50 mg sodium 1-13C-propionate) was administered to all subjects after the collection of blood samples.
2. Reproducibility of the BBT
A subset of all subjects (33 of 58) received a second breath test 1 to 3 days after initial testing to evaluate the reliability of the BBT. Both the blood and breath tests were repeated and compared to the first for congruency and variation(s) respectively.
3. Propionate Randomized Crossover Dose Ranging Study
Our study addressed the lowest amount of sodium 1-13C-propionate needed to be administered in order to detect vitamin B12 deficiency. Twenty-two subjects were studied on two occasions with 9 at-risk subjects for B12 deficiency and 13 subjects as healthy controls (<65 years of age). Subjects were randomized to receive either a 10 or 25 mg sodium 1-13C-propionate dose. Within 72 hours of the first test, subjects received a second breath test with the other 13C-propionate dose. The breath test results were compared to MMA as the reference vitamin B12 test. The results of the 10 and 25 mg dose were compared to the prior study with the 50 mg 13C-propionate dose.
4. Reduced Fasting Time
We also evaluated whether the 8-hour fast was required for the BBT (n=10). Results from the 8-hour and 1-hour fasts were compared respectively. The purpose of this phase was to demonstrate the feasibility of reducing the fasting requirement.
Study Group 2 was used to assess two more components:
5. Evaluation of B12 deficiency in subjects at-risk
One hundred and two subjects with a high-risk of vitamin B12 deficiency were fasted for greater than 8 hours. All subjects received a BBT consisting of an oral dose of 25 mg sodium 1-13C-propionate with breath collections via straw into an Exetainer tube at −5, and 10 and 20 minutes after dosing. Subjects were classified as an abnormal BBT if either the 10- and/or 20-minute samples had less than 42% of the propionate metabolized per hour.
6. Evaluation of treatment for B12 deficiency
Subjects testing positive with the BBT or MMA were treated with 1,000 ug vitamin B12 (cyanocobalamin) administered intramuscularly for 5 days. Subjects were retested two to six weeks after treatment. If treatment still resulted in a positive test, a second treatment of 1,000 ug cyanocobalamin for five days was administered with a third diagnostic assessment by the BBT.
Statistical Analysis
GraphPad Prism version 5.0 was used for statistical analysis and for generation of graphs. All data was expressed as mean ± SD with statistical significance indicated when p<0.05. Linear multiple regression analysis with the best subsets approach was used to further reduce the number of data points that could predict B12 deficiency. Minitab Version 13 software was used to select the smallest subset of predictors (breath 13C data points) that would predict vitamin B12 status with a clinically significant coefficient (R2 > 0.5 and P < 0.05). The time points with the highest correlation to the complete 120 minute AUC were the 10 and 20 minute breath collections.
Results
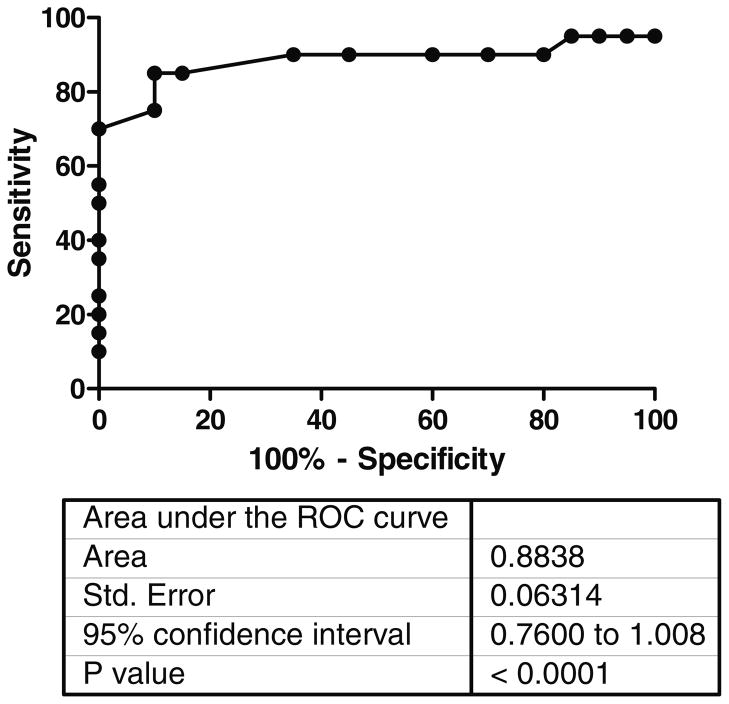
The rate of oxidation of 1-13C-propionate to 13CO2 in breath over 120 minutes for subjects with normal B12 status (N=49) and subjects with vitamin B12 deficiency (N=9) is shown in Figure 2. Significant differences (P <0.05) in the 13CO2 recovered between vitamin B12 deficiency and healthy controls occurred only between 10 and 30 minutes after dosing of 1-13C-propionate. Statistical analysis of the 120 minute collection period showed that the 10- and 20-minute time points gave the best diagnostic accuracy. Receiver Operating Characteristic (ROC) analysis of the data was performed to indicate the optimal cut-off value to minimize both false negative and false positive results, see figure 3. A cut-off value of >42% of the dose metabolized per hour at either 10 or 20 minutes indicated normal B12 status.
Figure 2.
Percent of 13C-propionate dose recovered per hour in breath as 13CO2 for subjects with normal B12 status (blue line) and for B12 deficient subjects (red line). The standard error of the mean (SEM) is shown at each point. There were 58 normal B12 subjects and 10 B12 deficient subjects evaluated.
Figure 3.
Receiver Operating Curve for the BBT using MMA to define B12 status. MMA was considered abnormal if greater than 243 mmol/liter.
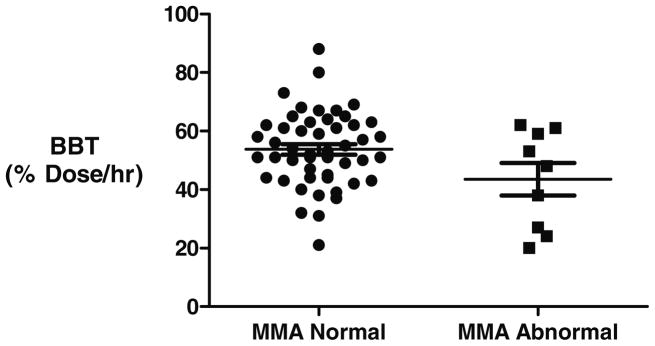
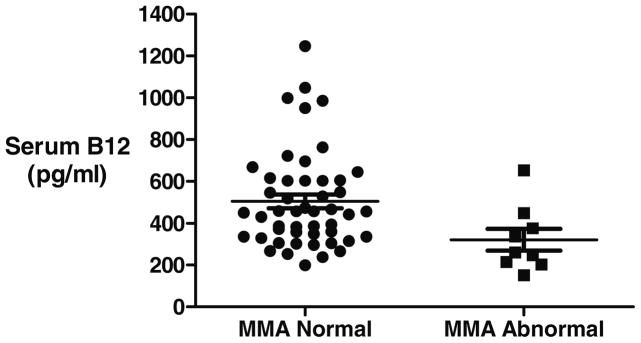
In the initial studies, nine subjects, 16% of Study Group 1 (n=58), had elevated MMA levels. The results of the BBT, serum B12 and serum HC with normal and abnormal MMA are shown in figures 4–6. Four out of 9 subjects as defined by an abnormal MMA level could be detected by the BBT. Five additional subjects had an abnormal BBT but normal MMA levels. Abnormal serum B12 and homocysteine levels were seen in only one of the 9 B12 deficient subjects with abnormal MMA levels. The sensitivity, specificity, positive and negative predictive values are for the three tests using MMA as the reference diagnosis is shown in Table 3. If MMA levels reflect true B12 deficiency, then serum B12 and HC showed little clinical utility to detect B12 deficiency but do show a high specificity
Figure 4.
BBT results of 58 subjects compared to serum MMA levels. BBT was expressed as the maximum percent dose recovered per hour at either 10 or 20 minutes. MMA was considered abnormal if greater than 243 mmol/liter.
Figure 6.
Serum homocysteine (HC) levels (μmol/l) of 58 subjects compared to serum MMA levels. MMA was considered abnormal if greater than 243 mmol/liter.
Table 3.
Accuracy of Detecting Abnormal B12 Status Utilizing MMA as the reference test.
| Serum B12 | B12 Breath Test | Serum HC | |
|---|---|---|---|
| Number detected as Abnormal Subjects | 1/9 | 4/9 | 1/9 |
| Sensitivity | 11% | 44% | 11% |
| Specificity | 98% | 90% | 81% |
| Positive Predictive Value | 50% | 44% | 20% |
| Negative Predictive Value | 85% | 90% | 85% |
| Likelihood Ratio + | 5.44 | 4.36 | 1.36 |
| Likelihood Ratio − | 1.10 | 1.62 | 1.03 |
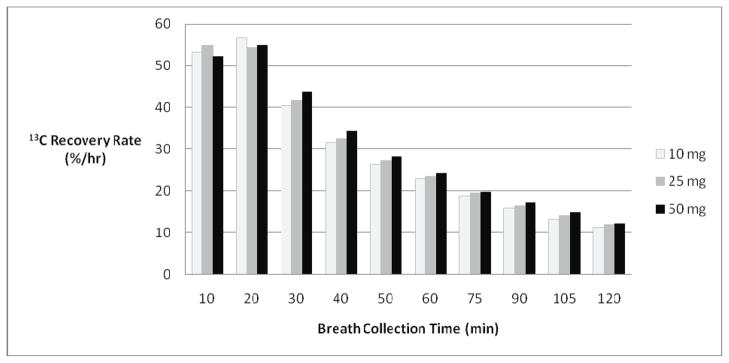
The dose ranging study (n=22) assessed the sensitivity and specificity of 10 and 25 mg doses of 13C-propionate to detect vitamin B12 deficiency using the BBT. The BBT results were compared to MMA as the reference standard. The dose ranging study is shown in Figure 7. Thirteen healthy controls and 9 patients with a comorbidity predisposing to B12 deficiency were evaluated. Three out of 22 subjects (14%) had abnormal MMA levels and were considered positive for B12 deficiency. The diagnostic results between 10, 25, and 50 mg propionate complement each other, although one true positive B12 deficient subject was not detected with the 10 mg dose. For the follow-up studies, we chose the 25 mg propionate dose since the amount of 13C appearing in breath after the 10 mg propionate dose was just barely detectable above natural 13C variability with an isotope ratio mass spectrometer.
Figure 7.
13CO2 recovery in the breath by varying sodium 1-13C-propionate dose size. The dose sizes administered were either 10, 25, and 50 milligrams.
There was a significant agreement for reproducibility of the BBT between test 1 and test 2. The Kappa value for the agreement between tests was 0.7415 (95% CI: 0.4664 – 1.0166). The BBT value of the two tests was on average within 10% of each other (95% CI: 7.5 – 9.8%).
Comparing the 8-hour and 1-hour fasts led us to the conclusion that it is best to run the BBT under an extended fast time (≥8 hours). The 1-hour fast compromised the specificity of the BBT, yielding several false positives (4/10).
The detection of B12 deficiency in 118 at-risk subjects (study group 2) is shown in Table 4. The highest incidence of B12 deficiency using the BBT was found in patients with chronic pancreatitis and Crohn’s disease, 33% and 24% respectively. Abnormal MMA levels were detected more often in patients with chronic pancreatitis and those over 65 years old, 22% and 13% respectively. We observed 16 to 44% of all subgroups had either test, BBT or MMA abnormal.
Table 4.
Incidence of B12 Deficiency in Subclasses of Study Group 2.
| Group | B12 Breath Test | MMA | Both Tests |
|---|---|---|---|
| SIBO | 11% (5/44) | 7% (3/44) | 16% (7/44) |
| Ileal Crohn’s Disease | 24% (8/33) | 9% (3/33) | 33% (11/33) |
| Chronic Pancreatitis | 33% (3/9) | 22% (2/9) | 44% (4/9) |
| Elderly | 6% (2/32) | 13% (4/32) | 16% (5/32) |
Treatment with cyanocobalamin (1,000 ug per day for 5 days) was performed in a subset of 11 subjects with an initial diagnosis of B12 deficiency by the BBT. Abnormal BBT results prior to B12 treatment changed to normal test results in 7 of 11 subjects.
Discussion
The BBT measures the metabolism of propionate to CO2. This pathway requires the enzyme methylmalonyl mutase and sufficient vitamin B12 as cofactor. The insufficiency of B12 limits formation of CO2 from propionate. The evidence for this pathway was first reported by Fish, Pollycove and Wallerstein using 2-14C-propionate (12) in both normal and vitamin B12 deficient subjects. Similarly, our studies using the non-radioactive tracer carbon-13 finds the in vivo oxidative metabolism of propionate to CO2 is decreased in B12 deficiency. The breath test we developed uses 10- and 20-minute breath samples. Only breath collections less than 30 minutes were decreased in B12 deficient subjects. This suggests that we are measuring first-pass clearance of propionate by the liver using an oral route of administration of propionate. Mouth to cecum transit time is typically 90–120 minutes. It would be unlikely that colonic bacteria would alter propionate metabolism within the 20 minute window we are measuring. Further, our bacterial overgrowth patients did not show enhanced propionate metabolism to CO2. Therefore, it is unlikely that bacterial metabolism of propionate accounts for our results.
Previous studies have also shown that the liver extracts 90% of propionate during a single pass (13,14). DeGrazia et al. using an intravenous bolus of 2-14C-propionate showed that 20% of the tracer dose was excreted as MMA in urine and 20% as CO2 (15). This suggested two disposal routes for propionate but does not account for the entire labeled dose administered. Burns et al. elucidated the propionate excretion disposal pathway by showing that propionate via propionyl-CoA can form propionyl carnitine (16). Propionate is converted to propionyl-CoA by a number of sources in the body such as metabolism of valine, methionine, and threonine, odd-chain fatty acids, cholesterol and gut propionate. Burns et al. suggest that high carnitine levels in extra-hepatic tissues facilitate the conversion of propionate to propionyl-CoA. Alternatively, propionyl-CoA produced endogenously in the liver does not get bound to carnitine.
Based on this evidence, we suggest that the BBT and MMA levels may be measuring different B12 deficiency pathways. We hypothesize that while the BBT may be measuring very rapid propionate metabolism (within 20 minutes) due to the liver’s first-pass effect of the orally administered propionate, MMA is gauging liver/intestinal metabolism as well as the extra-hepatic metabolism of propionate. We have found cases where MMA levels are normal but the BBT is abnormal, with an abnormal BBT normalizing following cobalamin treatment. These data may suggest that liver metabolism of propionate may be more sensitive to decreased vitamin B12 stores. Further studies should investigate the differences between the two B12 deficiency methods.
The pitfalls associated with using serum B12 concentrations as a functional marker of deficiency have long been known. Serum B12 levels can be affected by a plethora of confounding variables including the concentrations of B12 binding proteins, liver disease, and myeloproliferative disorders (2). Pregnancy and folate deficiency may also indicate a false diagnosis leading to over diagnosis and over treatment (3). Because of poor sensitivity and specificity, low serum B12 levels may not necessarily indicate vitamin B12 deficiency, while normal serum B12 levels fail to truly confirm normalcy (4). One study has shown that the limited positive predictive value of the test demonstrates that nearly 80% of indicated deficiencies in some 504 patients studied were false positives after a 5 year follow up (5). Other published reports demonstrate quite clearly that the serum B12 level is nonspecific with a low positive predictive value and should be abandoned as a test for vitamin B12 deficiency (4, 6, 17–18). Similarly serum homocysteine levels were also not sensitive to detect B12 deficiency which agrees with many published studies. In a urine MMA screening study, serum Hcys levels were above normal in only 9 of 16 subjects while another presented nine vegetarians with a high MMA and/or low serum B12 levels that all had normal Hcys levels (19, 20). Our results showed that serum B12 and Hcys had a low sensitivity to detect functional B12 deficiency compared to the BBT and MMA levels.
The clinical significance of increased MMA levels has also been questioned in 432 subjects followed for up to 4 years after initial MMA testing (21). Symptoms and neurological testing were used in the follow-up period. No association was found in the follow-up period between MMA and symptoms. MMA levels did not predict clinical manifestations related to vitamin B12 deficiency. In only 16% of participants, MMA levels increased substantially, whereas 44% showed a decrease even in the absence of B12 treatment. While we have used MMA levels as the gold standard, it certainly is not clear cut that MMA levels have clinical utility.
Conclusion
We studied healthy subjects over age 65, patients with Crohn’s disease of the terminal ileum, patients with small intestinal bacterial overgrowth and patients with chronic pancreatitis. These are not rare disorders and ongoing phases of our study continue to show a remarkable number of these individuals with B12 deficiency. We found a 16% incidence of B12 deficiency in our elderly subjects using both serum MMA levels and the BBT tests. This is similar to recent reports using MMA in larger cohorts by Johnson et al. (23%) and Morris et al. (20%) (18, 22). The United States is on the brink of a major rise in the number of older Americans. By 2030, the number of Americans over 65 years will double to 70 million. The clinical significance of B12 deficiency in these various at-risk populations presents a growing major and under-addressed public health problem. Should there be any suspicion that B12 deficiency is a possibility or the individual falls into any significant at-risk population, it is paramount that a more sensitive B12 test (i.e., MMA or BBT) be used in conjunction with a detailed clinical evaluation for any possible signs and symptoms of deficiency.
In this study we have developed and refined the B12 Breath Test to be a simple diagnostic test for B12 deficiency. Overall, these results indicate that the vitamin B12 breath test is a non-invasive, sensitive, specific, and reproducible diagnostic test to detect vitamin B12 deficiency. The ultimate prevention of devastating complications associated with B12 deficiency, particularly in the elderly population and malabsorption diseases, can be accomplished with tests such as the BBT and the MMA to more accurately diagnose B12 deficiency. More studies will be required to validate the BBT in classical B12 deficient subjects. If the BBT has a high sensitivity and specificity for B12 deficiency, it may be prudent to perform both the BBT and measure MMA levels in patients since alternate pathways may be operative with respect to the BBT or MMA levels.
Figure 5.
Serum vitamin B12 levels (pg/ml) of 58 subjects compared to serum MMA levels. MMA was considered abnormal if greater than 243 mmol/liter.
Acknowledgments
Grant Support: 2 R44AG021850-02 NIH NIA SBIR PHASE 2
Abbreviations
- AUC
Area under the curve
- BBT
Vitamin B12 Breath Test
- BMI
Body Mass Index
- BMR
Basal Metabolic Rate
- HC
Homocysteine
- MMA
Methylmalonic Acid
- ROC
Receiver Operating Characteristic
References
- 1.Tucker KL, Rich S, Rosenberg I, Jacques P, Dallal G, Wilson PWF, Seleb J. Plasma vitamin B-12 concentrations relate to intake source in the Framingham Offspring Study. Am J Clin Nutr. 2000;71:514–22. doi: 10.1093/ajcn/71.2.514. [DOI] [PubMed] [Google Scholar]
- 2.Klee GG. Cobalamin and folate evaluation: Measurement of methylmalonic acid and homcysteine vs. vitamin B(12) and folate. Clin Chem. 2000;46:1277–83. [PubMed] [Google Scholar]
- 3.Snow C. Laboratory diagnosis of vitamin B12 and folate deficiency. Arch Intern Med. 1999;159:1289–1298. doi: 10.1001/archinte.159.12.1289. [DOI] [PubMed] [Google Scholar]
- 4.Green R. Screening for B12 deficiency: Caveat emptor. Ann Int Med. 1996;124:509–11. doi: 10.7326/0003-4819-124-5-199603010-00009. [DOI] [PubMed] [Google Scholar]
- 5.Cooper BA, Fehedy V, Blanshay P. Recognition of deficiency of vitamin B12 using measurements of serum concentration. J Lab Clin Med. 1986;107:447–452. [PubMed] [Google Scholar]
- 6.Snow CF. Laboratory disgnosis of vitamin B12 and folate deficiency: a guide for the primary care physician. Arch Intern Med. 1999;159:1289–98. doi: 10.1001/archinte.159.12.1289. [DOI] [PubMed] [Google Scholar]
- 7.Lindenbaum J, Savage DG, Stabler SP, Allen RH. Diagnosis of cobalamin deficiency: Relative sensitivities of seum cobalamin, methylmalonic acid, and total homocysteine concentrations. Am J Hematol. 1990;34:99–107. doi: 10.1002/ajh.2830340205. [DOI] [PubMed] [Google Scholar]
- 8.Herrmann W, Schorr H, Geisel J, Riegel W. Homocysteine, cystathionine, methylmalonic acid and B-vitamins in patients with renal disease. Clin Chem Lab Med. 2001;239:739–46. doi: 10.1515/CCLM.2001.123. [DOI] [PubMed] [Google Scholar]
- 9.Magera MJ, Helgeson JK, Matern D, Rinaldo P. Methylmalonic acid measured in plasma and urine by stable-isotope dilution and electrospray tandem mass spectrometry. Clin Chem. 2000;46:1804–10. [PubMed] [Google Scholar]
- 10.Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr. 1985;39:5–41. [PubMed] [Google Scholar]
- 11.Elia M. Energy equivalents of CO2 and their importance in assessing energy expenditure when using tracer techniques. Am J Physiol. 1991;260:E75–E88. doi: 10.1152/ajpendo.1991.260.1.E75. [DOI] [PubMed] [Google Scholar]
- 12.Fish MB, Pollycove M, Wallerstein RO. In vivo oxidative metabolism of propionic acid in human vitamin B12 deficiency. J Lab Clin Med. 1968;72:767–77. [PubMed] [Google Scholar]
- 13.Dankert J, Zijlstra J, Wolthers B. Volatile fatty acids in human peripheral and portal blood: quantitative determination, vacuum distillation, and gas chromatrography. Clin Chim Acta. 1981;110:301–7. doi: 10.1016/0009-8981(81)90359-4. [DOI] [PubMed] [Google Scholar]
- 14.Peters S, Pomare E, Fisher C. Portal and peripheral blood short-chain fatty acid concentrations after caecal lactulose instillation at surgery. Gut. 1992;33:1249–52. doi: 10.1136/gut.33.9.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeGrazia JA, Fish MB, Pollycove M, Wasserstein RO, Hollander L. The role of propionic acid as a precursor of methylmalonic acid in normal and vitamin B12-deficient man. J Lab Clin Med. 1969;73:917–23. [PubMed] [Google Scholar]
- 16.Burns SP, Iles RA, Saudubray J-M, Chambers RA. Propionylcarnitine excretion is not affected by metronidazole administration to patients with disorders of propionate metabolism. Eur J Pediatr. 1996;155:31–35. doi: 10.1007/BF02115623. [DOI] [PubMed] [Google Scholar]
- 17.Stabler SP, Allen RH, Savage DG, et al. Clinical Spectrum and diagnosis of cobalamin deficiency. Blood. 1990;76:871–81. [PubMed] [Google Scholar]
- 18.Johnson MA, Hawthorne NA, Brackett WR, Fischer JG, Gunter EW, Allen RH, Stabler SP. Hyperhomocysteinemia and vitamin B-12 deficiency in elderly using Title IIIc nutrition services. Am J Clin Nutr. 2003;77:211–20. doi: 10.1093/ajcn/77.1.211. [DOI] [PubMed] [Google Scholar]
- 19.Norman EJ, Morrison JA. Screening elderly populations for cobalamin (vitamin B12) deficiency using the urinary methylmalonic acid assay by gas chromatography mass spectrometry. Am J Med. 1993;94:589–594. doi: 10.1016/0002-9343(93)90209-8. [DOI] [PubMed] [Google Scholar]
- 20.Elmadfa I, Singer I. Vitamin B-12 and homocysteine status among vegetarians: a global perspective. Am J Clin Nutr. 2009;89:1693S–98S. doi: 10.3945/ajcn.2009.26736Y. [DOI] [PubMed] [Google Scholar]
- 21.Hvas A-M, Ellegaard J, Nexo E. Increased plasma methylmalonic acid level does not predict clinical manifestations of vitamin B12 deficiency. Arch Intern Med. 2001;161:1534–41. doi: 10.1001/archinte.161.12.1534. [DOI] [PubMed] [Google Scholar]
- 22.Morris MS, Jacques PF, Rosenberg IH, Seleb J. Serum methylmalonic acid concentrations are common among elderly Americans. J Nutr. 2002;132:2799–803. doi: 10.1093/jn/132.9.2799. [DOI] [PubMed] [Google Scholar]