Abstract
Murine cytomegalovirus (MCMV) brain infection stimulates microglial cell-driven proinflammatory chemokine production which precedes the presence of brain-infiltrating systemic immune cells. Here, we show that in response to MCMV brain infection, antigen-specific CD8(+) T-cells migrated into the brain and persisted as long-lived memory cells. The role of these persistent T-cells in the brain is unclear because most of our understanding of antimicrobial T-cell responses comes from analyses of lymphoid tissue. Strikingly, memory T-cells isolated from the brain exhibited an effector phenotype and produced IFN-γ upon restimulation with viral peptide. Furthermore, we observed time-dependent and long-term activation of resident microglia, indicated by chronic MHC class II up-regulation and TNF-α production. The immune response in this immunologically restricted site persisted in the absence of active viral replication. Lymphocyte infiltrates were detected until 30 d p.i., with CD8(+) and CD4(+) T-cells present at a 3:1 ratio, respectively. We then investigated the role of IFN-γ in chronic microglial activation by using IFN-γ-knockout (GKO) mice. At 30 d p.i., GKO mice demonstrated a similar phenotypic brain infiltrate when compared to wild-type mice (Wt), however, MHC class II expression on microglia isolated from these GKO mice was significantly lower compared to Wt animals. When IFN-γ producing CD8(+) T-cells were reconstituted in GKO mice, MHC class II up-regulation on microglial cells was restored. Taken together, these results suggest that MCMV brain infection results in long-term persistence of antigen specific CD8(+) T-cells which produce IFN-γ and drive chronic microglial cell activation. This response was found to be dependent on IFN-γ production by viral Ag-specific T-cells during the chronic phase of disease.
Keywords: MCMV, Brain, Microglia, T-cells
Introduction
Infection of the central nervous system (CNS) with cytomegalovirus (CMV) is the leading cause of congenital brain abnormalities in children. The virus is also a common pathogen of AIDS patients, infecting more than 90% of the at-risk population. With disseminated disease, virtually all organ systems can be affected, leading to mononucleosis, severe respiratory infection, liver and kidney damage, and intestinal disease, as well as brain damage. In an immunocompetent host, after primary infection, the immune response effectively terminates virus replication; however, clearance of the viral genome is not achieved, and the virus establishes lifelong latency, with periodic reactivation and shedding (Fields et al, 2007). Using a murine model of CMV encephalitis, we have previously shown that murine cytomegalovirus (MCMV) brain infection induces an increase in chemokine production within the CNS, which precedes the infiltration of CD3(+) lymphocytes.
Neuroimmune responses to viral infections of the brain must clear the invading pathogen while simultaneously preventing extensive damage to this vital generally nonregenerating tissue (Patterson et al, 2002). T lymphocytes use different immune mechanisms tailored to control viral infections in particular organs, and it is becoming increasingly clear that target cell type–specific mechanisms within particular tissues also exist. For example, in the CNS, IFN-γ production by infiltrating T lymphocytes has been shown to play an important role in clearance of viral infections from neurons without concomitant neuronal loss (Binder and Griffin, 2001; Kundig et al, 1993).
CD8(+) T-cells are critical components in the many viral infections (Harty et al, 2000; Koszinowski et al, 1991), we have previously reported that CD8(+) T lymphocytes posses the ability to restrict intracerebral spread of MCMV brain infection through a perforin-dependent mechanism (Cheeran et al, 2004; Cheeran et al, 2005). Infection of both mice and humans with CMV elicits broad CD8(+) T-cell responses specific for an array of viral antigens (Holtappels et al, 2008; Munks et al, 2006; Sylwester et al, 2005). T-cell derived antimicrobial mediators include perforin, granzymes, and Fas-Fas ligand, as well as soluble agents such as IFN-γ, TNF-α, and selected chemokines. Cytokines are especially crucial because they exert antiviral actions in multiple ways involving induction of an antiviral state, direct cytolysis, and the up-regulation of MHC expression (Guidotti and Chisari, 2000; Slifka and Whitton, 2000).
MHC Class II expression is rare in the normal CNS, but elevated expression on certain populations of brain cells, predominantly microglia and occasionally astrocytes, has been observed during numerous neurologic disease states, including multiple sclerosis (MS), neuroAIDS, Alzheimer’s disease, and experimental allergic encephalomyelitis (EAE), as well as several other viral infections (Marques et al, 2008; McGeer et al, 1993; Perry and Gordon, 1988; Spencer and Price, 1992). Previous studies from our laboratory, using human microglia, have identified these cells as a source of proinflammatory cytokines and chemokines in response to viral infection (Cheeran et al, 2003; Cheeran et al, 2001). Activation of microglia and macrophages during CNS disease leads to production of pro-inflammatory cytokines, such as IL-1β, TNF-α and IL-6, as well as chemokines, which contribute to inflammation and myelin damage within the CNS (Benveniste, 1997). Production of TNF-α within the CNS potently induces inflammation by increasing antigen presentation, promoting astrocytic proliferation and altering chemokine and adhesion molecule expression, thus regulating cell trafficking into the brain (Quintana et al, 2009). Like other herpes viruses, reactivation of CMV can produce significant morbidity and mortality in immunocompromised hosts. However, the consequences of chronic CMV encephalitis have not been well studied and there is little available literature deciphering long-term host-pathogen interactions. In this study, using a well-defined murine model of encephalitis, we investigated long-term neuroimmune responses to MCMV encephalitis, which included the assessment of the cellular profile, function, and persistence of peripheral immune cells which traffic into the brain, and exert their effector functions.
Methods
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. These experiments were approved by the University of Minnesota Institutional Animal Care and Use Committee (Protocol Number: 0807A40181). All surgery was performed under ketamine/xylazine anesthesia, and all efforts were made to minimize suffering.
Virus and animals
RM461, a MCMV expressing Escherichia coli β-galactosidase under the control of the human ie1/ie2 promoter/enhancer (Stoddart et al, 1994) was kindly provided by Edward S. Mocarski. The virus was maintained by passage in weanling female BALB/c mice. Salivary gland-passed virus was then grown in NIH 3T3 cells for 2 passages, which minimized any carry over of salivary gland tissue. Infected 3T3 cultures were harvested at 80% to 100% cytopathic effect and subjected to three freeze–thaw cycles. Cellular debris was removed by centrifugation (1000g) at 4 °C, and the virus was pelleted through a 35% sucrose cushion (in Tris-buffered saline [50 mM Tris–HCl, 150 mM NaCl, pH 7.4]) at 23,000g for 2 h at 4 °C. The pellet was resuspended in Tris buffered saline containing 10%FBS. Viral stock titers were determined on 3T3 cells as 50% tissue culture infective doses (TCID50) per milliliter. Six to eight weeks old BALB/c mice were obtained from Charles River Laboratories (Wilmington, MA), while age-matched GKO animals were purchased from the Jackson Laboratory (Bar Harbor, ME).
Intracerebroventricular infection of mice
Infection of mice with MCMV was performed as previously described (Cheeran et al, 2004). Briefly, female mice (6–8 week old) were anesthetized using a combination of Ketamine and Xylazine (100 mg and 10 mg/kg body weight, respectively) and immobilized on a small animal stereotactic instrument equipped with a Cunningham mouse adapter (Stoelting Co., Wood Dale, IL). The skin and underlying connective tissue were reflected to expose reference sutures (sagittal and coronal) on the skull. The sagittal plane was adjusted such that the bregma and lambda were positioned at the same coordinates on the vertical plane. Virulent, salivary gland-passaged MCMV RM461 (1.5 × 105 TCID50 units in 10 μl), was injected into the right lateral ventricle at 0.9 mm lateral, 0.5 mm caudal to the bregma and 3.0 mm ventral to the skull surface using a Hamilton syringe (10 μl) fitted to a 27 G needle. The injection was delivered over a period of 3–5 min. The opening in the skull was sealed with bone wax and the skin was closed using 9 mm wound clips (Stoelting Co., Wood Dale, IL).
Isolation of brain leukocytes and FACS
Leukocytes were isolated from MCMV-infected murine brains using a previously described procedure with minor modifications (Cheeran et al, 2007; Ford et al, 1995; Marten et al, 2003; Mutnal et al). In brief, brain tissues harvested from four to six animals were minced finely in RPMI 1640 (2 g/L D-glucose and 10 mM HEPES) and digested in 0.0625% trypsin (in Ca/Mg-free HBSS) at room temperature for 20 min. Single cell preparations from infected brains were resuspended in 30% Percoll and banded on a 70% Percoll cushion at 900 × g at 15°C. Brain leukocytes obtained from the 30–70% Percoll interface were treated with Fc block (anti-CD32/CD16 in the form of 2.4G2 hybridoma culture supernatant with 2% normal rat and 2% normal mouse serum) to inhibit nonspecific Ab binding and were stained with anti-mouse immune cell surface markers for 45 min at 4°C (anti-CD45-allophycocyanin (eBioscience), anti-CD11b-FITC or anti-CD11b-allophycocyanin-CY7, anti-CD4-FITC, anti-Ly6G-FITC, anti-MHC class II-PE, anti-CD8-PE, and anti-CD3-PE-Cy7 (BD Biosciences)) and analyzed by flow cytometry. Control isotype Abs were used for all isotype and fluorochrome combinations to assess nonspecific Ab binding. Live leukocytes were gated using forward scatter and side scatter parameters on a BD FACSCanto flow cytometer (BD Biosciences). Data was analyzed using FlowJo software (TreeStar).
Synthesis of intracellular IFN-γ in response to stimulation with the αCD3/CD28 or MCMV-IE1 peptide (YPHFMPTNL) was determined by incubating 2 × 106 brain leukocytes in 200 μl of RPMI complete supplemented with 10% FCS, 1 μM peptide, and 1 μl/ml Golgistop (BD PharMingen) for 5 h at 37°C (Horner et al, 2001). Peptide was omitted in negative control samples. Cells were surface stained prior to fixation/permeabalization using the Cytofix/Cytosperm Kit (BD PharMingen). Cells were then stained with mAb specific for IFN-γ (XMG1.2) as recommended by the supplier (BD PharMingen). Resident microglial cells isolated from control and infected brains at the indicated time points were incubated ex-vivo with Golgistop (BD PharMingen) for 4 h prior to staining for intracellular TNF-α as described above.
Adoptive transfer
FVB/N luciferase transgenic mice (luciferase expression driven by the β-actin promoter; Xenogen) were backcrossed for 10 generations to BALB/c mice (Charles River Laboratories) and were bred in house. Luciferase activity of resulting BALB/c Luc+ mouse colony was confirmed for 5 generations prior to use. These BALB/c Luc+ mice were used as donors in adoptive transfer experiments. Spleen and lymph nodes (cervical, lumbar and inguinal) from MCMV primed (1 × 105 TCID50/mouse, i.p. injection) donor animals were collected aseptically at 7 days post-priming. Single cell suspensions of immunocytes were depleted of RBC by treatment with 0.87% ammonium chloride, washed twice, and cell viability was confirmed using trypan blue. CD8(+) T lymphocytes were enriched by negative selection using CD8(+) T cell purification kit, as per the manufacturer’s instructions (R&D systems, Minneapolis, MN USA). Immune cells were transferred (5 × 106 cells/mouse) via tail vein 1 d prior to infection with MCMV into syngenic recipients.
Bioluminescence imaging
Imaging of firefly luciferase expression in live animals was performed using an IVIS50 (Xenogen) equipped with a charge-coupled camera device, as previously described with minor modifications (Luker et al, 2003). In brief, 150 μg of D-luciferin (Gold Biotechnology) was administered to anesthetized mice by i.p. injection. Animals were imaged 5 min after D-luciferin administration and data were acquired using a 5-min exposure window. Bioluminescence imaging studies were conducted with age-matched 8- to 10-wk-old female Wt and Interferon-γ knock out (GKO) mice as recipients and MHC- matched female BALB/c luciferase transgenic mice (luciferase expression driven by the β-actin promoter; Xenogen) as leukocyte donors. Immune cells were derived from the spleen, inguinal, and lumbar lymphnodes of MCMV primed animals. Enriched CD8(+) T lymphocyte population was used for the adoptive transfer experiments to obtain a combination of activated cells that represent the two major lymphoid compartments, i.e., the spleen and the lymph nodes. Signal intensity of luciferase expression, as measured by the total amount of transmitted light, was quantified as photons/sec/cm2 using Living Image (Xenogen) and Igor (Wavemetrics) image analysis software. Four treatment groups were set up for these experiments: 1) MCMV-infected Wt mice that received virus-primed CD8(+) T-cells, 2) MCMV-infected GKO mice that received virus-primed CD8(+) T-cells, 3) Mock-infected Wt and 4) GKO mice were also given virus-primed CD8(+) T-cells.
Immunohistochemistry for MCMV-IE1 antigen
Brains were harvested from infected mice that were sacrificed and perfused with serial washes of 2% sodium nitrate and phosphate-buffered saline (PBS) to remove contaminating blood cells and prefixed with 4% paraformaldehyde. Murine brains were subsequently submerged in 4% paraformaldehyde for 24 h and transferred to 30% sucrose solution for 2 d. Brain tissue slices (30-μm) were stained with a monoclonal antibody to IE1 (MAb Croma 101, kindly provided by Dr. Stipan Jonjic, University of Rijeka, Croatia) followed by a fluorescein-labeled donkey anti-mouse immunoglobulin G (IgG) antibody (Jackson Immunoresearch, West Grove, PA). Immunofluorescent staining for MCMV-IE1 was performed and sections were then counter stained with 4′,6-diamidino-2-phenyindole (DAPI), a nucleic acid dye (Chemicon).
Real-time PCR
Total RNA was extracted from brain tissue homogenates with Trizol reagent (Invitrogen, Carlsbad, CA). One μg RNA was DNase (Ambion, Applied Biosystems, Austin, TX) treated and reverse transcribed to cDNA with SuperScript™ III (Invitrogen), dNTP (GE Healthcare, Piscataway, NJ) and oligo (dT)12–18 (Promega, Madison, WI). Real-time PCR was performed in Mx3000p (Stratagene, La Jolla, CA) with SYBR Advantage qPCR Premix (Clontech, Mountain View, CA), primers and cDNA according to manufacturer’s protocol. Reaction conditions for qPCR were as follows: initial denaturation at 95°C for 15 sec, amplification for 40 cycles at 95°C for 10 sec, 60°C for 10 sec and 72°C for 10 sec followed by dissociation curve analysis (1 cycle at 95°C for 60 sec, 55°C for 30 sec and 95°C for 30 sec) to verify PCR product specificity. After normalizing to HPRT-1 expression (ΔCt = target gene Ct – HPRT Ct) and then to control group (ΔΔCt = treatment ΔCt – C ΔCt), relative quantification using 2^−ΔΔCt was calculated as fold change of target mRNA expression vs. control. Primer sequences used are listed in Table 1.
Table 1.
List of primers used for real-time PCR.
| Name | GenBank | Primers (Forward/Reverse) | Product size(bp) |
|---|---|---|---|
| MCMV IE1 | M11788 | 5′-ATCTGAAACAGCCGTATATCATCTTG | 99 |
| 5′-TCAGCCATCAACTCTGCTACCAAC | |||
| mIL-15 | NM_008357 | 5′-GGAAAGAATCCACCTTGACACA | 243 |
| 5′-CTGCCATCCATCCAGAACTC | |||
| mCXCL10 | NM_021274 | 5′-GTCATTTTCTGCCTCATCCTGCT | 211 |
| 5′-GGATTCAGACATCTCTGCTCATCA | |||
| mCXCL9 | NM_008599 | 5′-GAGTTCGAGGAACCCTAGTGATAAGGAA | 164 |
| 5′-AGGTTTGATCTCCGTTCTTCAGTGTAGC | |||
| mIL-7 | NM_008371 | 5′-GGAGTGATTATGGGTGGTGAGA | 240 |
| 5′-TCAGTTCCTGTCATTTTGTCCA |
Results
Brain infiltrating leukocyte populations were phenotypically distinct during acute and chronic phases
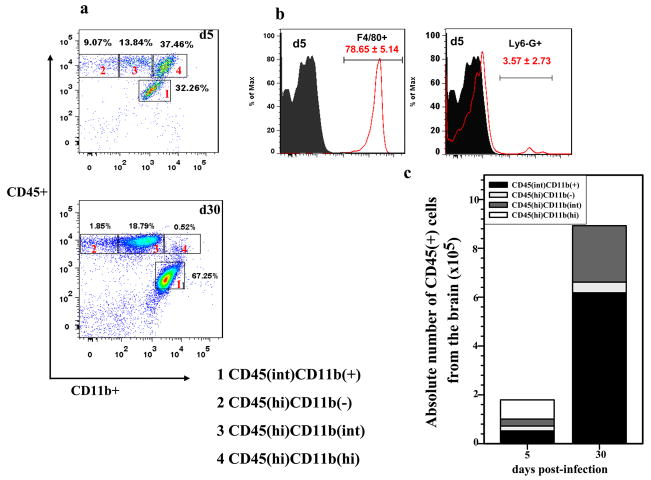
To identify cell types involved in the neuroimmune response to MCMV brain infection, leukocytes were isolated from the brains of infected BALB/c mice (Wt) at 5, 14, and 30 d p.i. These isolated cells were subsequently immunostained using markers that distinguished microglia, T lymphocytes, macrophages, and neutrophils (i.e., CD45, CD11b, CD4, CD8, F4/80, MHC class II, and Ly6G). At 5 d p.i., ~30% of the total brain leukocytes gated were identified as resident microglia by expression of a CD45(int)CD11b(+) phenotype. CD45(hi) expressing cells, indicative of brain-infiltrating peripheral myeloid cells (vs. resident myeloid cells, Fig. 1a), contributed to ~60% of the total leukocytes recovered, which include CD45(hi)CD11b(−), CD45(hi)CD11b(+) and CD45(hi)CD11b(hi) each represented with ~9%, ~14% and ~37%, respectively. Among the CD45(hi) population, a proportion of the leukocytes displayed a CD11b(int) (14.1 ± 2.5% at 5 d p.i.). This CD11b(int) cell population was found to persist up to 30 d p.i. (21.5 ± 7.2%). In these experiments, the phenotypic profiles of brain-infiltrating immune cells at 14 and 30 d p.i. were found to be identical, so data from the 14 d p.i. time point are not shown; therefore, data derived from the acute (5 d p.i.) and chronic (30 d p.i.) phases of disease are presented for the rest of the study. At 5 d p.i, approximately (41.6 ± 2.5%) of the CD45(hi) cells expressed CD11b(hi) and this specific cell population was missing at 30 d p.i. We then went on to further identify the phenotypes of these CD45(hi)CD11b(hi) cells at 5 d p.i. They constituted mainly macrophages (CD45(hi)CD11b(hi)F4/80(+), 78.65 ± 5.14%), with 3.57 ± 2.73% being identified as neutrophils (CD45(hi)CD11b(hi)Ly6G(+), Fig. 1b). The absolute number of macrophages and neutrophils at 5 d p.i. was also determined (4×105 ± 6.8×104 and 1×104 ± 6.7×103, respectively). One striking difference in leukocyte infiltration between the acute and chronic phases was an absence of innate components (i.e., macrophages and neutrophils) at 30 d p.i. In addition, a low percentage (2.1 ± 0.3) of the CD45(hi)CD11b(hi) cells expressed the NK cell marker CD49 at 5 d p.i., however, these cells were not detected at 30 d p.i. The absolute numbers of brain-infiltrating immune cells and resident microglial cells [CD45(int)CD11b(+)] were also determined at indicated time points (Fig. 1c). Taken together, these findings show that innate constituents (i.e. macrophages and neutrophils) make-up the majority of the infiltrate during the acute phase of disease (5 d p.i.).
Figure 1. Early leukocyte trafficking into the brain during MCMV encephalitis consists of predominantly macrophages and neutrophils.
Age-matched Wt mice were infected with 1.5 × 105 TCID50 of MCMV through the intracerebroventricular (i.c.v) route. Single cell suspensions of brain tissue obtained from MCMV-infected mice (2–4 animals) per time point were banded on a 70% Percoll cushion. Brain leukocytes at the 30–70% Percoll interface were collected, labeled with APC-conjugated Abs specific for CD45 and Cy7-labeled anti-CD11b Abs and analyzed using flow cytometry (Canto, BD, CA), with FlowJo software (TreeStar, Inc.). a. Dot plots shown are representative of 3 experiments at 5 and 30 d p.i.. The CD45(hi)CD11b(hi) population was significantly decreased at 30 d p.i. when compared to 5 d p.i. b. Histograms showing differential expression of Ly6-G and F4-80 among the CD45(hi)CD11b(hi); markers used to identify neutrophils and macrophages, respectively. Gray plot on the histograms denotes isotype Ab binding for each respective MAb, data expressed as mean (±SEM) percentage of cells infiltrating the brain were pooled from three independent experiments. c. Data showing absolute numbers of cells infiltrating the brain including CD45(int)CD11b(+), microglia at each indicated time point were pooled from three independent experiments.
Long-term presence of T lymphocytes following brain infection
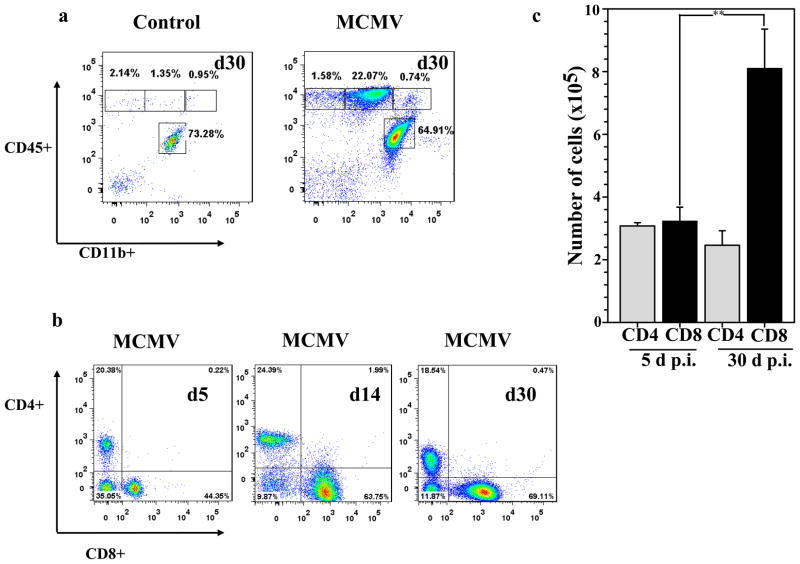
After clearance of an acute infection, the greatly expanded effector CD8(+) T-cell population dramatically contracts, leaving behind a small number of cells that enter the memory T-cell pool (Harty and Badovinac, 2008). Some of these memory cells persist in non-lymphoid tissues and form the front line of defense against reinfection (Hogan et al, 2001; Masopust et al, 2001). Our previous studies have shown the presence of T-cells in the brain during the acute phase of MCMV encephalitis (Cheeran et al, 2004; Cheeran et al, 2005), in this study we looked for these cells at a protracted time point, 30 d p.i. Results generated from these studies show the presence of a CD45 (hi)CD11b(−/int) population at 30 d p.i., these cells were first detected at 5 d p.i. The CD45(hi) population was completely absent in mock-infected controls at 30 d p.i., indicating there was no non-specific myelomonocytic cell infiltration. The only cell type that was recovered during mock-infection was brain resident microglia, CD45(int)CD11b(+). So, these mock-infected animals were not included in further studies. The percentage of CD45(hi)CD11b(int) expressing cells was significantly higher in brains obtained from MCMV-infected mice when compared to mock-infected mice at 30 d p.i. (21.07 ± 2.0% versus 1.14 ± 0.25%, respectively, p < 0.01 Student’s t test) (Fig. 2a). We then compared the percentage of CD4(+) and CD8(+) expressing cells from 5 and 30 d p.i., obtained from the CD45(hi)CD11b(−/int) population. The percentage of CD4(+) expressing cells remained unaltered throughout the time points studied (~20%), however the percent of CD8(+) T-cells was incremental and was significantly higher at 30 d p.i. when compared to 5 d p.i. (71.26 ± 3.10% versus 41.30 ± 2.90%, respectively, p < 0.01 Student’s t test) (Fig. 2b), we also observed CD8(+) T-cells outnumbered CD4(+) T-cells three to one. A significant increase in the absolute number of infiltrating CD8(+) T-cells was also observed at 30 d p.i., when compared to 5 d p.i. (8.1 × 105 ± 6.7 × 104 versus 1.19 × 105 ± 1.2 × 105, respectively, p = 0.01) (Fig. 2c).
Figure 2. Persistence of T lymphocytes in the MCMV-infected brain.
Single cell suspensions of brain tissue obtained from MCMV-infected mice (2–4 animals) per time point were banded on a 70% Percoll cushion. Brain leukocytes at the 30–70% Percoll interface were collected, labeled with APC-conjugated Abs specific for CD45 and Cy7-APC-labeled anti-CD11b Abs, and analyzed using flow cytometry and FlowJo software. a. Dot plots from uninfected control and MCMV-infected animals are shown and are representative of 3–5 experiments at 30 d p.i.. Control mice showed minimal peripheral cell trafficking. b. Representative dot plots showing the percentages of CD4(+) and CD8(+) T lymphocytes in infected brains at 5, 14 and 30 d p.i.. c. Data showing the mean (±SEM) absolute number of cells infiltrating the brain were pooled from 3 independent experiments. PE-labeled anti-CD8(+) and FITC-labeled anti-CD4 Abs were used to determine the total number of CD4(+) and CD8(+) T lymphocytes within the infiltrating CD45(hi) population. Data presented show the mean absolute number of cells in each population, **p < 0.01 versus MCMV-infected Wt.
Persistent brain-infiltrating CD8(+) T lymphocytes produce IFN-γ
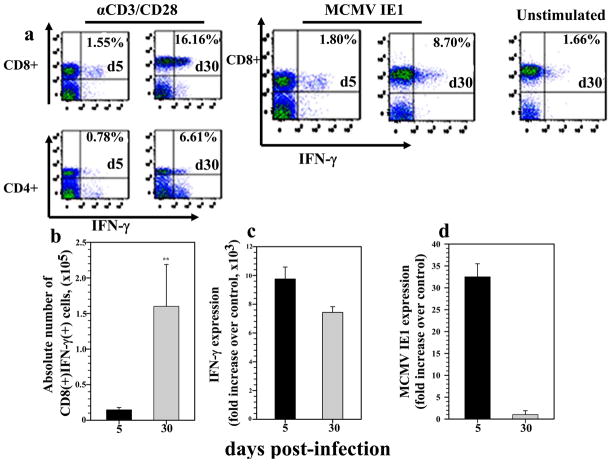
Long-term persisting CD8(+) T lymphocytes were further studied for their functional ability by assessing IFN-γ production in response to MCMV peptide, IE1, which has previously been identified as a MHC class I restricted epitope (Del Val et al, 1988; Holtappels et al, 2002). We excluded CD4(+)T-cells from this study because, to best of our knowledge, no MCMV MHC class II-restricted epitopes have been identified in BALB/c mice. Leukocytes derived from brains at 5 and 30 d p.i. were incubated either with CD3/CD28 antibodies or with MCMV IE1 peptides for 5 h and were subjected to intracellular staining for IFN-γ after staining for appropriate surface markers. When IFN-γ production by T-cells stimulated with anti-CD3 and anti-CD28 Abs was observed at 5 d p.i., there was no significant difference in the percentage of IFN-γ producing CD4(+) and CD8(+) T-cells (0.6 + 0.2% and 1.6 + 0.3%, respectively, Fig. 3a). In addition, CD8(+) T-cells derived from brains at 30 d p.i. were found to produce significantly higher amounts of IFN-γ compared to those isolated at 5 d p.i., when stimulated with IE1 peptide (9.1 ± 1.1% and 1.69 ± 0.45 %, respectively) (Fig. 3a). The absolute number of IFN-γ producing T-cells was also determined, CD8(+) T-cells producing IFN-γ were found in significantly higher numbers at 30 d p.i. when compared to those at 5 d p.i. (1.60 × 105 ± 5.8 × 104 versus 1.7 × 104 ± 5.9 × 103, respectively, p < 0.01 Student’s t test) (Fig. 3b). We also determined the levels of IFN-γ (Fig. 3c) and MCMV IE1 (Fig. 3d) mRNA expression in total brain homogenates at 5 and 30 d p.i. using real-time PCR. There was no significant difference in the expression level of IFN-γ between 5 and 30 d p.i.. The apparent discrepancy between data generated by PCR using total brain homogenates and the flow cytometry data, may be due to the presence of T-cells that are specific to other MCMV epitopes, another potential source of IFN-γ. IE1 mRNA levels were barely detectable at 30 d p.i. (< 1 fold increase), while expression was evident at 5 d p.i. (~ 32 fold increase).
Figure 3. Antigen-specific CD8(+) T lymphocytes are a major source of IFN-γ at 30 d p.i.
Single cell suspensions of brain tissue obtained from MCMV-infected mice (2–4 animals) per time point were banded on a 70% Percoll cushion. Brain leukocytes at the 30–70% Percoll interface were collected. For intracellular IFN-γ staining, brain infiltrating leukocytes (2 × 106 cells/ml) were pulsed with either CD3/CD28 antibodies or with a MCMV-specific IE1 peptide for 5 h at 37°C and cells were treated with Brefeldin A. After incubation, cells were washed in FACS buffer and stained for the surface molecules CD45, CD11b, CD4, CD8 and for intracellular IFN-γ using a Cytofix/Cytoperm kit (BD Pharmingen), before analysis by flow cytometry. a. Representative dot plots showing the percentage of CD8(+) and CD4(+) T lymphocytes producing IFN-γ at 5 and 30 d p.i. in response to αCD3/CD28 and MCMV-IE1 peptide treatment b. Absolute numbers of CD8(+) cells producing IFN-γ were determined within the infiltrating CD45(hi)CD3(+) population. Data showing the mean (±SEM) absolute number of cells infiltrating the brain were pooled from three to five experiments. **p < 0.01 versus MCMV infected Wt mice at 5 and 30 d p.i.. Total RNA extracted from the brain of three to five mice/time point was reverse transcribed and examined for the expression of IFN-γ (c) and MCMV IE1 (d) mRNA by real-time PCR. Mean RNA transcript levels normalized to HPRT expression and presented as fold increase over mock-infected controls from three to five animals per time point.
Persistent, brain-infiltrating T-cells displayed an effector memory phenotype
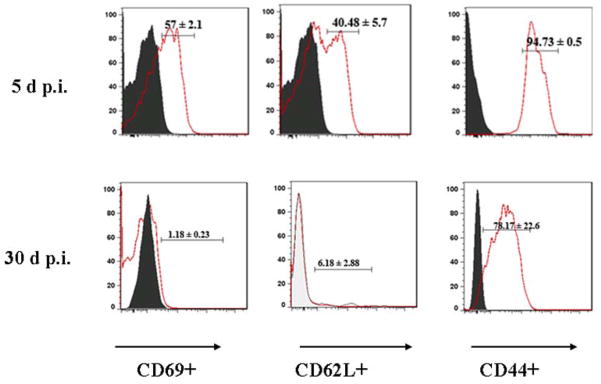
In our next set of experiments, brain infiltrating, long-term persisting T-cells were characterized. The cells isolated from brains at 5 and 30 d p.i. were incubated with CD45, CD11b, CD3, CD69, CD62L and CD44 monoclonal antibodies. The CD45(hi)CD11b(−/int)CD3(+) cell population was subsequently analyzed for CD69, CD62L and CD44. Analysis of the early activation marker CD69 revealed that there was markedly higher levels of expression on T-cells isolated at 5 d p.i. compared to those which chronically persisted in the brain (57.0 ± 2.1% and 1.18 ± 0.23%, respectively). CD62L (L-selectin) is expressed by central memory T-lymphocytes, which have encountered antigen. These cells express CD62L to localize in secondary lymphoid organs and prepare to proliferate upon re-encountering antigen. Effector/memory T-lymphocytes do not express L-selectin, as they circulate in the periphery and have immediate effector functions upon encountering antigen. As expected, brain-infiltrating T-cells at 30 d p.i. were found to have very minimal expression of CD62L compared to cells derived from brains at 5 d p.i. (6.18 ± 2.88% and 40.48 ± 5.7%, respectively). In contrast, CD44 expression is characteristic of effector memory T-cells that have seen antigen and is the most frequently used surface marker to identify effector-memory cells. In these experiments, the accumulation of CD44-expressing T-cells in the brain was then determined at 5 and 30 d p.i.. Infiltrating CD45(hi)CD11b(−/int)CD3(+) cells were analyzed for CD44 expression. The population studied included both CD4(+) and CD8(+) T-cells, however the percentage of CD4(+) T cells remained unchanged through the time-points studied. CD44 expression on T-cells was found to be 94.73 ± 0.5% and 78.17 ± 22.6%, respectively. Collectively, these findings confirm that the T lymphocytes persisting in the brain at 30 d p.i. were effector memory T-cells (Fig. 4).
Figure 4. Persistent, brain infiltrating T lymphocytes express an effector memory phenotype.
Single cell suspensions of brain tissue obtained from MCMV-infected mice (2–4 animals) per time point were banded on a 70% Percoll cushion. Cells were stained using MAbs against CD45-APC, CD11b-APC-cy7, CD3-PE-Cy7, CD44-PE, and CD62L-FITC or with CD69-FITC, and the percentages of these respective markers were determined among the CD45(hi)CD11b(int/hi)CD3(+) cells, at indicated time points. Histogram overlays from isotype control (grey, filled) and MCMV-infected mice (solid) are shown. Pooled data indicating percentages of the various cell populations, based on a leukocyte gate, are expressed as mean ± SEM.
Persistent microglial cell activation in MCMV-infected brains
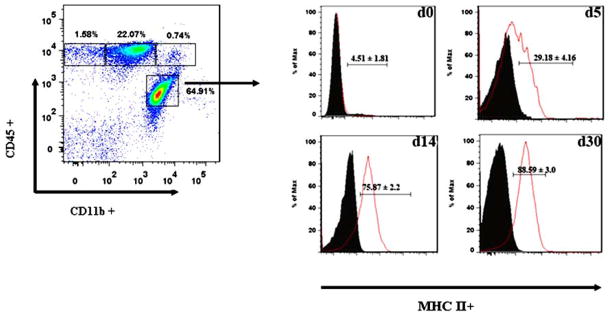
Previous studies from our laboratory have demonstrated that microglial cells respond strongly to stimulation with MCMV (Cheeran et al, 2003; Mutnal et al). To determine whether microglia were activated during MCMV encephalitis in vivo, the kinetics of MHC class II expression, as an indicator of activation, was assessed using flow cytometry. In these experiments, MHC class II expression on the CD45(int)CD11b(+) cell population was evaluated at 5, 14, and 30 d p.i.. While resting microglia (i.e., the CD45(int)CD11b(+) cell population) in the uninfected brain (d0) showed minimal expression of MHC class II (<5%), expression of this molecule was induced within 5 d following MCMV brain infection, at which time it was present on 29.18 ± 4.16% of the cells. Interestingly, additional experiments demonstrated that MHC class II expression on microglial cells remained highly elevated at both 14 and 30 d p.i., (75.87 ± 2.2% and 88.59 ± 3.0%, respectively) (Fig. 5).
Figure 5. Up-regulation of MHC class II expression on resident microglial cells in response to MCMV brain infection.
CD45(int)CD11b(+), microglia in single cell suspensions of brain tissue obtained from MCMV-infected mice at the indicated time points were stained with PE-labeled anti-MHC class II Abs and analyzed for expression using flow cytometry. Pooled data indicating the percentage of cells expressing MHC class II, based on a CD45(int)CD11b(+) gate, are presented as mean ± SEM. Histogram overlays from isotype control (grey, filled) and MCMV-infected mice (solid) are shown.
Long-term activated microglia are functionally competent
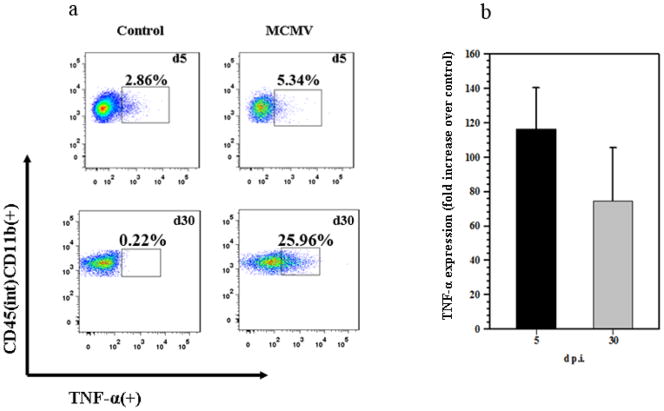
To further investigate the functional significance of this microglial cell activation, leukocytes isolated from MCMV-infected murinebrains (5 and 30 d p.i.) were stained for intracellular TNF-α and analyzed using flow cytometry. Microglial cells expressing CD45(int)CD11b(+) were gated to identify TNF-α producing cells. We observed, considerably higher levels of TNF-α production at 30 d p.i. when compared to microglia isolated from infected brains at 5 d p.i. We included mock-infected controls in this set of experiments as resident microglia can be isolated from a resting brain as well (Fig. 6a). Additionally, we determined expression levels of TNF-α mRNA from total brain homogenates at 5 and 30 d p.i. using quantitative real-time PCR (Fig. 6b). TNF-α mRNA expression was found to be higher at 5 d p.i. when compared to 30 d p.i., however, this discrepancy found using total brain homogenates could be due to the presence of macrophages at 5 d p.i. that are another potential source of TNF-α.
Figure 6. Chronic TNF-α production by activated microglia.
CD45(int)CD11b(+), microglia in single cell suspensions of brain tissue obtained from MCMV-infected mice at the indicated time points were stained with FITC-labeled anti-TNF-α Abs and analyzed for expression using flow cytometry. a. Representative dot plots showing the percentage of TNF-α producing CD45(int)CD11b(+), microglial cells at the indicated time points. b. TNF-α expression from total brain homogenate obtained at 5 and 30 d p.i. is shown.
Persistent MHC Class II expression on microglia is IFN-γ dependent
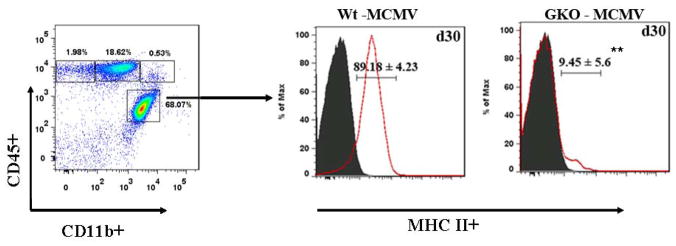
In addition to its role as a potent antiviral mediator, IFN-γ enhances both MHC class I and class II expression and Ag presentation (Fruh and Yang, 1999; Guidotti and Chisari, 2000). CNS cells are inherently limited in MHC expression in the quiescent state and dependent upon activation for effective Ag presentation (Hickey, 2001). To determine whether MHC class II expression is IFN-γ dependent, we infected IFN-γ knockout mice (GKO) along with Wt mice, as a loss of function approach. GKO mice survived the infection and at 30 d p.i., their microglial cells were analyzed for MHC class II expression. In these experiments, GKO mice were found to have significantly lower expression of MHC class II on microglia when compared to Wt mice that were identically processed (9.45 ± 5.6% versus 89.18 ± 4.23%, respectively, p < 0.01 Student’s t test) (Fig. 7).
Figure 7. Up-regulation of MHC class II expression on resident microglial cells is IFN-γ-dependent.
CD45(int)CD11b(+) microglia in single cell suspensions of brain tissue obtained from MCMV-infected Wt and GKO mice at 30 d p.i. were stained with PE-labeled anti-MHC class II Abs and analyzed for expression using flow cytometry. Pooled data indicating percentages of the MHC class II expressing cells, based on a CD45(int)CD11b(+) gate, are expressed as mean ± SEM. **p < 0.01 versus MCMV infected Wt at 30 d p.i..
Reconstitution with CD8 lymphocytes induces MHC class II expression on microglia from GKO animals
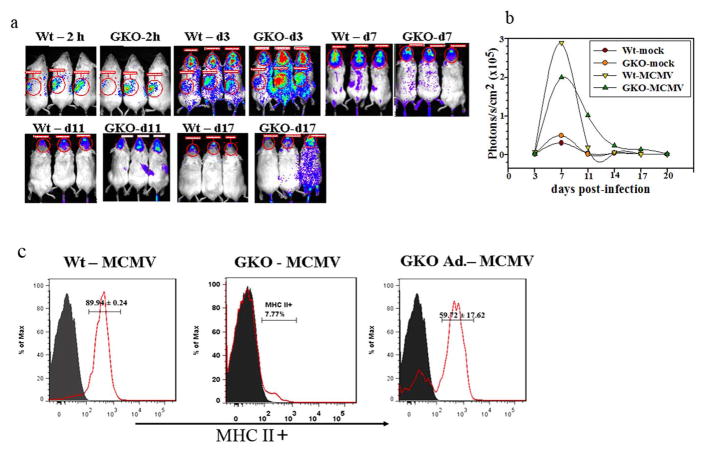
To evaluate the contributions of IFN-γ to microglial activation, we went on to perform adoptive transfer experiments. In this next approach, we transferred MCMV-primed CD8(+) T-cells from β-actin promoter-luciferase expressing transgenic mice into GKO mice. GKO and Wt mice received 5 × 106 primed CD8(+) T-cells 1 d prior to infection. Using bioluminescent imaging of live animals, we examined the kinetics of CD8 lymphocyte infiltration into the brain longitudinally in both MCMV-infected Wt and GKO mice. Within 2 h of adoptive transfer, luciferase-positive cells were detected in the spleen. Movement of the CD8(+) T-cells into the brain was observed within 3 d after infection in both Wt and GKO mice (Fig. 8a). Signal intensity of the luminescence from Wt and GKO mice that were infected with MCMV peaked between 7 and 11 d p.i., and remained noticeably elevated until 17 d p.i. By 20 d post-transfer, the overall signal generated by the luciferase-positive CD8(+) T-cells markedly decreased (Fig. 8b). The Wt and GKO mock-infected control animals had very low signal intensity through out the study period. At 30 d p.i., we harvested brains from both Wt and GKO mice, and the brain infiltrating leukocytes were purified using a percoll gradient. These cells were then analyzed using flow cytometry following the appropriate immunostaining. Interestingly, reconstitution of IFN-γ producing CD8(+) T-cells restored MHC class II expression on microglial cells isolated from GKO mice (59.72 ± 17.62% versus 9.45 ± 5.6%), indicating that persistent IFN-γ signaling is the mechanism driving chronic microglial cell activation.
Figure 8. Microglial cell activation is restored following adoptive transfer of Wt CD8(+) T-cells in GKO mice.
a. MCMV-primed splenocytes and lymph node cells from β-actin-luciferase transgenic BALB/c mice were enriched for CD8(+) T-cells by using negative selection kit as described in the methods. The primed CD8(+) T-cells were adoptively transferred via tail vein injection into MHC-matched recipients, 1 d prior to the infection with MCMV. Dorsal bioluminescence images of recipient mice are shown at 2 h post-transfer (p.t.), 3 d p.i./4 d p.t., 7d p.i./8d p.t., 11 d p.i./12 d p.t., and 17 d p.i./18 d p.t.. b. Signal intensity of the luciferase expression, indicative of the number of immune cells present, was quantified in the brain as photons/sec/cm2 at each time point. c. Histogram overlays with pooled data from Wt (Wt - MCMV), GKO (GKO – MCMV), and GKO mice that received CD8(+) T-cells (GKO Ad.-MCMV) are shown for MHC class II up-regulation on CD45(int)CD11b(+), microglial cells at 30 d p.i.. Grey line (filled) represents isotype control and red line (solid) for MHC class II expression.
Discussion
Results generated from this study clearly demonstrate that MCMV brain infection induces neuroimmune responses which persist in the absence of detectable virus replication. Early during infection, neuroimmune responses were dominated by the influx of macrophages and neutrophils, which constituted the prominent component of the cellular infiltrate at 5 d p.i. These innate components were completely absent at 30 d p.i. In addition to macrophages and neutrophils, the infiltrating cell profile included some CD4(+) and CD8(+) T lymphocytes during the acute phase, which became the predominant leukocyte infiltrate at 30 d p.i. The brain lymphocyte population predominantly consisted of CD8(+) T-cells at 30 d p.i. and these cells persisted at a time when there was no substantial presence of viral gene products such as IE1 (Fig. 3d and Fig. S1). In addition, long-term microglial cell activation was observed, indicated by MHC class II up-regulation. Further, this study showed that long-term microglial cell activation was driven by IFN-γ production by persistent T-cells.
Early neutrophil and macrophage responses play a critical role in the pathogenesis of MCMV infection. We have previously shown a paradoxical role of neutrophils where they contribute to both defense and neuropathogenesis during absence of the anti-inflammatory cytokine IL-10 (Mutnal et al). In addition, infiltrating macrophages have been reported to carry virus into the brain. Also, innate immune responses mediated by nitric oxide (NO) derived from macrophages seem to contribute to viral elimination from the brain, as they have been shown in other organs such as spleen and liver (Kosugi et al, 2002). In the present study, macrophages and neutrophils were present in the brain at 5 d p.i., but these cell types did not participate in chronic neuroimmune responses.
In this report, we also observed that peak lymphocyte infiltration was seen at 14 d p.i. More than 70% of the infiltrating CD3(+) cells at 30 d p.i. were CD8(+) T lymphocytes. There is still ambiguity regarding the type of T-cell that provides protection against MCMV in the brain, CD4(+) T-cells are necessary for viral clearance primarily in the salivary gland (Jonjic et al, 1990), while transfer of immune CD4(+) T-cells is not protective against systemic disease (Reddehase et al, 1987; Reddehase et al, 1985). Studies from our laboratory suggested that CD8(+) T-cells play a critical role in controlling MCMV brain infection, with viral clearance being perforin-mediated (Cheeran et al, 2005). The continued long-term detection of lymphocytes, primarily CD8(+) cells in the brain indicates ongoing neuroimmune responses.
It is generally believed that IFN-γ production by effector T-cells would cease following the loss of contact with antigen (Badovinac et al, 2000; Slifka et al, 1999). Using our previously described model of MCMV brain infection (Cheeran et al, 2004; Cheeran et al, 2007) we have shown that active viral infection could be detected up to 10 d p.i. Interestingly, in the present study CD8(+) T-cells isolated from the brains of infected animals at 30 d p.i. were found to produce IFN-γ when stimulated with viral peptide (IE1). These MCMV IE1-specific CD8(+) T-cells have shown to be protective during MCMV infection (Cheeran et al, 2005; Reddehase et al, 1987). In addition to detection of mRNA expression for IFN-γ, indirect evidence of in-vivo secretion was also demonstrated by analysis of IFN-γ inducible genes such as CXCL9 and CXCL10 at 30 d p.i. in the total brain homogenates (Fig. S2a). These chemokines are best known for their ability to attract activated lymphocytes to sites of viral infection (Farber, 1997). In this study, we demonstrated that persistent T-cells express an effector memory phenotype at 30 d p.i. These cells down-regulated CD62L and CD69; and retained CD44 expression during the chronic phase of viral infection. Similar observations have been reported during other viral brain infections such as Vesicular stomatitis virus (VSV) where resident memory T-cells persist long after viral products have been cleared. This long-term persistence of T-cells in the brain was, however, independent of Ag presence (Wakim et al). Factors governing a memory CD8(+) T cell pool are dependent on the history and environment of Ag exposure, as well as the tissue of residence (Bachmann et al, 2005; Masopust et al, 2006). Despite elevated levels of CXCL9 and CXCL10 at 30 d p.i., we believe that the brain’s T-cell population is not replenished from the peripheral immune system based on the observation of CD62L, CD69 and CD44 expression. Our finding suggests that T-cell survival in the brain is fostered by cytokines IL-7 and IL-15 (Fig. S2b). The role of these cytokines, specifically, IL-15 is well documented in T-cell homeostasis, survival and even cytotoxicity (Becker et al, 2002; Saito et al, 2006; Yajima et al, 2006).
Under normal physiological conditions there is entry of immune cells into the CNS for the purpose of immune surveillance (Prendergast and Anderton, 2009). In addition to persistence of lymphocytes in the brain, our study also demonstrated long-term activation of resident microglia. Up-regulated MHC class II expression on microglial cells was observed until 30 d p.i. It is well known that IFN-γ induces MHC class II expression, but it is important to note that the turnover of these molecules on resident microglia may be slow and may persist in the absence of IFN-γ (Hamo et al, 2007) Previous studies from our laboratory have identified microglia as an important source of immune mediators such as cytokines and chemokines (Cheeran et al, 2003; Cheeran et al, 2001; Mutnal et al). Activated microglia are known to participate in neurodegenerative processes by releasing cytotoxic factors including nitric oxide (NO) and several pro-inflammatory cytokines (Chao et al, 1992; Matsuoka et al, 1999). In this study, we show that microglial cells are a source of TNF-α at 30 d p.i. This continued production of proinflammatory mediator could have adverse effects on the brain, keeping it in a state of constant inflammation. The robust up-regulation of MHC class II on microglia was coincident with maximal IFN-γ detection at 30 d p.i., implicating IFN-γ as a driving force for activation and production of proinflammatory mediators. The increased persistence of CD8(+) T-cells in the CNS, compared with CD4(+) T-cells, in response to MCMV infection was obvious and suggests that CD8(+) T-cells make the prominent contribution to IFN-γ secretion in the local environment. Further analysis of MHC class II expression on microglia derived from MCMV-infected GKO mice supported our finding that MHC Class II expression is driven predominantly by IFN-γ production by persisting virus-specific CD8(+) T-cells. The GKO mice, when infected with MCMV showed similar characteristics when compared to Wt mice, viz. there was no significant difference in the amount of T-cell infiltration between these groups (Fig. S3a). However, viral mRNA expression for the IE1 gene was detected at significantly higher amounts in the brains of GKO mice at 5 d p.i. (Fig. S3b). The fact that IFN-γ drives MHC class II expression on microglia was additionally confirmed by gain of function experiments in which Ag-experienced CD8(+) T-cells filled the IFN-γ compartment in GKO mice and induced MHC class II expression on microglia. Similar IFN-γ driven microglial cell activation has been shown in other viral infections of the brain (Bergmann et al, 2003; Marques et al, 2008).
Although the immune system serves to both protect and defend the host from invading pathogens, uncontrolled inflammatory responses can be deleterious. For example, the synergistic effect of TNF-α and IFN-γ is well known to exacerbate NO-induced neurodegeneration and demyelination in murine brains (Blais and Rivest, 2004). Further studies to evaluate the extent and effects of prolonged neuroimmune activation are necessaryto determine its contribution to long-term neuropathological sequelae following viral encephalitis.
Supplementary Material
MCMV-infected brains were harvested at the indicated time points and were fixed and sectioned as described in the methods. Brain tissue slices (30-μm) were stained with a monoclonal antibody to IE1 followed by a fluorescein-labeled donkey anti-mouse immunoglobulin G (IgG) antibody (Jackson Immunoresearch, West Grove, PA). Immunofluorescent staining for MCMV-IE1 was performed and sections were then counter stained with 4′,6-diamidino-2-phenyindole (DAPI), a nucleic acid dye (Chemicon). Shown are the immunofluorescent staining for MCMV IE1 antigen at 5 d p.i., (a–d) and 30 d p.i., (e–h), representing different brain regions.
Brains from MCMV-infected Wt mice were harvested at 5 and 30 d p.i. Total RNA was extracted, DNAse treated and reverse transcribed. The cDNA obtained was analyzed by quantitative real-time PCR using primers specific to a. CXCL9 and CXCL10 b. IL7 and IL15. mRNA expression was normalized to HPRT. Mean data from 3–6 animals per time point are expressed as fold increase in relative mRNA induction over mock-infected controls.
Wt and GKO mice were compared for their response to MCMV brain infection. a. T-cell infiltration was determined in GKO and Wt mice at 30 d p.i.. Results obtained from these experiments suggested that there was no significant difference in the T-cell infiltration. b. MCMV-IE1 mRNA was determined in the total brain homogenates from Wt and GKO mice at 5 and 30 d p.i. by real-time PCR. At 5 d p.i., we detected significantly higher levels of IE1 expression in the brains of GKO mice when compared to Wt mice. Data are expressed as mean (± SEM) of three to five animals per group (*p < 0.05 versus MCMV infected Wt).
Acknowledgments
This project was supported by Award Number R01 NS-038836 from the National Institute of Neurological Disorders and Stroke. The funding agency had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of interest statement: All authors declare that there are no conflicts of interest.
References
- Bachmann MF, Wolint P, Schwarz K, Oxenius A. Recall proliferation potential of memory CD8+ T cells and antiviral protection. J Immunol. 2005;175:4677–85. doi: 10.4049/jimmunol.175.7.4677. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Corbin GA, Harty JT. Cutting edge: OFF cycling of TNF production by antigen-specific CD8+ T cells is antigen independent. J Immunol. 2000;165:5387–91. doi: 10.4049/jimmunol.165.10.5387. [DOI] [PubMed] [Google Scholar]
- Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, Ahmed R. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med. 2002;195:1541–8. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste EN. Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J Mol Med. 1997;75:165–73. doi: 10.1007/s001090050101. [DOI] [PubMed] [Google Scholar]
- Bergmann CC, Parra B, Hinton DR, Chandran R, Morrison M, Stohlman SA. Perforin-mediated effector function within the central nervous system requires IFN-gamma-mediated MHC up-regulation. J Immunol. 2003;170:3204–13. doi: 10.4049/jimmunol.170.6.3204. [DOI] [PubMed] [Google Scholar]
- Binder GK, Griffin DE. Interferon-gamma-mediated site-specific clearance of alphavirus from CNS neurons. Science. 2001;293:303–6. doi: 10.1126/science.1059742. [DOI] [PubMed] [Google Scholar]
- Blais V, Rivest S. Effects of TNF-alpha and IFN-gamma on nitric oxide-induced neurotoxicity in the mouse brain. J Immunol. 2004;172:7043–52. doi: 10.4049/jimmunol.172.11.7043. [DOI] [PubMed] [Google Scholar]
- Chao CC, Hu S, Molitor TW, Shaskan EG, Peterson PK. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol. 1992;149:2736–41. [PubMed] [Google Scholar]
- Cheeran MC, Gekker G, Hu S, Min X, Cox D, Lokensgard JR. Intracerebral infection with murine cytomegalovirus induces CXCL10 and is restricted by adoptive transfer of splenocytes. J Neurovirol. 2004;10:152–62. doi: 10.1080/13550280490441130. [DOI] [PubMed] [Google Scholar]
- Cheeran MC, Gekker G, Hu S, Palmquist JM, Lokensgard JR. T cell-mediated restriction of intracerebral murine cytomegalovirus infection displays dependence upon perforin but not interferon-gamma. J Neurovirol. 2005;11:274–80. doi: 10.1080/13550280590952808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeran MC, Hu S, Palmquist JM, Bakken T, Gekker G, Lokensgard JR. Dysregulated interferon-gamma responses during lethal cytomegalovirus brain infection of IL-10-deficient mice. Virus Res. 2007;130:96–102. doi: 10.1016/j.virusres.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeran MC, Hu S, Sheng WS, Peterson PK, Lokensgard JR. CXCL10 production from cytomegalovirus-stimulated microglia is regulated by both human and viral interleukin-10. J Virol. 2003;77:4502–15. doi: 10.1128/JVI.77.8.4502-4515.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeran MC, Hu S, Yager SL, Gekker G, Peterson PK, Lokensgard JR. Cytomegalovirus induces cytokine and chemokine production differentially in microglia and astrocytes: antiviral implications. J Neurovirol. 2001;7:135–47. doi: 10.1080/13550280152058799. [DOI] [PubMed] [Google Scholar]
- Del Val M, Volkmer H, Rothbard JB, Jonjic S, Messerle M, Schickedanz J, Reddehase MJ, Koszinowski UH. Molecular basis for cytolytic T-lymphocyte recognition of the murine cytomegalovirus immediate-early protein pp89. J Virol. 1988;62:3965–72. doi: 10.1128/jvi.62.11.3965-3972.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber JM. Mig and IP-10: CXC chemokines that target lymphocytes. J Leukoc Biol. 1997;61:246–57. [PubMed] [Google Scholar]
- Fields BN, Knipe DM, Howley PM. Fields’ virology. 5. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia: 2007. [Google Scholar]
- Ford AL, Goodsall AL, Hickey WF, Sedgwick JD. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J Immunol. 1995;154:4309–21. [PubMed] [Google Scholar]
- Fruh K, Yang Y. Antigen presentation by MHC class I and its regulation by interferon gamma. Curr Opin Immunol. 1999;11:76–81. doi: 10.1016/s0952-7915(99)80014-4. [DOI] [PubMed] [Google Scholar]
- Guidotti LG, Chisari FV. Cytokine-mediated control of viral infections. Virology. 2000;273:221–7. doi: 10.1006/viro.2000.0442. [DOI] [PubMed] [Google Scholar]
- Hamo L, Stohlman SA, Otto-Duessel M, Bergmann CC. Distinct regulation of MHC molecule expression on astrocytes and microglia during viral encephalomyelitis. Glia. 2007;55:1169–77. doi: 10.1002/glia.20538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–19. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- Harty JT, Tvinnereim AR, White DW. CD8+ T cell effector mechanisms in resistance to infection. Annu Rev Immunol. 2000;18:275–308. doi: 10.1146/annurev.immunol.18.1.275. [DOI] [PubMed] [Google Scholar]
- Hickey WF. Basic principles of immunological surveillance of the normal central nervous system. Glia. 2001;36:118–24. doi: 10.1002/glia.1101. [DOI] [PubMed] [Google Scholar]
- Hogan RJ, Zhong W, Usherwood EJ, Cookenham T, Roberts AD, Woodland DL. Protection from respiratory virus infections can be mediated by antigen-specific CD4(+) T cells that persist in the lungs. J Exp Med. 2001;193:981–6. doi: 10.1084/jem.193.8.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtappels R, Grzimek NK, Simon CO, Thomas D, Dreis D, Reddehase MJ. Processing and presentation of murine cytomegalovirus pORFm164-derived peptide in fibroblasts in the face of all viral immunosubversive early gene functions. J Virol. 2002;76:6044–53. doi: 10.1128/JVI.76.12.6044-6053.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtappels R, Simon CO, Munks MW, Thomas D, Deegen P, Kuhnapfel B, Daubner T, Emde SF, Podlech J, Grzimek NK, Oehrlein-Karpi SA, Hill AB, Reddehase MJ. Subdominant CD8 T-cell epitopes account for protection against cytomegalovirus independent of immunodomination. J Virol. 2008;82:5781–96. doi: 10.1128/JVI.00155-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner AA, Datta SK, Takabayashi K, Belyakov IM, Hayashi T, Cinman N, Nguyen MD, Van Uden JH, Berzofsky JA, Richman DD, Raz E. Immunostimulatory DNA-based vaccines elicit multifaceted immune responses against HIV at systemic and mucosal sites. J Immunol. 2001;167:1584–91. doi: 10.4049/jimmunol.167.3.1584. [DOI] [PubMed] [Google Scholar]
- Jonjic S, Pavic I, Lucin P, Rukavina D, Koszinowski UH. Efficacious control of cytomegalovirus infection after long-term depletion of CD8+ T lymphocytes. J Virol. 1990;64:5457–64. doi: 10.1128/jvi.64.11.5457-5464.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi I, Kawasaki H, Arai Y, Tsutsui Y. Innate immune responses to cytomegalovirus infection in the developing mouse brain and their evasion by virus-infected neurons. Am J Pathol. 2002;161:919–28. doi: 10.1016/S0002-9440(10)64252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koszinowski UH, Reddehase MJ, Jonjic S. The role of CD4 and CD8 T cells in viral infections. Curr Opin Immunol. 1991;3:471–5. doi: 10.1016/0952-7915(91)90005-l. [DOI] [PubMed] [Google Scholar]
- Kundig TM, Hengartner H, Zinkernagel RM. T cell-dependent IFN-gamma exerts an antiviral effect in the central nervous system but not in peripheral solid organs. J Immunol. 1993;150:2316–21. [PubMed] [Google Scholar]
- Luker GD, Prior JL, Song J, Pica CM, Leib DA. Bioluminescence imaging reveals systemic dissemination of herpes simplex virus type 1 in the absence of interferon receptors. J Virol. 2003;77:11082–93. doi: 10.1128/JVI.77.20.11082-11093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques CP, Cheeran MC, Palmquist JM, Hu S, Urban SL, Lokensgard JR. Prolonged microglial cell activation and lymphocyte infiltration following experimental herpes encephalitis. J Immunol. 2008;181:6417–26. doi: 10.4049/jimmunol.181.9.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marten NW, Stohlman SA, Zhou J, Bergmann CC. Kinetics of virus-specific CD8+-T-cell expansion and trafficking following central nervous system infection. J Virol. 2003;77:2775–8. doi: 10.1128/JVI.77.4.2775-2778.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–7. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J Immunol. 2006;176:2079–83. doi: 10.4049/jimmunol.176.4.2079. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Kitamura Y, Takahashi H, Tooyama I, Kimura H, Gebicke-Haerter PJ, Nomura Y, Taniguchi T. Interferon-gamma plus lipopolysaccharide induction of delayed neuronal apoptosis in rat hippocampus. Neurochem Int. 1999;34:91–9. doi: 10.1016/s0197-0186(98)00053-9. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Kawamata T, Walker DG, Akiyama H, Tooyama I, McGeer EG. Microglia in degenerative neurological disease. Glia. 1993;7:84–92. doi: 10.1002/glia.440070114. [DOI] [PubMed] [Google Scholar]
- Munks MW, Gold MC, Zajac AL, Doom CM, Morello CS, Spector DH, Hill AB. Genome-wide analysis reveals a highly diverse CD8 T cell response to murine cytomegalovirus. J Immunol. 2006;176:3760–6. doi: 10.4049/jimmunol.176.6.3760. [DOI] [PubMed] [Google Scholar]
- Mutnal MB, Cheeran MC, Hu S, Little MR, Lokensgard JR. Excess neutrophil infiltration during cytomegalovirus brain infection of interleukin-10-deficient mice. J Neuroimmunol. 2010;227:101–10. doi: 10.1016/j.jneuroim.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson CE, Lawrence DM, Echols LA, Rall GF. Immune-mediated protection from measles virus-induced central nervous system disease is noncytolytic and gamma interferon dependent. J Virol. 2002;76:4497–506. doi: 10.1128/JVI.76.9.4497-4506.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Gordon S. Macrophages and microglia in the nervous system. Trends Neurosci. 1988;11:273–7. doi: 10.1016/0166-2236(88)90110-5. [DOI] [PubMed] [Google Scholar]
- Prendergast CT, Anderton SM. Immune cell entry to central nervous system--current understanding and prospective therapeutic targets. Endocr Metab Immune Disord Drug Targets. 2009;9:315–27. doi: 10.2174/187153009789839219. [DOI] [PubMed] [Google Scholar]
- Quintana A, Muller M, Frausto RF, Ramos R, Getts DR, Sanz E, Hofer MJ, Krauthausen M, King NJ, Hidalgo J, Campbell IL. Site-specific production of IL-6 in the central nervous system retargets and enhances the inflammatory response in experimental autoimmune encephalomyelitis. J Immunol. 2009;183:2079–88. doi: 10.4049/jimmunol.0900242. [DOI] [PubMed] [Google Scholar]
- Reddehase MJ, Mutter W, Munch K, Buhring HJ, Koszinowski UH. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J Virol. 1987;61:3102–8. doi: 10.1128/jvi.61.10.3102-3108.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddehase MJ, Weiland F, Munch K, Jonjic S, Luske A, Koszinowski UH. Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J Virol. 1985;55:264–73. doi: 10.1128/jvi.55.2.264-273.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Yajima T, Kumabe S, Doi T, Yamada H, Sad S, Shen H, Yoshikai Y. Impaired protection against Mycobacterium bovis bacillus Calmette-Guerin infection in IL-15-deficient mice. J Immunol. 2006;176:2496–504. doi: 10.4049/jimmunol.176.4.2496. [DOI] [PubMed] [Google Scholar]
- Slifka MK, Rodriguez F, Whitton JL. Rapid on/off cycling of cytokine production by virus-specific CD8+ T cells. Nature. 1999;401:76–9. doi: 10.1038/43454. [DOI] [PubMed] [Google Scholar]
- Slifka MK, Whitton JL. Antigen-specific regulation of T cell-mediated cytokine production. Immunity. 2000;12:451–7. doi: 10.1016/s1074-7613(00)80197-1. [DOI] [PubMed] [Google Scholar]
- Spencer DC, Price RW. Human immunodeficiency virus and the central nervous system. Annu Rev Microbiol. 1992;46:655–93. doi: 10.1146/annurev.mi.46.100192.003255. [DOI] [PubMed] [Google Scholar]
- Stoddart CA, Cardin RD, Boname JM, Manning WC, Abenes GB, Mocarski ES. Peripheral blood mononuclear phagocytes mediate dissemination of murine cytomegalovirus. J Virol. 1994;68:6243–53. doi: 10.1128/jvi.68.10.6243-6253.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, Nelson JA, Picker LJ. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202:673–85. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci U S A. 107:17872–9. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima T, Yoshihara K, Nakazato K, Kumabe S, Koyasu S, Sad S, Shen H, Kuwano H, Yoshikai Y. IL-15 regulates CD8+ T cell contraction during primary infection. J Immunol. 2006;176:507–15. doi: 10.4049/jimmunol.176.1.507. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MCMV-infected brains were harvested at the indicated time points and were fixed and sectioned as described in the methods. Brain tissue slices (30-μm) were stained with a monoclonal antibody to IE1 followed by a fluorescein-labeled donkey anti-mouse immunoglobulin G (IgG) antibody (Jackson Immunoresearch, West Grove, PA). Immunofluorescent staining for MCMV-IE1 was performed and sections were then counter stained with 4′,6-diamidino-2-phenyindole (DAPI), a nucleic acid dye (Chemicon). Shown are the immunofluorescent staining for MCMV IE1 antigen at 5 d p.i., (a–d) and 30 d p.i., (e–h), representing different brain regions.
Brains from MCMV-infected Wt mice were harvested at 5 and 30 d p.i. Total RNA was extracted, DNAse treated and reverse transcribed. The cDNA obtained was analyzed by quantitative real-time PCR using primers specific to a. CXCL9 and CXCL10 b. IL7 and IL15. mRNA expression was normalized to HPRT. Mean data from 3–6 animals per time point are expressed as fold increase in relative mRNA induction over mock-infected controls.
Wt and GKO mice were compared for their response to MCMV brain infection. a. T-cell infiltration was determined in GKO and Wt mice at 30 d p.i.. Results obtained from these experiments suggested that there was no significant difference in the T-cell infiltration. b. MCMV-IE1 mRNA was determined in the total brain homogenates from Wt and GKO mice at 5 and 30 d p.i. by real-time PCR. At 5 d p.i., we detected significantly higher levels of IE1 expression in the brains of GKO mice when compared to Wt mice. Data are expressed as mean (± SEM) of three to five animals per group (*p < 0.05 versus MCMV infected Wt).