Abstract
Glutathione (GSH) conjugating enzymes, glutathione S-transferases (GSTs) are present in different subcellular compartments including cytosol, mitochondria, endoplasmic reticulum, nucleus and plasma membrane. The regulation and function of GSTs have implications in cell growth, oxidative stress, as well as in disease progression and prevention. Of the several mitochondria localized forms, GSTK (GST kappa) is mitochondria-specific since it contains N-terminal canonical and cleavable mitochondria targeting signal. Other forms, like GST alpha, mu and pi purified from mitochondria are similar to the cytosolic molecular forms or “echoproteins”. Altered GST expression has been implicated in hepatic, cardiac and neurological diseases. Mitochondria-specific GSTK has also been implicated in obesity, diabetes and related metabolic disorders. Studies have shown that silencing the GSTA4 (GST alpha) gene resulted in mitochondrial dysfunction, as was also seen in GSTA4 null mice which could contribute to insulin resistance in type 2 diabetes. This review highlights the significance of mitochondrial GST pool, particularly the mechanism and significance of dual targeting of GSTA4-4 under in vitro and in vivo conditions. GSTA4-4 is targeted in the mitochondria by activation of the internal cryptic signal present at the C-terminus of the protein by protein kinase-dependent phosphorylation and cytosolic heat shock protein (Hsp70) chaperon. Mitochondrial GSTpi, on the other hand, has been shown to have two uncleaved cryptic signals rich in positively charged amino acids at the N-terminal region. Both physiological and pathophysiological implications of GST translocation to mitochondria have been discussed in this review.
Introduction
The induced expression of multiple forms of glutathione S-transferase (GST; EC 2.5.1.18) appears to be an evolutionary response of cells for protection against chemical toxicity and oxidative stress. The tissue and species specific expression and distribution of GSTs are considered to be an adaptive response against the toxicity of endogenous and exogenous metabolites [1, 2], oxidative stress related degenerative disorders, and in drug resistance seen in cancer therapy. GST isoenzymes have also been implicated in the progression of cancer [1-4]. GSTs also play an important role in the activation of signals by mitogen-activated protein (MAP) kinases and various transcription factors that regulate apoptosis and cell survival [4-5]. Maintenance of the cellular antioxidant, glutathione (GSH), in different cellular compartments is also critically regulated by GSTs. GST is a multifunctional enzyme involved in cellular detoxification of endogenous toxic metabolites, superoxide radicals and exogenous toxic chemicals [3-4]. The ubiquitous distribution of GSTs in microbes, animals and plants signifies the physiological importance of this multigene family of enzyme.
GSTs are divided into soluble cytosolic (cGST alpha (A, α), mu (M, μ), Pi (pi, π), omega (ω), theta (T, θ), delta (δ), sigma (σ), zeta (ζ)), mitochondrial (mGST α, μ, π and kappa (K)) and structurally distinct membrane bound microsomal GST (MGST). The MGST family contains six members including 1, 2, 3, leukotriene C-4 synthase, 5-lipoxygenase activating protein and prostaglandin E synthase, now referred to as membrane associated proteins in eicosanoid and glutathione metabolism (MAPEG) members [1-2]. Each member of the family has multiple isoenzymes with overlapping substrate specificity. GSTs with some specific catalytic properties have also been reported in the plasma membrane, outer mitochondrial membrane as well as in the nucleus [1-3]. Several groups, including ours, have successfully purified and characterized multiple forms of GSTs from hepatic mitochondria [6-10]. Recently, dual localization of GSTK in the peroxisome and mitochondria has been reported [10]. These mitochondrial GST (mGST) proteins are coded by nuclear genes, synthesized in the cytoplasm, and then transported to mitochondria utilizing two separate mechanisms: 1) the mitochondria specific form, GSTK-1 is expressed with an N-terminal extension which contains a putative mitochondria targeting signal [10] and 2) in the other cases, the nearly intact and unprocessed GST proteins are translocated to mitochondria using signal sequences resident within the protein. The transport of nearly intact GST proteins to the mitochondrial compartment requires the help of chaperons and mitochondrial membrane bound translocases. The mechanism of import of GSTs into mitochondria will be discussed later.
Mitochondrial GSH pool and its regulation
Despite its exclusive synthesis in the cytosol [11], GSH is distributed in other intracellular organelles. Almost 85-90% of cellular GSH is present in the cytosol, 10-15% in the mitochondria (equivalent to 10-12 mM, considering the volume of mitochondrial matrix) and a small percentage is in the endoplasmic reticulum (ER) and nucleus [11]. GSH exists in the reduced thiol (GSH) and oxidized- disulfide (GSSG) forms. The compartmentalization of GSH constitutes distinct redox pools in terms of balance between oxidized and reduced forms and their turnover rates. Cytosolic GSH (cGSH) has a rapid turnover of 2-3h while mitochondrial GSH (mGSH) has relatively longer half-life of 30h. GSH is predominantly (~98-99%) found in the reduced form in most cell compartments, with the exception in the ER, where it exists mainly in the oxidized form, GSSG. A shift in this balance is a good indicator of cellular redox stress.
Mitochondria are the primary site of oxygen metabolism and their proper function is closely linked to maintenance of the GSH pool. Mitochondrial GSH pool is exclusively derived from the cytosol since these organelles lack the enzyme system for GSH synthesis. The cytosol to mitochondrial translocation of GSH is dependent on a functional transport system. At the physiological pH 7.4, GSH exists as an anion and hence permeable to cross the outer mitochondrial membrane. In liver and kidney mitochondria, there exists two major anion carriers, dicarboxylate and oxoglutarate along with other anions (glutamate, citrate, aspartate, tricorboxylate) which mediate the exchange of GSH for phosphate and dicarboxylate across the inner mitochondrial membrane. The uptake of GSH by rat kidney mitochondria appears to be saturable (Km=1.3 mM, Vmax= 5.59 nmol/min/mg protein). However, it is not clear if this is a general mechanism of GSH import in all tissues [11, 13]. Mitochondrial membrane fluidity, especially cholesterol content, is also a key factor regulating GSH import into mitochondria. Increase in cholesterol content of the inner mitochondrial membrane, as seen in rodents under chronic treatment with alcohol or under certain oxidative stress conditions such as hypoxia, diminish GSH import into mitochondria. It is noteworthy that GSH transport to mitochondria is unidirectional since no back transport of mitochondrial GSH to the cytosol has been reported. Increasing the fluidity of mitochondrial inner membrane increases cytosolic GSH (cGSH) transport into mitochondria [12, 13].
Mitochondrial GSH pool in pathology
Mitochondrial GSH status is closely associated with mitochondrial oxygen consumption, reactive oxygen species (ROS) production and redox status. A majority of mitochondrial respiratory and transport enzymes contain critical sulfhydryl groups that must be maintained in the reduced form. Alteration in mitochondrial GSH (mGSH) concentration has been associated with numerous oxidative stress related disorders including aging, cancer, diabetes, hypoxia, ischemia/injury and other diseases associated with cardiac, hepatic and neurological functions [12-15]. Under experimental or pathophysiological conditions, mGSH and cGSH pools can be selectively depleted and upon depletion, recovery of mGSH pool takes significantly longer time [10, 12] than that of cGSH. Our previous studies have also shown that in cells treated with an oxidant lipid aldehyde, 4-hydroxynonenal (4-HNE), the cGSH and mGSH pools are differently affected and the recovery of mGSH pool was significantly delayed compared to cGSH pool [8]. The mGSH pool size is also dependent upon the turnover and metabolism of GSH in mitochondria [13-14]. Under oxidative stress conditions, as seen in ischemia-associated cardiovascular and neurological disorders, altered mGSH content and ratio of mGSH/GSSG are directly associated with increased production of ROS [12-15]. Therefore, a sustained mGSH pool in the mitochondrial matrix would be advantageous to minimize the potential ROS-induced oxidative insults during physiological and pathophysiological metabolism of oxygen in the mitochondria [15].
Both under physiological and pathological conditions, the mGSH pool is regulated by a combination of mitochondrial GSH transport activity and GSH metabolism. The important enzymes of GSH metabolism inside the mitochondria are glutathione peroxidases (GSHPX1 and 4) which protect (detoxify the endogenous and exogenous toxic peroxides by conjugating with GSH) the mitochondria against oxidative degeneration. The other GSH-metabolizing enzymes are mitochondrial GSTs, which play a significant role in protecting mitochondrial functions by enzymatic transfer of GSH to proteins and metabolites. mGSH is also utilized for glutathionylation of proteins by glutaredoxin-2/thioltransferases. Mitochondrial GSH-reductase, which recycles GSSG back to GSH also plays a significant role in protecting mitochondria against oxidative stress. These enzymes are differentially regulated in the cytosol and mitochondria when exposed to chemicals, biological or physical insults [13-16].
Mitochondrial GST pool
The mitochondrial GST (GST13-13) was first identified in rat liver mitochondria by Harris et al. [6]. This enzyme was later characterized as GST kappa (GSTK1-1) and the human (hGSTK1-1) and murine (mGSTK1-1) homologues have since been identified [10, 17]. Over the years, multiple forms of GST in hepatic mitochondria have been characterized in rats, mice and humans [6-10]. However, very little is known about their physiological properties, functions and regulation. Mitochondria are the main site for ROS production during respiration coupled oxygen metabolism that may ultimately damage membrane lipids, DNA and proteins. Consequently, it is believed that GSTs play a key role in protecting mitochondrial genetic and metabolic machinery against oxidative insults. Mitochondrial GSTs are also presumed to render protection against cardiolipin oxidation in the mitochondrial inner membrane, which in turn prevents the release of cytochrome c and initiation of apoptosis. We have previously reported presence of class alpha and mu GSTs in rat and mouse liver mitochondria and also in rat brain mitochondria [7, 8, 18]. Gallagher et al. [9] showed the presence of alpha GSTA4-4 only in the mitochondria but not in the cytosol. In contrast, we and others have shown the presence of GSTA4-4 in both cytosol and mitochondria [8, 17].
Our studies have shown that preferred substrate for mitochondrial GSTA4-4 is 4-HNE, an endogenous toxic product of lipid peroxidation under oxidative stress conditions. Our recent studies, using immunolabelling and confocal microscopy have indicated increased translocation of GSTA4-4 from cytosol to mitochondria when cells were incubated with 4-HNE, tumor promoter phorbol ester (PMA), or cAMP (protein kinase A activator) suggesting its role in cancer and in protecting mitochondrial functions under oxidative stress conditions [8, 19]. Since 4-HNE is considered as a signaling molecule, preferential metabolism of this molecule in the mitochondria may modulate mitochondrial signaling pathways. We have also identified other GST isoenzymes, GSTA1/2 and GSTM1/2, in mouse liver mitochondria [8], which were later confirmed by Thomson et al. [17] and in human liver by Gallagher et al. [9]. Gallagher et al. [9] have also described the occurrence of trace amounts of GSTPi in the human liver mitochondria. A recent study by Goto et al. [20] has shown the presence of GSTPi in the mitochondria as well as nucleus of mammalian cells.
Mitochondrial Targeting of GSTs
Several studies have shown that GST isoenzymes in mitochondria are structurally and catalytically similar to their cytosolic counterparts [6,7,8, 9, 17,18,19,20].These proteins are now often termed as “echoproteins”. These studies indicate that the import of mitochondrial GST isoforms depends on the internal cryptic signals, without any proteolytic processing or alternate translation of the protein, similar to that has been established for some of the mitochondrial cytochrome P450s (CYPs) and other proteins [21-23].Mitochondrial specific GST Kappa (GSTK1-1) is, however, a unique GST, distinct from the cytosolic GSTs as it has a putative cleavable N-terminal signal for mitochondrial translocation and a C-terminal signal sequence, Ala-Arg-Leu, for peroxisomal targeting [10]. In general, the majority of mitochondrial imported proteins have 15-40 N-terminal cleavable residues rich in positively charged and hydroxylated amino acids which form an amphipathic helix essential for interaction with negatively charged residues on the translocase of the outer membrane of mitochondrial complex and import of proteins from the cytosol to the mitochondrial matrix [22, and Avadhani et al. “Bimodal protein targeting to endoplasmic reticulum and mitochondria: the concept of chimeric signals” in this series]. However, a number of mitochondria targeted proteins, particularly the xenobiotic inducible cytochrome P450s lack a cleavable N-terminal presequence [21,23-29, and reviews by Knockaert et al., “Mechanism of mitochondrial targeting of cytochrome P450 2E1: physiopathological role in liver injury and obesity” and Yogev et al. “Fumarase: a paradigm of dual targeting and dual localized functions” in this series]. In these cases, the bimodal targeting of proteins to ER and mitochondria is catalyzed by the chimeric signals they carry. Furthermore, the bimodal targeting of these predominantly microsomal CYPs is facilitated by post-translational phosphorylation by kinases under physiological and pathological conditions [24-29, review by Knockaert et al. in this series]. Protein kinase A mediated phosphorylation of serine residues of CYPs increases the affinity of proteins for binding to cytoplasmic chaperons such as heat shock proteins, Hsp70/Hsp90, resulting in increased mitochondrial translocation [27, 28, 29].
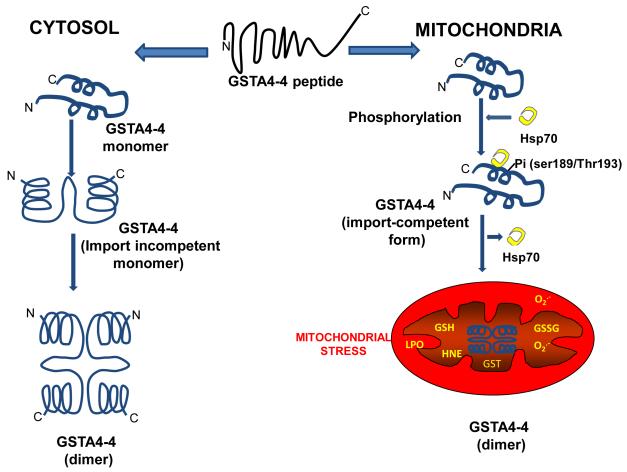
The molecular mechanism by which recombinant mouse mGSTA4-4 is targeted to mitochondria was investigated using a combination of in vitro mitochondrial import assay and in vivo targeting in COS cells transfected with GSTA4-4 cDNA. Results showed that mitochondrial GSTA4-4 is hyperphosphorylated compared to cytosolic GSTA4-4. Both cAMP and PMA, markedly increased the import of GSTA4-4 from cytosol to mitochondria. Mutational analysis show that the putative mitochondrial targeting signal in GSTA4-4 resides within C-terminal 20 amino acids and Ser-189 and Thr-193 are the sites for phosphorylation activation of the import signal [24]. The targeting function of the C-terminal sequence was further confirmed in experiments showing that the C-terminal 172-222 sequence of GSTA4-4 was able to target the N-terminally fused, but not C-terminally fused dihdrofolate reductase (DHFR, a cytosolic protein) to mitochondria. In addition, we have also provided evidence that hyperphosphorylated mitochondrial GSTA4-4 has an increased affinity for molecular chaperon Hsp70 compared to the hypophosphorylated cytosolic GSTA4-4. Our hypothesis is that the newly synthesized GSTA4-4 subunits have two fates: 1) cytosolic retention due to inefficient phosphorylation and Hsp70 binding which results in rapid folding and dimerization of subunits which make them incompetent for import; 2) mitochondrial import due to hyperphosphorylation and Hsp70 binding preventing rapid dimerization in the cytosol and making them import competent [24, Figure 1]. The import competent conformational change in GSTA4-4 is augmented under oxidative stress conditions, due to increased ROS production as seen in numerous diseases, suggesting a physiological role of mitochondrial GSTA4-4 (Figure 2a and 2b).
Figure 1. Bimodal targeting of GSTA4-4 in the cytosol and mitochondria.
Model showing Ser189/Thr193 protein kinase-dependent phosphorylation of GST A4-4 has increased affinity for chaperon Hsp70 which activates mitochondrial competent import signals for GSTA4-4. Nonphosphorylated form is retained in the cytosol.Increased oxidative stress and activated protein kinase enhances mitochondrial import of GSTA4-4.
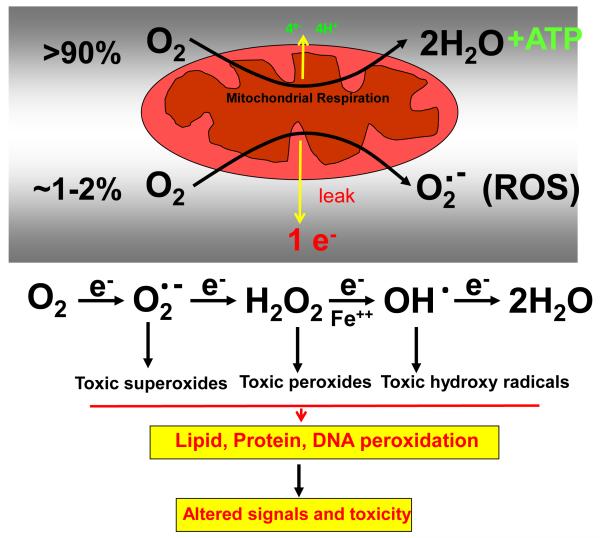
Figure 2a.
Mitochondrial oxygen metabolism and electron leakage in ROS production and toxicity
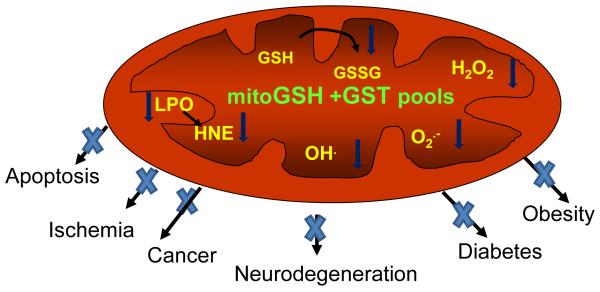
Figure 2b.
Mitochondrial GSH/GST pools implicated in the protection of oxidative stress related complications
In a recent study, Goto et al. [20] have reported that mitochondrial targeting of GSTPi also involves no detectable protease processing. They observed no difference in the size of cGSTPi and mGSTPi forms and the mitochondrial translocation depended on an internal signal located at its N-terminal region. They identified two clusters (1-19 and 71-84) of positively charged amino acid-rich regions as possible mitochondria targeting signals of mGSTPi. The reasons for the observed difference in the location of signal from C-terminal in the case of GSTA4-4 to N-terminal in the case of GSTPi remain unclear. Nevertheless, it is clear from these studies that cryptic mitochondria targeting signals of GST isoforms are used for their bimodal targeting to mitochondria.
Physiological role of mitochondrial GSH and GSTs
ROS production in the mitochondria at the sites of complex I and complex III of the respiratory chain is a physiological process occurring during oxygen reduction and ATP synthesis. ROS at lower level may function as signaling intermediates for cell survival [30]. ROS, at higher level caused oxidative stress and cell death and have been implicated in the pathogenesis of many diseases, notably neurodegeneration, aging, cancer, ischemia and diabetes [30, 31, Figure 2a and Figure 2b]. GSH is critical for cellular functions, cell growth and cell death. GSH also regulates signaling pathways mostly by maintaining redox status, ROS, sulfhydryl groups of cellular proteins, and oxidative stress which activates various signal transduction and transcriptional pathways [10, 13, 15, 16, 23]. As mentioned above, mitochondria lack GSH synthesis, and therefore depend on the import of cytosolic GSH. Increased cellular GSH promotes the growth of normal as well as cancer cells presumably by modulating rate-limiting enzymes in DNA synthesis and cell cycle progression [10, 13, 33]. GSH also modulates cell death by redox regulation of mitochondrial functions, ATP synthesis and thiol-contents of signaling molecules like NFk-B, stress kinases and caspases [10, 13, 33].
As mentioned above, the mGSH pool (10-15%) is metabolically separate from cGSH pool in terms of synthesis and turnover. Increased mitochondrial stress has been reported when mGSH level is below a critical level (i.e. 2-3 nmol/mg protein) [15]. Recent studies have shown that mGSH also play a significant role in trafficking antiapoptotic Bcl-2 and proton transporter uncoupler protein 2 (UCP2) and therefore are involved critically in cell survival and cell death mechanisms [15]. Additionally, mGSH also interacts with nitric oxide (NO) by formation of S-nitroglutathione and thus mGSH may serve as a NO donor or reservoir. The high concentration of mGSH and mGSTs and the presence of NO synthase in mitochondria suggest the physiological role of mGSH [15, 31]. GSH-dependent protein S-glutathionylation of mitochondrial complex I is involved in shifting the balance of mGSH/GSSG physiologically and also implicated in oxidative damage in many pathologies and altered mitochondrial bioenergetics [32]. Similarly, glutathionylation of mitochondrial complex II has been implicated in post ischemic heart diseases [33]. Glutathionylation of adenine nucleotide translocase has been reported to prevent mitochondrial membrane permeabilization and apoptosis [34]. Furthermore, GSH contributes to the reduction of physiological hydroperoxides, including the products of lipid peroxidation, through GST and GSHPx which will be discussed later in this review. These studies have shown that mGSH play multiple roles in maintaining mitochondrial bioenergetics in normal and disease conditions.
Pathophsyiological role of mGSH and GSTs
The mGSH pool, mitochondrial ROS production and mitochondrial metabolic and oxidative stress have been consistently implicated in physiological aging and many hepatic, cardiac and neurological diseases and ischemia/reperfusion associated disorders [15]. Dynamics of mGSH pool play a central role in these processes. mGSH, besides playing a critical role in mitochondrial bioenergetics, cell survival and apoptosis, is critically involved in the etiology and pathophysiology of numerous oxidative stress related disorders [12-16,30,35,36]. A number of mitochondria-targeted antioxidants have been developed which efficiently scavenge ROS from dysfunctional mitochondria. These antioxidants may prove effective in the treatment of these disorders [37, 38]. Permeabilization of mitochondrial membrane has been extensively implicated with apoptotic and necrotic cell death as seen during drug/chemical -induced toxicity, cancer and therapeutic interventions. A depleted mGSH promotes the oxidation of critical thiols of mitochondrial membrane and leads to mitochondrial permeabilization and cytochrome c release associated with apoptosis [15]. Gradual loss of mGSH and mitochondrial physiological functions has also been a hallmark of the aging process [39]. Alterations in mGSH pool and its metabolism and altered ROS productions have also been associated with the initiation and progression of diabetes and insulin sensitivity [40-42]. Recent studies from our laboratory have also demonstrated the protective role of mGSH and GSTs in ischemia/reperfusion and hypoxia induced injuries by ROS, alcohol and other toxic agents [28, 43, 44]. Our studies and others have also shown that mitochondrial oxidative and metabolic stress due to altered respiration and ROS production play an important role in cancer progression and metastasis [45, 46].
Previous studies have shown high specificity of mitochondrial GSTA4-4 for the metabolism of an endogenous signaling molecule, 4-HNE [8, 24]. Additionally, increased translocation of GSTA4-4 to the mitochondria under increased oxidative and chemical stress was suggested as a possible mechanism for protection against oxidative damage [8, 18, 24]. A recent study by Curtis et al. [47] has also demonstrated that down regulation of adipose tissue GSTA4-4 leads to increased mitochondrial dysfunctions and oxidative stress leading to reduced insulin sensitivity. 4-HNE mediated action on cellular metabolism is concentration dependent (toxic at 10 μM or higher and signal regulatory at lower level) depending on the makeup of transcription factors bound to cis-acting elements. Signaling by growth regulatory ERK, Nrf1/Nrf2, AP-1, TNFα and NFk-B and selective regulation by Keap1 pathways have all been implicated in GSH/GST regulation [5, 44, 48]. Mitochondrial ROS and 4-HNE are also potent activators of proton conductance by mitochondrial uncoupling proteins, UCP2 and UCP3. The uncoupling of mitochondria may be a mechanism to defend against oxidative stress by diminishing ROS production [49]. In addition to GSTA4-4, mitochondria also possess multiple other isoforms of GSTs which might also play a role in redox-regulated signaling, cell survival and death. GST isoenzymes, GSTM1 and GSTPi, have been shown to protect cells against TGF beta-induced apoptosis by MAP kinase and JNK dependent pathways.
The accumulation of ceramide in the mitochondria which inhibits GST alpha gene transactivation and Bax translocation presumably through Nrf2 and HNF1 also seems to play a role in apoptosis [50]. GST superfamily has also been implicated in the storage of NO in the form of dinitro-diglutathionyl-iron complex. NO is known to regulate a number of physiological functions including inhibition of cytochrome c oxidase, formation of peroxynitrate with mitochondrial superoxide and S-nitrosylation of proteins. Mitochondrial NO has also been shown to compensate for depleted GSH and thus prevents cell death by preserving protein thiols [51-52]. GSH conjugation with NO appears to play a critical role in hypoxia induced cell signaling. Mitochondrial GSH is also implicated in protecting mitochondria against the deleterious effects of peroxinitrate. Reports also suggest that mitochondrial calcium accumulation and release are responsible for many pathologies and apoptosis. Mitochondrial GSH pool and S-glutathionylation of specific proteins acts as a molecular linker between calcium and redox signaling [53]. Recent studies by Shield et al. [54] and Morel and Aninat [55] have shown that polymorphism in human GSTK1 gene plays an important role in lipid metabolism, obesity and insulin resistance in type 2 diabetes. Dual localization of GSTK1 enzyme in peroxisomes and mitochondria, the two compartments involved in lipid metabolism, support the regulatory function of this enzyme in energy metabolism. GST kappa is also a key regulator of adiponectin biosynthesis and since adiponectin expression has been correlated with insulin resistance, obesity and diabetes, GSTK1 expression level which is negatively correlated with obesity in human adipose tissues may be an important factor in these metabolic disorders. Polymorphism in numerous cGST isoenzymes have also been implicated in the development of specific cancers in human. However, the precise role of mGSTs in cancer and implications of individual variations are not clear.
Conclusion
Alterations in mitochondrial bioenergetics and redox metabolism by modulation of mitochondrial GSH pool have been implicated in several diseases. Mitochondrial GSH directly regulates ROS production, protein glutathionylation, protein/enzyme sulfhydryl modification, and calcium homeostasis which in turn affect cell physiology, aging and pathophysiology of diseases such as diabetes, cancer and ischemia/reperfusion-associated damage. A number of therapeutic approaches can be appropriately designed to increase the mGSH pool by increasing the expression of mGSH carriers and /or by selectively manipulating GSH metabolism [10-16]. Unfortunately, mitochondrial electron transport complexes and other enzymes that contribute to mitochondrial ROS production are not appropriate therapeutic targets for protection against oxidative stress without extreme physiological consequences. Mitochondria-targeted antioxidants and prodrugs are probably better suited for this purpose. Pharmacological or physiological interference that enhance mitochondrial GSH level presents a potential way by which oxidative damage can be directly countered. In this respect, studies on mechanisms of mitochondria targeting of GSTs and other enzymes involved in GSH metabolism may present important therapeutic targets.
Acknowledgement
Supports from Terry Fox Cancer Research Fund and grants from Research Committee, FMHS- UAE University (HR) and a support from NIH grant GM-34883 (principal investigator, NG Avadhani, University of Pennsylvania, Philadelphia, USA) are acknowledged.
References
- 1.Hayes JD, Flanagan JU, Jowsey IR. Glutathione S-transferases. Ann Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 2.Zimniak P, Singh SP. Families of glutathione transferases. In: Taylor Awasthi YC., editor. Toxicology of glutathione transferases. Francis CRC Press; Boca Raton, Fl,USA: 2006. pp. 11–26. [Google Scholar]
- 3.Allocati N, Federici L, Masulli M, Di Ilio C. Glutathione transferases in bacteria. FEBS J. 2009;276:58–75. doi: 10.1111/j.1742-4658.2008.06743.x. [DOI] [PubMed] [Google Scholar]
- 4.Zimniak P. Substrate and reaction mechanism of GSTs. In: Taylor Awasthi YC., editor. Toxicology of glutathione transferases. Francis CRC Press; Boca Raton, Fl,USA: 2006. pp. 71–102. [Google Scholar]
- 5.Yang Y, Awasthi YC. Glutathione S-transferases as modulators of signal transduction. In: Taylor Awasthi YC., editor. Toxicology of glutathione transferases. Francis CRC Press; Boca Raton, Fl, USA: 2006. pp. 205–230. [Google Scholar]
- 6.Harris JM, Meyer DJ, Coles B, Kettere B. A novel glutathione transferase (13-13) isolated from the matrix of rat liver mitochondria having structural similarity to class theta enzymes. Biochem J. 1991;278:137–141. doi: 10.1042/bj2780137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Addya S, Mullick J, Fang JK, Avadhani NG. Purification and characterization of a hepatic mitochondrial glutathione S-transferase exhibiting immunochemical relationship to the alpha-class of cytosolic isoenzyme. Arch Biochem Biophys. 1994;310:82–88. doi: 10.1006/abbi.1994.1143. [DOI] [PubMed] [Google Scholar]
- 8.Raza H, Robin MA, Fang JK, Avadhani NG. Multiple isoforms of mitochondrial glutathione S-transferases and their differential induction under oxidative stress. Biochem J. 2002;366:45–55. doi: 10.1042/BJ20020533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallagher EP, Gardner JL, Barber DS. Several glutathione S-transferase isoenzymes that protect against oxidative injury are expressed in human liver mitochondria. Biochem Pharmacol. 2006;71:1619–1628. doi: 10.1016/j.bcp.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Morle FP, Rauch C, Petit E, Piton A, Theret N, Coles B, Guillouzo A. Gene and protein characterization of the human glutathione S-transferase kappa and evidence for a peroxisomal localization. J Biol Chem. 2004;279:16246–16253. doi: 10.1074/jbc.M313357200. [DOI] [PubMed] [Google Scholar]
- 11.Lu SC. Regulation of glutathione synthesis. Mol Aspect Med. 2009;30:42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan L, Kaplowitz N. Glutathione in liver diseases and hepatotoxicity. Mol Aspect Med. 2009;30:29–41. doi: 10.1016/j.mam.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Lash LH. Mitochondrial glutathione transport: physiological, pathological and toxicological implications. Chem Biol Interact. 2006;163:54–67. doi: 10.1016/j.cbi.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulinsky VI, Kolesnichenko LS. Mitochondrial glutathione. Biochemistry (Moscow) 2007;72:698–701. doi: 10.1134/s0006297907070024. [DOI] [PubMed] [Google Scholar]
- 15.Mari M, Morales A, Colell A, Montfort CV, Garcia-Ruiz C, Fernandez-Checa JC. Antioxidants and Redox signaling: Redox control of liver function in health and disease. Antioxid Redox Signal. 2010;12:1295–1331. doi: 10.1089/ars.2009.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez-Checa JC, Kaplowitz N. Hepatic mitochondrial glutathione: transport and role in disease and toxicity. Toxicol Appl Pharmacol. 2005;204:263–273. doi: 10.1016/j.taap.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Thomson RE, Bigley AL, Foster JR, Jowsey IR, Elcombe CR, Orton TC, Hayes JD. Tissue-specific expression and subcellular distribution of murine glutathione S-transferase calss Kappa. J Histo Cyto. 2004;52:653–662. doi: 10.1177/002215540405200509. [DOI] [PubMed] [Google Scholar]
- 18.Bhagwat SV, Vijayasarathy C, Raza H, Mullick J, Avadhani NG. Preferential effects of nicotine and 4-N-methyl-N-nitrosamine 1-(3-pyridyl)-1-butanone on mitochondrial glutathione S-transferase A4-4 induction and increased oxidative stress in the rat brain. Biochem Pharmacol. 1998;56:831–839. doi: 10.1016/s0006-2952(98)00228-7. [DOI] [PubMed] [Google Scholar]
- 19.Raza H, Avadhani NG. Mitochondrial glutathione S-transferase pool in health and disease. In: Taylor Awasthi YC., editor. Toxicology of glutathione transferases. Francis CRC Press; Boca Raton, Fl,USA: 2006. pp. 277–292. [Google Scholar]
- 20.Goto S, Kawakatsu M, Izumi SI, Urata Y, Kageyama K, Ihara Y, Koji T, Kondo T. Glutathione S-transferase π localizes in mitochondria and protect against oxidative stress. Free Radic Biol Med. 2009;46:1392–1403. doi: 10.1016/j.freeradbiomed.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 21.Robin MA, Anandatheerathavarada HK, Fang JK, Cudic M, Otvos L, Avadhani NG. Mitochondrial targeted cytochrome P450 2E1 (P450 MT5) contain an intact N terminus and requires mitochondrial specific electron transfer proteins for activity. J Biol Chem. 2001;276:24680–24689. doi: 10.1074/jbc.M100363200. [DOI] [PubMed] [Google Scholar]
- 22.Wiedemann N, Frazier AE, Pfanner N. The protein import machinery of mitochondria. J Biol Chem. 2004;279:14473–14476. doi: 10.1074/jbc.R400003200. [DOI] [PubMed] [Google Scholar]
- 23.Anandatheerathavarada HK, Biswas G, Mullick J, Babu SVN, Laszlo O, Pain D, Avadhani NG. Dual targeting of cytochrome P450 2B1 to mitochondria and microsomes involves a novel signal activation by phosphorylation at Ser 128. EMBO J. 1999;18:5494–5504. doi: 10.1093/emboj/18.20.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robin MA, Prabu SK, Raza H, Anandatheerathavarada HK, Avadhani NG. Phosphorylation enhances mitochondrial targeting of GSTA4-4 through increased affinity for binding to cytoplasmic Hsp70. J Biol Chem. 2003;278:18960–18970. doi: 10.1074/jbc.M301807200. [DOI] [PubMed] [Google Scholar]
- 25.Dasari VR, Anandatheerthavarada HK, Robin MA, Boopathi E, Biswas G, Fang JK, Nebert DW, Avadhani NG. Role of protein kinase C-mediated protein phosphorylation in mitochondrial translocation of mouse CYP 1A1, which contains a non-canonical targeting signal. J Biol Chem. 2006;281:30834–30847. doi: 10.1074/jbc.M510725200. [DOI] [PubMed] [Google Scholar]
- 26.Sepuri NB, Yadav S, Anandatheerathavarada HK, Avadhani NG. Mitochondrial targeting of intact CYP2B1 and CYP2E1 and N-terminal truncated CYP1A1 proteins in Sacchromyces cerevisiae: role of protein kinase A in the mitochondrial targeting of CYP 2E1. FEBS J. 2007;274:4615–4630. doi: 10.1111/j.1742-4658.2007.05990.x. [DOI] [PubMed] [Google Scholar]
- 27.Sangar MC, Bansal S, Avadhani NG. Bimodal targeting of microsomal cytochrome P450s to mitochondria: implications in drug metabolism and toxicity. Expert Opin Drug Metab Toxicol. 2010;6:1–21. doi: 10.1517/17425255.2010.503955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bansal S, Liu C-P, Sepuri NB, Anandatheerthavarada HK, Selvaraj V, Hoek J, Milne GL, Guengerich FP, Avadhani NG. Mitochondria-targeted cytochrome P450 2E1 induces oxidative damage and augment alcohol-mediated oxidative stress. J Biol Chem. 2010;285:24609–24619. doi: 10.1074/jbc.M110.121822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Biswas G, Prabu SK, Avadhani NG. Modulation of mitochondrial metabolic function by phorbol 12-myristate 13-acetate through increased mitochondrial translocation of protein kinase C alpha in C2C12 myocytes. Biochem Pharmacol. 2006;72:881–892. doi: 10.1016/j.bcp.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 30.Lluis JM, Buricchi F, Chiarugi P, Morales A, Fernandez-Checa JC. Dual role of mitochondrial reactive oxygen species in hypoxia signaling: activation of nuclear factor-kappa B via c-SRC and oxidant dependent cell death. Cancer Res. 2007;67:7368–7377. doi: 10.1158/0008-5472.CAN-07-0515. [DOI] [PubMed] [Google Scholar]
- 31.Townsend DM, Manevich Y, He L, Hutchens S, Pazoles CJ, Tew KD. Novel role for glutathione S-transferase pi. Regulator of protein S-Glutathionylation following oxidative and nitrosative stress. J Biol Chem. 2009;284:436–445. doi: 10.1074/jbc.M805586200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hurd TR, Requejo R, Filipovska A, Brown S, Prime TA, Robinson AJ, Feanley IM, Murphy MP. Complex I within oxidatively stressed bovine heart mitochondria is glutathionylated on Cys-531 and Cys-704 of 75-kDa subunit: potential role of Cys residues in decreasing oxidative damage. J Biol Chem. 2008;283:24801–2015. doi: 10.1074/jbc.M803432200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen YR, Chen CL, Pfeiffer DR, Zweir JL. Mitochondrila complex II in the post-ischemic heart: oxidative injury and the role of protein S-glutathionylation. J Biol Chem. 2007;282:32640–32654. doi: 10.1074/jbc.M702294200. [DOI] [PubMed] [Google Scholar]
- 34.Queiroga CS, Almeida AS, Martel C, Brenner C, Alves PM, Vieira HL. Glutathionylation of adenine nucleotide translocase induced by carbon monoxide prevents mitochondrial membrane permeabilization and apoptosis. J Biol Chem. 2010;285:17077–17088. doi: 10.1074/jbc.M109.065052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adam-Vizi V, Chinopoulos C. Bioenergetics and formation of mitochondrial reactive oxygen species. Trends Pharmacol Sci. 2006;27:639–645. doi: 10.1016/j.tips.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Enns GM. The contribution of mitochondria to common disorders. Mol Gen Metab. 2003;80:11–26. doi: 10.1016/j.ymgme.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 37.Wanagat J, Dai D-F, Rabinovitch P. Mitochondrial oxidative stress and mammalian health. Mech. Ageing Dev. 2010;131:527–535. doi: 10.1016/j.mad.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy MP, Smith RA. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- 39.Wei YH, Wu SB, Ma YS, Lee HC. Respiratory function decline and DNA mutation in mitochondria, oxidative stress and altered gene expression during aging. Chang Gung Med J. 2009;32:113–132. [PubMed] [Google Scholar]
- 40.Raza H, Prabu SK, Robin MA, Avadhani NG. Elevated mitochondrial cytochrome P450 2E1 and glutathione S-transferase A4-4 in streptozotocin-induced diabetic rats: tissue-specific variations and roles in oxidative stress. Diabetes. 2004;53:185–194. doi: 10.2337/diabetes.53.1.185. [DOI] [PubMed] [Google Scholar]
- 41.Sivitz WI, Yorek MA. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxd Redox Signal. 2010;12:537–577. doi: 10.1089/ars.2009.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim J-a, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circulation Res. 2008;102:401–414. doi: 10.1161/CIRCRESAHA.107.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prabu SK, Anandatheerathavarada HK, Raza H, Srinivasan S, Spear JF, Avadhani NG. Protein kinase A-mediated phosphorylation modulates cytochrome c oxidase function and augments hypoxia and myocardial ischemia-related injury. J Biol Chem. 2006;281:2061–2070. doi: 10.1074/jbc.M507741200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raza H, John A, Brown EM, Benedict S, Kambal A. Alterations in mitochondrial respiratory functions, redox metabolism and apoptosis by oxidant 4-hydroxynonenal and antioxidants curcumin and melatonin in PC12 cells. Toxicol Appl Pharmacol. 2008;226:161–168. doi: 10.1016/j.taap.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H, Nakada K, Honma Y, Hayashi J. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008;320:661–664. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- 46.Biswas G, Srinivasan S, Anandatheerthavarada HK, Avadhani NG. Dioxin-mediated tumor progression through activation of mitochondria-to-nucleus. Proc Natl Acad Sci (U S A) 2008;105:186–191. doi: 10.1073/pnas.0706183104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Curtis JM, Grimsrud PA, Wright WS, Xu X, Foncea RE, Graham DW, Brestoff JR, Wiczer BM, Ilkayeva O, Cianflone K, Muoio DE, Arriaga EA, Bernlohr DA. Downregulation of adipose glutathione S-transferase A4 leads to increased protein carbonylation, oxidative stress and mitochondrial dysfunction. Diabetes. 2010;59:1132–1142. doi: 10.2337/db09-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Awasthi YC, Sharma R, Sharma A, Yadav S, Singhal SS, Chaudhary P, Awasthi S. Self-regulatory role of 4-hydroxynonenal in signaling for stress-induced programmed cell death. Free Rad Biol Med. 2008;45:111–118. doi: 10.1016/j.freeradbiomed.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brand MD, Affortit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL, Parker N. Mitochondrial superoxide: production, biological effects and activation of uncoupling proteins. Free Rad Biol Med. 2004;37:755–767. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 50.Birbes H, Luberto C, HSU YT, El Bawab S, Hannun YA, Obeid LM. A mitochondrial pool of sphingomyelin is involved in TNFalpha-induced Bax translocation to mitochondria. Biochem J. 2005;386:445–451. doi: 10.1042/BJ20041627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whiteman M, Chua YL, Zhang D, Duan W, Liou YC, Armstrong JS. Nitric oxide protects against mitochondrial permeabilization induced by glutathione depletion: role of S-nitrosylation? Biochim Biophys Res Commun. 2006;339:255–262. doi: 10.1016/j.bbrc.2005.10.200. [DOI] [PubMed] [Google Scholar]
- 52.Turella P, Pedersen JZ, caccuri AM, Maria FD, Mastroberardino P, Bello ML, Federici G, Ricci G. Glutathione transferase superfamily behaves like storage proteins for dinitro-diglutathionyl-iron complex in heterogenous systems. J Biol Chem. 2003;278:42294–42299. doi: 10.1074/jbc.M305569200. [DOI] [PubMed] [Google Scholar]
- 53.Frosali S, Leonini A, Ettorre A, Di Maio G, Nuti S, Tavarini S, Di Simplicio P, Di Stefano A. Role of intracellular calcium and S-glutathionylation in cell death induced by a mixture of isothiazolinones in HL60 cells. Biochim Biophys Acta. 2009;1793:572–583. doi: 10.1016/j.bbamcr.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 54.Shield AJ, Murray TP, Cappello JY, Coggan M, Board PG. Polymorphism in the human glutathione transferase kappa (GSTK1) promoter alter gene expression. Genomics. 2010;95:299–305. doi: 10.1016/j.ygeno.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 55.Morel F, Aninat C. The glutathione transferase kappa family. Drug Metab Rev. 2011;43:281–291. doi: 10.3109/03602532.2011.556122. [DOI] [PubMed] [Google Scholar]