Abstract
Nicotine vaccines have shown preliminary evidence of efficacy for enhancing smoking cessation rates, but the serum nicotine-specific antibody (NicAb) concentrations produced are highly variable and many subjects do not develop effective levels. As an alternative to vaccination, passive immunization with nicotine-specific monoclonal antibodies could produce more uniform serum NicAb concentrations, but its use is limited by their high cost and shorter elimination half-life. This study investigated supplementing vaccination with monoclonal antibodies in a targeted fashion to increase vaccine efficacy while minimizing the required monoclonal antibody dose. Rats were vaccinated and then given individualized supplemental doses of the nicotine-specific monoclonal antibody Nic311 to achieve a target total serum NicAb concentration known to be effective for blocking locomotor sensitization (LMS) to nicotine. Rats received vaccine, Nic311, both, or neither, followed by 0.3 mg/kg nicotine s.c. for 10 days to produce LMS. Combination immunotherapy completely blocked the development of LMS, while monotherapy with vaccine or Nic311 alone were only minimally effective. Lower brain nicotine levels were associated with reduced locomotor activity averaged over days 7-10. Despite its greater efficacy, combination immunotherapy did not reduce the variability in the resulting total serum NicAb concentrations. Variability in total serum NicAb concentrations was contributed to by both vaccine-generated antibody and by Nic311. These data show that combination immunotherapy, using a Nic311 dose that is by itself only minimally effective, can substantially enhance nicotine vaccine efficacy. However, variability in serum NicAb levels with combination immunotherapy may make translation of this approach challenging.
Keywords: nicotine, immunotherapy, locomotor sensitization, vaccine, monoclonal antibody, pharmacokinetics
1. Introduction
Immunization is being studied as a potential treatment for drug addiction. Vaccination with a suitably designed drug-protein conjugate vaccine stimulates the production of drug-specific antibodies that bind and sequester drug in serum, reducing or slowing drug distribution to brain and attenuating behavioral effects [1-4]. Vaccines directed against nicotine and cocaine have entered clinical trials and have provided early evidence of efficacy with no important side effects [5-7]. Vaccination for the treatment of nicotine or other drug addictions has a number of attractive features. Because the antibodies generated are highly specific for the target drug and do not bind endogenous compounds or structures, they appear to be quite safe. In principle, even very high antibody concentrations should be well tolerated. Vaccine-generated antibody is long lasting so that, in contrast to most other addiction medications, daily dosing is not required. In clinical trials, 3-5 initial monthly injections followed by a booster dose months later provided a sustained antibody response. Immunotherapies for opiate, amphetamine, and phencyclidine abuse are also being developed [8-10].
Efficacy of addiction vaccines in animals is closely correlated with the serum concentration of drug-specific antibody present; initial observations suggest the same in humans. Two common themes have emerged from clinical trials. First, efficacy of vaccination is largely confined to subjects achieving the highest serum antibody concentrations [5-7]. In a phase II trial of a nicotine vaccine, efficacy for enhancing smoking cessation rates was entirely attributable to the 30% of subjects with the highest serum nicotine-specific antibody (NicAb) concentrations [7]. Second, the serum drug-specific antibody concentrations achieved are highly variable and substantially lower than those achieved in animals [5-7, 11]. Providing a robust and reproducible immune response with consistently high serum antibody concentrations has emerged as the principle challenge for successful translation of addiction immunotherapy into clinical use.
Immunization may be achieved actively through vaccination or passively through the administration of drug-specific monoclonal antibodies. Clinical trials have focused on vaccination because of its excellent safety profile, long duration of action, and relatively low cost. Passive immunization with drug-specific monoclonal antibodies provides efficacy in animals similar to that of vaccination and has advantages that could address the limitations of vaccination. Passive immunization allows for control of the antibody dose and more uniform initial serum antibody concentrations [12]. Because monoclonal antibodies are well tolerated [13], high doses can be administered to achieve higher serum antibody concentrations than can be produced by vaccination. However, passive immunization is substantially more expensive than vaccination. In addition, the serum half-life of passively administered IgG is shorter than IgG generated by vaccination (3 weeks v. several months or longer in humans) [14], and even humanized or fully human monoclonal antibodies may themselves be immunogenic [15].
An alternative to using vaccination or passive immunization alone is to combine these treatments in an effort to exploit the advantages of each while minimizing their limitations. Combining these treatments could provide higher serum NicAb concentrations than vaccination alone while decreasing the cost and use of the monoclonal antibody, because smaller doses would be necessary than if used alone. In a proof-of-concept study, Roiko, et al. (2008) showed that combining vaccination with a fixed and partially effective dose of the nicotine-specific monoclonal antibody Nic311 produced significantly higher total NicAb concentrations, greater reductions of nicotine distribution to brain, and greater attenuation of locomotor sensitization to nicotine than vaccination alone. Because there was considerable variability in vaccine-generated NicAb concentrations, some rats achieved an effective serum NicAb concentration with vaccination alone and presumably did not require combined therapy for efficacy. This finding suggests that instead of administering a fixed dose of Nic311 to all subjects, an individualized targeted approach to Nic311 supplementation could be used to selectively supplement only those subjects requiring it while tailoring the Nic311 dose to their vaccine-generated NicAb response.
The purpose of the current study was to assess the feasibility and efficacy of a target antibody concentration strategy for supplementing vaccination against nicotine with Nic311. The vaccination schedule was chosen to provide sub-maximal efficacy to avoid a ceiling effect that could mask an increase in efficacy due to supplemental Nic311 administration. Each vaccinated animal received only enough Nic311 to increase its serum NicAb level to the anticipated effective target concentration. Immunization effects were evaluated by measuring serum NicAb concentrations, LMS to nicotine, and serum and brain nicotine concentrations.
2. Materials and Methods
2.1 Animals
Male Holtzman Sprague Dawley rats (Harlan, Indianapolis, IN) weighing 275-300 g were individually housed in temperature- and humidity-controlled rooms and maintained on a 12 h light/dark cycle, with testing taking place during hours 4-6 of the light phase. Animals were restricted to 18 g/day of rat chow to minimize weight gain and prevent catheter migration. All protocols were approved by the Minneapolis Medical Research Foundation Animal Care and Use Committee and were in accordance with National Institute of Health guidelines.
2.2 Vaccine
The nicotine immunogen 3′-AmNic-rEPA is a 3′-aminomethyl-nicotine hapten conjugated via a succinic acid linker to the carrier protein recombinant Pseudomonas exoprotein A. This immunogen generates antibodies that have a high affinity for nicotine (Kd= 20 nM) and <1% cross-reactivity with similar compounds including acetylcholine, the major nicotine metabolites cotinine and nicotine-N-oxide, and other neurotransmitters and medications [16]. Control immunogen was the carrier protein rEPA alone. The vaccine dose was 25 μg of immunogen in complete Freund’s adjuvant for the initial immunization and incomplete Freund’s adjuvant for subsequent immunizations in a volume of 0.4 ml.
2.3 Monoclonal Antibody
The monoclonal antibody Nic311 was prepared from mice immunized with 3′-AmNic-rEPA. Nic311 was purified by protein G chromatography to ≥ 95% protein content with endotoxin levels of < 0.2 enzyme unit/mg and diluted in phosphate-buffered saline. Nic311 has previously been characterized as an IgG1κ with Kd = 60 nM for nicotine and <1% cross-reactivity with cotinine, nicotine-N-oxide or acetylcholine [17]. Control IgG was human polyclonal IgG (Gammagard; Baxter Healthcare Corp., Westlake Village, CA) that does not bind nicotine or influence nicotine pharmacokinetics or behavior [18].
2.4 Nicotine
(-)-Nicotine Bitartrate (Sigma-Aldrich, St. Louis, MO) was dissolved in sterile saline and adjusted to a pH of 7.4 with 1M NaOH. Nicotine doses are expressed as weight of the base. Serum and brain nicotine levels were measured by solvent extraction of serum or tissue followed by gas chromatography with nitrogen-phosphorus detection [19]. This assay measures total nicotine in serum; nicotine bound to antibody as well as unbound (free) nicotine. Brain nicotine concentrations were corrected for brain blood content [20].
2.5 Immunologic Assays
Serum nicotine-specific antibody (NicAb) concentrations produced by vaccination were measured by ELISA using 3′-AmNic-polyglutamate as the coating antigen and goat anti-rat horseradish peroxidase as the detecting antibody [21]. Nic311 concentrations were determined using goat anti-mouse IgG horseradish peroxidase as the detecting antibody [18]. Because Nic311 levels in the combination immunotherapy group were measured in the presence of antibodies generated by vaccination, these levels were corrected for the minimal (3.2%) cross reactivity of vaccine-generated antibodies with the coating antigen used to quantitate Nic311.
2.6 Locomotor Activity
Locomotor activity sessions were conducted in open-field activity chambers (MED Associates, St. Albans, VT) each measuring 43 cm × 43 cm. Horizontal activity was measured using a 16 × 16 photocell array placed 2.5 cm above the chamber floor. Interruptions in photocell transmission were measured as horizontal activity and were recorded using open-field activity software (MED Associates). Chambers were placed inside sound-attenuating boxes with ambient lighting and exhaust fans to provide white noise.
2.7 Experimental Protocol
2.7.1 Overview
Five groups of rats (n = 8-10 per group) were used (Table 1). A non-immunized nicotine only group served as a positive control to demonstrate LMS to nicotine in the absence of immunotherapy, and a non-immunized saline only group served as a negative control. Those groups receiving vaccine had serum NicAb concentrations measured prior to beginning the LMS protocol to assure that they were within the desired range. Rats in the combination group were supplemented with Nic311 after vaccination and prior to the LMS protocol as needed in order to reach their target total serum NicAb concentration. Nic311 doses for rats in the Nic311 alone group were matched to the Nic311 doses administered to the combination group.
Table 1.
Group design
| Group | Active Immunization (Vaccine) |
Passive Immunization (Nic311) |
Nicotine |
|---|---|---|---|
| Nicotine control | − | − | + |
| Vaccine only | + | − | + |
| Nic311 only | − | + | + |
| Combination | + | + | + |
| Saline control | − | − | − |
2.7.2 Vaccination
Rats were immunized with 3′-AmNic-rEPA or control vaccine on days 0, 21, and 42 (Fig 1). One week after the third vaccine dose, an indwelling catheter was implanted in the right jugular vein and blood was obtained to measure vaccine-generated serum NicAb concentrations prior to starting the LMS protocol. Animals with a pre-LMS NicAb concentration of 100-150 μg/ml began the LMS protocol one week after blood sampling. Animals with pre-LMS vaccine-generated NicAb concentrations >150 μg/ml were monitored every two weeks until NicAb levels had decreased to within the desired range before starting the LMS protocol. Animals with vaccine-generated NicAb concentrations <100 μg/ml received a fourth vaccine dose on day 63 and serum NicAb concentrations were again determined one week later and monitored until in the desired range.
Fig. 1.
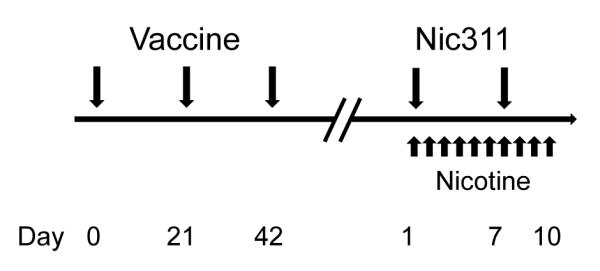
Schedule of treatments. Animals were assessed for serum NicAb level starting at 1 week after the third vaccine dose. If serum NicAb levels were below the intended range after the third vaccine dose, a fourth dose was administered at day 63. When the serum NicAb level was within the intended range, the animal underwent 2 days of habituation, received the first dose of Nic311, and began nicotine dosing for the LMS protocol.
The pre-LMS NicAb concentration range of 100-150 μg/ml was selected because, based on an expected decline in serum NicAb over the study period (unpublished data), it would result in a post-LMS serum NicAb concentration range of 80-120 μg/ml. This post-LMS NicAb concentration range was shown in pilot studies to produce minimal attenuation of LMS to nicotine. A partial effect from vaccination was necessary so that any additional effect of Nic311 in the combination immunotherapy group could be detected.
2.7.3 Passive Immunization
Rats in the combination immunotherapy group received Nic311 when the vaccine-generated pre-LMS serum NicAb was within the desired range as above. Nic311 doses were calculated to provide a target post-LMS total serum NicAb concentration (vaccine-generated NicAb + Nic311) of 200 μg/ml, based on pilot data suggesting that this total serum NicAb concentration should be sufficient to markedly suppress LMS to nicotine. Nic311 doses were calculated as a proportionality based on preliminary data showing that a dose of 27 mg/kg produces a serum Nic311 concentration of 100 μg/ml 24 hours after dosing. The difference between each rat’s measured vaccine-generated serum NicAb concentration and the target of 200 μg/ml was used to determine the required Nic311 dose. Rats began the first locomotor activity test session 60 minutes after receiving their initial supplemental dose of Nic311. Because Nic311 has a half-life of 7 days in the rat, an additional 50% of the initial Nic311 dose was administered on day 7 of the LMS protocol to maintain the desired total serum NicAb concentration [22]. Rats were studied in 6 cohorts of 6-12 animals each using a balanced design. Rats in the Nic311 only group of each cohort received a dose of Nic311 matching the mean of the individual Nic311 doses administered to rats in the combination immunotherapy group of that cohort (cohorts 1-6 received 25, 32, 22, 32, 30.5, and 30 mg/kg Nic311, respectively).
2.7.4 Locomotor Activity Measurement
Locomotor sensitization, or the progressive increase in locomotor activity elicited by repeated nicotine exposure, was used to examine the ability of NicAbs to attenuate the behavioral effects of nicotine. The locomotor sensitization protocol consisted of two habituation sessions in the locomotor chambers followed by 10 consecutive test sessions; all sessions lasted 30 minutes each. All animals received saline s.c. immediately prior to the habituation sessions. During test sessions, the saline control group continued to receive saline while all other groups received 0.3 mg/kg nicotine s.c. [22]. Following the tenth test session (40 min after nicotine dosing), rats received pentobarbital 50 mg/kg to confirm catheter patency and were sacrificed with brain and serum samples collected for analysis.
2.8 Statistical Analyses
Serum NicAb concentrations, serum nicotine levels, and brain nicotine levels were analyzed using separate one-way ANOVAs followed by Bonferroni’s post test for between-group comparisons. Locomotor activity was measured as total horizontal distance traveled over each 30-min session. Data during habituation and test sessions were analyzed using separate two-way ANOVAs with group as a between-subjects factor and day as a within-subjects factor. The primary outcome for this study was locomotor activity on days 7-10 as used previously [22]. To confirm that the non-immunized nicotine control group exhibited sensitization, a paired-samples t-test was used to compare activity in this group during the first (test day 1) and final (mean of test days 7-10) days of sensitization. Mean distance traveled over test days 7-10 was compared among groups using a one-way ANOVA followed by Bonferroni’s post-test. Within-session data on test days 1 and 10 were analyzed as 5-minute blocks using separate two-way ANOVAs with group as a between-subjects factor and time as a within-subjects factor, followed by Bonferroni’s post-test for between-group comparisons.
Relationships between total serum NicAb concentrations, serum nicotine levels, brain nicotine levels, and mean locomotor activity over days 7 to 10 were analyzed using linear regression with Pearson’s correlation coefficient for normally distributed data or Spearman’s correlation coefficient for non-normally distributed data.
2.9 Exclusions
Two rats receiving only Nic311 and one rat receiving the combination of treatments were excluded from all analyses due to technical problems. Two non-immunized rats were excluded only from serum nicotine concentration analyses due to loss of sample.
3. Results
3.1 Serum Antibody Concentrations
Six of 10 rats in the vaccine only group had post-LMS serum NicAb concentrations within the intended range of 80-120 μg/ml. The mean serum NicAb concentration for this group of 81±24 μg/ml was somewhat lower than anticipated but still within the intended range (Table 2). Seven of 9 rats in the combination immunotherapy group had post-LMS total serum NicAb concentrations (vaccine-generated NicAb + Nic311) of >160 μg/ml and the mean concentration of 192±75 μg/ml was close to the targeted 200 μg/ml. The individual contributions of vaccination and Nic311 to the total serum NicAb concentration in the combination group are shown in Table 2. There was considerable variability in the total serum NicAb concentration in the combination group (range 58-274 μg/ml). The Nic311 alone group did not have a specific intended serum NicAb range since Nic311 doses in this group were matched to those in the combination group. The mean Nic311 dose administered to the combination and Nic311 groups was 30±4 mg/kg (range 21-38 mg/kg).
Table 2.
Serum NicAb and nicotine concentrations, mean ± SD (range)
| NicAb (μg/ml) | Nicotine | ||||
|---|---|---|---|---|---|
| Vaccine | Nic311 | Total | Serum (ng/ml) | Brain (ng/g) | |
| Nicotine control | -- | -- | -- | 70 ± 10 | 380 ± 80 |
| Vaccine only | 81 ± 24 | -- | 81 ± 24 (39 – 106) |
580 ± 260 | 260 ± 50 |
| Nic311 only | -- | 150 ± 32 | 150 ± 32 (116 – 199) |
340 ± 80 | 290 ± 130 |
| Combination | 83 ± 39 (32 – 144) |
109 ± 45 (26 – 144) |
192 ± 75 (58 – 274) |
720 ± 450 | 160 ± 90 |
| Saline control | -- | -- | -- | -- | -- |
3.2 Locomotor Activity
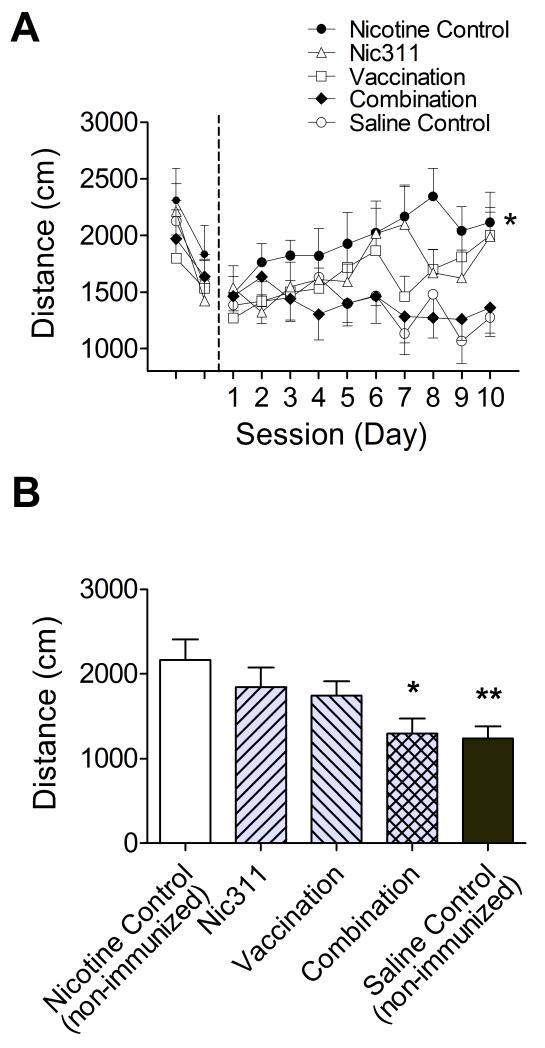
3.2.1 Sensitization to nicotine (Fig 2a)
Fig. 2.
a) Locomotor activity (mean ± SE) measured across all days for the entire 30 minute session. Initial unmarked days are non-nicotine habituation sessions and days 1 through 10 are nicotine test sessions. The non-immunized nicotine control group showed sensitization to nicotine, * p < 0.05, mean across days 7-10 compared to day 1. b) Effects of treatment on the primary outcome, mean distance traveled over the 30 minute session across days 7-10 (mean ± SE), * p < 0.05, ** p < 0.001 compared to non-immunized nicotine control group.
During habituation there was a significant effect of day (p = 0.0004), reflecting a decrease in activity over the habituation sessions for all groups, but no effect of group or interaction. Over all 10 days of sensitization there was a significant effect of day (p = 0.009) and group (p = 0.04) and interaction between the two (p = 0.005). The non-immunized nicotine control group exhibited a significant increase in activity between the first and final days of testing (p = 0.02), confirming that this nicotine dosing regimen produced sensitization.
3.2.2 Treatment effects on days 7-10 (Fig 2b)
There was an effect of treatment (p = 0.007) on activity averaged over days 7-10. Only the non-immunized saline control and combination immunotherapy groups showed significantly lower mean activity scores than the non-immunized nicotine control group; the monotherapy groups (vaccine only and Nic311 only) did not differ from the non-immunized nicotine control group. The 3 treatment groups (vaccine only, Nic311 only, combination immunotherapy) did not differ from each other.
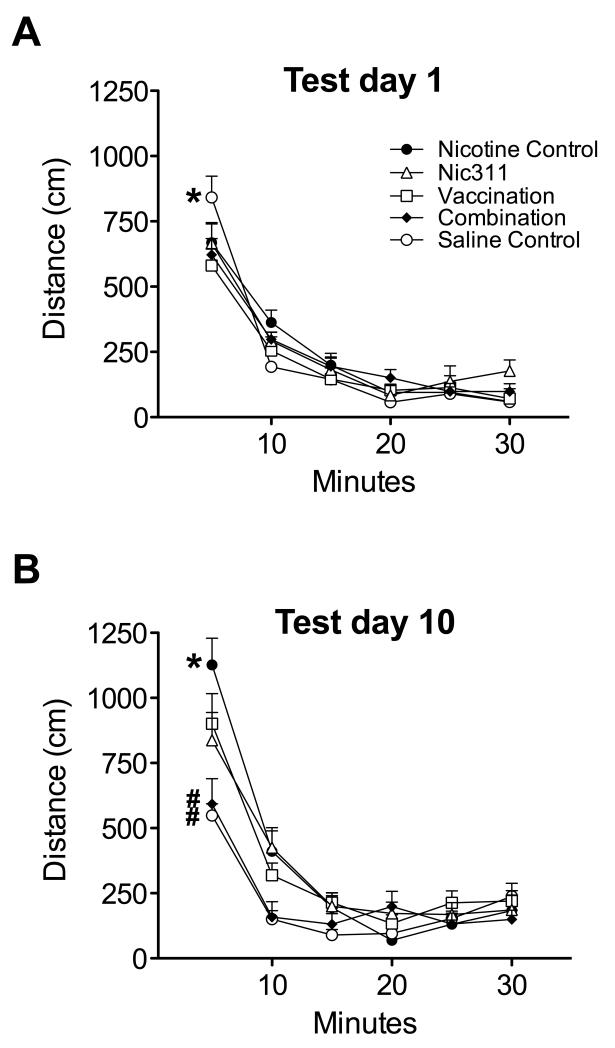
3.2.3 Within session effects (Fig 3)
Fig. 3.
Within-session locomotor activity (mean ± SE) in 5-minute blocks. a) Day 1; All groups receiving nicotine had lower activity than the saline control group, reflecting an initial decrease in activity due to nicotine, * p < 0.05. b) Day 10; All immunized groups as well as the non-immunized saline control group had lower locomotor activity than the non-immunized nicotine alone group, * p < 0.05. The combination immunotherapy and non-immunized saline control groups had lower activity than either of the monotherapy groups, # p <0.05.
Within session analysis on day 1 showed no effects of treatment, but an effect of time (p < 0.0001) and interaction (p = 0.002). Over the first 5-minute block of the session, the non-immunized saline control group showed significantly greater activity than all other groups, showing an initial suppression of activity in animals receiving nicotine (Fig 3a). Within session analysis of day 10 indicated a significant effect of group (p = 0.02), time (p < 0.0001), and interaction (p < 0.0001). Over the first 5-minute block, activity in the non-immunized nicotine control group was greater than in all other groups (Fig 3b). Activity levels in the combination immunotherapy group and the non-immunized saline control group were lower than either of the monotherapy groups.
3.3 Serum and Brain Nicotine Concentrations
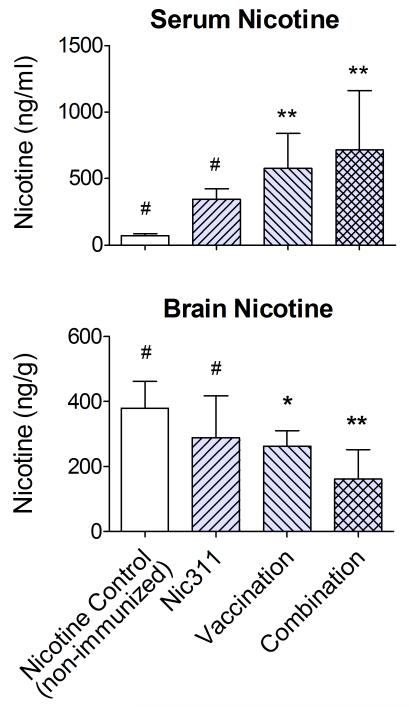
The combination immunotherapy and vaccine alone groups had higher total serum nicotine concentrations (bound + free) and lower brain nicotine concentrations than the non-immunized nicotine control group (Fig 4 and Table 2). Serum and brain nicotine levels in the Nic311 alone group did not differ from the non-immunized nicotine control group. The brain nicotine level in the combination immunotherapy group was lower than that of the Nic311 only group or the non-immunized nicotine control group (p < 0.05). The difference in brain nicotine levels between the combination immunotherapy and vaccine alone groups approached significance (p = 0.07).
Fig. 4.
Nicotine concentrations obtained 40 min after the final nicotine dose of the LMS protocol (mean ± SD). * p < 0.05, ** p < 0.001 compared to non-immunized nicotine control group; # p < 0.05 compared to combination group.
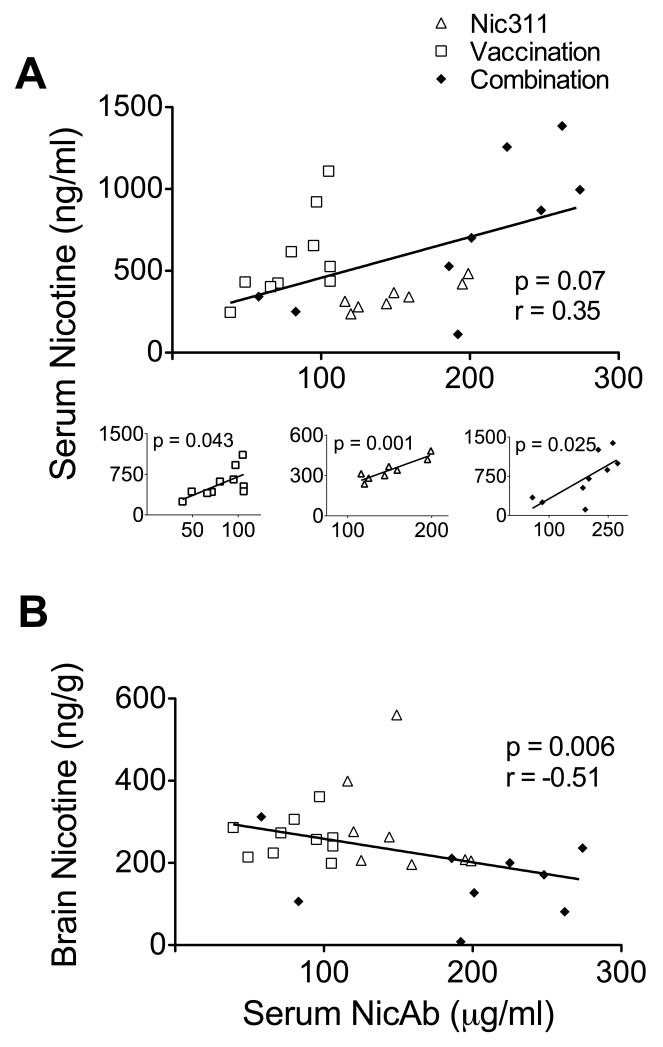
3.4 Correlations
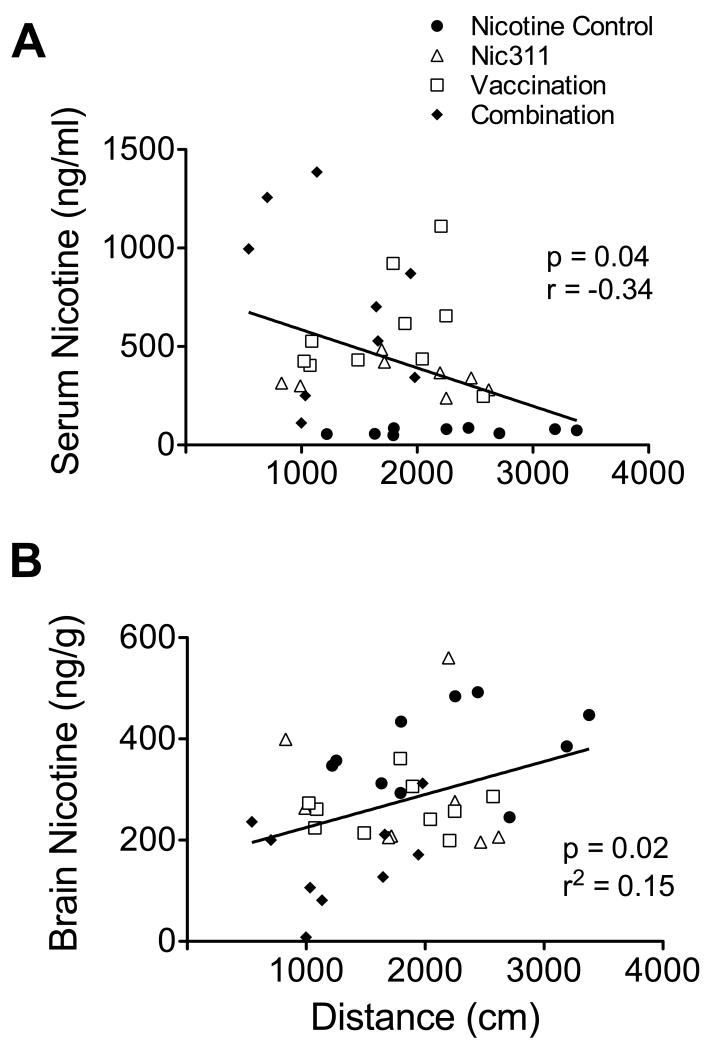
Higher serum NicAb concentrations were associated with larger effects on nicotine distribution. There was a significant negative correlation between serum NicAb and brain nicotine concentrations (Fig 5). There was a trend toward a positive correlation between serum NicAb and serum nicotine concentrations overall; however, these correlations for each individual treatment group were highly significant (Fig 5a). Nicotine concentrations were correlated with the mean distance traveled across days 7 to 10 (Fig 6), with lower serum levels and higher brain levels associated with greater distance traveled. There was no correlation between serum NicAb concentration and distance traveled on days 7 to 10.
Fig. 5.
a) Relationship of serum nicotine concentration to serum NicAb concentration across all groups; smaller figures show the relationship of serum nicotine concentration to serum NicAb concentration within individual treatment groups. b) Relationship of brain nicotine concentration to serum NicAb concentration.
Fig. 6.
Relationship of mean distance traveled on days 7 through 10 to serum (a) and brain (b) nicotine concentrations.
4. Discussion
Combination immunotherapy using a target serum NicAb concentration strategy provided substantially greater attenuation of LMS to nicotine than vaccination alone. Enhanced efficacy was achieved using a mean supplemental Nic311 dose that was by itself only minimally effective. These data support the potential use of targeted combination immunotherapy to improve the efficacy of vaccination against nicotine while minimizing the required monoclonal antibody dose.
The use of drug-specific monoclonal antibodies alone to block the behavioral effects of addictive drugs has been well studied in rodents and is remarkably effective, but high doses are generally needed [17, 22-26]. Nic311 doses required to attenuate or block LMS, nicotine discrimination, or the re-acquisition of nicotine self-administration in rats when Nic311 is used alone have ranged from 80-160 mg/kg [22, unpublished data]. The primary impediment to using monoclonal antibodies as a monotherapy for addiction is the cost of such high doses. The use of similar doses of monoclonal antibodies has clinical precedent in the treatment of some cancers or immunological disorders, but is less appealing for a widespread problem such as tobacco addiction [27, 28]. The possible need for repeated administration of monoclonal antibody to sustain its effects could further increase the required dose. Occasional immune responses to the monoclonal antibody, even if it is humanized, may also reduce its efficacy [15, 29]. Minimizing the required monoclonal antibody dose through targeted combination immunotherapy could address these concerns to some extent. While the mean initial Nic311 dose of 30 mg/kg used in this study was still appreciable, it was lower than the 80 mg/kg used in a prior study of combination immunotherapy in which supplemental Nic311 was administered as a fixed dose rather than in a targeted manner [22].
Nic311 is a murine monoclonal antibody and would need to be humanized or replaced with a fully human monoclonal to be suitable for clinical use. Nevertheless Nic311 provides a convenient experimental tool to investigate the combination immunotherapy concept and how to optimize it. Extrapolation of required antibody doses from rats to humans is difficult but it is possible that the Nic311 doses required by smokers would be lower than those suggested by the rat model. Clinical trials of nicotine vaccines show that smoking cessation can be enhanced with lower vaccine-generated serum NicAb concentrations (40-100 μg/ml) than are typically required in rat behavioral studies of nicotine vaccines (100-300 μg/ml) [7, 22, 30, 31]. It is also possible that the current study overestimated the required Nic311 dose because immunotherapy produced essentially complete blockade of LMS; a lower Nic311 dose might have been effective as well.
Blockade of LMS by combination immunotherapy in this study was essentially complete, producing activity levels comparable to that of saline controls. It is interesting that both Nic311 alone and vaccine alone were only marginally effective, yet the serum NicAb concentrations in the Nic311 alone group were nearly twice those of vaccine-generated NicAb in the vaccine alone group. This apparent difference in potency between Nic311 and vaccine-generated antibodies was likely due to their different affinities for nicotine (Nic311 Kd = 60 nM; vaccine-generated NicAb Kd = 20 nM) [16, 18].
A limitation of the experimental design is that vaccine-generated serum NicAb concentrations were selected to be within a relatively narrow range so that vaccine alone would have the desired minimal efficacy in the LMS model. As a result, the twofold range of Nic311 doses required in the combination immunotherapy group was not as wide as might otherwise have been the case. It could be argued that a fixed Nic311 dose of 30 mg/kg (the mean dose required) would have produced the same result. However, it is unlikely that a fixed dose approach would be as useful if the vaccine-generated NicAb concentrations had been as variable as the antibody levels reported in nicotine vaccine clinical trials.
The trends in the pharmacokinetic data were consistent with the LMS data but by several analyses did not reach statistical significance. This result was anticipated and is not surprising. The primary outcome measure for this study was LMS and the protocol was designed to optimize this behavioral measure rather than effects on drug levels, which are greatest shortly (1-25 min) after a nicotine dose and are less apparent with repeated or chronic nicotine dosing [32, 33]. Nevertheless, the trends in the serum and brain nicotine levels were consistent with the behavioral data. In addition, expected correlations were observed between the serum NicAb and nicotine concentrations as well as between serum or brain nicotine concentrations and distance traveled in the LMS protocol across days 7-10.
Despite its efficacy, the targeted combination immunotherapy approach did not reduce overall variability in the total serum NicAb concentrations measured at the end of the LMS protocol (range 58-274 μg/ml). Variability in the vaccine-generated contribution to the serum NicAb concentrations was expected, but variability in Nic311 levels was also considerable. Nic311 concentration variability likely represented individual differences in both the volume of distribution and clearance of Nic311 over the 10 day protocol. The elimination half-life of Nic311 in rats is 7 days so that the 10 day protocol was long enough for differences in Nic311 elimination to have a substantial effect [22]. The elimination half-life of monoclonal antibodies in humans is 3 weeks [34], longer than in rats, but because the usual duration of treatment for tobacco dependence (e.g. with counseling or marketed medications) is 6-12 weeks or longer, variability in monoclonal antibody elimination could be an issue for human use as well.
The current study extends earlier findings on combination immunotherapy by showing that an individualized target concentration strategy can be used to produce complete blockade of LMS to nicotine and that this can be achieved using a Nic311 dose that is by itself only minimally effective. This approach to immunotherapy has the potential to produce greater efficacy than is possible with vaccination alone while minimizing the Nic311 dose required. A possible limitation to this approach is that the resulting total serum NicAb concentrations are highly variable and may require monitoring to assure they remain above the targeted level.
Highlights.
Combination immunotherapy was studied to enhance nicotine vaccine efficacy Vaccination was supplemented with a nicotine-specific monoclonal antibody A target concentration strategy was used to minimize the required antibody dose The combination of treatments attenuated locomotor sensitization to nicotine in rats Combination immunotherapy was more effective than either therapy alone
Acknowledgements
Supported by NIH grants R01 DA10714 and T32 DA07097, and the Minneapolis Medical Research Foundation Translational Addiction Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].LeSage MG, Keyler DE, Pentel PR. Current status of immunologic approaches to treating tobacco dependence: vaccines and nicotine-specific antibodies. AAPS J. 2006;8:E65–75. doi: 10.1208/aapsj080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2].Elkashef A, Biswas J, Acri JB, Vocci F. Biotechnology and the treatment of addictive disorders - New opportunities. Biodrugs. 2007;21:259–67. doi: 10.2165/00063030-200721040-00006. [DOI] [PubMed] [Google Scholar]
- [3].Moreno AY, Janda KD. Immunopharmacotherapy: Vaccination strategies as a treatment for drug abuse and dependence. Pharmacol Biochem Behav. 2009;92:199–205. doi: 10.1016/j.pbb.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Orson FM, Kinsey BM, Singh RAK, Wu Y, Gardner T, Kosten TR. Addiction Reviews 2008. Blackwell Publishing; Oxford: 2008. Substance Abuse Vaccines; pp. 257–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cornuz J, Zwahlen S, Jungi WF, Osterwalder J, Klingler K, van Melle G, et al. A vaccine against nicotine for smoking cessation: A randomized controlled trial. Plos One. 2008:3. doi: 10.1371/journal.pone.0002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Martell BA, Orson FM, Poling J, Mitchell E, Rossen RD, Gardner T, et al. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatry. 2009;66:1116–23. doi: 10.1001/archgenpsychiatry.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hatsukami DK, Jorenby DE, Gonzales D, Rigotti NA, Glover ED, Oncken CA, et al. Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin Pharmacol Ther. 2011;89:392–9. doi: 10.1038/clpt.2010.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pitas G, Laurenzana EM, Williams DK, Owens SM, Gentry WB. Anti-phencyclidine monoclonal antibody binding capacity is not the only determinant of effectiveness, disproving the concept that antibody capacity is easily surmounted. Drug Metab Dispos. 2006;34:906–12. doi: 10.1124/dmd.105.005934. [DOI] [PubMed] [Google Scholar]
- [9].Anton B, Salazar A, Flores A, Matus M, Marin R, Hernandez JA, et al. Vaccines against morphine/heroin and its use as effective medication for preventing relapse to opiate addictive behaviors. Hum Vaccines. 2009;5:214–29. doi: 10.4161/hv.5.4.7556. [DOI] [PubMed] [Google Scholar]
- [10].Laurenzana EM, Hendrickson HP, Carpenter D, Peterson EC, Gentry WB, West M, et al. Functional and biological determinants affecting the duration of action and efficacy of anti-(+)-methamphetamine monoclonal antibodies in rats. Vaccine. 2009;27:7011–20. doi: 10.1016/j.vaccine.2009.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pentel PR, Keyler DE. Vaccines to treat drug addiction. In: Levine MM, editor. New Generation Vaccines. 3rd ed Dekker; New York: 2004. pp. 1057–66. [Google Scholar]
- [12].Keyler DE, Roiko SA, Benlhabib E, Lesage MG, St Peter JV, Stewart S, et al. Monoclonal nicotine-specific antibodies reduce nicotine distribution to brain in rats: Dose- and affinity-response relationships. Drug Metab Disposition. 2005;33:1056–61. doi: 10.1124/dmd.105.004234. [DOI] [PubMed] [Google Scholar]
- [13].Hardin JS, Wessinger WD, Wenger GR, Proksch JW, Laurenzana EM, Owens SM. A single dose of monoclonal anti-phencyclidine IgG offers long-term reductions in phencyclidine behavioral effects in rats. J Pharmacol Exp Ther. 2002;302:119–26. doi: 10.1124/jpet.302.1.119. [DOI] [PubMed] [Google Scholar]
- [14].Bazin-Redureau MI, Renard CB, Scherrmann JG. Pharmacokinetics of heterologous and homologous immunoglobulin G, F(ab’)2 and Fab after intravenous administration in the rat. Journal of Pharmacy and Pharmacology. 1997;49:277–81. doi: 10.1111/j.2042-7158.1997.tb06795.x. [DOI] [PubMed] [Google Scholar]
- [15].Getts DR, Getts MT, McCarthy DP, Chastain EML, Miller SD. Have we overestimated the benefit of human(ized) antibodies? Mabs. 2010;2:682–94. doi: 10.4161/mabs.2.6.13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pentel PR, Malin DH, Ennifar S, Hieda Y, Keyler DE, Lake JR, et al. A nicotine conjugate vaccine reduces nicotine distribution to brain and attenuates its behavioral and cardiovascular effects in rats. Pharmacol Biochem Behav. 2000;65:191–8. doi: 10.1016/s0091-3057(99)00206-3. [DOI] [PubMed] [Google Scholar]
- [17].Pentel PR, Dufek MB, Roiko SA, LeSage MG, Keyler DE. Differential effects of passive immunization with nicotine-specific antibodies on the acute and chronic distribution of nicotine to brain in rats. J Pharmacol Exp Ther. 2006;317:660–6. doi: 10.1124/jpet.105.097873. [DOI] [PubMed] [Google Scholar]
- [18].Keyler DE, Roiko SA, Benlhabib E, LeSage MG, St Peter JV, Stewart S, et al. Monoclonal nicotine-specific antibodies reduce nicotine distribution to brain in rats: Dose- and affinity-response relationships. Drug Metab Dispos. 2005;33:1056–61. doi: 10.1124/dmd.105.004234. [DOI] [PubMed] [Google Scholar]
- [19].Jacob P, 3rd, Wilson M, Benowitz NL. Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. J Chromatogr. 1981;222:61–70. doi: 10.1016/s0378-4347(00)81033-6. [DOI] [PubMed] [Google Scholar]
- [20].Hieda Y, Keyler DE, VanDeVoort JT, Niedbala RS, Raphael DE, Ross CA, et al. Immunization of rats reduces nicotine distribution to brain. Psychopharmacology (Berl) 1999;143:150–7. doi: 10.1007/s002130050930. [DOI] [PubMed] [Google Scholar]
- [21].Keyler DE, Shoeman D, Lesage MG, Calvin AD, Pentel PR. Maternal vaccination against nicotine reduces nicotine distribution to fetal brain in rats. J Pharmacol Exp Ther. 2003;305:587–92. doi: 10.1124/jpet.102.046805. [DOI] [PubMed] [Google Scholar]
- [22].Roiko SA, Harris AC, Keyler DE, LeSage MG, Zhang Y, Pentel PR. Combined active and passive immunization enhances the efficacy of immunotherapy against nicotine in rats. J Pharmacol Exp Ther. 2008;325:985–93. doi: 10.1124/jpet.107.135111. [DOI] [PubMed] [Google Scholar]
- [23].Daniels JR, Wessinger WD, Hardwick WC, Li M, Gunnell MG, Hall CJ, et al. Effects of anti-phencyclidine and anti-(+)-methamphetamine monoclonal antibodies alone and in combination on the discrimination of phencyclidine and (+)-methamphetamine by pigeons. Psychopharmacology. 2006;185:36–44. doi: 10.1007/s00213-005-0299-6. [DOI] [PubMed] [Google Scholar]
- [24].Gentry WB, Laurenzana EM, Williams DK, West JR, Berg RJ, Terlea T, et al. Safety and efficiency of an anti-(+)-methamphetamine monoclonal antibody in the protection against cardiovascular and central nervous system effects of (+)-methamphetamine in rats. Int Immunopharmacol. 2006;6:968–77. doi: 10.1016/j.intimp.2006.01.008. [DOI] [PubMed] [Google Scholar]
- [25].Norman AB, Norman MK, Buesing WR, Tabet MR, Tsibulsky VL, Ball WJ. The effect of a chimeric human/murine anti-cocaine monoclonal antibody on cocaine self-administration in rats. J Pharmacol Exp Ther. 2009;328:873–81. doi: 10.1124/jpet.108.146407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Carrera MR, Ashley JA, Hoffman TZ, Isomura S, Wirsching P, Koob GF, et al. Investigations using immunization to attenuate the psychoactive effects of nicotine. Bioorg Med Chem. 2004;12:563–70. doi: 10.1016/j.bmc.2003.11.029. [DOI] [PubMed] [Google Scholar]
- [27].Tabrizi MA, Roskos LK. Preclinical and clinical safety of monoclonal antibodies. Drug Discovery Today. 2007;12:540–7. doi: 10.1016/j.drudis.2007.05.010. [DOI] [PubMed] [Google Scholar]
- [28].Dierickx D, Delannoy A, Saja K, Verhoef G, Provan D. Anti-CD20 monoclonal antibodies and their use in adult autoimmune hematological disorders. Am J Hematol. 2010;86:278–91. doi: 10.1002/ajh.21939. [DOI] [PubMed] [Google Scholar]
- [29].Presta LG. Engineering of therapeutic antibodies to minimize immunogenicity and optimize function. Advanced Drug Delivery Reviews. 2006;58:640–56. doi: 10.1016/j.addr.2006.01.026. [DOI] [PubMed] [Google Scholar]
- [30].Hatsukami DK, Rennard S, Jorenby D, Fiore M, Koopmeiners J, de Vos A, et al. Safety and immunogenicity of a nicotine conjugate vaccine in current smokers. Clin Pharmacol Ther. 2005;78:456–67. doi: 10.1016/j.clpt.2005.08.007. [DOI] [PubMed] [Google Scholar]
- [31].LeSage MG, Keyler DE, Hieda Y, Collins G, Burroughs D, Le C, et al. Effects of a nicotine conjugate vaccine on the acquisition and maintenance of nicotine self-administration in rats. Psychopharmacology (Berl) 2006;184:409–16. doi: 10.1007/s00213-005-0027-2. [DOI] [PubMed] [Google Scholar]
- [32].Satoskar SD, Keyler DE, LeSage MG, Raphael DE, Ross CA, Pentel PR. Tissue-dependent effects of immunization with a nicotine conjugate vaccine on the distribution of nicotine in rats. Int Immunopharmacol. 2003;3:957–70. doi: 10.1016/S1567-5769(03)00094-8. [DOI] [PubMed] [Google Scholar]
- [33].Hieda Y, Keyler DE, Ennifar S, Fattom A, Pentel PR. Vaccination against nicotine during continued nicotine administration in rats: immunogenicity of the vaccine and effects on nicotine distribution to brain. Int J Immunopharmacol. 2000;22:809–19. doi: 10.1016/s0192-0561(00)00042-4. [DOI] [PubMed] [Google Scholar]
- [34].Vincenti F, Kirkman R, Light S, Bumgardner G, Pescovitz M, Halloran P, et al. Interleukin-2-receptor blockade with daclizumab to prevent acute rejection in renal transplantation. New England Journal of Medicine. 1998;338:161–5. doi: 10.1056/NEJM199801153380304. [DOI] [PubMed] [Google Scholar]