Abstract
Ample evidence indicates that dietary fatty acids alters the plasma levels of HDL-C. However, the mechanisms underlying the effects of fatty acids still remain elusive. Recent advances in our understanding of ATP binding cassette transporter A1 (ABCA1) function and regulation have provided a valuable insight into the mechanisms by which fatty acids may affect plasma HDL-C levels. ABCA1 mediates the assembly of phospholipids and free cholesterol with apolipoprotein A-I, which is a critical step for HDL biogenesis. Studies have shown that unsaturated fatty acids, but not saturated fatty acids, repress the expression of ABCA1 in vitro. Although information on mechanisms for the fatty acid-mediated regulation of ABCA1 expression is still limited and controversial, recent evidence suggests that unsaturated fatty acids inhibit the expression of ABCA1 at the transcriptional and posttranscriptional levels. The transcriptional repression of ABCA1 expression by unsaturated fatty acids is likely LXR-dependent. Evidence also suggests that histone deacetylation may play a role in the repression. Posttranscriptionally, unsaturated fatty acids may facilitate ABCA1 protein degradation, which may involve phosphorylation of ABCA1 by protein kinases. Further studies are warranted to better understand the role of dietary fatty acids in HDL metabolism and their effects on cardiovascular health.
Keywords: ATP-binding cassette transporter AI, high density lipoprotein, fatty acids, regulation
1. Introduction
Epidemiological studies have consistently shown a strong inverse relationship between plasma high-density lipoprotein cholesterol (HDL-C) concentrations and the incident of coronary heart disease (CHD). High-density lipoproteins (HDL) are known to be antiatherogenic as they play a key role in reverse cholesterol transport (RCT), inhibit cytokine-induced expression of adhesion molecules by endothelial cells, and prevent the oxidation of low-density lipoproteins (LDL). In particular, the RCT pathway, by which excess cholesterol is delivered from the periphery to the liver for excretion, is considered to be a primary mechanism for the cardioprotective effect of HDL.
HDL particles present in human plasma are heterogeneous and classified based on density, size, electophoretic mobility, and apolipoprotein content. Apolipoprotein A-I (apoA-I) is a structurally and functionally important component of HDL particles and accounts for ~80% of the total protein in the particle. By functioning as an acceptor of cholesterol and phospholipid effluxed from cells and as an activator of lecithin:cholesterol acyltransferase (LCAT), apoA-I plays a critical role in RCT. Lipidation of apoA-I with phospholipid and cholesterol produces discoidal preβ-HDL particles that migrate to the pre-β position on agarose gel electrophoresis. Earlier, the preβ-HDL particles were thought to be produced in the circulation during remodeling of spherical mature HDL by cholesteryl ester transfer protein (CETP), phospholipid transfer protein (PLTP) and hepatic lipase. However, studies showed that the preβ-HDL are nascent HDL particles that are subsequently transformed into mature spherical HDL particles by the action of LCAT. The mature HDL particles are further remodeled during circulation by CETP and PLTP. Although the formation of preβ-HDL is a prerequisite step toward the formation of mature HDL particles, the process has been obscure until the discovery of ATP-binding cassette transporter AI (ABCA1).
Current evidence indicates that ABCA1 is the major determinant for the formation of preβ-HDL and hence plasma HDL-C concentrations. Among many dietary factors, dietary fat or fatty acids have long been known to alter the metabolism and circulating levels of LDL and HDL. Key and Hegsted et al. first developed predictive equations for changes in plasma total cholesterol concentrations in response to dietary fatty acids and cholesterol. The equations indicated that in general, most saturated fatty acid (SFA) are hypercholesterolemic, whereas stearic acid and monounsaturated fatty acids (MUFA) are neutral or mildly hypocholesterolemic, and polyunsaturated fatty acids (PUFA) are hypocholesterolemic. The equations also provided insight into how dietary fatty acids may alter cholesterol levels in different lipoprotein classes including LDL and HDL [22, 23]. However, the relative contributions of changes in these lipoproteins by dietary fatty acids to CHD risk have not been fully resolved. More recent studies have shown that dietary fatty acids may alter the formation and metabolism of HDL by modulating the expression of ABCA1 that facilitates the efflux of cellular phospholipid and cholesterol to extracellular acceptors such as apo A-I. This review focuses on the regulation of ABCA1 expression and potential mechanisms by which fatty acids alter ABCA1 expression and plasma HDL-C.
2. Dietary fatty acids and HDL-C
Studies with non-human primates and humans showed that isocaloric substitution of n-3 PUFA for SFA decreased plasma total cholesterol, LDL-C, and HDL-C. Also, in African green monkeys fed atherogenic diets containing PUFA, the plasma levels of total cholesterol and HDL-C as well as apoA-I were significantly decreased, compared with animals fed SFA and MUFA. A meta-analysis of 60 controlled trials with human subjects indicated that SFA, MUFA and PUFA significantly increased plasma HDL-C when they replaced carbohydrate. However, the increase was the least with PUFA. Predictive equations based on several meta-analyses of human studies also support the similar effect of fatty acid classes on plasma HDL-C. Additionally, a recent study with human subjects showed that plasma HDL-C levels were decreased in subjects consuming a PUFA-rich diet, compared with a SFA-rich diet. Thus, the above-cited studies support the notion that in general, PUFA lower HDL-C relative to SFA and MUFA and are least effective in raising HDL-C with its isocaloric substitution for carbohydrates. However, further studies are needed to evaluate effects of individual fatty acids on plasma HDL-C using a fatty acid as a reference for comparison. This is an important area that requires further evaluation, particularly, in relation to their effects on mechanisms of HDL assembly/biogenesis and, ultimately, plasma HDL-C.
3. ABCAI and HDL metabolism
3.1. Role of ABCA1 in HDL formation
In 1961, Fredrickson et al. reported a new malady discovered in Tangier island of Chesapeake Bay in Virginia and the disease was named as Tangier disease. Individuals with Tangier disease have severe HDL deficiency with less than 5% of normal plasma HDL-C levels and higher incidence of premature CHD. Etiology of the disease was identified in the late 1990’s that mutations in the gene encoding ABCA1 present in Tangier patients are responsible for the extremely low plasma HDL-C. Subsequent studies with mice [39, 40] and chickens lacking ABCA1 [41, 42] demonstrated that the animals also exhibit HDL and apoA-I deficiencies, substantiating a pivotal role of ABCA1 in HDL formation.
ABCA1 is an integral membrane protein consisting of twelve transmembrane domains and two ATP-binding domains. The primary function of ABCA1 is to facilitate the efflux of cellular phospholipid and cholesterol to extracellular acceptors. Interaction of apoA-I, the major protein in HDL particles, with ABCA1 produces preβ-HDL. Lipid-free or lipid-poor apoA-I is the initial acceptor of cellular phospholipid and cholesterol in ABCA1-mediated efflux.
In several studies, the formation of heterogeneous nascent preβ-HDL particles was observed during incubation of apoA-I with Chinese hamster ovary (CHO) cells and THP-1 macrophages, presumably by the interaction of apoA-I with cell surface binding sites or through retroendocytosis. In J774 macrophages, in which ABCA1 expression was up regulated by cAMP, the presence of extracellular apoA-I induced formation of 9 and 12 nm sizes of particles containing ~3:1 and 1:1 phospholipid/free cholesterol (mol/mol), respectively. The interaction of apoA-I with ABCA1 under in vitro conditions produces pre-β migrating nascent HDL subpopulations (preβ1 to preβ4) that vary in size, lipid and apoA-I content. The differing sizes of preβ HDL have different metabolic fates depending on the degree of lipidation; less lipidated preβ1 HDL are destined to be rapidly removed from the circulation by kidney, whereas other preβ HDL with more lipid are able to participate in the maturation process. The studies suggest that lipidation of apoA-I via the interaction with ABCA1 is an essential initial step for the formation of HDL that ultimately determines plasma HDL-C levels.
3.2. Sites of HDL formation
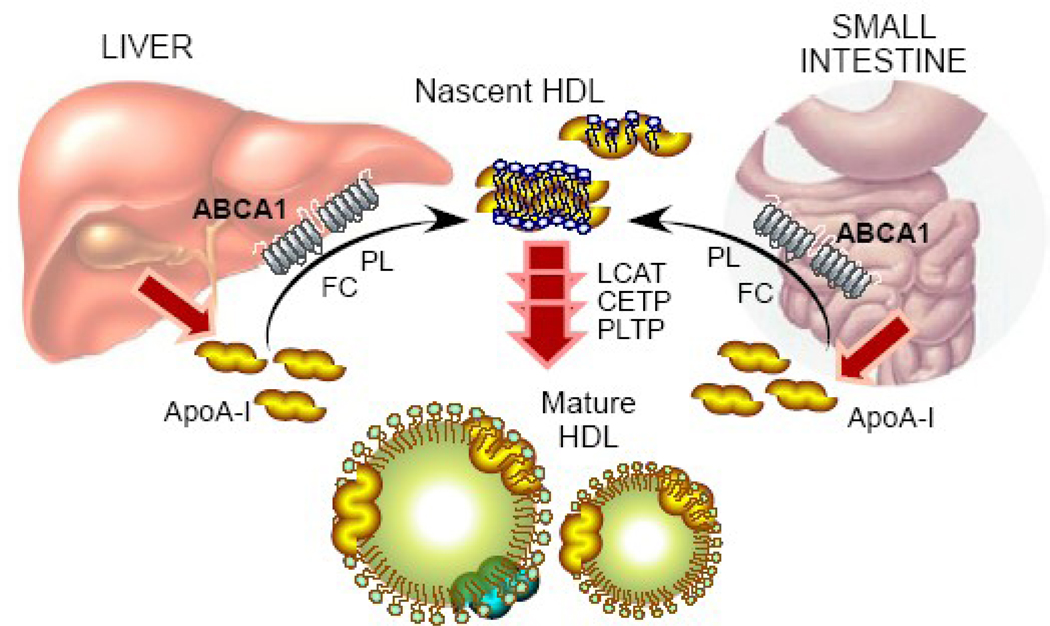
ApoA-I is primarily synthesized in the liver and intestine that highly express ABCA1. Thus, it is reasonable to expect that liver and intestine have a major role in producing nascent HDL and modulating plasma HDL-C levels. Several strategies have been tried to determine the contribution of hepatic ABCA1 to plasma HDL-C levels. When human ABCA1 was overexpressed in mouse liver and macrophages under the control of apolipoprotein E promoter, plasma HDL-C and apoA-I were increased in the transgenic mice with an elevation in preβ-HDL particles. Given the minimal effect of macrophage ABCA1 on HDL-C levels, the human ABCA1 overexpressed in the mouse liver was likely responsible for the increased HDL-C levels. To further define the role of hepatic ABCA1 expression on plasma HDL-C, Basso et al. infused adenovirus expressing ABCA1 into mice. The hepatic overexpression of ABCA1 by this method induced apoA-I-dependent cholesterol efflux from primary hepatocytes and also increased plasma HDL-C and apoA-I levels. Another study employing adenoviral-mediated overexpression of human ABCA1 in the liver also showed an increase in plasma HDL-C. To examine the role of the intestine in HDL biogenesis, Murthy et al. demonstrated that in Caco-2 cells, ABCA1 mRNA expression and cholesterol efflux from the basolateral membrane to apoA-I or HDL particles were enhanced by liver X receptor (LXR)/retinoid X receptor (RXR) activation, suggesting that ABCA1 plays an important role in intestinal HDL production. The role of ABCA1 in the formation of HDL particles was further studied by using mice with tissue-specific deletion of Abca1 gene [57]. Mice with liver-specific Abca1 knockout (Abca1−/−) mice exhibited marked reductions in plasma HDL-C and apoA-I by ~80% and 98%, respectively, compared with the wild-type. The deletion of intestinal Abca1 gene elicited ~30% reduction in plasma HDL-C and the mice without functional ABCA1 in both liver and intestine had only ~10% of the wild-type plasma HDL-C levels . These studies strongly suggest that liver and intestine are quantitatively the most important sites for producing and modulating plasma HDL-C levels (Figure 1).
Figure 1. Role of ABCA1 in HDL formation.
Newly secreted apoA-I from the liver and small intestine interacts with ABCA1 and acquires phospholipids (PL) and free cholesterol (FC). The lipidation of apoA-I produce nascent HDL that subsequently undergoes maturation process by several factors including LCAT, CETP and PLTP to become spherical HDL.
Macrophages were presumed to play a role in HDL formation. However, the role of macrophage ABCA1 in maintaining the plasma HDL-C pool is considered minimal. Mice expressing ABCA1 only in macrophages and those with selected inactivation of ABCA1 in macrophages were generated using bone marrow transplantation. Restoration of macrophage ABCA1 by transplantation of the wild-type bone marrow into in Abca1−/−mice minimally increased plasma HDL-C and apoA-I levels, whereas mice with the selective inactivation of ABCA1 in macrophages had normal HDL-C levels. Furthermore, Abca1 deletion in macrophages did not alter plasma HDL-C levels in mice, supporting that macrophage ABCA1 is not a major contributor to plasma HDL-C levels. Thus, it is evident that the liver and intestine produce nascent preβ-HDL particles that function as an acceptor of cellular cholesterol in the RCT pathway and that ABCA1-mediated efflux from macrophages to lipid-free apoA-I plays a quantitatively less important role in RCT.
3.3. Role of ABCA1 in HDL catabolism
As discussed above, a large body of evidence has shown that ABCA1 plays a pivotal role in HDL formation, particularly, in the liver and small intestine. In addition, hepatic ABCA1 is thought to be involved in the catabolism of HDL particles as well. An in vivo kinetic study demonstrated that liver-specific Abca1−/− mice has a significantly higher fractional catabolic rate of 125I-radio labeled mature HDL as well as lipid-free apoA-I than the wild-type counterparts, suggesting that HDL catabolism occurs at a faster rate in the absence of hepatic ABCA1. The tracer uptake by the liver was not different between liver specific Abca1−/− mice and the wild type, where as a significantly higher tracer up take by the kidney was noted in the liver specific Abca1−/− mice. Based on the observations, it is presumed that ABCA1 is critical in lipidating not only newly-synthesized apoA-I, but also apoA-I released from mature HDL. Thus, in the absence of ABCA1, apoA-I generated in the liver would fail to acquire lipid and consequently be rapidly cleared by the kidney without being recycled.
4. Regulation of ABCA1 by fatty acids
4.1. Regulation of ABCA1 expression
Transcription of ABCA1 is primarily under the control of liver X receptors (LXR), a major transcription factor that senses high cellular cholesterol levels and triggers the expression of genes involved in the removal of excess cholesterol from the cells. Considering the critical role of ABCA1 in the efflux of cellular cholesterol, it is not surprising that the expression of ABCA1 is under control of LXR at the transcriptional level. LXR is a ligand-activated transcription factor that forms a heterodimer with retionoid X receptor (RXR) to transactivate gene expression. Evidence shows the presence of a cis-element for LXR/RXR heterodimer binding in the Abca1 promoter and up-regulation of ABCA1 expression by LXR results in a concomitant increase in cellular cholesterol efflux to apoA-I.
ABCA1 protein levels do not always correlate with ABCA1 mRNA, suggesting the presence of posttranscriptional regulation. The turnover of ABCA1 protein is rapid with a half-life of less than 1 hr in macrophages, which indicates posttranscriptional regulation of ABCA1 could be an important determinant for its function. Binding of helical apolipoproteins, e.g. apoA-I and apoA-II, but not HDL, to ABCA1 retarded the degradation of ABCA1 protein in THP-1 cells by protecting ABCA1 from degradation by a thiol protease. Wang et al. identified a sequence rich in proline, glutamic acid, serine and threonine (PEST) present in the cytoplasmic region of ABCA1, which facilitated ABCA1 protein degradation by calpain protease. The presence of extracellular apoA-I abolished the effect of calpain protease and increased efflux of phospholipids and cholesterol. The finding suggests that a positive feedback regulation of ABCA1 protein expression exists where the interaction of apoA-I with ABCA1 inhibits PEST sequence-mediated calpain proteolysis, consequently increasing the efflux of cellular phospholipid and cholesterol to apoA-I. Subsequently, the same research group reported that phosphorylation of the PEST sequence in ABCA1 protein directed the protein to calpain proteolysis whereas extracellular apoA-I diminished the phosphorylation of PEST sequence, resulting in increased cell surface expression of ABCA1. Although phosphorylation of Thr-1286 and Thr-1305 in the PEST sequence of ABCA1 increases the protein degradation by calpain, ABCA1 protein levels are positively correlated with its phosphorylation in THP-1 cells. Therefore, the regulation of ABCA1 protein levels via phosphorylation likely depends on the sites of phosphorylation in the protein.
4.2. Transcriptional regulation of ABCA1 by fatty acids
Considerable evidence exists that fatty acids modulate the expression of ABCA1 and potentially influence plasma HDL-C levels. In LXR or RXR ligand-treated HepG2 cells and RAW264.7 macrophages, unsaturated fatty acids reduced the expression of ABCA1 mRNA and protein with a concomitant decrease in cholesterol efflux, where as palmitic acid failed to diminish ABCA1 expression and activity. Also, in human monocyte-derived macrophages, ABCA1 mRNA abundance was significantly decreased by linoleic acid whereas palmitic acid significantly increased its expression compared with control. In mouse small intestinal epithelial cells, MUFA and PUFA such as oleic, linoleic, linolenic and docosahexaenoic acids significantly reduced ABCA1 mRNA abundance, where as SFA such as palmitic and stearic acids did not alter its expression. The repressive effect of unsaturated fatty acids on ABCA1 mRNA was also observed in vivo. In duodenal mucosal cells of dogs, PUFA-rich diet significantly down-regulated ABCA1 mRNA expression. Similarly, ABCA1 mRNA levels were significantly reduced in the small intestines of SV129 mice gavaged with triacylglycerols of unsaturated fatty acids.
Further, mutations or deletion of direct repeat 4, a cis-acting element for LXR/RXR heterodimer binding, in Abca1 promoter abolished the suppressive effects of unsaturated fatty acids on its expression in RAW 264.7 macrophages, suggesting that unsaturated fatty acids inhibit ABCA1 expression in an LXR-dependent manner. However, their precise modes of action are not well understood. In our recent study, we observed that unsaturated fatty acids significantly reduced ABCA1 mRNA levels in RAW 264.7 macrophages but the repression was completely abolished in the presence of an LXR agonist. This finding further supports that the effect of unsaturated fatty acids on ABCA1 expression is likely LXR-dependent.
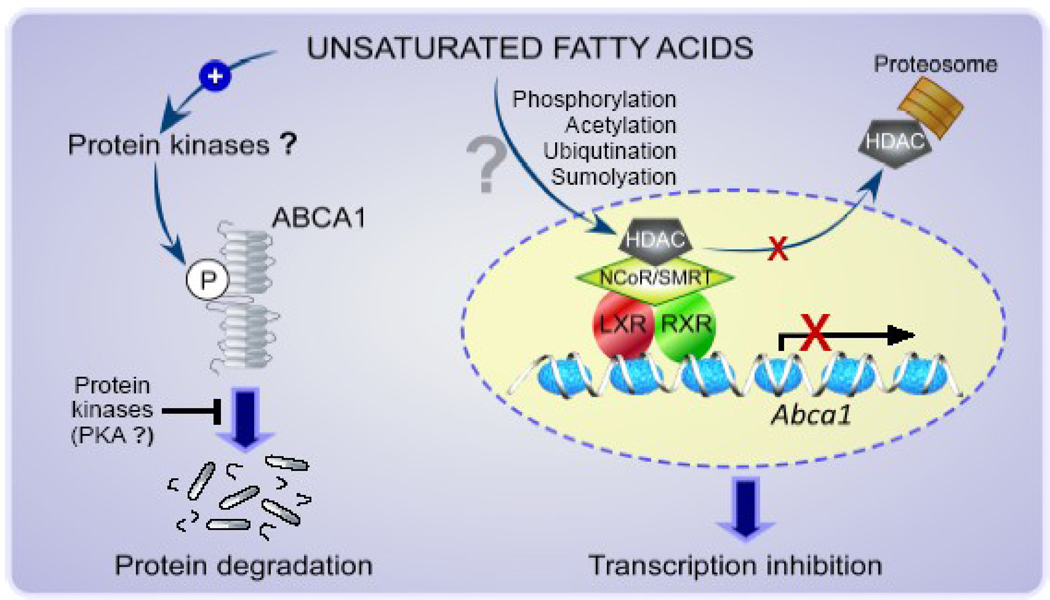
In addition, our recent finding suggests the possibility that histone acetylation/deacetylation may be involved in the transcriptional regulation of ABCA1. We observed that trichostatin A, a pan-histone deacetylase (HDAC) inhibitor, reversed the linoleic acid-induced reduction in ABCA1 mRNA in macrophages. Histone is a component of nucleosome, a unit of chromatin, and its posttranslational modifications, e.g., acetylation and methylation, largely influence gene expression. In general, transcriptionally active DNA regions are associated with hyperacetylated histone 3 and 4, resulting in local expansion of chromatin for increased accessibility of regulatory proteins to DNA. Certain HDACs, HDAC3 in particular, have shown to be actively involved in transcriptional regulation of genes as a component of a corepressor complex, commonly consisting of nuclear hormone receptor corepressor (NCoR) or silencing mediator of retinoid and thyroid hormone receptors (SMRT). Interestingly, LXR is one of the transcription factors that have been shown to interact with the corepressor complex for the transcriptional repression. HDACs are subject to posttranslational modifications, such as acetylation, phosphorylation, ubiquitination, and sumoylation, that determine stability, localization, activity, and protein-protein interaction. As such, the modifications are important determinants for HDAC activity. Our preliminary data [73], together with the available information above cited, suggest that the LXR-dependent transcription repression of ABCA1 by unsaturated fatty acids may be mediated at least partly via posttranslational modifications in an HDAC as a component of a corepressor complex associated with a LXR/RXR heterodimer in the promoter of Abca1. Further studies are warranted to identify a specific HDAC isoform(s) involved and to determine the modes of action for the regulation of HDAC activity by fatty acids and consequently ABCA1 expression. Hypothetical regulatory mechanisms for the transcriptional repression of ABCA1 by unsaturated fatty acids are summarized in Figure 2.
Figure 2. Hypothetical mechanisms for the regulation of ABCA1 expression by unsaturated fatty acids.
Post-transcriptional regulation of ABCA1 by unsaturated fatty acids may also occur via mechanisms involving protein kinases that phosphorylate (P) ABCA1 to facilitate the protein degradation. In contrast, other types of kinases such as PKA may inhibit the action of unsaturated fatty acids and stabilize the protein. Unsaturated fatty acids may also repress ABCA1 transcription in a LXR-dependent manner. Activity or recruitment of an HDAC as a component of NCoR/SMRT coprepressor complex that is associated with a LXR/RXR heterodimer in the promoter of Abca1 may be altered by unsaturated fatty acids via post transcriptional histone modifications, such as phosphorylation, acetylation, ubiqutination and sumolyation.
4.3. Posttranscriptional regulation of ABCA1 by fatty acids
Wang et al. first showed that in cAMP-activated murine macrophages, unsaturated fatty acids decreased ABCA1 protein levels, lowering the efflux of free cholesterol and phospholipid to lipid-free apoA-I, whereas SFA did not. The decrease in ABCA1 by unsaturated fatty acids was attributed to increased ABCA1 protein turnover, but not to the modulation of transcription, mRNA decay, or impaired translation efficiency. Wang et al. further demonstrated that when macrophages were incubated with LXR/RXR agonists, treatment with SFA also increased ABCA1 protein degradation and reduced the efflux of cholesterol and phospholipid to apoA-I to a similar extent to that observed with unsaturated fatty acids. Facilitated ABCA1 protein degradation by SFA in the presence of LXR agonists was due to the increased expression of stearoyl-CoA desaturase that converts SFA to MUFA. In HepG2 cells, MUFA such as palmitoleic and oleic acids as well as linoleic acid, significantly reduced ABCA1 protein levels and cholesterol efflux compared with palmitic acid, but without altering mRNA abundance. In Caco-2 cells incubated with LXR agonists, PUFA decreased the efflux of cholesterol from the basolateral membrane and the effects of fatty acids were mediated primarily by decreased ABCA1 protein with minimal change in mRNA levels. In HepG2 cells as well as FHs 74 Int cells, a human small intestine cell line, we observed ABCA1 protein levels were diminished by unsaturated fatty acids without a change in mRNA levels, compared with SFA (unpublished data). The reduction was greater with PUFA, such as linoleic acid and eicosapentaenoic acid (EPA), than with MUFA.
The enhanced degradation of ABCA1 protein by unsaturated fatty acids was shown to be mediated through serine phosphorylation of ABCA1 by intracellular unsaturated acyl-CoA derivatives generated by the activation of phospholipase D2. Also, chemical inhibition with rottlerin and genetic inhibition using small interfering RNA (siRNA) of protein kinase C delta (PKCδ) abolished the ABCA1-destabilizing effects of unsaturated fatty acids, suggesting that serine phosphorylation of ABCA1 by PKCδ may be partly responsible for the facilitated degradation of ABCA1 protein by unsaturated fatty acids. However, in RAW 264.7 macrophages, we were not able to observe the similar role of PKCδ in the reduction of ABCA1 protein by unsaturated fatty acids. In our study, knock down of PKCδ by ~80% using siRNA did not reverse the linoleic acid-induced reduction in ABCA1 protein. Rather, when PKCδ was knocked down, ABCA1 protein was reduced in control, palmitic acid and linoleic acid-treated cells, suggesting that PKCδ may not be involved in the fatty acid regulation of the protein, but play a role in stabilization of ABCA1 protein. A potential involvement of protein kinase A (PKA) in the regulation has also been suggested. EPA was shown to significantly destabilize ABCA1 protein and reduce ABCA1-dependent cholesterol efflux without altering ABCA1 mRNA expression in THP-1 cell-derived macrophages, but the activation of PKA attenuated the repressive effect of EPA without affecting ABCA1 degradation rate. Interestingly, in contrast to RAW 264.7 macrophages in which unsaturated fatty acids reduced ABCA1 expression at the transcriptional as well as posttranscriptional level, only ABCA1 protein levels were reduced by unsaturated fatty acids with a minimal effect on mRNA abundance in HepG2 cells. Therefore, cell type-specific regulation of ABCA1 expression by fatty acids is likely present. Although the mechanisms still remain unclear, evidence thus far suggests that the degradation/stabilization of ABCA1 protein involves several protein kinases. Further studies are necessary to identify types of kinases and phosphorylation sites in ABCA that are sensitive to the regulation by fatty acids.
4. Conclusion
Current evidence indicates that ABCA1, particularly in the liver and intestine, plays a critical role in the formation and metabolism of HDL. Newly secreted apoA-I from the liver and small intestine acquires phospholipid and cholesterol via the interaction with ABCA1, producing nascent HDL particles that subsequently undergo the process of maturation. As such, nascent HDL formation via ABCA1 is critical in RCT. Although the mechanisms involved in the regulation of ABCA1 are not well understood, considerable evidence from in vitro studies indicates that unsaturated fatty acids reduce ABCA1 expression at the transcriptional as well as posttranscriptional levels as depicted in Figure 2. The inhibitory effect of unsaturated fatty acids, PUFA in particular, on ABCA1 expression and HDL formation is contradictory to a well-known atheroprotective effect of oleic acid and PUFA compared with SFA. Atheroprotection imparted by MUFA and PUFA, however, may be attributable to their favorable effects on very-low density lipoproteins, LDL, plasma triglyceride, anti-inflammation, and anti-thrombosis. These benefits could outweigh the inhibition of ABCA1 expression by these fatty acids in protecting against atherosclerosis. Further studies are needed to verify the inhibition of ABCA1 expression by individual unsaturated fatty acids under in vivo conditions and to elucidate the mechanisms involved. Nonetheless, the diminished ABCA1 expression by unsaturated fatty acids could account at least in part for lower HDL-C levels by unsaturated fatty acid than SFA.
Acknowledgements
This work was supported in part by NIH Grant R21AT005152 to J. Lee
Abbreviations
- ApoA-I
apolipoprotein A-I
- ABCA1
ATP binding cassette transporter A1
- CETP
cholesterol ester transfer protein
- CHD
coronary heart disease
- EPA
eicosapentaenoic acid
- HDAC
histone deacetylase
- HDL
high-density lipoprotein
- HDL-C
HDL cholesterol
- LCAT
lecithin:cholesterol acyltransferase
- LDL
low-density lipoprotein
- LDL-C
LDL cholesterol
- LXR
liver X receptor
- MUFA
monounsaturated fatty acids
- NCoR
nuclear hormone receptor corepressor
- PEST
proline, glutamic acid, serine and threonine
- PKA
protein kinase A
- PKCδ
protein kinase C δ
- PLTP
phospholipid transfer protein
- PUFA
polyunsaturated fatty acids
- RCT
reverse cholesterol transport
- RXR
retinoid X receptor
- SFA
saturated fatty acids
- siRNA
small interfering RNA
- SMRT
silencing mediator of retinoid and thyroid hormone receptors
- SR-BI
scavenger receptor type B, class I
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62:707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 3.Miller NE. The evidence for the antiatherogenicity of high density lipoprotein in man. Lipids. 1978;13:914–919. doi: 10.1007/BF02533850. [DOI] [PubMed] [Google Scholar]
- 4.Fielding CJ, Fielding PE. Molecular physiology of reverse cholesterol transport. J Lipid Res. 1995;36:211–228. [PubMed] [Google Scholar]
- 5.Barter PJ. Inhibition of endothelial cell adhesion molecule expression by high density lipoproteins. Clinical and Experimental Pharmacology & Physiology. 1997;24:286–287. doi: 10.1111/j.1440-1681.1997.tb01821.x. [DOI] [PubMed] [Google Scholar]
- 6.Parthasarathy S, Barnett J, Fong LG. High-density lipoprotein inhibits the oxidative modification of low-density lipoprotein. Biochim Biophys Acta. 1990;1044:275–283. doi: 10.1016/0005-2760(90)90314-n. [DOI] [PubMed] [Google Scholar]
- 7.Glomset JA. The plasma lecithins:cholesterol acyltransferase reaction. J Lipid Res. 1968;9:155–167. [PubMed] [Google Scholar]
- 8.Pieters MN, Schouten D, Van Berkel TJ. In vitro and in vivo evidence for the role of HDL in reverse cholesterol transport. Biochim Biophys Acta. 1994;1225:125–134. doi: 10.1016/0925-4439(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 9.Anderson DW, Nichols AV, Forte TM, Lindgren FT. Particle distribution of human serum high density lipoproteins. Biochim Biophys Acta. 1977;493:55–68. doi: 10.1016/0005-2795(77)90259-8. [DOI] [PubMed] [Google Scholar]
- 10.Blanche PJ, Gong EL, Forte TM, Nichols AV. Characterization of human high-density lipoproteins by gradient gel electrophoresis. Biochim Biophys Acta. 1981;665:408–419. doi: 10.1016/0005-2760(81)90253-8. [DOI] [PubMed] [Google Scholar]
- 11.Noble RP, Hatch FT, Mazrimas JA, Lindgren FT, Jensen LC, Adamson GL. Comparison of lipoprotein analysis by agarose gel and paper electrophoresis with analytical ultracentrifugation. Lipids. 1969;4:55–59. doi: 10.1007/BF02531795. [DOI] [PubMed] [Google Scholar]
- 12.Cheung MC, Albers JJ. Characterization of lipoprotein particles isolated by immunoaffinity chromatography. Particles containing A-I and A-II and particles containing A-I but no A-II. J Biol Chem. 1984;259:12201–12209. [PubMed] [Google Scholar]
- 13.Schaefer EJ, Zech LA, Jenkins LL, Bronzert TJ, Rubalcaba EA, Lindgren FT, et al. Human apolipoprotein A-I and A-II metabolism. J Lipid Res. 1982;23:850–862. [PubMed] [Google Scholar]
- 14.Jauhiainen M, Metso J, Pahlman R, Blomqvist S, van Tol A, Ehnholm C. Human plasma phospholipid transfer protein causes high density lipoprotein conversion. J Biol Chem. 1993;268:4032–4036. [PubMed] [Google Scholar]
- 15.Pussinen P, Jauhianinen M, Metso J, Tyynela J, Ehnholm C. Pig plasma phospholipid transfer protein facilitates HDL interconversion. J Lipid Res. 1995;36:975–985. [PubMed] [Google Scholar]
- 16.Tu AY, Nishida HI, Nishida T. High density lipoprotein conversion mediated by human plasma phospholipid transfer protein. J Biol Chem. 1993;268:23098–23105. [PubMed] [Google Scholar]
- 17.Thuren T. Hepatic lipase and HDL metabolism. Curr Opin Lipidol. 2000;11:277–283. doi: 10.1097/00041433-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Ha YC, Gorjatschko L, Barter PJ. Changes in the density distribution of pig high density lipoproteins during incubation in vitro. Influence of esterified cholesterol transfer activity. Atherosclerosis. 1983;48:253–263. doi: 10.1016/0021-9150(83)90043-6. [DOI] [PubMed] [Google Scholar]
- 19.Kunitake ST, Mendel CM, Hennessy LK. Interconversion between apolipoprotein A-I-containing lipoproteins of pre-beta and alpha electrophoretic mobilities. J Lipid Res. 1992;33:1807–1816. [PubMed] [Google Scholar]
- 20.Keys A. Effects of different dietary fats on plamsa lipid levels. Lancet. 1965;1:318–319. doi: 10.1016/s0140-6736(65)91053-6. [DOI] [PubMed] [Google Scholar]
- 21.Hegsted D, McGandy RB, Myers ML, Stare FJ. Quantative effects of dietary fat on serum cholesterol in man. Am J Clin Nutr. 1965;17:281–293. doi: 10.1093/ajcn/17.5.281. [DOI] [PubMed] [Google Scholar]
- 22.Francone OL, Subbaiah PV, van Tol A, Royer L, Haghpassand M. Abnormal phospholipid composition impairs HDL biogenesis and maturation in mice lacking Abca1. Biochemistry (Mosc) 2003;42:8569–8578. doi: 10.1021/bi034540v. [DOI] [PubMed] [Google Scholar]
- 23.McNeish J, Aiello RJ, Guyot D, Turi T, Gabel C, Aldinger C, et al. High density lipoprotein deficiency and foam cell accumulation in mice with targeted disruption of ATP-binding cassette transporter-1. Proc Natl Acad Sci U S A. 2000;97:4245–4250. doi: 10.1073/pnas.97.8.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parks JS, Rudel LL. Effect of fish oil on atherosclerosis and lipoprotein metabolism. Atherosclerosis. 1990;84:83–94. doi: 10.1016/0021-9150(90)90077-v. [DOI] [PubMed] [Google Scholar]
- 25.Harris WS. n-3 fatty acids and lipoproteins: comparison of results from human and animal studies. Lipids. 1996;31:243–252. doi: 10.1007/BF02529870. [DOI] [PubMed] [Google Scholar]
- 26.Johnson FL, Babiak J, Rudel LL. High density lipoprotein accumulation in perfusates of isolated livers of African green monkeys. Effects of saturated versus polyunsaturated dietary fat. J Lipid Res. 1986;27:537–548. [PubMed] [Google Scholar]
- 27.Mensink RP, Zock PL, Kester ADM, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- 28.Hegsted DM, Ausman LM, Johnson JA, Dallal GE. Dietary fat and serum lipids: an evaluation of the experimental data. Am J Clin Nutr. 1993;57:875–883. doi: 10.1093/ajcn/57.6.875. [DOI] [PubMed] [Google Scholar]
- 29.Mensink RP, Katan MB. Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler Thromb. 1992;12:911–919. doi: 10.1161/01.atv.12.8.911. [DOI] [PubMed] [Google Scholar]
- 30.Kris-Etherton PM, Yu S. Individual fatty acid effects on plasma lipids and lipoproteins: human studies. Am J Clin Nutr. 1997;65:1628S–1644S. doi: 10.1093/ajcn/65.5.1628S. [DOI] [PubMed] [Google Scholar]
- 31.Howell WH, McNamara DJ, Tosca MA, Smith BT, Gaines JA. Plasma lipid and lipoprotein responses to dietary fat and cholesterol: a meta-analysis. Am J Clin Nutr. 1997;65:1747–1764. doi: 10.1093/ajcn/65.6.1747. [DOI] [PubMed] [Google Scholar]
- 32.Kralova Lesna I, Suchanek P, Kovar J, Stavek P, Poledne R. Replacement of dietary saturated FAs by PUFAs in diet and reverse cholesterol transport. J Lipid Res. 2008;49:2414–2418. doi: 10.1194/jlr.M800271-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Fredrickson DS, Altrocchi PH, Avioli LV, Goodman DS, Goodman HC. Tangier disease. Ann Intern Med. 1961;55:1016–1031. [Google Scholar]
- 34.Serfaty-Lacrosniere C, Civeira F, Lanzberg A, Isaia P, Berg J, Janus ED, et al. Homozygous Tangier disease and cardiovascular disease. Atherosclerosis. 1994;107:85–98. doi: 10.1016/0021-9150(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 35.Oram JF. Tangier disease and ABCA1. Biochim Biophys Acta. 2000;1529:321–330. doi: 10.1016/s1388-1981(00)00157-8. [DOI] [PubMed] [Google Scholar]
- 36.Bodzioch M, Orso E, Klucken J, Langmann T, Bottcher A, Diederich W, et al. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet. 1999;22:347–351. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- 37.Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, et al. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet. 1999;22:336–345. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- 38.Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette JC, et al. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet. 1999;22:352–355. doi: 10.1038/11921. [DOI] [PubMed] [Google Scholar]
- 39.Luciani MF, Denizot F, Savary S, Mattei MG, Chimini G. Cloning of two novel ABC transporters mapping on human chromosome 9. Genomics. 1994;21:150–159. doi: 10.1006/geno.1994.1237. [DOI] [PubMed] [Google Scholar]
- 40.Castro GR, Fielding CJ. Early incorporation of cell-derived cholesterol into pre-beta-migrating high-density lipoprotein. Biochemistry (Mosc) 1988;27:25–29. doi: 10.1021/bi00401a005. [DOI] [PubMed] [Google Scholar]
- 41.Huang Y, von Eckardstein A, Assmann G. Cell-derived unesterified cholesterol cycles between different HDLs and LDL for its effective esterification in plasma. Arterioscler Thromb. 1993;13:445–458. doi: 10.1161/01.atv.13.3.445. [DOI] [PubMed] [Google Scholar]
- 42.Forte TM, Goth-Goldstein R, Nordhausen RW, McCall MR. Apolipoprotein A-I-cell membrane interaction: extracellular assembly of heterogeneous nascent HDL particles. J Lipid Res. 1993;34:317–324. [PubMed] [Google Scholar]
- 43.Forte TM, Bielicki JK, Knoff L, McCall MR. Structural relationships between nascent apoA-I-containing particles that are extracellularly assembled in cell culture. J Lipid Res. 1996;37:1076–1085. [PubMed] [Google Scholar]
- 44.Bielicki JK, McCall MR, Forte TM. Apolipoprotein A-I promotes cholesterol release and apolipoprotein E recruitment from THP-1 macrophage-like foam cells. J Lipid Res. 1999;40:85–92. [PubMed] [Google Scholar]
- 45.Francis GA, Knopp RH, Oram JF. Defective removal of cellular cholesterol and phospholipids by apolipoprotein A-I in Tangier Disease. J Clin Invest. 1995;96:78–87. doi: 10.1172/JCI118082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takahashi Y, Smith JD. Cholesterol efflux to apolipoprotein AI involves endocytosis and resecretion in a calcium-dependent pathway. Proc Natl Acad Sci U S A. 1999;96:11358–11363. doi: 10.1073/pnas.96.20.11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu L, Bortnick AE, Nickel M, Dhanasekaran P, Subbaiah PV, Lund-Katz S, et al. Effects of apolipoprotein A-I on ATP-binding cassette transporter A1-mediated efflux of macrophage phospholipid and cholesterol: formation of nascent high density lipoprotein particles. J Biol Chem. 2003;278:42976–42984. doi: 10.1074/jbc.M308420200. [DOI] [PubMed] [Google Scholar]
- 48.Mulya A, Lee JY, Gebre AK, Thomas MJ, Colvin PL, Parks JS. Minimal lipidation of pre-beta HDL by ABCA1 results in reduced ability to interact with ABCA1. Arterioscler Thromb Vasc Biol. Arterioscler Thromb Vasc Biol 2007;27:1828–1836. doi: 10.1161/ATVBAHA.107.142455. [DOI] [PubMed] [Google Scholar]
- 49.Mulya A, Lee JY, Gebre AK, Boudyguina EY, Chung SK, Smith TL, et al. Initial interaction of ApoA-I with ATP binding cassette transporter A1 (ABCA1) impacts in vivo metabolic fate of nascent HDL. J Lipid Res. 2008;49:2390–2401. doi: 10.1194/jlr.M800241-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaisman BL, Lambert G, Amar M, Joyce C, Ito T, Shamburek RD, et al. ABCA1 overexpression leads to hyperalphalipoproteinemia and increased biliary cholesterol excretion in transgenic mice. J Clin Invest. 2001;108:303–309. doi: 10.1172/JCI12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haghpassand M, Bourassa PA, Francone OL, Aiello RJ. Monocyte/macrophage expression of ABCA1 has minimal contribution to plasma HDL levels. J Clin Invest. 2001;108:1315–1320. doi: 10.1172/JCI12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Basso F, Freeman L, Knapper CL, Remaley A, Stonik J, Neufeld EB, et al. Role of the hepatic ABCA1 transporter in modulating intrahepatic cholesterol and plasma HDL cholesterol concentrations. J Lipid Res. 2003;44:296–302. doi: 10.1194/jlr.M200414-JLR200. [DOI] [PubMed] [Google Scholar]
- 53.Wellington CL, Brunham LR, Zhou S, Singaraja RR, Visscher H, Gelfer A, et al. Alterations of plasma lipids in mice via adenoviral-mediated hepatic overexpression of human ABCA1. J Lipid Res. 2003;44:1470–1480. doi: 10.1194/jlr.M300110-JLR200. [DOI] [PubMed] [Google Scholar]
- 54.Murthy S, Born E, Mathur SN, Field FJ. LXR/RXR activation enhances basolateral efflux of cholesterol in CaCo-2 cells. J Lipid Res. 2002;43:1054–1064. doi: 10.1194/jlr.m100358-jlr200. [DOI] [PubMed] [Google Scholar]
- 55.Brunham LR, Kruit JK, Iqbal J, Fievet C, Timmins JM, Pape TD, et al. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J Clin Invest. 2006;116:1052–1062. doi: 10.1172/JCI27352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu X, Lee JY, Timmins JM, Brown JM, Boudyguina E, Mulya A, et al. Increased Cellular Free Cholesterol in Macrophage-specific Abca1 Knock-out Mice Enhances Pro-inflammatory Response of Macrophages. J Biol Chem. 2008;283:22930–22941. doi: 10.1074/jbc.M801408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Timmins JM, Lee JY, Boudyguina E, Kluckman KD, Brunham LR, Mulya A. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J Clin Invest. 2005;115:1333–1342. doi: 10.1172/JCI23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee JY, Parks JS. ATP-binding cassette transporter AI and its role in HDL formation. Curr Opin Lipidol. 2005;16:19–25. doi: 10.1097/00041433-200502000-00005. [DOI] [PubMed] [Google Scholar]
- 59.Naik SU, Wang X, Da Silva JS, Jaye M, Macphee CH, Reilly MP, et al. Pharmacological Activation of Liver X Receptors Promotes Reverse Cholesterol Transport In Vivo. Circulation. 2006;113:90–97. doi: 10.1161/CIRCULATIONAHA.105.560177. [DOI] [PubMed] [Google Scholar]
- 60.Tontonoz P, Mangelsdorf DJ. Liver X receptor signaling pathways in cardiovascular disease. Mol Endocrinol. 2003;17:985–993. doi: 10.1210/me.2003-0061. [DOI] [PubMed] [Google Scholar]
- 61.Costet P, Luo Y, Wang N, Tall AR. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J Biol Chem. 2000;275:28240–28245. doi: 10.1074/jbc.M003337200. [DOI] [PubMed] [Google Scholar]
- 62.Venkateswaran A, Laffitte BA, Joseph SB, Mak PA, Wilpitz DC, Edwards PA, et al. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc Natl Acad Sci U S A. 2000;97:12097–12102. doi: 10.1073/pnas.200367697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wellington CL, Walker EK, Suarez A, Kwok A, Bissada N, Singaraja R, et al. ABCA1 mRNA and protein distribution patterns predict multiple different roles and levels of regulation. Lab Invest. 2002;82:273–283. doi: 10.1038/labinvest.3780421. [DOI] [PubMed] [Google Scholar]
- 64.Arakawa R, Yokoyama S. Helical apolipoproteins stabilize ATP-binding cassette transporter A1 by protecting it from thiol protease-mediated degradation. J Biol Chem. 2002;277:22426–22429. doi: 10.1074/jbc.M202996200. [DOI] [PubMed] [Google Scholar]
- 65.Oram JF, Lawn RM, Garvin MR, Wade DP. ABCA1 is the cAMP-inducible apolipoprotein receptor that mediates cholesterol secretion from macrophages. J Biol Chem. 2000;275:34508–34511. doi: 10.1074/jbc.M006738200. [DOI] [PubMed] [Google Scholar]
- 66.Wang N, Chen W, Linsel-Nitschke P, Martinez LO, Agerholm-Larsen B, Silver DL, et al. A PEST sequence in ABCA1 regulates degradation by calpain protease and stabilization of ABCA1 by apoA-I. J Clin Invest. 2003;111:99–107. doi: 10.1172/JCI16808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martinez LO, Agerholm-Larsen B, Wang N, Chen W, Tall AR. Phosphorylation of a pest sequence in ABCA1 promotes calpain degradation and is reversed by ApoA-I. J Biol Chem. 2003;278:37368–37374. doi: 10.1074/jbc.M307161200. [DOI] [PubMed] [Google Scholar]
- 68.Arakawa R, Hayashi M, Remaley AT, Brewer BH, Yamauchi Y, Yokoyama S. Phosphorylation and stabilization of ATP binding cassette transporter A1 by synthetic amphiphilic helical peptides. J Biol Chem. 2004;279:6217–6220. doi: 10.1074/jbc.C300553200. [DOI] [PubMed] [Google Scholar]
- 69.Uehara Y, Engel T, Li Z, Goepfert C, Rust S, Zhou X, et al. Polyunsaturated fatty acids and acetoacetate down-regulate the expression of the ATP-binding cassette transporter A1. Diabetes. 2002;51:2922–2928. doi: 10.2337/diabetes.51.10.2922. [DOI] [PubMed] [Google Scholar]
- 70.Mauerer R, Ebert S, Langmann T. High glucose, unsaturated and saturated fatty acids differentially regulate expression of ATP-binding cassette transporters ABCA1 and ABCG1 in human macrophages. Exp Mol Med. 2009;41:126–132. doi: 10.3858/emm.2009.41.2.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Vogel-van den Bosch HM, de Wit NJW, Hooiveld GJEJ, Vermeulen H, van der Veen JN, Houten SM, et al. A cholesterol-free, high-fat diet suppresses gene expression of cholesterol transporters in murine small intestine. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2008;294:G1171–G1180. doi: 10.1152/ajpgi.00360.2007. [DOI] [PubMed] [Google Scholar]
- 72.Ontsouka CE, Burgener IA, Mani O, Albrecht C. Polyunsaturated fatty acid-enriched diets used for the treatment of canine chronic enteropathies decrease the abundance of selected genes of cholesterol homeostasis. Domest Anim Endocrinol. 2010;38:32–37. doi: 10.1016/j.domaniend.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 73.Uehara Y, Miura Si, von Eckardstein A, Abe S, Fujii A, Matsuo Y, et al. Unsaturated fatty acids suppress the expression of the ATP-binding cassette transporter G1 (ABCG1) and ABCA1 genes via an LXR/RXR responsive element. Atherosclerosis. 2007;191:11–21. doi: 10.1016/j.atherosclerosis.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 74.Ku CS, Park YK, Coleman SL, Seo JM, Lee J-Y. Regulation of ATP binding cassette transporter A1 (ABCA1) and ABCG1 by fatty acids in RAW 264.7 macrophages. Journal. 2010 Volumn:pages. [Google Scholar]
- 75.Roth SY, Denu JM, Allis CD. Histone acetyltransferases. Annu Rev Biochem. 2001;70:81–120. doi: 10.1146/annurev.biochem.70.1.81. [DOI] [PubMed] [Google Scholar]
- 76.Jones DR, Divecha N. Linking lipids to chromatin. Curr Opin Genet Dev. 2004;14:196–202. doi: 10.1016/j.gde.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 77.Guenther MG, Lane WS, Fischle W, Verdin E, Lazar MA, Shiekhattar R. A core SMRT corepressor complex containing HDAC3 and TBL1, a WD40-repeat protein linked to deafness. Genes Dev. 2000;14:1048–1057. [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang J, Kalkum M, Chait BT, Roeder RG. The N-CoR-HDAC3 nuclear receptor corepressor complex inhibits the JNK pathway through the integral subunit GPS2. Mol Cell. 2002;9:611–623. doi: 10.1016/s1097-2765(02)00468-9. [DOI] [PubMed] [Google Scholar]
- 79.Perissi V, Aggarwal A, Glass CK, Rose DW, Rosenfeld MG. A corepressor/coactivator exchange complex required for transcriptional activation by nuclear receptors and other regulated transcription factors. Cell. 2004;116:511–526. doi: 10.1016/s0092-8674(04)00133-3. [DOI] [PubMed] [Google Scholar]
- 80.Lee SK, Kim JH, Lee YC, Cheong J, Lee JW. Silencing mediator of retinoic acid and thyroid hormone receptors, as a novel transcriptional corepressor molecule of activating protein-1, nuclear factor-kappaB, and serum response factor. J Biol Chem. 2000;275:12470–12474. doi: 10.1074/jbc.275.17.12470. [DOI] [PubMed] [Google Scholar]
- 81.Baek SH, Ohgi KA, Rose DW, Koo EH, Glass CK, Rosenfeld MG. Exchange of N-CoR corepressor and Tip60 coactivator complexes links gene expression by NF-kappaB and beta-amyloid precursor protein. Cell. 2002;110:55–67. doi: 10.1016/s0092-8674(02)00809-7. [DOI] [PubMed] [Google Scholar]
- 82.Rosenfeld MG, Glass CK. Coregulator codes of transcriptional regulation by nuclear receptors. J Biol Chem. 2001;276:36865–36868. doi: 10.1074/jbc.R100041200. [DOI] [PubMed] [Google Scholar]
- 83.Naar AM, Lemon BD, Tjian R. Transcriptional coactivator complexes. Annu Rev Biochem. 2001;70:475–501. doi: 10.1146/annurev.biochem.70.1.475. [DOI] [PubMed] [Google Scholar]
- 84.McKenna NJ, O'Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell. 2002;108:465–474. doi: 10.1016/s0092-8674(02)00641-4. [DOI] [PubMed] [Google Scholar]
- 85.Wagner BL, Valledor AF, Shao G, Daige CL, Bischoff ED, Petrowski M, et al. Promoter-Specific Roles for Liver X Receptor/Corepressor Complexes in the Regulation of ABCA1 and SREBP1 Gene Expression. Mol Cell Biol. 2003;23:5780–5789. doi: 10.1128/MCB.23.16.5780-5789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brandl A, Heinzel T, Kramer OH. Histone deacetylases: salesmen and customers in the post-translational modification market. Biol Cell. 2009;101:193–205. doi: 10.1042/BC20080158. [DOI] [PubMed] [Google Scholar]
- 87.Wang Y, Oram JF. Unsaturated fatty acids inhibit cholesterol efflux from macrophages by increasing degradation of ATP-binding cassette transporter A1. J Biol Chem. 2002;277:5692–5697. doi: 10.1074/jbc.M109977200. [DOI] [PubMed] [Google Scholar]
- 88.Wang Y, Kurdi-Haidar B, Oram JF. LXR-mediated activation of macrophage stearoyl-CoA desaturase generates unsaturated fatty acids that destabilize ABCA1. J Lipid Res. 2004;45:972–980. doi: 10.1194/jlr.M400011-JLR200. [DOI] [PubMed] [Google Scholar]
- 89.Yang Y, Jiang Y, Wang Y, An W. Suppression of ABCA1 by unsaturated fatty acids leads to lipid accumulation in HepG2 cells. Biochimie. 2010;92:958–963. doi: 10.1016/j.biochi.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 90.Murthy S, Born E, Mathur SN, Field FJ. Liver-X-receptor-mediated increase in ATP-binding cassette transporter A1 expression is attenuated by fatty acids in CaCo-2 cells: effect on cholesterol efflux to high-density lipoprotein. Biochem J. 2004;377:545–552. doi: 10.1042/BJ20030903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Y, Oram JF. Unsaturated fatty acids phosphorylate and destabilize ABCA1 through a phospholipase D2 pathway. J Biol Chem. 2005;280:35896–35903. doi: 10.1074/jbc.M506210200. [DOI] [PubMed] [Google Scholar]
- 92.Wang Y, Oram JF. Unsaturated fatty acids phosphorylate and destabilize ABCA1 through a protein kinase C delta pathway. J Lipid Res. 2007;48:1062–1068. doi: 10.1194/jlr.M600437-JLR200. [DOI] [PubMed] [Google Scholar]
- 93.Hu Y-W, Ma X, Li X-X, Liu X-H, Xiao J, Mo Z-C, et al. Eicosapentaenoic acid reduces ABCA1 serine phosphorylation and impairs ABCA1-dependent cholesterol efflux through cyclic AMP/protein kinase A signaling pathway in THP-1 macrophage-derived foam cells. Atherosclerosis. 2009;204:e35–e43. doi: 10.1016/j.atherosclerosis.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 94.Park YK, Rasmussen HE, Ehlers SJ, Ku CS, Jesch ED, Carr TP, et al. Down-regulaiton of ATP-binding cassette transporter A1 (ABCA1) and ABCG1 expression by unsaturated fatty acids in vivo and in vitro. Journal. 2008 Volumn:pages. [Google Scholar]
- 95.Demaison L, Moreau D. Dietary n-3 polyunsaturated fatty acids and coronary heart disease-related mortality: a possible mechanism of action. Cell Mol Life Sci. 2002;59:463–477. doi: 10.1007/s00018-002-8439-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schaefer EJ. Effects of dietary fatty acids on lipoproteins and cardiovascular disease risk: summary. Am J Clin Nutr. 1997;65:1655S–1656S. doi: 10.1093/ajcn/65.5.1655S. [DOI] [PubMed] [Google Scholar]
- 97.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]