Abstract
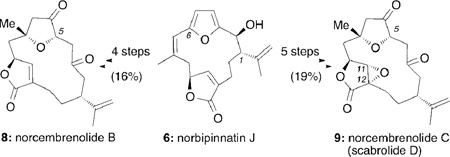
An efficient stereoselective synthesis of norcembrenolide B (8) and scabrolide D (9) is reported. The strategy is inspired by biogenetic relationships of related cembrenoids. Central to this approach is the construction of norbipinnatin J which upon selective C2 deoxygenation and C8 oxygenation produces norrubifolide and norcoralloidolide A. A sequence of site-selective oxidations and skeletal reorganizations then yields in a divergent manner, compounds 8 and 9. The studies allow revision of the proposed structure of scabrolide D (9), which is identical to norcembrenolide C.
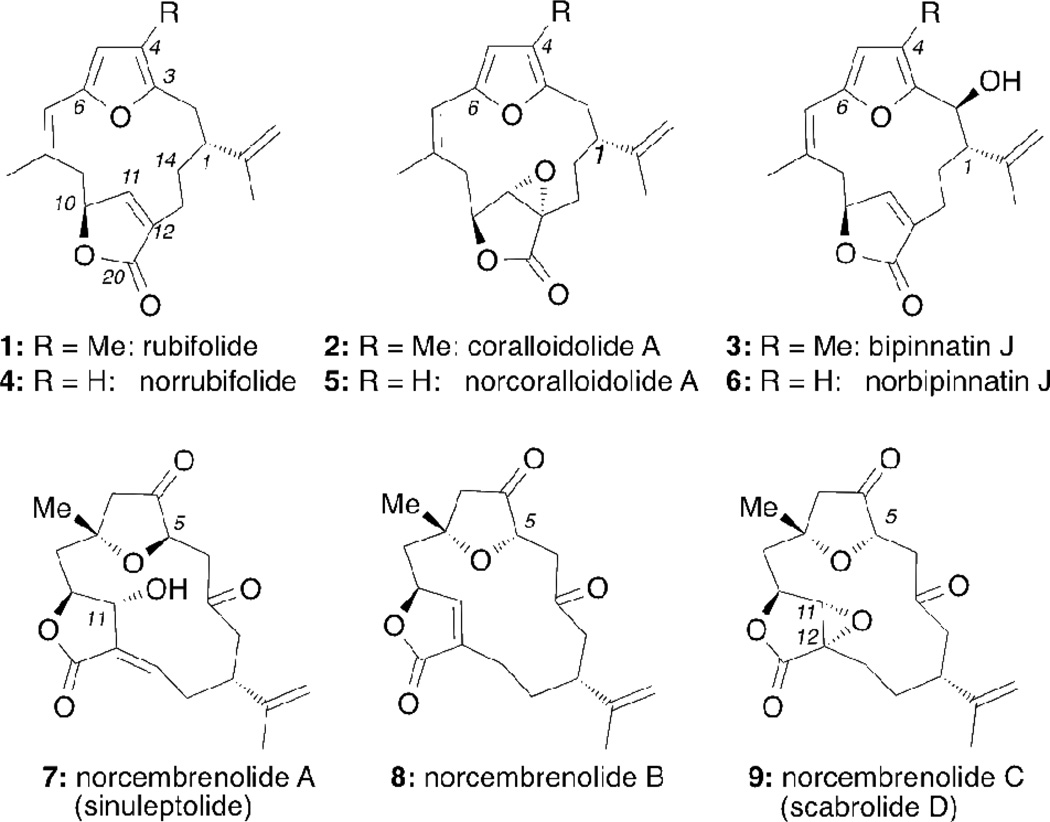
Gorgonian octocorals and soft corals, particularly those of the genus Sinularia, have been recognized as a rich source of natural products containing the 14-membered cembrane skeleton (Figure 1).1 Proposed to be utilized by the corals as chemical defense against predation, these natural products display an intriguing array of biological and pharmacological activities.2 For example, members of the bipinnatin subfamily have been evaluated as active-site-directed inhibitors of nicotinic acetylcholine receptors.2b Various norcembrenolides were found to exhibit low micromolar cytotoxicities against several cancer cell lines.3 More recently, sinuleptolide (7) and scabrolide D (9) were shown to inhibit LPS-induced TNF-α production in a dose-dependent manner.4
Figure 1.
Chemical structures of selected cembrenoids
Norcembrenolides A (7),5 B (8) and C (9) were isolated by Fenical et al. from several Sinularia species collected in Palau.6 Certain members of this family were also isolated by Sheu et al. from the Taiwanese soft coral S. scabra and were named scabrolides.3a,7 From a biosynthetic standpoint, these compounds are proposed to derive from the furanocembrenoids, in which a furan (C3–C6) and a butenolide C10–C20) are embedded in the 14-membered cembrane macrocycle (see structure of rubifolide, 1).8 Oxidations of the carbocyclic framework of 1 are proposed to give access to oxygenated furanocembrenoids like 29 and 3.10 Further oxidation at the furan ring followed by oxidative decarboxylation of the C4 methyl group could account for the formation of norcembrenolides.1d
Inspired by the combination of interesting chemical structures and unexplored bioactivities and guided by the proposed biosynthesis, we sought to develop a divergent synthesis toward this family of compounds.11 Here we describe the first synthesis of norcembrenolides B (8) and C (9). Our results also revise the proposed structure of scabrolide D, which is in fact identical to that of norcembrenolide C.
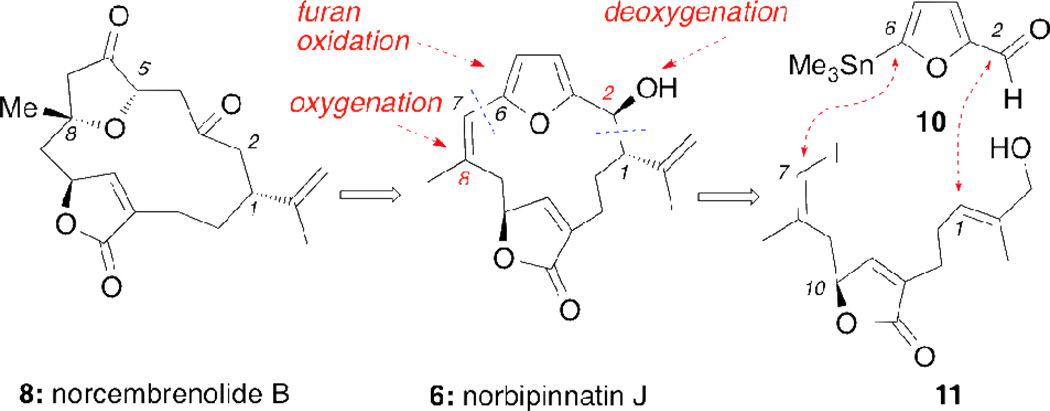
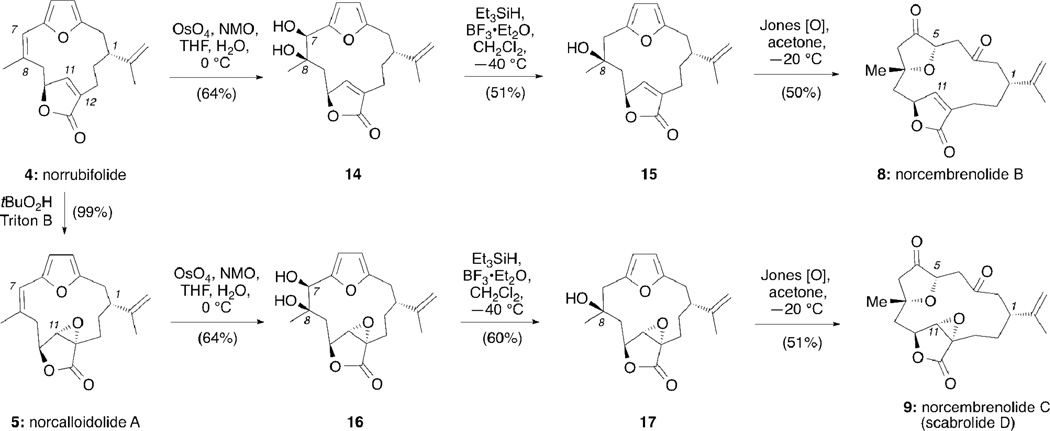
Scheme 1 highlights the key elements of the strategy as applied to the synthesis of norcembrenolide B (8). A sequence of deoxygenation at the C2 center followed by a selective oxygenation at the C8 center and furan oxidation/cyclization would form 8 from norbipinnatin J (6). The central cembrane skeleton could be constructed from aldehyde 10 and butenolide 11 using well established Stille and Kishi-Nozaki couplings.12
Scheme 1.
Retrosynthetic analysis of norcembrenolide B (8)
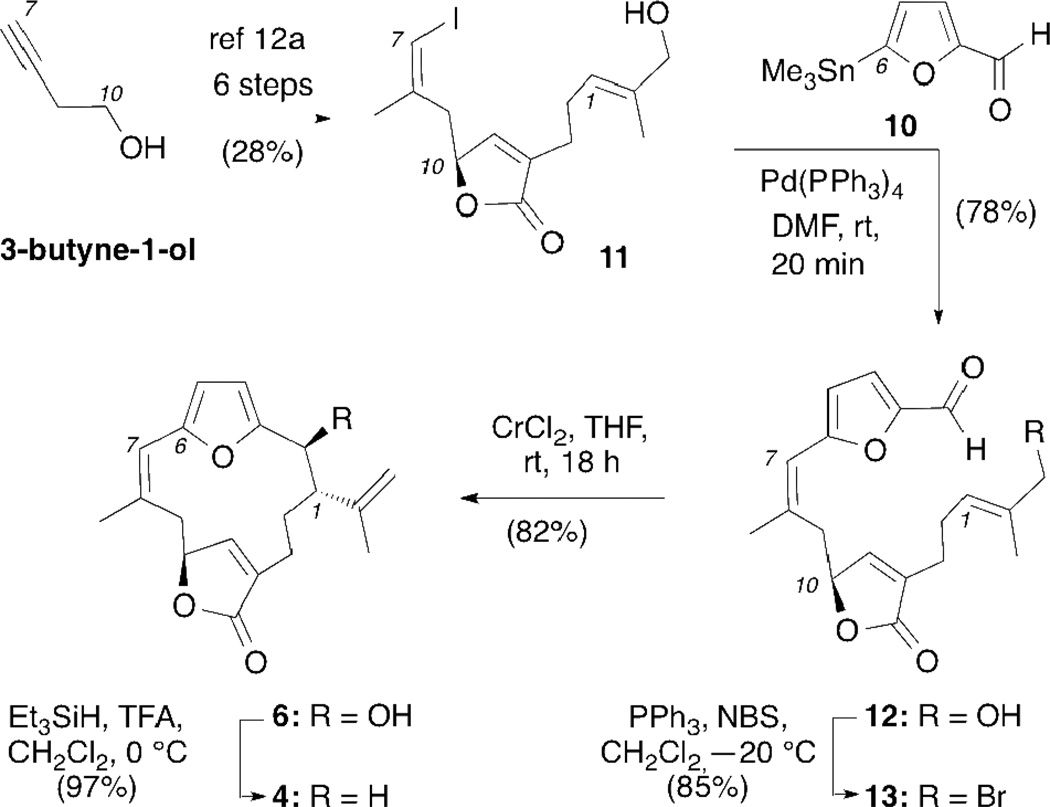
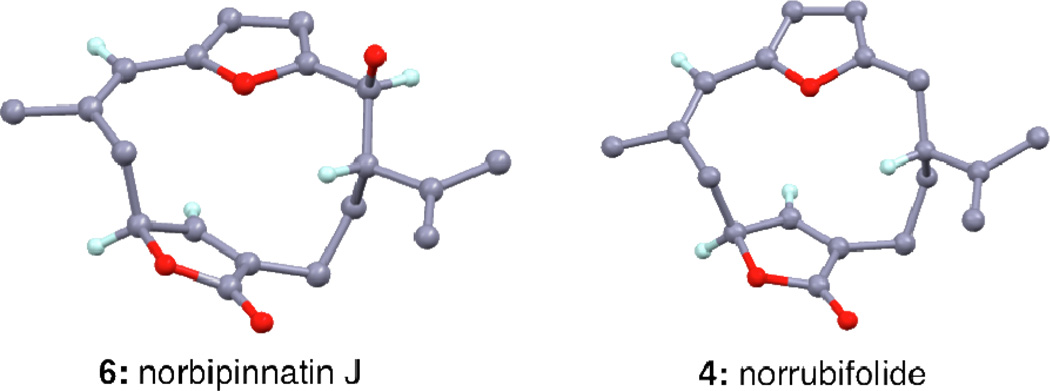
The synthesis began with 3-butyne-1-ol, containing the C7–C10 cembrane fragment (Scheme 2). A sequence of 6 steps, based on Trauner’s strategy,12a afforded the racemic vinyl iodide 11 in 28% overall yield.13 Coupling of 11 with furfural stannane 10 under Pd(0) conditions produced aldehyde 12 in 78% yield. In preparation for the Kishi-Nozaki coupling the allylic alcohol of 12 was first converted to allyl bromide 13 using Appel bromination14 that upon treatment with CrCl2/NiCl2 produced norbipinnatin J (6) as the major diastereomer in 82% yield.15 The relative stereochemistry of 6 was unambiguously confirmed via a single crystal X-ray analysis (Figure 2).16 Deoxygenation of the C2 hydroxyl group was achieved using TFA/Et3SiH12a,17 to afford norrubifolide (4) in 97% yield.
Scheme 2.
Synthesis of norbipinnatin J (6) and norrubifolide (4)
Figure 2.
X-ray structures of compounds 6 and 4
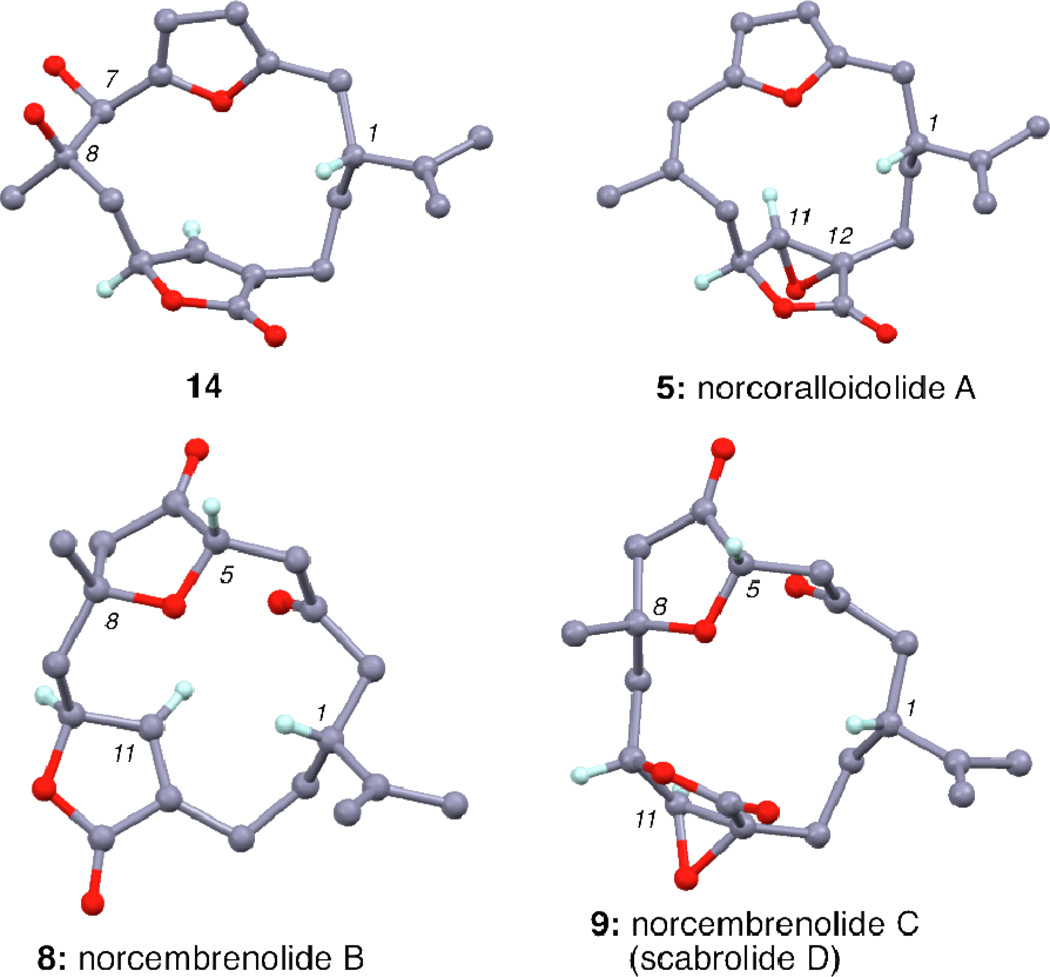
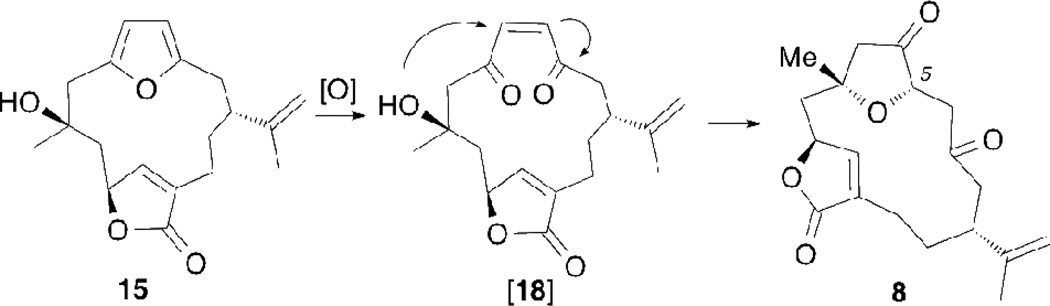
Compound 4 represents a branching point of our strategy (Scheme 3). The X-ray of 4 shows a rigid structure that is amenable to regioselective functionalizations at both the C7–C8 and C11–C12 double bonds (Figure 2). The best way to achieve a selective oxygenation at C8 was found to be a dihydroxylation of the C7–C8 double bond, followed by deoxygenation of the C7 hydroxyl group. The dihydroxylation reaction proceeded best under Upjohn conditions18 (OsO4, NMO) and afforded diol 14 as a single isomer in 64% yield. As predicted, the hydroxyl groups were introduced from the sterically more accessible β-face of the cembrane ring (Figure 3). Deoxygenation under Et3SiH/BF3•Et2O conditions19 then produced compound 15 in 51% yield. Conversion of the furan to the β-keto-tetrahydrofuranone was accomplished utilizing the Jones reagent. It is believed that the transformation begins with an initial oxidation of the furan to an intermediate Z-ene-dione structure (Figure 4).20 The tertiary alcohol, under acidic conditions, then quickly cyclizes in a 5-exo-trig fashion, producing norcembrenolide B (8) in 50% yield. The structure of 8 was confirmed via crystallographic studies (Figure 3).
Scheme 3.
Synthesis of norcembrenolide B (8) and norcembrenolide C (scabrolide D, 9)
Figure 3.
X-rays of compounds 14, 5, 8 and 9.
Figure 4.
Conversion of 15 to 8 via the Jones oxidation.
Our synthesis diverges with a selective oxidation of 4 using anhydrous TBHP and catalytic Triton B21 affording norcoralloidolide A (5) in 99% yield. As expected this epoxidation proceeded selectively from the α–face of the butenolide motif. The structure of 5 was unambiguously confirmed via a single crystal X-ray analysis (Figure 3). Further manipulation of 5 using the conditions described above gave rise to norcembrenolide C (9) in 3 steps and 17% overall yield.
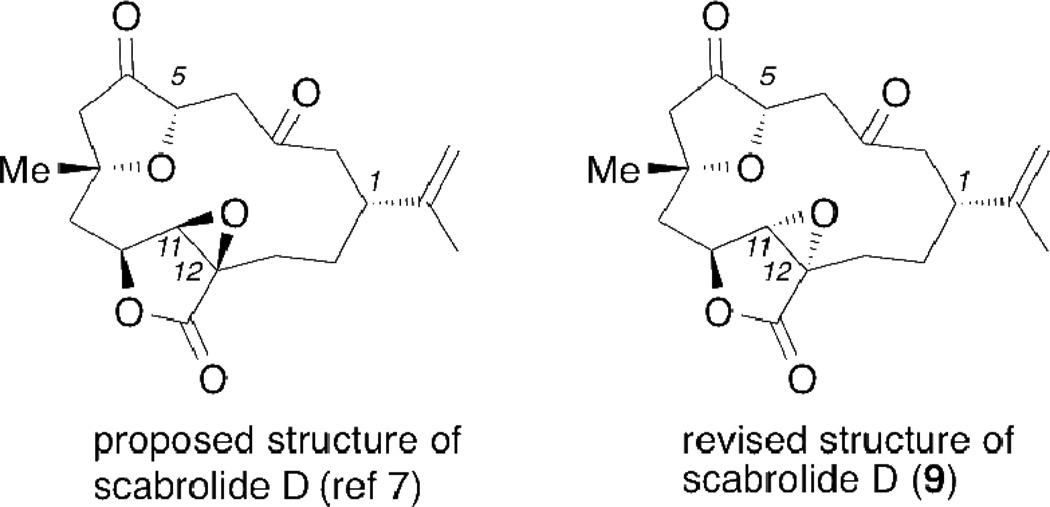
Interestingly, the proposed structure of scabrolide D, as reported by Sheu et al,7 had a relative stereochemistry in which the epoxide moiety is on the same face as the lactone oxygen (see Figure 5). Spectroscopic (1H and 13C NMR) comparison of synthetic 9 taken in CDCl3 with the data reported for scabrolide D7 reveals that the structures are identical. Moreover, X-ray crystallographic analysis of synthetic 9 confirmed the structural identity of scabrolide D leading to the revision of its relative stereochemistry at the C11 and C12 centers, with the epoxide occuring on the opposite face in regard to the lactone oxygen. It was previously unrealized, but in fact, scabrolide D is identical to norcembrenolide C (9) which was studied in benzene-d6 and was first reported by the Fenical group6 several years earlier.
Figure 5.
Proposed and revised structures of scabrolide D (9).
In conclusion, we present here a divergent strategy for the synthesis of norcembrenolides B (8) and C (9), two members of a family of structurally complex marine natural products with potent and largely unexplored biological properties. Our approach utilizes highly substrate-controlled modifications and allows an efficient, stereoselective and protecting-group-free access to this scaffold. Our results also establish that norcembrenolide C (9) is structurally identical to scabrolide D. The overall strategy paves the way for a methodical evaluation of the structure-activity relationship and chemical biology of this family of natural products.
Supplementary Material
Acknowledgment
We gratefully acknowledge the National Institutes of Health (NIH) for financial support of this work through Grant Number R01 GM081484. We thank the National Science Foundation for instrumentation grants CHE9709183 and CHE0741968. We also thank Dr. Anthony Mrse (UCSD NMR Facility), Dr. Yongxuan Su (UCSD MS Facility) and Dr. Arnold L. Rheingold and Dr. Curtis E. Moore (UCSD X-Ray Facility).
Footnotes
Dedicated to Professor W. Fenical (Scripps Institution of Oceanography, UCSD) on his 70th Birthday.
Supporting Information Available Detailed experimental procedures, spectral characterization, and copies of 1H and 13C NMR data. This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.(a) Roethle PA, Trauner D. Nat. Prod. Rep. 2008;25:298–317. doi: 10.1039/b705660p. [DOI] [PubMed] [Google Scholar]; (b) Lakshmi V, Kumar R. Nat. Prod. Res. 2009;23:801–850. doi: 10.1080/14786410802137135. [DOI] [PubMed] [Google Scholar]; (c) Li Y, Pattenden G. Nat. Prod. Rep. 2011;28:429–440. doi: 10.1039/c0np00029a. [DOI] [PubMed] [Google Scholar]; (d) Li Y, Pattenden G. Nat. Prod. Rep. 2011;28:1269–1310. doi: 10.1039/c1np00023c. [DOI] [PubMed] [Google Scholar]
- 2.(a) Kamel HN, Slattery M. Pharmac. Biol. 2005;43:253–269. [Google Scholar]; (b) Abramson SN, Fenical W, Taylor P. Drug Dev. Res. 1991;24:297–312. [Google Scholar]; (c) Su J-H, Wen Z-H. Mar. Drugs. 2011;9:944–951. doi: 10.3390/md9060944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Ahmed AF, Su J-H, Kuo Y-H, Sheu J-H. J. Nat. Prod. 2004;67:2079–2082. doi: 10.1021/np040112u. [DOI] [PubMed] [Google Scholar]; (b) Ahmed AF, Shiue R-T, Wang G-H, Dai C-F, Kuo Y-H, Sheu J-H. Tetrahedron. 2003;59:7337–7344. [Google Scholar]
- 4.(a) Takaki H, Koganemaru R, Iwakawa Y, Higuchi R, Miyamoto T. Biol. Pharm. Bull. 2003;26:380–382. doi: 10.1248/bpb.26.380. [DOI] [PubMed] [Google Scholar]; (b) Cheng S-Y, Chuang C-T, Wen Z-H, Wang S-K, Chiou S-F, Hsu C-H, Dai C-F, Duh C-Y. Biorg. & Med. Chem. 2010;18:3379–3386. doi: 10.1016/j.bmc.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Norcembrenolide A (7), later named sinuleptolide, was initially isolated from the Australian soft-coral Sinularia leptoclados: Bowden BF, Coll JC, Mitchell SJ, Mulder J, Stokie GJ. Aust. J. Chem. 1978;31:2049–2056. For the revision of its structure see: Shoji N, Umeyama A, Arihara S. J. Nat. Prod. 1993;56:1651–1653.
- 6.Sato A, Fenical W, Qi-tai Z, Clardy J. Tetrahedron. 1985;41:4303–4308. [Google Scholar]
- 7.Sheu J-H, Ahmed AF, Shiue R-T, Dai C-F, Kuo Y-H. J. Nat. Prod. 2002;65:1904–1908. doi: 10.1021/np020280r. [DOI] [PubMed] [Google Scholar]
- 8.Williams D, Andersen RJ, Van Duyne GD, Clardy JJ. Org. Chem. 1987;52:332–335. [Google Scholar]
- 9.D’ Ambrosio M, Fabbri D, Guerriero A, Pietra F. Helv. Chim. Acta. 1987;70:63–70. [Google Scholar]
- 10.(a) Rodriguez AD, Shi J-G. J. Org. Chem. 1998;63:420–421. doi: 10.1021/jo971884g. [DOI] [PubMed] [Google Scholar]; (b) Rodriguez AD, Shi J-G, Huang SD. J. Nat. Prod. 1999;62:1228–1237. doi: 10.1021/np990064r. [DOI] [PubMed] [Google Scholar]
- 11.For related divergent strategies see: Li Y, Pattenden G. Tetrahedron Lett. 2011;52:3315–3319. Kimbrough TJ, Roethle PA, Mayer P, Trauner D. Angew. Chem. Int. Ed. 2010;49:2619–2621. doi: 10.1002/anie.200906126. Roethle PA, Hernandez PT, Trauner D. Org. Lett. 2006;8:5901–5904. doi: 10.1021/ol062581o. Tang B, Bray CD, Pattenden G. Org. Biomol. Chem. 2009;7:4448–4457. doi: 10.1039/b910572g. Tang B, Bray CD, Pattenden G. Tetrahedron Lett. 2006;47:6401–6404.
- 12.(a) Roethle PA, Trauner D. Org. Lett. 2006;8:345–347. doi: 10.1021/ol052922i. [DOI] [PubMed] [Google Scholar]; (b) Huang Q, Rawal VH. Org. Lett. 2006;8:543–545. doi: 10.1021/ol053054s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Detailed experimental procedures and data for all synthesis steps are shown in the Supporting Information.
- 14.Appel R. Angew. Chem. Int. Ed. 1975;14:801–811. [Google Scholar]
- 15.For the use of this reaction to the synthesis of related natural products see: Rayner CM, Astles PC, Paquette LA. J. Am. Chem. Soc. 1992;114:3926–3936. Paquette LA, Astles PC. J. Org. Chem. 1993;58:165–169.
- 16.CCDC 831509 (14), 831510 (8), 831511 (6), 831512 (5), 831515 (9) and 831516 (4) contain the supplementary crystallographic data. This information can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/products/csd/request/. In the figures some hydrogen atoms have been omitted for clarity.
- 17.Kursanov DN, Parnes ZN, Loim NM. Synthesis. 1974;9:633–651. [Google Scholar]
- 18.VanRheenen V, Kelly RC, Cha DY. Tetrahedron. 1976;17:1973–1976. [Google Scholar]
- 19.Nomura S, Sakamaki S, Hongu M, Kawanishi E, Koga Y, Sakamoto T, Yamamoto Y, Ueta K, Kimata H, Nakayama K, Tsuda-Tsukimoto M. J. Med. Chem. 2010;53:6355–6360. doi: 10.1021/jm100332n. [DOI] [PubMed] [Google Scholar]
- 20.Hayes SJ, Knight DW, Smith AWT, O'Halloran MJ. Tetrahedron Lett. 2010;51:720–723. [Google Scholar]
- 21.Yoshimitsu T, Nojima S, Hashimoto M, Tsukamoto K, Tanaka T. Synthesis. 2009;17:2963–2969. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.