SUMMARY
Purpose
Increased activity of mTOR Complex 1 (mTORC1) has been demonstrated in cortical dysplasia and tuberous sclerosis complex, as well as in animal models of epilepsy. Recent studies in such models revealed that inhibiting mTORC1 with rapamycin effectively suppressed seizure activity. However, seizures can recur after treatment cessation, and continuous rapamycin exposure can adversely affect animal growth and health. Here, we evaluated the efficacy of an intermittent rapamycin treatment protocol on epilepsy progression using neuron subset-specific-Pten (NS-Pten) conditional knockout mice.
Methods
NS-Pten knockouts were treated with a single course of rapamycin during postnatal weeks four and five, or intermittently over a period of five months. Epileptiform activity was monitored using video-EEG recordings, and mossy fiber sprouting was evaluated using Timm staining. Survival and body weight were assessed in parallel.
Key Findings
NS-Pten knockouts treated with a single course of rapamycin had recurrence of epilepsy four to seven weeks after treatment ended. In contrast, epileptiform activity remained suppressed, and survival increased if knockout mice received additional rapamycin during weeks 10–11 and 16–17. Aberrant mossy fiber sprouting, present by four weeks of age and progressing in parallel with epileptiform activity, was also blocked by rapamycin.
Significance
These findings demonstrate that a single course of rapamycin treatment suppresses epileptiform activity and mossy fiber sprouting for several weeks before epilepsy recurs. However, additional intermittent treatments with rapamycin prevented this recurrence and enhanced survival without compromising growth. Thus, these studies add to the growing body of evidence implicating an important role for mTORC1 signaling in epilepsy.
Keywords: mTORC1, Pten, cortical dysplasia, TSC, mossy fiber sprouting, mTOR
INTRODUCTION
Ubiquitously expressed and highly conserved, the mammalian target of rapamycin complex 1 (mTORC1) signaling pathway is involved in protein translation, cell growth, and proliferation (reviewed in Hay & Sonenberg, 2004). Recently however, this pathway has received much attention due to the specialized roles it plays in the nervous system. Activation of mTORC1 signaling mediates axonal branching and growth (Park et al., 2008; Grider et al., 2009), growth cone collapse (Campbell & Holt, 2001), dendritic arborization, and spine density (Jaworski et al., 2005; Kumar et al., 2005; Tavazoie et al., 2005; Chow et al., 2009). In addition, mTORC1 signaling participates in various aspects of synaptic plasticity including both long-term potentiation (LTP) and long-term depression (LTD; reviewed in Hoeffer & Klann, 2010). Thus, aberrant regulation of this pathway could lead to a variety of neuropathologies. We and a number of other investigators have focused on the mTORC1 signaling pathway as a candidate molecular player in epilepsy (Uhlmann et al., 2002; Erbayat-Altay et al., 2007; Meikle et al., 2007; Meikle et al., 2008; Zeng et al., 2008; Buckmaster et al., 2009; Ljungberg et al., 2009; Way et al., 2009; Zeng et al., 2009).
Recently, several studies have implicated mTORC1 as playing a crucial role in the epileptogenesis seen in acquired models of temporal lobe epilepsy (TLE; Buckmaster et al., 2009; Zeng et al., 2009; Huang et al., 2010). Following chemo-convulsant status epilepticus, these studies revealed a dramatic increase in phosphorylation of ribosomal protein S6, an indication of mTORC1 activity, which could be effectively blocked with the specific mTORC1 inhibitor, rapamycin. Rapamycin also reduced both aberrant sprouting of mossy fibers (Buckmaster et al., 2009; Zeng et al., 2009; Huang et al., 2010; Buckmaster & Lew, 2011) and spontaneous seizures (Zeng et al., 2009; Huang et al., 2010). Frequently observed in both patients and animal models of TLE, mossy fiber sprouting is defined by the abnormal sprouting of hippocampal dentate granule cell axons which then form aberrant synapses within the granule cell layer (reviewed in Koyama & Ikegaya, 2004). Some investigators have even correlated the degree of mossy fiber sprouting with the severity of epilepsy (Cavazos et al., 1991; Wuarin & Dudek, 2001). Although the precise role of mossy fiber sprouting in epilepsy is controversial, many studies have suggested these abnormal connections form a recurrent loop of excitation, initiating or perhaps exacerbating seizure activity and epileptogenesis (for comprehensive reviews, see Nadler, 2003; Sutula & Dudek, 2007).
Malformations of cortical development, a common cause of pediatric intractable epilepsy, have also been associated with increased mTOR signaling. The most well-characterized disorder is Tuberous Sclerosis Complex (TSC). TSC results from mutations leading to the loss of function in either the TSC1 or TSC2 genes (European Chromosome 16 TS Consortium, 1993; van Slegtenhorst et al., 1997), whose protein products (hamartin or tuberin, respectively) complex together to indirectly inhibit mTOR (for reviews see Kwiatkowski, 2003; Orlova & Crino, 2010). In the presence of disease-rendering mutations in TSC1 or TSC2 the molecular association between these two molecules is disrupted leading to a loss of mTOR inhibition and thereby hyperactivity of the pathway. Interestingly, the characteristic features of dislamination, cytomegalic neurons, and abnormal glioneuronal cell types present in TSC are often shared by cortical dysplasia (CD) patients who lack a defined genetic etiology. In fact, dysplastic tissue resected from both TSC and CD patients display increased mTORC1 activity, as measured by increased phosphorylation of downstream targets (Baybis et al., 2004; Miyata et al., 2004; Ljungberg et al., 2006). These shared phenotypes and molecular markers implicate aberrant mTORC1 signaling as a major player in the pathology of both disorders, and suggests a common epileptic substrate.
Moreover, recent studies with conditional knockout mice of TSC1 or Pten (another, more upstream regulator of mTOR signaling) have shown that subsequent inhibition of mTORC1 with rapamycin rescues many of the TSC and CD-like phenotypes recapitulated in these mice, including increased mTORC1 pathway activity, hypertrophy, and epilepsy (Meikle et al., 2008; Zeng et al., 2008; Ljungberg et al., 2009). When TSC1 was selectively knocked out of either neurons or astrocytes in these mouse models, many of the abnormal phenotypes were suppressed during ongoing rapamycin treatment, and re-appeared shortly after treatment was halted (Meikle et al., 2008; Zeng et al., 2008). Similarly, when Pten was selectively deleted in a subset of neurons (NS-Pten knockout mice), hypertrophy also began to recur within three weeks after a two-week treatment with rapamycin. In contrast to the TSC1 conditional knockouts, however, the epileptiform activity in NS-Pten conditional knockout mice remained significantly suppressed for at least three weeks after rapamycin treatment was stopped (Ljungberg et al., 2009). The precise duration of this suppression and whether the seizure activity ultimately recurred was not examined.
In the studies presented here, we further characterized a role for mTORC1 in the progression of epilepsy in NS-Pten conditional knockout mice by using video-EEG recordings to assess the duration of epilepsy suppression after rapamycin treatment, and determined whether intermittent treatment could prevent epilepsy recurrence. Additionally, since NS-Pten knockout mice lack Pten expression in the majority of hippocampal dentate granule cells (Backman et al., 2001; Kwon et al., 2001), we used the Timm stain technique to assess the effects of inherent mTOR up-regulation, and subsequent pharmacological mTORC1 inhibition on aberrant mossy fiber sprouting.
METHODS
Mice
Neuron subset-specific Pten (NS-Pten) conditional knockout mice were a gift from S. Baker (St. Jude Children’s Research Hospital, Memphis, TN), and have been described previously as GFAP-Cre;PtenloxP/loxP (Backman et al., 2001; Kwon et al., 2001). They exist on a unique, FVB-based mixed background strain. We used NS-PtenloxP/+ (heterozygote) animals for breeding to generate NS-Pten+/+ (wild type) and NS-PtenloxP/loxP (knockouts). With the exception of the body weight study, all experiments reported here utilized mice of both genders. Animal housing and use were in compliance with the NIH Guidelines for the Care and Use of Laboratory Animals and were approved by the institutional animal care committee at Baylor College of Medicine.
Rapamycin treatment
Rapamycin (LC Laboratories) was dissolved in a vehicle solution of 4% ethanol, 5% polyethylene glycol 400 (Sigma), and 5% Tween 80 (Sigma), as previously described (Eshleman et al., 2002). Animals received a first course of daily 10 mg/kg intraperitoneal injections five times per week of either rapamycin or vehicle during the fourth and fifth postnatal weeks. A subset of knockout mice also received a second and third course of rapamycin treatment during postnatal weeks 10–11 and 16–17.
Electrode implantation
Cortical EEG electrodes were implanted prior to treatment in three-week-old knockout animals as previously described, or in adult knockouts after a first course of rapamycin (n=3) in order to increase the longevity of the electrodes (Ljungberg et al., 2009). Briefly, animals were anaesthetized with a ketamine/xylazine/acepromazine mixture (obtained from the Baylor College of Medicine Center for Comparative Medicine), placed in a stereotaxic frame fitted with a mouse adaptor, and four stainless steel electrodes (Plastics One) were placed bilaterally over the cortex whilst a reference was placed anterior to bregma and a ground placed in the cervical paraspinous area. A subset of pre-treatment animals had placement of two hippocampal depth electrodes in addition to two cortical electrodes, as previously described (Anderson et al., 1997). Cortical electrodes were placed 0.1mm posterior and 1.8mm lateral to bregma, whilst depth electrodes were placed 1.6mm posterior and 1.8mm lateral to bregma at a depth of 1.8mm. Animals were allowed to recover for 4–7 days before video-EEG recording.
EEG acquisition and analysis
Video-EEGs were recorded and analyzed essentially as described previously (Ljungberg et al., 2009). Digital video-EEG systems (Nicolet or Stellate) were used to record approximately four hours of synchronized video-EEGs at four, six, and nine weeks of age, then once every week thereafter. To assess and quantify the severity of epileptiform activity, we selected 30-minute epochs of EEG traces after a one-hour acclimation in the recording chamber, and quantified the amount of time (in seconds) spent in epileptiform activity (as defined in Ljungberg et al., 2009; Supplemental Figure 1) and reported this data as a percent of the total 30-minute (1800 second) observation time. All scoring was performed blinded to treatment.
Timm staining and analysis
Timm staining was accomplished using a protocol modified from Anderson et al. (1997). Animals were deeply anaesthetized with a ketamine/xylazine/acepromazine mixture and transcardially perfused with sodium sulfide solution (1.2% Na2S·9H20, 1% NaH2PO4·H20) for ten minutes (~20 ml), or until extremities turned blue/gray and livers black. Brains were removed and placed in sulfide perfusate for an additional 45–60min before fixation in 10% neutral buffered formalin (Richard Allan Scientific) for 24–72 hours. Brains were then transferred for 90 minutes to a solution containing 2.5% gluteraldehyde (Sigma) and 24% dextrose, then back to formalin for a final 24hrs before an alcohol dehydration series and paraffin embedding. Coronal sections were cut 12µm thick, mounted on gelatin-coated slides, and dried at 60°C overnight. Sections from each treatment group were stained in parallel with Timm stain solution (120ml of 50% gum arabic, 20ml of 2M citrate buffer, 60ml of hydroquinone, and 1ml of 17% silver nitrate) in the dark at room temperature for 45min, then at 60°C for 20min. Slides were washed with deionized water, counterstained with cresyl violet, dehydrated, and sealed with a coverslip. Pictures were taken with an Olympus DP70 digital color camera fitted to an Olympus BX51 microscope.
Severity of mossy fiber sprouting was analyzed for two to four sections per mouse by three independent and blinded investigators using a slightly modified scale from Cavazos et al. (1991). These scores were then averaged to obtain a single value for each animal. The scale was as follows: 0 – hilar region only stained, 1 – sparse and patchy distribution of staining extending into the granule cell layer (GCL), 2 – more staining in the GCL which may extend into the supragranular layer (SGL), 3 – prominent staining within the GCL and SGL, 4 – prominent staining within the GCL and SGL resulting in a confluent dense laminar band, 5 – a confluent dense laminar band that extends beyond the SGL into the inner molecular layer. Examples of each score can be found in Supplemental Figure 2.
Statistical Analyses
Unless otherwise stated, all data was analyzed with Student’s t-test or One-Factor ANOVA with Neuman-Keuls post-hoc test, and data presented as mean ± SEM. Analyses were carried out using GraphPad Prism software with significance placed at p<0.05.
RESULTS
NS-Pten knockout mice exhibit progressive aberrant mossy fiber sprouting
Aberrant axonal sprouting of dentate gyrus mossy fibers has been linked to recurrent excitation in temporal lobe epilepsy and could play a potential role in epileptogenesis (Cavazos et al., 1991; Wuarin & Dudek, 2001; Nadler, 2003; Sutula & Dudek, 2007). The mTOR pathway is a key regulator of both neuronal polarity and axonal growth (Choi et al., 2008; Grider et al., 2009; Morita & Sobue, 2009), and is highly upregulated in the majority of dentate granule cell neurons of conditional NS-Pten knockout mice due to high levels of expression of the transgene in this area (Kwon et al., 2001). As reported previously, the knockout mice also exhibit progressive epilepsy (Ljungberg et al., 2009). In the current studies we implanted a subgroup of the NS-Pten knockout mice with both cortical and depth electrodes (n=3) to assess localization of epileptiform activity in these regions using EEG. As with the knockout mice implanted with only cortical electrodes, spike and polyspike activity (interictal), subclinical continuous polyspike activity (subclinical seizures), and electroclinical seizures (electrographic seizures with associated tonic clonic behavior seizures) were observed in the knockouts with cortical and hippocampal depth electrodes (for examples of each type of epileptiform activity see Supplemental Figure 1). Furthermore, interictal and ictal activity were present both synchronously as well as independently from all four recording regions. Of the four electroclinical seizures recorded in these animals, the seizure onset was characterized as follows: 1) in the left cortex, 2) synchronously in the right cortex and hippocampus, 3&4) synchronously in all recording electrodes (right and left cortical and hippocampal depth electrodes). After onset with each of the electrographic seizures there was spread to involve all recording electrodes and at this point an associated tonic clonic seizure. Seizures three and four which arose synchronously in all recording electrodes may represent focal onset that was missed with our recording setup, perhaps representing rapid bilateral synchrony instead of generalized onset. Based on these findings there are multifocal irritative zones within the brains of NS-Pten knockout mice, involving both cortical and hippocampal regions. Given the hippocampal involvement, we evaluated knockout mice for aberrant mossy fiber sprouting, which we hypothesized would likely progress in severity over time in parallel with epileptiform activity.
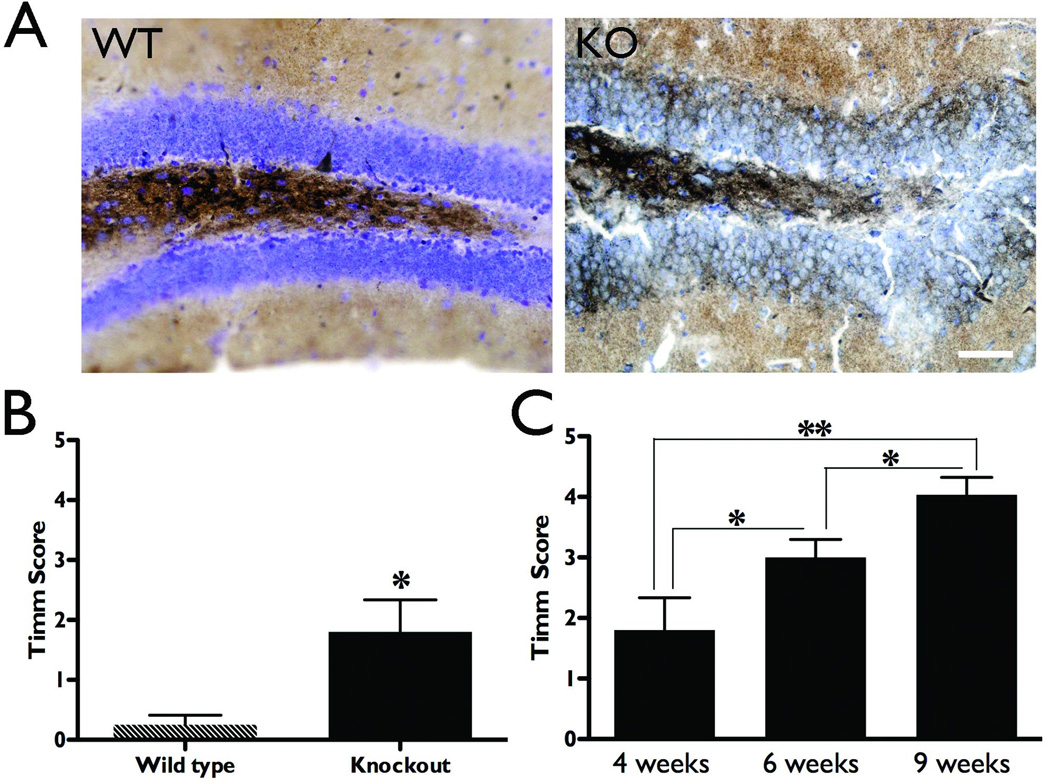
Wild type and knockout animals were sacrificed at four, six, or nine weeks of age to visualize hippocampal mossy fiber terminals using the Timm staining technique (Figure 1A, representative sections from wild type and NS-Pten knockout mice at four weeks old). The degree of mossy fiber sprouting was assessed at these time points using a modified scale from Cavazos et al. (1991), where a score of zero indicated normal staining, and a five indicated severe mossy fiber sprouting infiltrating the inner molecular layer (Supplemental Figure 2). At four weeks of age, aberrant sprouting was already apparent in NS-Pten conditional knockout mice as compared to wild type controls (1.8 ± 0.5 and 0.3 ± 0.2, respectively, p<0.05, Figure 1B). Mossy fiber sprouting was progressive in the knockouts, becoming significantly more severe with each age sampled (n=5–9, p<0.05 between successive ages and p<0.01 between four and nine weeks of age, Figure 1C). These findings provide a neuroanatomical correlate to the neurophysiology findings of progressive epilepsy that we have previously reported in these mice (Ljungberg et al., 2009).
Figure 1.
Aberrant mossy fiber sprouting increases with age in NS-Pten knockouts. Wild type and knockout animals were sacrificed and their brains processed for Timm staining to detect mossy fiber terminals. Sections were scored by three independent investigators on a scale of 0–5, where zero indicates no abnormal staining outside of the hilus, and 5 indicates severely abnormal staining that penetrates through the granule cell layer and into the inner molecular layer. A) Timm stained coronal sections at the level of the dentate gyrus reveal aberrant mossy fiber sprouting in NS-Pten conditional knockout animals compared to wild type mice at four weeks of age (scale bar = 50µm). B) Summary data reveal significantly increased Timm scores in the knockout compared to the wild type mice at four weeks of age (n=4–5). C) Timm scores significantly increased with age in NS-Pten knockout mice (n=5–9), indicating a progressive increase in aberrant mossy fiber sprouting. *p<0.05, **p<0.01.
Rapamycin treatment blocks aberrant mossy fiber sprouting in NS-Pten knockout mice
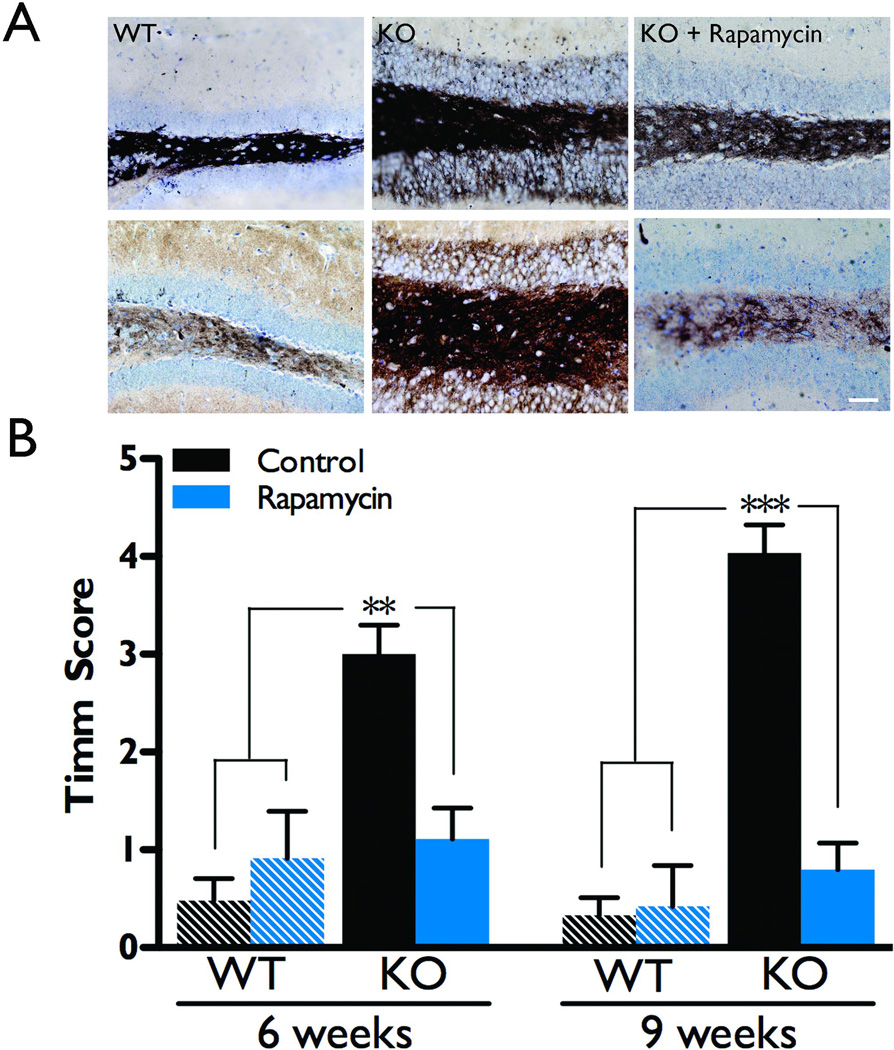
We recently reported that rapamycin treatment significantly attenuated epileptiform activity in the NS-Pten conditional knockout mice (Ljungberg et al., 2009). Thus, next we investigated whether rapamycin also blocked aberrant mossy fiber sprouting in these mice. Wild type and knockout mice were treated with rapamycin or vehicle during postnatal weeks four and five, then were sacrificed at six or nine weeks of age to visualize hippocampal mossy fiber terminals using the Timm staining technique (Figure 2A). Since there was no statistically significant difference between naïve and vehicle-treated groups (p>0.05 by t-test), they were pooled into a control group for each genotype.
Figure 2.
Rapamycin treatment suppresses aberrant mossy fiber sprouting. Wild type and knockout animals were treated with either vehicle or rapamycin during postnatal weeks 4–5 and then were sacrificed at 6 or 9 weeks of age to evaluate mossy fiber sprouting with Timm staining. Sections were scored by three independent investigators on a scale of 0–5, where zero indicates no abnormal staining outside of the hilus, and 5 indicates severely abnormal staining that penetrates through the granule cell layer and into the inner molecular layer. As there was no statistical difference between them, naïve and vehicle-treated groups were combined into one control group for each genotype and age. A) Representative examples of Timm stained sections at both 6 and 9 weeks of age reveal aberrant mossy fiber sprouting in control NS-Pten conditional knockouts as compared to wild types of the same age, which is dramatically decreased with rapamycin treatment (scale bar = 50µm). B) Summary data reveal that control knockouts had significantly elevated Timm scores compared to both control and rapamycin-treated wild type mice. Furthermore, knockouts treated with rapamycin exhibited significantly lower Timm scores than control knockouts and had Timm scores that were not significantly different from the wild type groups (n=4–9 per group). **p<0.01, ***p<0.001.
NS-Pten knockouts that received rapamycin treatment during postnatal weeks four and five had a significant decrease in mossy fiber sprouting as compared to knockout controls at both six (p<0.01) and nine weeks of age (p<0.001, Figure 2B), similar to the decrease in epileptiform activity previously observed with rapamycin treatment at these same time points (Ljungberg et al., 2009). Mossy fiber sprouting was essentially reversed in these animals, as there was no significant difference between rapamycin-treated knockouts and wild type controls at either age (p>0.05).
Epileptiform activity recurs following a single course of rapamycin treatment in the NS-Pten knockout mice
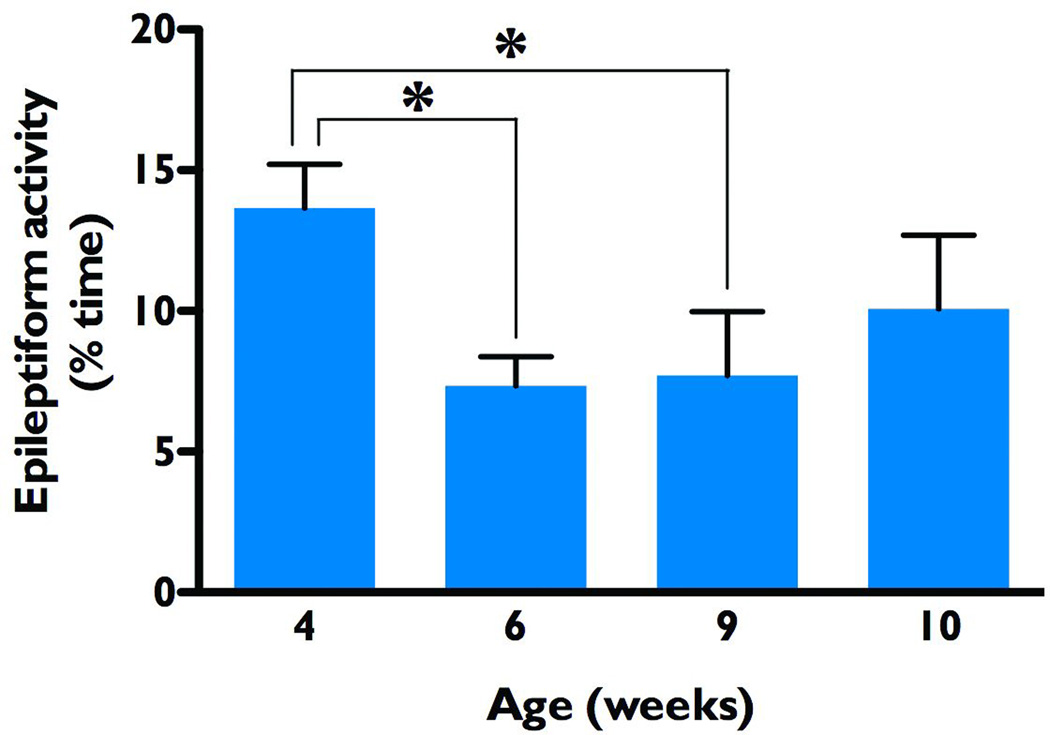
NS-Pten conditional knockout mice treated with rapamycin during postnatal weeks four and five had significantly attenuated epileptiform activity that persisted for at least three weeks after treatment withdrawal (Ljungberg et al., 2009). The precise duration of this effect, however, was not examined in the previous study. Thus, here we performed video-EEG studies after transient rapamycin treatment to evaluate if the epileptiform activity recurred. We implanted knockout mice with cortical EEG electrodes during postnatal week three, and after an initial video-EEG recording, rapamycin treatment was administered during weeks four and five. Video-EEG recordings were taken at postnatal weeks six and nine, and then once per week thereafter until the animals either died or the electrodes stopped working. Concordant with our previous observations, time spent in epileptiform activity was significantly decreased relative to the four-week baseline (13.7 ± 1.5% of the time) both immediately following treatment (six weeks of age, 7.3 ± 1.0%, p<0.05) and three weeks later (nine weeks of age, 7.7 ± 2.3%, p<0.05 by Repeated Measures ANOVA). By ten weeks however, this reduction was no longer significant (10.1 ± 2.6%), indicating that epileptiform activity had recurred in a significant number of the knockout animals examined (n=11, Figure 3). These findings indicate that while rapamycin effectively suppressed epileptiform activity in the NS-Pten knockout mice, epileptiform activity eventually recurred following cessation of treatment, supporting the concept that long-term or repeated treatments with rapamycin are necessary to suppress epileptiform activity in this model.
Figure 3.
Epileptiform activity recurs after a single course of rapamycin treatment. Baseline EEG recordings were taken from NS-Pten conditional knockout mice at 4 weeks of age, before receiving a single course of rapamycin treatment during postnatal weeks 4–5. As compared to baseline, epileptiform activity was significantly reduced at weeks 6 and 9, but not at 10 weeks of age (Repeated Measures ANOVA, n=11), indicating a recurrence of epileptiform activity within 4 weeks after rapamycin withdrawal. *p<0.05.
Long-term intermittent rapamycin treatment suppresses epileptiform activity in the NS-Pten knockout mice
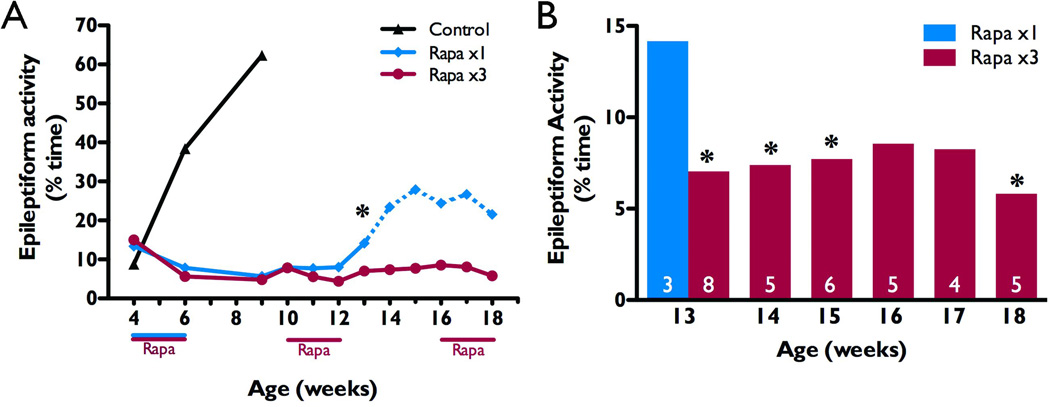
We next evaluated whether recurrence of epileptiform activity in the Pten mutant mice could be prevented with additional rapamycin treatment. For these studies we designed a novel dosing schedule of repeated, intermittent two week courses of rapamycin. Knockout mice were randomly selected to receive additional courses of rapamycin treatment during postnatal weeks 10–11 and 16–17, in a two weeks on, four weeks off pattern (n=8, Rapa x3), while others served as a single course comparison group (n=6, Rapa x1). By 13 weeks of age, NS-Pten conditional knockout mice that had not received a second course of rapamycin treatment were spending significantly more time in epileptiform activity (median of 14.2%) than those that received the additional treatment (median of 7.0%, p<0.05, Mann-Whitney U test due to unequal variances; Figure 4A,B). Due to low sample sizes from premature deaths in the Rapa x1 group, statistical comparisons could not be made past 13 weeks. However, the EEG data for the one remaining Rapa x1 knockout mouse demonstrate a rapid increase in epileptiform activity from 5.9% of the time at 12 weeks to 25% between weeks 15–18. This increase was similar, although not as dramatic, to what we have previously reported in naïve and vehicle-treated knockouts from four to nine weeks of age (plotted for comparison in Figure 5A; Ljungberg et al., 2009). NS-Pten conditional knockouts that received additional courses of rapamycin treatment, on the other hand, maintained suppression of epileptiform activity for as long as we could obtain EEG recordings (n=5–8 up to week 13 and n=4–6 for weeks 14–18). When compared to the Rapa x1 group at 13 weeks, Rapa x3 knockouts continued to spend significantly less time in epileptiform activity at weeks 14 and 15 (p<0.05 by Mann-Whitney U test due to unequal variances, Figure 4B). In fact, the only time this difference was not significant was during the final course of treatment at weeks 16 and 17 (p=0.14 and p=0.23), returning to significance again by week 18 (p<0.05). These results further illustrate the power and utility of the intermittent treatment schedule employed in these studies.
Figure 4.
Repeated rapamycin treatment maintains suppression of epileptiform activity. NS-Pten knockouts received a single course of rapamycin during postnatal weeks 4–5 and a subset received additional courses of treatment during weeks 10–11 and 16–17 (Rapa x3, red). A) Knockouts that only received the initial treatment (Rapa x1, blue) showed a significant increase in epileptiform activity at 13 weeks (n=6 for weeks 4–10, n=3–4 for weeks 11–13, and n=1 for weeks 14–18; low sample size is due to premature death; see Figure 5), as compared to the Rapa x3 group (n=5–8 for weeks 4–13, and n=4–6 for weeks 14–18). For comparison, previous data from naïve and vehicle-treated knockouts (Ljungberg et al., 2009) are also plotted, but eliminated from statistical analyses (Control, n=12–14, black). Note that statistical analyses between the rapamycin-treated groups could not be made after 13 weeks due to premature deaths in the Rapa x1group resulting in a sample size <3 (represented by a dotted line), however all available data are plotted. B) Rapa x3 knockouts demonstrated continued suppression of epileptiform activity as compared to the Rapa x1 group at 13 weeks. Data are presented as the median for each age and treatment group. Analysis was performed using the Mann-Whitney U test due to unequal variances. For weeks 4–13, comparisons were made between treatment groups (A); for weeks 14–18, comparisons were made against the Rapa x1 group at 13 weeks (B). *p<0.05.
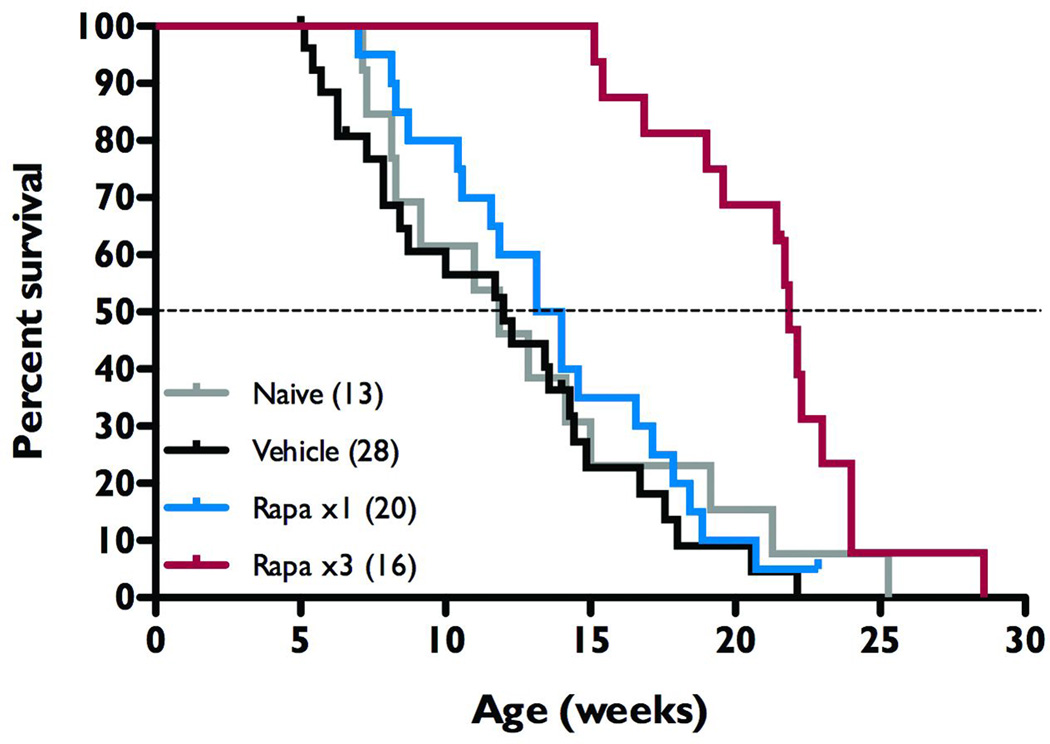
Figure 5.
Intermittent rapamycin treatment increases survival in NS-Pten conditional knockout mice. During postnatal weeks 4–5, NS-Pten knockouts were treated with vehicle solution (Vehicle, n=28, black trace), rapamycin (Rapa x1, n=20, blue), or left untreated (Naïve, n=13, grey). Additional knockout mice were treated with three courses of rapamycin during weeks 4–5, 10–11, and 16–17 (Rapa x3, n=16, red). Kaplan-Meier Logrank survival plot and analysis reveals Rapa x3 animals to live significantly longer than all other treatment groups (p=0.001), with a median age of 22 weeks as compared to 14 weeks or less in the vehicle and Rapa x1 groups.
Intermittent rapamycin treatment increases survival in the Pten knockout mice
NS-Pten conditional knockout mice are known to suffer premature death (Backman et al., 2001; Kwon et al., 2001; Kwon et al., 2003) with an average lifespan of 13.1 weeks (range=7.1 to 25.3 weeks; n=13). Kwon et al. (2003) have previously shown that during the period of administration of the rapamycin analog, CCI-779 there was a reduction in premature death in the NS-Pten knockout mice. In our studies we treated with either a single two week course of rapamycin (Rapa x1) or repeated intermittent courses of rapamycin (Rapa x3) and followed the mice long-term to assess the effects of the two rapamycin treatment protocols compared to vehicle-treated and naïve knockout mice. Although a few animals with EEG implants were included in this survival study, there was no correlation between EEG implants and longevity, and mice that died as a direct result of surgery or anesthesia were not included in these analyses. We found that following a single two-week course of rapamycin treatment survival was not significantly improved in NS-Pten knockout mice as compared to both naïve and vehicle-treated controls. In fact, of the six Rapa x1 knockouts monitored for epileptiform activity, two of them died before their eleventh week and three more died before postnatal week 14, leaving just one that managed to survive for 23 weeks. Those receiving multiple two-week courses of rapamycin treatment, however, lived significantly longer than all other groups of knockouts (p<0.001 by Kaplan-Meier Logrank survival test, Figure 5). The Rapa x3 treatment group lived an average of 21 weeks, more than 50% longer than those that received just one course of rapamycin treatment, indicating that intermittent rapamycin treatment is sufficient to extend lifespan in these animals.
Rapamycin treatment has no long-lasting effects on growth in NS-Pten knockout mice
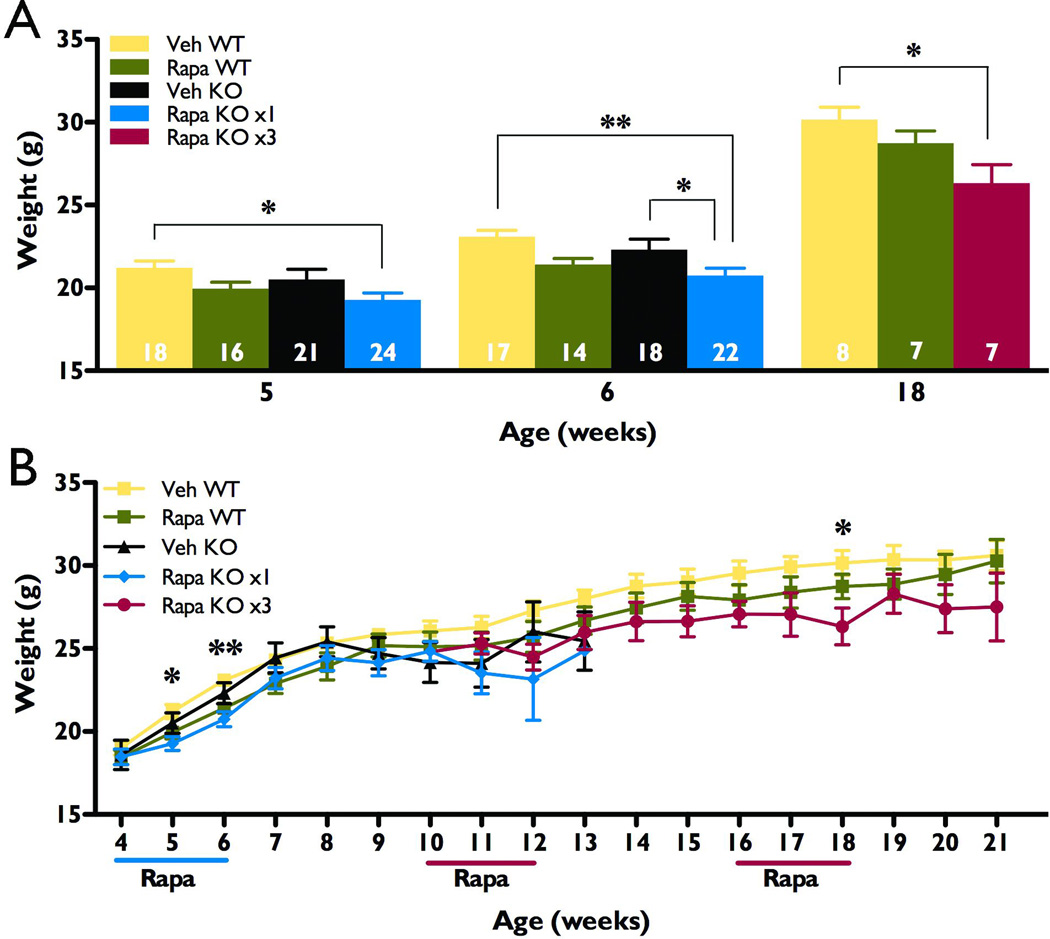
The mTORC1 pathway is a known regulator of cellular growth (reviewed in Hay & Sonenberg, 2004) and continuous rapamycin treatment in mice has previously been shown to cause a decrease in weight gain (Meikle et al., 2008; Zeng et al., 2008; Zhou et al., 2009). The impact of mTORC1 inhibition on growth is an important potential adverse effect of long-term therapy with an mTORC1 inhibitor such as rapamycin. Thus we sought to evaluate the effects of the two rapamycin protocols used in these studies on body weight, using this parameter as an index of growth. To avoid potential interactions between gender and treatment, or gender and genotype, only male mice were used for this portion of the study. Wild type and knockout males were treated with an initial course of either rapamycin or vehicle during postnatal weeks four and five. A subset of knockout mice also received additional courses of treatment during weeks 10–11 and 16–17 (Rapa KO x3). At week five, male rapamycin-treated knockouts weighed significantly less than vehicle-treated wild type controls, and by week six, weighed significantly less than the vehicle-treated knockouts, as well (p<0.05, Figure 6A). However, rapamycin treatment had no such effect on wild types and by seven weeks, no significant differences could be found between any of the groups, regardless of treatment or genotype (Figure 6B). Due to premature death of NS-Pten conditional knockouts treated with only vehicle or just one course of rapamycin, data from these two groups could not be analyzed past 13 weeks. However, except for a significant difference at 18 weeks of age (p<0.05 as compared to vehicle-treated wild types), intermittent rapamycin treatment did not significantly impact the weight of knockout males at any of these later time points (n=5–10 for all three groups at ages 14–21 weeks, Figure 6A, B). Taken together, these data indicate that while body weight and growth may be transiently affected by acute rapamycin treatment, intermittent treatment has no long-lasting effect on these parameters of growth.
Figure 6.
Intermittent rapamycin treatment had no long-lasting effect on growth as assessed by body weight measures. Wild type (WT) and NS-Pten conditional knockout (KO) male mice received either vehicle (Veh) or rapamycin (Rapa) treatment during postnatal weeks 4–5. A subset of knockout mice received additional rapamycin treatment during weeks 10–11, and 16–17. All knockouts receiving rapamycin were combined into one group up through week 10, then randomly split into Rapa KO x1 (blue) and Rapa KO x3 (red). Due to inadequate sample sizes from premature death after week 13 in the Rapa x1 and Veh knockout groups, these mice were eliminated from analyses at the later ages (n=9–24 for each group for weeks 4–7, n=3–18 for weeks 8–13, and n=5–10 for remaining treatment groups at weeks 14–21). A) Acute treatment with rapamycin during weeks 4–5 is associated with a transient decrease in the weight of knockout animals. At five weeks of age, rapamycin-treated knockouts weighed significantly less than vehicle-treated wild type controls, but not vehicle-treated knockouts. By six weeks of age, they weighed significantly less than both vehicle-treated groups. Comparisons at 18 weeks of age also revealed a significant decrease in body weight for the Rapa KO x3 group as compared to vehicle-treated wild type controls, but earlier and subsequent comparisons showed no significant difference between these groups (see Figure 7B). Sample sizes are indicated at the base of each bar. B) Rapamycin treatment had no long lasting effect on bodyweight, regardless of treatment protocol or genotype (p>0.05 across all groups at ages 7–21 weeks, except as noted in A). *p<0.05, **p<0.01.
DISCUSSION
In the studies presented here, we further characterized the effects of mTORC1 inhibition in NS-Pten conditional knockout mice. A single, two week course of rapamycin suppressed epileptiform activity and aberrant mossy fiber sprouting. However, the epileptiform activity recurred as early as four weeks after withdrawal of rapamycin treatment. Thus we established that a novel treatment protocol of intermittent two week courses of rapamycin treatment was effective at maintaining suppression of epileptiform activity and significantly extending lifespan without compromising growth.
Previous studies in mice with conditional knockouts of TSC1 or Pten utilized continuous rapamycin treatment to maintain seizure suppression, eventually leading to growth retardation (Meikle et al., 2008; Zeng et al., 2008; Zhou et al., 2009). Once treatment was stopped, the seizures recurred within weeks, similar to what we report here for the NS-Pten knockouts. However, in these studies we demonstrate that continuous treatment was not essential to maintain the suppression of epileptiform activity, and that this intermittent treatment also improved longevity. Furthermore, knockouts treated intermittently with rapamycin suffered no lasting compromise in their ability to gain and maintain a normal bodyweight, as compared to wild type mice (Figure 6A,B). This may have implications in a clinical setting, as rapamycin is not only a known growth inhibitor, but also an immunosuppressant (reviewed in Sehgal, 2003). Thus, a periodic dosing schedule may be associated with fewer side effects than traditional, continuous treatment paradigms, particularly in the pediatric population.
Exactly how mTORC1 inhibition suppresses epileptiform activity in NS-Pten knockout mice, and why the effect lasts for at least four weeks after withdrawal of the inhibitor, is still unknown. Since activation of the mTORC1 pathway is involved in both axonal growth and neuronal polarity (Choi et al., 2008; Grider et al., 2009; Morita & Sobue, 2009), as well as dendritic arborization (Jaworski et al., 2005; Kumar et al., 2005; Chow et al., 2009), one possible explanation lies in the time required to establish aberrant connections such as mossy fiber sprouting. These abnormal connections could potentially lead to activation of hyperexcitable networks. Research from human CD tissue supports this idea, as cytomegalic neurons with hyperexcitable electrical properties (Cepeda et al., 2003) not only express increased levels of mTORC1 targets (Miyata et al., 2004; Ljungberg et al., 2006), but also express markers of axonal growth and often possess a convoluted tangle of dendrites (Kerfoot et al., 1999; Cepeda et al., 2003).
One of the major findings of this study is that a brief course of treatment with rapamycin during postnatal weeks four and five successfully blocked aberrant mossy fiber sprouting. Since NS-Pten knockout mice already demonstrate mossy fiber sprouting at four weeks of age (Figure 2), these findings suggest that rapamycin does not simply prevent the formation of these abnormal projections, but may actually cause them to retract. Interestingly, Zhou et al. (2009) have already demonstrated that mTORC1 inhibition prevents mossy fiber sprouting in the Nse-Pten conditional knockout mice used in their studies; however once sprouting was well-established, reversal or suppression of this phenotype could not be achieved. Similar to the NS-Pten knockouts in this study, Nse-Pten knockout mice also lose Pten expression in dentate gyrus, however deletion is not complete until the fourth postnatal week, as opposed to within days after birth for the NS-Pten knockouts used in our study (Kwon et al., 2001; Kwon et al., 2006a; Kwon et al., 2006b). This difference in timing of Pten loss could account, in part, for the difference in effectiveness of mTORC1 inhibition to suppress mossy fiber sprouting in these models. Nse-Pten knockouts do not exhibit seizures and mossy fiber sprouting until around 10 weeks of age or later (Ogawa et al., 2007; Zhou et al., 2009), unlike in NS-Pten knockouts, which exhibit both as early as four weeks. Perhaps increased neural plasticity at younger ages allows for a better response to treatment. Another possibility for this discrepancy is the severity of mossy fiber sprouting observed in NS-Pten mice at four weeks, as compared to that of adult Nse-Pten mice. Four-week old NS-Pten knockouts are just beginning to exhibit abnormal sprouting (Figure 2), but by 10 weeks in Nse-Pten mice (the earliest age for which mossy fiber sprouting was reported), sprouting is much more obvious (Zhou et al., 2009). Further studies will be required to assess whether aberrant sprouting can still be suppressed at later stages of epileptogenesis in NS-Pten knockout mice.
The precise role of mossy fiber sprouting in epileptogenesis is highly controversial and heavily debated, however it is frequently observed in both epileptic animals and humans with TLE (reviewed in Nadler, 2003; Koyama & Ikegaya, 2004; Sutula & Dudek, 2007). The severity of sprouting has also been correlated with the progression of epilepsy (Cavazos et al., 1991; Wuarin & Dudek, 2001). Lending to the controversy, attempts to separate the two phenomena have been extremely difficult and have yielded contradictory results (Longo & Mello, 1997; Ikegaya et al., 2000; Williams et al., 2002; Buckmaster, 2004; Toyoda & Buckmaster, 2005; Ingram et al., 2009; Buckmaster & Lew, 2011). Furthermore, whereas some recent studies in models of acquired epilepsy have implicated increased activation of mTORC1 in the hippocampus as a common mediator in the development of both epilepsy and mossy fiber sprouting (Zeng et al., 2009; Huang et al., 2010), Buckmaster & Lew (2011) have demonstrated that chronic mTORC1 inhibition reduces mossy fiber sprouting without altering seizure frequency or severity. This apparent discrepancy may be explained by the modest, but not complete reduction in mossy fiber sprouting observed by Buckmaster & Lew (2011), as compared to the dramatic return to wild type levels observed in the current study. Together with the results from Zeng et al. (2009), these data suggest that even small amounts of mossy fiber sprouting may contribute to the generation of epilepsy, and that given the right circumstances, inhibition of mTORC1 signaling can successfully block the occurrence of both mossy fiber sprouting and seizures, but the precise role of mossy fiber sprouting in epilepsy remains unanswered.
It should also be noted that recent studies have indicated that high doses or long durations of treatment with rapamycin may also inhibit the other mTOR complex, mTORC2, in addition to mTORC1 (Sarbassov et al., 2006). Since mTORC2 has been shown to play a role in regulating cytoskeletal dynamics and neuronal polarity (Sarbassov et al., 2004; reviewed in Read & Gorman, 2009) it is possible that some of the effects observed with rapamycin treatment in epilepsy models are mediated at least in part, through mTORC2. In fact, studies in a different neuron-specific Pten knockout mouse (Nse-Pten) reveal that just one week of daily 10mg/kg rapamycin treatment significantly reduces phosphorylation of both mTORC1 and mTORC2 targets (Zhou et al., 2009). However, in conditional TSC1 knockout mice, a lower dose of rapamycin that does not appear to inhibit mTORC2 activity (Meikle et al., 2008) resulted in a similar decrease in cellular hypertrophy, phospho-S6 levels, and seizure activity to those observed in both Nse-Pten (Zhou et al., 2009) and NS-Pten (Ljungberg et al., 2009) conditional knockout mice. Further studies will be required to determine the precise role of each complex in the pathogenesis of epilepsy.
The findings of this study, combined with those from TSC1 conditional knockouts, Nse-Pten conditional knockouts, and acquired models of TLE, support a critical role for increased mTOR signaling in the development and progression of epilepsy. The exact molecular mechanisms underlying the role of this pathway in epilepsy are likely to be complex. Additional studies will be required to identify the downstream targets of the mTOR pathway directly responsible for establishing hyperexcitable networks in order to develop more directed therapies. For now, these findings and others support mTOR inhibition as a promising treatment in epilepsy, particularly for disorders of mTOR regulation, such as TSC and CD, and possibly also for other forms of epilepsy as well.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by NIH R01NS 39943 and 49427 (A.E.A), NIH R01NS 042616 (G.D.), T32NS 43124 and a 2011 post-doctoral fellowship from the Epilepsy Foundation (A.L.B), F32NS 56664 and a 2007 post-doctoral fellowship from the Epilepsy Foundation (J.N.L), a 2004 Research Award and a 2007 Challenge Award from the Citizens United for Research in Epilepsy (CURE; to G.D.), a 2007 pre-doctoral fellowship from the Epilepsy Foundation (C.N.S), and in part by NIH P30HD 024064 from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (D.P.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the NIH. The authors would like to thank Dr. S. Baker for the generous gift of the NS-Pten mice and Dr. J. Swann for critical reading of the manuscript.
Footnotes
DISCLOSURE OF CONFLICTS OF INTEREST
None of the authors have any conflicts of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- Anderson AE, Hrachovy RA, Swann JW. Increased susceptibility to tetanus toxin-induced seizures in immature rats. Epilepsy Res. 1997;26:433–442. doi: 10.1016/s0920-1211(96)01010-8. [DOI] [PubMed] [Google Scholar]
- Backman SA, Stambolic V, Suzuki A, Haight J, Elia A, Pretorius J, Tsao MS, Shannon P, Bolon B, Ivy GO, Mak TW. Deletion of Pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte-Duclos disease. Nat Genet. 2001;29:396–403. doi: 10.1038/ng782. [DOI] [PubMed] [Google Scholar]
- Baybis M, Yu J, Lee A, Golden JA, Weiner H, McKhann G, 2nd, Aronica E, Crino PB. mTOR cascade activation distinguishes tubers from focal cortical dysplasia. Ann Neurol. 2004;56:478–487. doi: 10.1002/ana.20211. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS. Prolonged infusion of tetrodotoxin does not block mossy fiber sprouting in pilocarpine-treated rats. Epilepsia. 2004;45:452–458. doi: 10.1111/j.0013-9580.2004.67103.x. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Ingram EA, Wen X. Inhibition of the mammalian target of rapamycin signaling pathway suppresses dentate granule cell axon sprouting in a rodent model of temporal lobe epilepsy. J Neurosci. 2009;29:8259–8269. doi: 10.1523/JNEUROSCI.4179-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Lew FH. Rapamycin suppresses mossy fiber sprouting but not seizure frequency in a mouse model of temporal lobe epilepsy. J Neurosci. 2011;31:2337–2347. doi: 10.1523/JNEUROSCI.4852-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DS, Holt CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32:1013–1026. doi: 10.1016/s0896-6273(01)00551-7. [DOI] [PubMed] [Google Scholar]
- Cavazos JE, Golarai G, Sutula TP. Mossy fiber synaptic reorganization induced by kindling: time course of development, progression, and permanence. J Neurosci. 1991;11:2795–2803. doi: 10.1523/JNEUROSCI.11-09-02795.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Hurst RS, Flores-Hernandez J, Hernandez-Echeagaray E, Klapstein GJ, Boylan MK, Calvert CR, Jocoy EL, Nguyen OK, Andre VM, Vinters HV, Ariano MA, Levine MS, Mathern GW. Morphological and electrophysiological characterization of abnormal cell types in pediatric cortical dysplasia. J Neurosci Res. 2003;72:472–486. doi: 10.1002/jnr.10604. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Di Nardo A, Kramvis I, Meikle L, Kwiatkowski DJ, Sahin M, He X. Tuberous sclerosis complex proteins control axon formation. Genes Dev. 2008;22:2485–2495. doi: 10.1101/gad.1685008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow DK, Groszer M, Pribadi M, Machniki M, Carmichael ST, Liu X, Trachtenberg JT. Laminar and compartmental regulation of dendritic growth in mature cortex. Nat Neurosci. 2009;12:116–118. doi: 10.1038/nn.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbayat-Altay E, Zeng LH, Xu L, Gutmann DH, Wong M. The natural history and treatment of epilepsy in a murine model of tuberous sclerosis. Epilepsia. 2007;48:1470–1476. doi: 10.1111/j.1528-1167.2007.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshleman JS, Carlson BL, Mladek AC, Kastner BD, Shide KL, Sarkaria JN. Inhibition of the mammalian target of rapamycin sensitizes U87 xenografts to fractionated radiation therapy. Cancer Res. 2002;62:7291–7297. [PubMed] [Google Scholar]
- European Chromosome 16 Tuberous Sclerosis Consortium. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell. 1993;75:1305–1315. doi: 10.1016/0092-8674(93)90618-z. [DOI] [PubMed] [Google Scholar]
- Grider MH, Park D, Spencer DM, Shine HD. Lipid raft-targeted Akt promotes axonal branching and growth cone expansion via mTOR and Rac1, respectively. J Neurosci Res. 2009;87:3033–3042. doi: 10.1002/jnr.22140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Zhang H, Yang J, Wu J, McMahon J, Lin Y, Cao Z, Gruenthal M, Huang Y. Pharmacological inhibition of the mammalian target of rapamycin pathway suppresses acquired epilepsy. Neurobiol Dis. 2010;40:193–199. doi: 10.1016/j.nbd.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegaya Y, Nishiyama N, Matsuki N. L-type Ca(2+) channel blocker inhibits mossy fiber sprouting and cognitive deficits following pilocarpine seizures in immature mice. Neuroscience. 2000;98:647–659. doi: 10.1016/s0306-4522(00)00188-3. [DOI] [PubMed] [Google Scholar]
- Ingram EA, Toyoda I, Wen X, Buckmaster PS. Prolonged infusion of inhibitors of calcineurin or L-type calcium channels does not block mossy fiber sprouting in a model of temporal lobe epilepsy. Epilepsia. 2009;50:56–64. doi: 10.1111/j.1528-1167.2008.01704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski J, Spangler S, Seeburg DP, Hoogenraad CC, Sheng M. Control of dendritic arborization by the phosphoinositide-3'-kinase-Akt-mammalian target of rapamycin pathway. J Neurosci. 2005;25:11300–11312. doi: 10.1523/JNEUROSCI.2270-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerfoot C, Vinters HV, Mathern GW. Cerebral cortical dysplasia: giant neurons show potential for increased excitation and axonal plasticity. Dev Neurosci. 1999;21:260–270. doi: 10.1159/000017405. [DOI] [PubMed] [Google Scholar]
- Koyama R, Ikegaya Y. Mossy fiber sprouting as a potential therapeutic target for epilepsy. Curr Neurovasc Res. 2004;1:3–10. doi: 10.2174/1567202043480242. [DOI] [PubMed] [Google Scholar]
- Kumar V, Zhang MX, Swank MW, Kunz J, Wu GY. Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways. J Neurosci. 2005;25:11288–11299. doi: 10.1523/JNEUROSCI.2284-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski DJ. Tuberous sclerosis: from tubers to mTOR. Ann Hum Genet. 2003;67:87–96. doi: 10.1046/j.1469-1809.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- Kwon CH, Zhu X, Zhang J, Knoop LL, Tharp R, Smeyne RJ, Eberhart CG, Burger PC, Baker SJ. Pten regulates neuronal soma size: a mouse model of Lhermitte-Duclos disease. Nat Genet. 2001;29:404–411. doi: 10.1038/ng781. [DOI] [PubMed] [Google Scholar]
- Kwon CH, Zhu X, Zhang J, Baker SJ. mTor is required for hypertrophy of Pten-deficient neuronal soma in vivo. Proc Natl Acad Sci U S A. 2003;100:12923–12928. doi: 10.1073/pnas.2132711100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, Li Y, Baker SJ, Parada LF. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006a;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon CH, Zhou J, Li Y, Kim KW, Hensley LL, Baker SJ, Parada LF. Neuron-specific enolase-cre mouse line with cre activity in specific neuronal populations. Genesis. 2006b;44:130–135. doi: 10.1002/gene.20197. [DOI] [PubMed] [Google Scholar]
- Ljungberg MC, Bhattacharjee MB, Lu Y, Armstrong DL, Yoshor D, Swann JW, Sheldon M, D'Arcangelo G. Activation of mammalian target of rapamycin in cytomegalic neurons of human cortical dysplasia. Ann Neurol. 2006 doi: 10.1002/ana.20949. [DOI] [PubMed] [Google Scholar]
- Ljungberg MC, Sunnen CN, Lugo JN, Anderson AE, D'Arcangelo G. Rapamycin suppresses seizures and neuronal hypertrophy in a mouse model of cortical dysplasia. Dis Model Mech. 2009;2:389–398. doi: 10.1242/dmm.002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo BM, Mello LE. Blockade of pilocarpine- or kainate-induced mossy fiber sprouting by cycloheximide does not prevent subsequent epileptogenesis in rats. Neurosci Lett. 1997;226:163–166. doi: 10.1016/s0304-3940(97)00267-x. [DOI] [PubMed] [Google Scholar]
- Meikle L, Talos DM, Onda H, Pollizzi K, Rotenberg A, Sahin M, Jensen FE, Kwiatkowski DJ. A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J Neurosci. 2007;27:5546–5558. doi: 10.1523/JNEUROSCI.5540-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle L, Pollizzi K, Egnor A, Kramvis I, Lane H, Sahin M, Kwiatkowski DJ. Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J Neurosci. 2008;28:5422–5432. doi: 10.1523/JNEUROSCI.0955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata H, Chiang AC, Vinters HV. Insulin signaling pathways in cortical dysplasia and TSC-tubers: tissue microarray analysis. Ann Neurol. 2004;56:510–519. doi: 10.1002/ana.20234. [DOI] [PubMed] [Google Scholar]
- Morita T, Sobue K. Specification of neuronal polarity regulated by local translation of CRMP2 and Tau via the mTOR-p70S6K pathway. J Biol Chem. 2009;284:27734–27745. doi: 10.1074/jbc.M109.008177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler JV. The recurrent mossy fiber pathway of the epileptic brain. Neurochem Res. 2003;28:1649–1658. doi: 10.1023/a:1026004904199. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Kwon CH, Zhou J, Koovakkattu D, Parada LF, Sinton CM. A seizure-prone phenotype is associated with altered free-running rhythm in Pten mutant mice. Brain Res. 2007;1168:112–123. doi: 10.1016/j.brainres.2007.06.074. [DOI] [PubMed] [Google Scholar]
- Orlova KA, Crino PB. The tuberous sclerosis complex. Ann N Y Acad Sci. 2010;1184:87–105. doi: 10.1111/j.1749-6632.2009.05117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read DE, Gorman AM. Involvement of Akt in neurite outgrowth. Cell Mol Life Sci. 2009;66:2975–2984. doi: 10.1007/s00018-009-0057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc. 2003;35:7S–14S. doi: 10.1016/s0041-1345(03)00211-2. [DOI] [PubMed] [Google Scholar]
- Sutula TP, Dudek FE. Unmasking recurrent excitation generated by mossy fiber sprouting in the epileptic dentate gyrus: an emergent property of a complex system. Prog Brain Res. 2007;163:541–563. doi: 10.1016/S0079-6123(07)63029-5. [DOI] [PubMed] [Google Scholar]
- Tavazoie SF, Alvarez VA, Ridenour DA, Kwiatkowski DJ, Sabatini BL. Regulation of neuronal morphology and function by the tumor suppressors Tsc1 and Tsc2. Nat Neurosci. 2005;8:1727–1734. doi: 10.1038/nn1566. [DOI] [PubMed] [Google Scholar]
- Toyoda I, Buckmaster PS. Prolonged infusion of cycloheximide does not block mossy fiber sprouting in a model of temporal lobe epilepsy. Epilepsia. 2005;46:1017–1020. doi: 10.1111/j.1528-1167.2005.04605.x. [DOI] [PubMed] [Google Scholar]
- Uhlmann EJ, Wong M, Baldwin RL, Bajenaru ML, Onda H, Kwiatkowski DJ, Yamada K, Gutmann DH. Astrocyte-specific TSC1 conditional knockout mice exhibit abnormal neuronal organization and seizures. Ann Neurol. 2002;52:285–296. doi: 10.1002/ana.10283. [DOI] [PubMed] [Google Scholar]
- van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S, Lindhout D, van den Ouweland A, Halley D, Young J, Burley M, Jeremiah S, Woodward K, Nahmias J, Fox M, Ekong R, Osborne J, Wolfe J, Povey S, Snell RG, Cheadle JP, Jones AC, Tachataki M, Ravine D, Sampson JR, Reeve MP, Richardson P, Wilmer F, Munro C, Hawkins TL, Sepp T, Ali JB, Ward S, Green AJ, Yates JR, Kwiatkowska J, Henske EP, Short MP, Haines JH, Jozwiak S, Kwiatkowski DJ. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277:805–808. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- Way SW, McKenna J, 3rd, Mietzsch U, Reith RM, Wu HC, Gambello MJ. Loss of Tsc2 in radial glia models the brain pathology of tuberous sclerosis complex in the mouse. Hum Mol Genet. 2009;18:1252–1265. doi: 10.1093/hmg/ddp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PA, Wuarin JP, Dou P, Ferraro DJ, Dudek FE. Reassessment of the effects of cycloheximide on mossy fiber sprouting and epileptogenesis in the pilocarpine model of temporal lobe epilepsy. J Neurophysiol. 2002;88:2075–2087. doi: 10.1152/jn.2002.88.4.2075. [DOI] [PubMed] [Google Scholar]
- Wuarin JP, Dudek FE. Excitatory synaptic input to granule cells increases with time after kainate treatment. J Neurophysiol. 2001;85:1067–1077. doi: 10.1152/jn.2001.85.3.1067. [DOI] [PubMed] [Google Scholar]
- Zeng LH, Xu L, Gutmann DH, Wong M. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol. 2008;63:444–453. doi: 10.1002/ana.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LH, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci. 2009;29:6964–6972. doi: 10.1523/JNEUROSCI.0066-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Blundell J, Ogawa S, Kwon CH, Zhang W, Sinton C, Powell CM, Parada LF. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice. J Neurosci. 2009;29:1773–1783. doi: 10.1523/JNEUROSCI.5685-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.