Summary
Purpose
We evaluated the ability of the ketogenic diet (KD) to improve thresholds to flurothyl-induced seizures in two mouse lines with Scn1a mutations: one that models Dravet syndrome (DS) and another that models genetic (generalized) epilepsy with febrile seizures plus (GEFS+).
Methods
At postnatal day 21, mouse models of DS and GEFS+ were fasted for 12–14 hours and then placed on either a 6:1 KD or a standard diet (SD) for two weeks. At the end of the two-week period, we measured thresholds to seizures induced by the chemiconvulsant flurothyl. Body weight, β-hydroxybutyrate (BHB) levels, and glucose levels were also recorded every two days over a two-week period in separate cohorts of mutant and wild-type mice that were either on the KD or the SD.
Key Findings
Mice on the KD gained less weight and exhibited significantly higher BHB levels compared to mice on the SD. Importantly, thresholds to flurothyl-induced seizures were restored to more normal levels in both mouse lines after two weeks on the KD.
Significance
These results indicate that the KD may be an effective treatment for refractory patients with SCN1A mutations. The availability of mouse models of DS and GEFS+ also provides an opportunity to better understand the mechanism of action of the KD, which may facilitate the development of improved treatments.
Keywords: Dravet syndrome, genetic epilepsy with febrile seizures plus, mouse models, ketogenic diet, SCN1A
Introduction
Among the growing number of epilepsy genes identified via mutation analysis studies are several members of the voltage-gated sodium channel (VGSC) gene family. The most frequently mutated VGSC gene is SCN1A, which encodes the α-subunit of the Nav1.1 channel. Mutations in SCN1A are responsible for a number of epilepsy disorders, including Dravet syndrome (also known as severe myoclonic epilepsy of infancy) and genetic (generalized) epilepsy with febrile seizures plus (GEFS+) (Escayg et al., 2000; Claes et al., 2001). Dravet syndrome (DS) is characterized by febrile seizures in the first year of life, with progression to partial and/or generalized afebrile epilepsy, moderate to severe intellectual disability, and ataxia. GEFS+ is characterized by febrile seizures that persist beyond six years of age and afebrile seizures in adulthood. DS, the more severe disorder, is often caused by loss-of-function SCN1A mutations, while GEFS+ results from subtle changes in the biophysical properties of Nav1.1 channels (Escayg & Goldin, 2010). SCN1A mutations account for approximately 85% and 10% of DS and GEFS+ cases, respectively (Lossin, 2009; Claes et al., 2009; Escayg & Goldin, 2010).
Seizures in patients with DS are often inadequately managed with available antiepileptic drugs (AEDs), and although GEFS+ is a less severe disorder, some members of GEFS+ families can also have severe, refractory epilepsy (Grant & Vazquez, 2005; Barela et al., 2006; Mahoney et al., 2009). Since many patients with SCN1A mutations do not respond to current AEDs, there is an urgent need for more effective treatments.
The ketogenic diet (KD), which is high in fat and low in carbohydrates and protein, is frequently used as an alternative treatment for refractory epilepsy. The KD causes elevated circulating levels of the C4 ketone bodies, acetoacetate and β-hydroxybutyrate (BHB), and acetone. The C4 ketone bodies are metabolized to two acetyl-CoA molecules that then enter the tricarboxylic acid (TCA) cycle to provide cellular energy. The mechanism by which the KD exerts its anticonvulsant action is not fully understood, but it has been suggested that ketosis results in more available glutamate for the glutamate decarboxylase (GAD) enzyme to use in GABA synthesis, potentially facilitating increased GABAergic inhibition (Yudkoff et al., 2005).
Analysis of the biophysical properties of dissociated cortical and hippocampal neurons from mouse models of DS and GEFS+ revealed reduced excitability of inhibitory interneurons. This reduction in inhibitory tone likely contributes to seizure generation in SCN1A-derived epilepsy (Yu et al., 2006; Ogiwara et al., 2007; Tang et al., 2009; Martin et al., 2010; Ohno et al., 2010). Since the KD may act, in part, by increasing GABAergic inhibition, we hypothesized that it would be efficacious in the treatment of SCN1A-derived epilepsy. Indeed, the administration of the KD for two weeks to mouse models of DS (Scn1a+/−) and GEFS+ (Scn1aRH/+) resulted in elevated seizure thresholds. These findings provide support for use of the KD in the treatment of DS and GEFS+ and highlights the usefulness of these mouse models to better understand the mechanism of the KD.
Materials and methods
Animals
Heterozygous Scn1a knockout mice (Scn1a+/−, a model of DS) and heterozygous knock-in mice with the human SCN1A GEFS+ mutation R1648H (Scn1aRH/+, a model of GEFS+) were generated as previously described (Yu et al., 2006; Martin et al., 2010). Scn1a+/− mice are maintained by backcrossing to the FVB/NJ background and Scn1aRH/+ mice are on a mixed 129X1/SvJ X C57BL/6J background. Wild-type (WT) littermates from each line were used as controls for all experiments to minimize variation due to differences in genetic background and rearing conditions. Mice were reared on a 12-h light/dark cycle and food and water were available ad libitum. The Emory University IACUC committee approved all experimental protocols involving mice.
Diet administration
Prior to administration of the KD, mouse pups were housed with dams that were fed a standard diet (5001; Purina Mills, St Louis, MO, USA). On postnatal day 21 (P21), mice were separated from their dams, group-housed by sex, and fasted overnight (12–14 h). The mice were then weighed and assigned to one of two diet groups: (1) DS or WT littermates (WTDS) and GEFS+ or WT littermates (WTGEFS+) on KD; (2) DS or WTDS and GEFS+ or WTGEFS+ on the standard diet (SD). We chose to administer the diets for 14 days based on previous studies that showed an anticonvulsant effect in rodents after two weeks on the KD (Appleton & DeVivo, 1974; Rho et al., 1999; Samala et al., 2008). The mice were allowed free access to water and food during the 14-day period. The KD used in the present study (6:1 ratio of fat to proteins + carbohydrates; TD.07797; Harlan Teklad, Chicago, IL, USA) has been described previously (Samala et al., 2008). The average daily consumption of each diet was determined using a separate cohort of individually housed mice (three mice per genotype) from the GEFS+ line.
Weight, β-Hydroxybutyrate (BHB) and glucose measurements
Body weight, and BHB and glucose levels were measured every two days from male mice of both lines (three mice per genotype) during the 14-day period of diet administration. Plasma BHB (mM) and glucose levels (mg/dL) were obtained from blood samples collected from the facial vein of each mouse using a test strip system and reader (Precision Xtra Advance Diabetes Management System with Precision Xtra blood ketone test strips and blood glucose test strips; Abbott Diabetes Care Inc., Alameda, CA, USA).
Flurothyl seizure induction
We measured latencies to flurothyl-induced seizures in additional cohorts of mice from each line that had been on either the KD or SD for 14 days. Mice were placed in a clear Plexiglas chamber, and flurothyl (2,2,2-trifluroethylether; Sigma-Aldrich, St Louis, MO, USA) was slowly dripped into the chamber via a syringe pump at a rate of 20 µl/min and allowed to volatilize. Thresholds to flurothyl-induced seizures are typically determined by measuring latencies to the first myoclonic jerk (MJ) and to the generalized tonic-clonic seizure (GTCS). However, previously we saw no differences in latencies to the MJ in the Scn1a mutants compared with WT littermates (Martin et al., 2007; Martin et al., 2010), so we chose not to evaluate the latency to the MJ in the present study. The GTCS is characterized by convulsions of the entire body and a loss of posture. Data from males and females were analyzed separately, but no sex differences were seen; therefore, the data from both sexes were combined.
Statistics
A repeated measures ANOVA (rANOVA) was used to detect differences in body weight, and plasma BHB and glucose levels between genotypes and diet groups over the 14-day period of diet administration. A two-way ANOVA was used to evaluate latencies to GTCS between and within genotypes and diet groups. When statistically significant differences were found, Tukey’s post hoc test was used for pairwise comparisons of the means. Differences were considered statistically significant when the probability of error was less than 0.05 (p < 0.05). All values are expressed as mean ± standard error of the mean (SEM).
Results
Mice on the KD show less weight gain
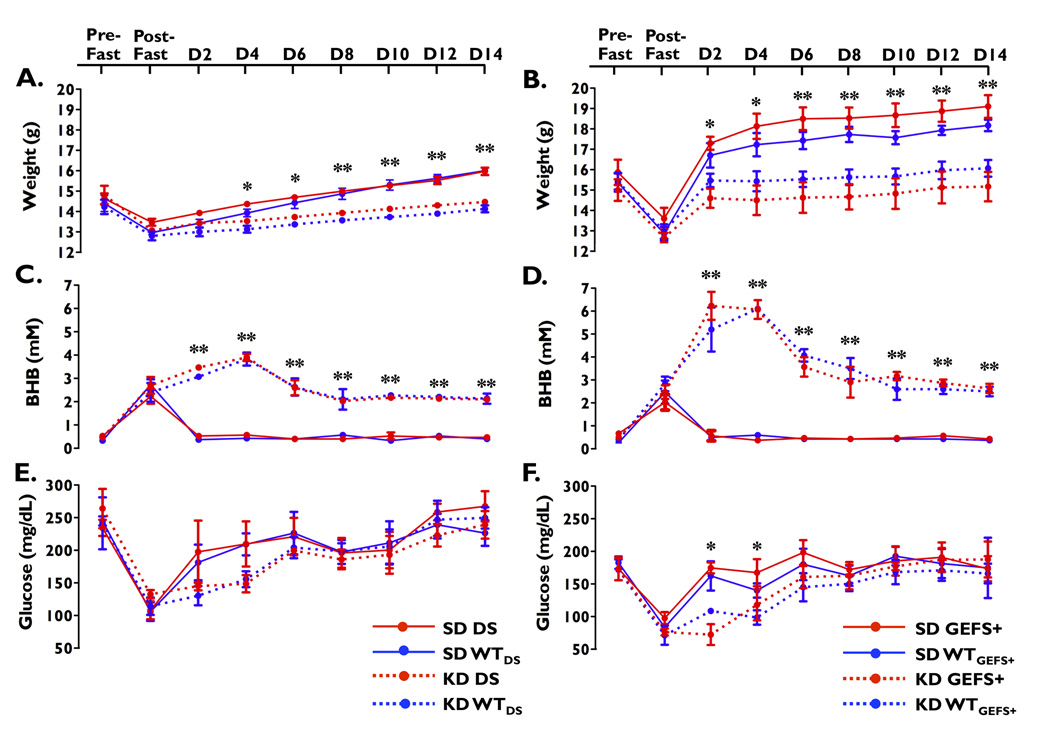
Both mutants (DS and GEFS+) and their respective WT littermates lost similar amounts of weight after the overnight fast (Figure 1A–B). There were no statistically significant differences in average weight gain between mutants (DS and GEFS+) and their respective WT littermates on the same diet during the 14-day period, indicating that weight gain was not affected by the heterozygous Scn1a mutations. Analysis of daily food consumption from singularly housed mice from the GEFS+ line showed that there was no difference in the amount of food consumed between the GEFS+ mutants and their WTGEFS+ littermates within either the SD or the KD groups. However, mice on the KD consumed less food and consequently fewer calories (KD: 12.4 ± 0.2 kcal vs. SD: 17.9 ± 0.2 kcal). As a result, the type of diet had a major effect on weight gain: mice from both lines gained significantly more weight on the SD compared with mice on the KD, regardless of genotype (DS line: main effect of diet: F(1,8) = 37.8, p < 0.001; GEFS+ line: main effect of diet: F(1,8) = 22.0, p < 0.001).
Figure 1.
Changes in weight, BHB and glucose levels. (A, B) Mice on the KD gained less weight than mice on the SD. (C, D) Mice on the KD had higher BHB levels than those on the SD. (E, F) While glucose levels in mice from the DS line on the KD remained similar to mice on the SD (E), mice from the GEFS+ line on the KD had lower average glucose levels than mice on the SD (F). * p < 0.005 between diet groups (SD vs. KD). **p < 0.001 between diet groups (SD vs. KD). Three mice of each genotype on each diet were evaluated at each time point. Error bars represent mean ± SEM.
Mice on the KD show increased BHB levels
To determine the level of ketosis induced by the diets, plasma BHB levels were examined throughout the 14-day period. After the overnight fast, both mutants (DS and GEFS+) and their respective WT littermates showed an increase in BHB levels (Figure 1C–D). During the 14-day period, there were no statistically significant differences in average BHB levels between DS and GEFS+ mutants and their respective WT littermates that were on the same diet, indicating that the Scn1a mutations do not affect ketone metabolism. However, for both lines, average BHB levels differed significantly between SD- and KD-fed mice (DS line: main effect of diet: F(1,8) = 251.8, p < 0.0001; GEFS+ line: main effect of diet: F(1,8) = 555.9, p < 0.001). BHB levels peaked within four days, and then began to decline for both mutants and WT littermates on the KD; however, plasma BHB levels did not decline to baseline levels and remained elevated for the duration of the study in all mice on the KD compared with groups on the SD (Figure 1C–D).
Variable affect of the KD on glucose levels
Mice within each line showed a decrease in glucose levels following the overnight fast (Figure 1E–F). During the 14-day period, there were no statistically significant differences in average glucose levels between mutants (DS and GEFS+) and their respective WT littermates that were on the same diet, indicating that the Scn1a mutations do not affect glucose metabolism. However, the effect of the diet on glucose levels differed between the lines. While the DS line showed no statistically significant differences in average glucose levels between SD- and KD-fed mice (main effect of diet: F(1,8) = 2.8, p = 0.13), GEFS+ mutants and WTGEFS+ littermates on the KD showed significantly lower average glucose levels compared to levels on the SD (main effect of diet: F(1,8) = 7.9, p < 0.05) (Figure 1E–F). Post-hoc analysis revealed statistically significant hypoglycemia on days 2 and 4 in KD-fed mice from the GEFS+ line (Figure 1F).
The KD elevates seizure thresholds
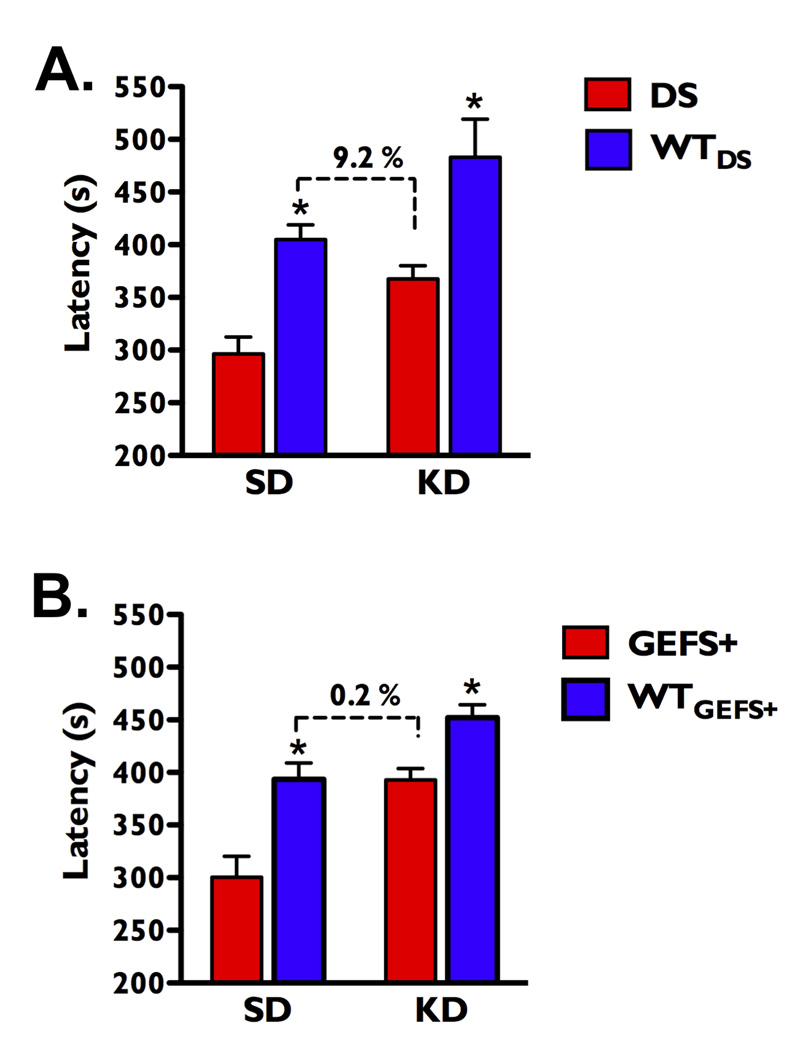
The average latency to flurothyl-induced GTCS in DS mice on the SD was 26.7% lower than WTDS littermates on the SD (main effect of genotype: F(1,99) = 16.3, p < 0.005, Figure 2A), and the average latency to the GTCS in GEFS+ mice on the SD was 23.7% lower than WTGEFS+ littermates on the SD (main effect of genotype: F(1,97) = 28.0, p < 0.001; Figure 2B). The KD was successful in significantly increasing the average latency to GTCS in both mutants and WT littermates from the DS line (main effect of diet: F(1,99) = 10.2, p < 0.002, Figure 2A) and the GEFS+ line (main effect of diet: F(1,97) = 28.0, p < 0.001, Figure 2B).
Figure 2.
The KD elevates seizure thresholds in Scn1a mutant mice. (A) On the SD, the average latency to flurothyl-induced GTCS was 26.7% lower in DS mutants compared with WTDS littermates. After 14 days on the KD, the average seizure latency of DS mice was 16% lower than WTDS littermates. Average seizure latency of the DS mutants on the KD was 9.2% lower than WTDS on the SD. n = 24–31 mice in each group. (B) On the SD, the average latency to the GTCS was 23.7% lower in the GEFS+ mice compared with WTGEFS+ littermates. After 14 days on the KD, average seizure latency of the GEFS+ mice was 13% lower than WTGEFS+ littermates. Average latency to the GTCS in the GEFS+ mutants on the KD was comparable to WTGEFS+ littermates on the SD. n = 20–29 mice per genotype on each diet. *p < 0.001 for mutants vs. WT littermates within a diet group. Error bars represent mean ± SEM.
To determine whether the KD could elevate thresholds to flurothyl-induced GTCS in the mutants to that of WT littermates on the SD, we compared the average latencies to GTCS of the mutant mice on the KD to their respective WT littermates on the SD. The average latency for DS mice on the KD (368 ± 13 s) was 9.2% lower than WTDS mice on the SD (405 ± 14 s), which is not a statistically significant difference (Tukeys post-hoc, p > 0.05, Figure 2A). Likewise, the average latency for GEFS+ mice on the KD (393 ± 11 s) was almost identical to the WTGEFS+ mice on the SD (394 ± 16 s, Tukeys post-hoc, p > 0.05, Figure 2B). Thus, the KD increased thresholds to flurothyl-induced GTCS in both Scn1a mutants to the levels seen in their respective WT littermates on the SD.
Discussion
Under normal physiological conditions, cells derive most of their energy from glucose or other carbohydrates, but when carbohydrates are not readily available, as happens during the KD or fasting, blood glucose levels fall, and cells rely on ketone bodies such as BHB for energy. Consumption of the KD typically leads to less weight gain compared to subjects on normal diets (Thio et al., 2006). In addition, robust elevations in plasma BHB levels are usually observed during KD administration (Bough et al., 1999; Thavendiranathan et al., 2003), and higher ratios of fat:carbohydrate in the KD result in greater anticonvulsant effect (Livingston & Berman, 1972; Bough et al., 2000). The transition from carbohydrates to ketones as the primary energy source is thought to contribute to the anticonvulsant mechanism of the KD (Schwartzkroin, 1999). As a result, clinical and experimental studies suggest that reduced body weight, increased plasma BHB levels and reduced glucose levels may be indicators of KD efficacy.
To compare these expected metabolic endpoints with actual changes seen in our mice, we measured weight and plasma BHB and glucose levels every two days during the administration of the diet in both Scn1a mutants and their respective WT littermates. In agreement with clinical and experimental observations, KD-fed mice from both lines gained less weight compared to littermates on the SD. Also as expected, all mice on the KD exhibited higher BHB levels compared to littermates on the SD. However, although average pre-fast BHB levels were similar for both lines, during KD administration we saw higher peak and average BHB levels in mice from the GEFS+ line. The KD also resulted in a statistically significant reduction in plasma glucose levels on days 2 and 4 in mice from the GEFS+ line, while glucose levels were not significantly altered in mice from the DS line. Since the Scn1a mutations do not appear to affect BHB or glucose metabolism, the observed differences in these parameters between the two lines likely reflect the contribution of the different genetic backgrounds to KD metabolism. We previously demonstrated that the efficacy of the KD in mice is influenced by genetic background (Dutton & Escayg, 2008).
Despite the ability of the KD to cause a greater degree of ketonemia in the GEFS+ mice, the average latency to flurothyl-induced GTCS was successfully elevated in both mutants. The average latency of the GEFS+ mice on the KD was almost identical to WTGEFS+ mice on the SD (0.2% difference). DS mice on the KD had thresholds that differed from WTDS mice on the SD by 9.2%; however, this difference was not statistically significant. We chose to induce seizures with flurothyl because it provides a sensitive and reliable method of examining seizure susceptibility in mice and we have previously demonstrated that both Scn1a mutants have reduced thresholds to this chemiconvulsant compared to their respective WT littermates (Martin et al., 2007; Martin et al., 2010; Hawkins et al., 2011). Furthermore, the KD has been shown to be effective at increasing thresholds to flurothyl-induced seizures in some inbred strains of mice (Rho et al., 1999; Dutton & Escayg, 2008) as well as heterozygous dopamine β-hydroxylase knockout mice (Szot et al., 2001).
Mice on the KD showed an approximately 31% reduction in calorie intake when compared to those on the SD, indicating that the KD may have resulted in moderate calorie restriction. However, food consumption was based on measurements from individually housed mice which typically do not feed as well as group housed mice. Therefore, a more modest level of calorie restriction would likely have occurred in the group-housed mice that were used for measurements of metabolic endpoints and determination of seizure thresholds. Nevertheless, there is data to suggest that calorie restriction may contribute to the anticonvulsant mechanism of the KD. Similar to our findings, Samala et al. (2008) found that without intentionally restricting food intake, mice on the 6:1 KD gained less weight. In addition, calorie-restricted standard and ketogenic diets were shown to be anticonvulsive compared to unrestricted diets in EL mice (Greene et al., 2001; Mantis et al., 2004). As a result, we cannot exclude the possibility that calorie restriction may have contributed to the effects of the KD in our mice.
The exact mechanism by which the KD produces its anticonvulsant effect is unclear, although several mechanisms have been hypothesized. Proposed mechanisms include direct modulation of ion channels via ketone bodies (Likhodii et al., 2008) activation of ATP-sensitive potassium (KATP) channels via glucose restriction (Ma et al., 2007), increased noradrenergic tone (Szot et al., 2001), and increased GABAergic inhibitory signaling (Erecinska et al., 1996; Yudkoff et al., 1997). Ketone bodies have also been reported to increase the amount of GABA in synaptosomes isolated from rat brains (Erecinska et al., 1996; Yudkoff et al., 1997), and elevated GAD expression levels are seen in forebrain and cerebellar homogenates of calorie-restricted and KD-fed mice (Cheng et al., 2004). In addition, two clinical studies report significant increases in GABA levels in cerebrospinal fluid (CSF) following KD administration, further supporting the hypothesis that the KD may be functioning through enhanced GABAergic inhibition (Wang et al., 2003; Dahlin et al., 2005). Conversely, in several studies, brain GABA levels were unchanged in KD-fed mice and rats (Appleton & DeVivo, 1974; Al-Mudallal et al., 1996; Melo et al., 2006). In addition, Dahlin et al. (2005) found elevated CSF GABA levels only when the KD was administered to children less than 5.5 years of age; however, the KD is known to be effective in patients of all ages (Sirven et al., 1999). Since Scn1a mutants are predicted to have reduced GABAergic inhibitory signaling, we speculate that the restoration of more normal seizure thresholds by the KD is due, in part, to enhancement of the GABAergic system. Nevertheless, because the exact mechanism of the KD remains unclear, it may be that multiple systems are working synergistically to improve seizure thresholds in the Scn1a mutant mice.
To date, the efficacy of the KD in DS patients has been examined in a small number of clinical studies (Caraballo et al., 2005; Caraballo & Fejerman, 2006; Kroll-Seger et al., 2006; Korff et al., 2007; Dressler et al., 2010). In one study, 77% of DS patients on the KD saw their seizure frequency decrease by over 75% (Caraballo & Fejerman, 2006); however, whether these patients were screened for SCN1A mutations is unknown. Similarly, Dressler at al. (2010) reported favorable treatment outcomes in 62.5% of DS on the KD; likewise, it is unknown whether these patients were screened for SCN1A mutations. Korff et al. (2007) performed a retrospective study on 16 patients with DS, in which six patients were treated with the KD. Of the 16 patients, six tested positive for SCN1A mutations, but it is unclear whether the KD was administered to the patients with SCN1A mutations. Nevertheless, taken together, the published clinical data and the results presented in the current study provide support for the potential benefit of using the KD in the treatment of patients with AED-resistant SCN1A-derived epilepsy, but prospective studies on well-defined groups of patients with known SCN1A mutations are required to firmly establish the efficacy of this treatment. In light of the influence of genetic background seen in both the current study and in our prior study (Dutton & Escayg, 2008), genetic variation at other loci in patients with SCN1A mutations is also likely to affect the magnitude of the protective effect conferred by the KD. Finally, the availability of mouse models of SCN1A dysfunction that respond positively to the KD now provides additional opportunities to explore the mechanism of action of the KD and to develop improved treatment strategies for these severe disorders.
Acknowledgements
We would like to thank Cheryl Strauss for editorial assistance. This study was supported by grants from the NIH (R21 NS06615 to AE, R01 NS25704 to WAC, 1F31 NS065694 to SBBD, and K01 NS062862 to FK) and by a predoctoral fellowship from the Epilepsy Foundation (SBBD).
Footnotes
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosure of conflicts of interest: Andrew Escayg has licensed the mouse model of GEFS+ described in this manuscript to Allergan. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policy. The remaining authors have no conflict of interest to disclose.
References
- Al-Mudallal AS, LaManna JC, Lust WD, Harik SI. Diet-induced ketosis does not cause cerebral acidosis. Epilepsia. 1996;37:258–261. doi: 10.1111/j.1528-1157.1996.tb00022.x. [DOI] [PubMed] [Google Scholar]
- Appleton DB, DeVivo DC. An animal model for the ketogenic diet. Epilepsia. 1974;15:211–227. doi: 10.1111/j.1528-1157.1974.tb04943.x. [DOI] [PubMed] [Google Scholar]
- Barela AJ, Waddy SP, Lickfett JG, Hunter J, Anido A, Helmers SL, Goldin AL, Escayg A. An epilepsy mutation in the sodium channel SCN1A that decreases channel excitability. J Neurosci. 2006;26:2714–2723. doi: 10.1523/JNEUROSCI.2977-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bough KJ, Valiyil R, Han FT, Eagles DA. Seizure resistance is dependent upon age and calorie restriction in rats fed a ketogenic diet. Epilepsy Res. 1999;35:21–28. doi: 10.1016/s0920-1211(98)00125-9. [DOI] [PubMed] [Google Scholar]
- Bough KJ, Yao SG, Eagles DA. Higher ketogenic diet ratios confer protection from seizures without neurotoxicity. Epilepsy Res. 2000;38:15–25. doi: 10.1016/s0920-1211(99)00077-7. [DOI] [PubMed] [Google Scholar]
- Caraballo RH, Cersosimo RO, Sakr D, Cresta A, Escobal N, Fejerman N. Ketogenic diet in patients with Dravet syndrome. Epilepsia. 2005;46:1539–1544. doi: 10.1111/j.1528-1167.2005.05705.x. [DOI] [PubMed] [Google Scholar]
- Caraballo RH, Fejerman N. Dravet syndrome: a study of 53 patients. Epilepsy Res. 2006;70 Suppl 1:S231–S238. doi: 10.1016/j.eplepsyres.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Cheng CM, Hicks K, Wang J, Eagles DA, Bondy CA. Caloric restriction augments brain glutamic acid decarboxylase-65 and -67 expression. J Neurosci Res. 2004;77:270–276. doi: 10.1002/jnr.20144. [DOI] [PubMed] [Google Scholar]
- Claes L, Del-Favero J, Ceulemans B, Lagae L, Van Broeckhoven C, De Jonghe P. De novo mutations in the sodium-channel gene SCN1A cause severe myoclonic epilepsy of infancy. Am J Hum Genet. 2001;68:1327–1332. doi: 10.1086/320609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes LR, Deprez L, Suls A, Baets J, Smets K, Van Dyck T, Deconinck T, Jordanova A, De Jonghe P. The SCN1A variant database: a novel research and diagnostic tool. Hum Mutat. 2009;30:E904–E920. doi: 10.1002/humu.21083. [DOI] [PubMed] [Google Scholar]
- Dahlin M, Elfving A, Ungerstedt U, Amark P. The ketogenic diet influences the levels of excitatory and inhibitory amino acids in the CSF in children with refractory epilepsy. Epilepsy Res. 2005;64:115–125. doi: 10.1016/j.eplepsyres.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Dressler A, Stocklin B, Reithofer E, Benninger F, Freilinger M, Hauser E, Reiter-Fink E, Seidl R, Trimmel-Schwahofer P, Feucht M. Long-term outcome and tolerability of the ketogenic diet in drug-resistant childhood epilepsy--the Austrian experience. Seizure. 2010;19:404–408. doi: 10.1016/j.seizure.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Dutton SB, Escayg A. Genetic influences on ketogenic diet efficacy. Epilepsia. 2008;49 Suppl 8:67–69. doi: 10.1111/j.1528-1167.2008.01839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecinska M, Nelson D, Daikhin Y, Yudkoff M. Regulation of GABA level in rat brain synaptosomes: fluxes through enzymes of the GABA shunt and effects of glutamate, calcium, and ketone bodies. J Neurochem. 1996;67:2325–2334. doi: 10.1046/j.1471-4159.1996.67062325.x. [DOI] [PubMed] [Google Scholar]
- Escayg A, MacDonald BT, Meisler MH, Baulac S, Huberfeld G, An-Gourfinkel I, Brice A, LeGuern E, Moulard B, Chaigne D, Buresi C, Malafosse A. Mutations of SCN1A, encoding a neuronal sodium channel, in two families with GEFS+2. Nat Genet. 2000;24:343–345. doi: 10.1038/74159. [DOI] [PubMed] [Google Scholar]
- Escayg A, Goldin AL. Sodium channel Scn1a and epilepsy: mutations and mechanisms. Epilepsia. 2010;51:1650–1658. doi: 10.1111/j.1528-1167.2010.02640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant AC, Vazquez B. A case of extended spectrum GEFS+ Epilepsia. 2005;46 Suppl 10:39–40. doi: 10.1111/j.1528-1167.2005.00357.x. [DOI] [PubMed] [Google Scholar]
- Greene AE, Todorova MT, McGowan R, Seyfried TN. Caloric restriction inhibits seizure susceptibility in epileptic EL mice by reducing blood glucose. Epilepsia. 2001;42:1371–1378. doi: 10.1046/j.1528-1157.2001.17601.x. [DOI] [PubMed] [Google Scholar]
- Hawkins NA, Martin MS, Frankel WN, Kearney JA, Escayg A. Neuronal voltage-gated ion channels are genetic modifiers of generalized epilepsy with febrile seizures plus. Neurobiol Dis. 2011;41:655–660. doi: 10.1016/j.nbd.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korff C, Laux L, Kelley K, Goldstein J, Koh S, Nordli D., Jr Dravet syndrome (severe myoclonic epilepsy in infancy): a retrospective study of 16 patients. J Child Neurol. 2007;22:185–194. doi: 10.1177/0883073807300294. [DOI] [PubMed] [Google Scholar]
- Kroll-Seger J, Portilla P, Dulac O, Chiron C. Topiramate in the treatment of highly refractory patients with Dravet syndrome. Neuropediatrics. 2006;37:325–329. doi: 10.1055/s-2007-964867. [DOI] [PubMed] [Google Scholar]
- Likhodii S, Nylen K, Burnham WM. Acetone as an anticonvulsant. Epilepsia. 2008;49 Suppl 8:83–86. doi: 10.1111/j.1528-1167.2008.01844.x. [DOI] [PubMed] [Google Scholar]
- Livingston S, Berman W. Checking compliance of epileptic patients. N Engl J Med. 1972;287:934. doi: 10.1056/nejm197211022871818. [DOI] [PubMed] [Google Scholar]
- Lossin C. A catalog of SCN1A variants. Brain Dev. 2009;31:114–130. doi: 10.1016/j.braindev.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Ma W, Berg J, Yellen G. Ketogenic diet metabolites reduce firing in central neurons by opening K(ATP) channels. J Neurosci. 2007;27:3618–3625. doi: 10.1523/JNEUROSCI.0132-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney K, Moore SJ, Buckley D, Alam M, Parfrey P, Penney S, Merner N, Hodgkinson K, Young TL. Variable neurologic phenotype in a GEFS+ family with a novel mutation in SCN1A. Seizure. 2009;18:492–497. doi: 10.1016/j.seizure.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Mantis JG, Centeno NA, Todorova MT, McGowan R, Seyfried TN. Management of multifactorial idiopathic epilepsy in EL mice with caloric restriction and the ketogenic diet: role of glucose and ketone bodies. Nutr Metab (Lond) 2004;1:11. doi: 10.1186/1743-7075-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MS, Tang B, Papale LA, Yu FH, Catterall WA, Escayg A. The voltage-gated sodium channel Scn8a is a genetic modifier of severe myoclonic epilepsy of infancy. Hum Mol Genet. 2007;16:2892–2899. doi: 10.1093/hmg/ddm248. [DOI] [PubMed] [Google Scholar]
- Martin MS, Dutt K, Papale LA, Dube CM, Dutton SB, de Haan G, Shankar A, Tufik S, Meisler MH, Baram TZ, Goldin AL, Escayg A. Altered function of the SCN1A voltage-gated sodium channel leads to gamma-aminobutyric acid-ergic (GABAergic) interneuron abnormalities. J Biol Chem. 2010;285:9823–9834. doi: 10.1074/jbc.M109.078568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo TM, Nehlig A, Sonnewald U. Neuronal-glial interactions in rats fed a ketogenic diet. Neurochem Int. 2006;48:498–507. doi: 10.1016/j.neuint.2005.12.037. [DOI] [PubMed] [Google Scholar]
- Ogiwara I, Miyamoto H, Morita N, Atapour N, Mazaki E, Inoue I, Takeuchi T, Itohara S, Yanagawa Y, Obata K, Furuichi T, Hensch TK, Yamakawa K. Na(v)1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J Neurosci. 2007;27:5903–5914. doi: 10.1523/JNEUROSCI.5270-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno Y, Sofue N, Ishihara S, Mashimo T, Sasa M, Serikawa T. Scn1a missense mutation impairs GABAA receptor-mediated synaptic transmission in the rat hippocampus. Biochem Biophys Res Commun. 2010;400:117–122. doi: 10.1016/j.bbrc.2010.08.021. [DOI] [PubMed] [Google Scholar]
- Rho JM, Kim DW, Robbins CA, Anderson GD, Schwartzkroin PA. Age-dependent differences in flurothyl seizure sensitivity in mice treated with a ketogenic diet. Epilepsy Res. 1999;37:233–240. doi: 10.1016/s0920-1211(99)00068-6. [DOI] [PubMed] [Google Scholar]
- Samala R, Willis S, Borges K. Anticonvulsant profile of a balanced ketogenic diet in acute mouse seizure models. Epilepsy Res. 2008;81:119–127. doi: 10.1016/j.eplepsyres.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA. Mechanisms underlying the anti-epileptic efficacy of the ketogenic diet. Epilepsy Res. 1999;37:171–180. doi: 10.1016/s0920-1211(99)00069-8. [DOI] [PubMed] [Google Scholar]
- Sirven J, Whedon B, Caplan D, Liporace J, Glosser D, O'Dwyer J, Sperling MR. The ketogenic diet for intractable epilepsy in adults: preliminary results. Epilepsia. 1999;40:1721–1726. doi: 10.1111/j.1528-1157.1999.tb01589.x. [DOI] [PubMed] [Google Scholar]
- Szot P, Weinshenker D, Rho JM, Storey TW, Schwartzkroin PA. Norepinephrine is required for the anticonvulsant effect of the ketogenic diet. Brain Res Dev Brain Res. 2001;129:211–214. doi: 10.1016/s0165-3806(01)00213-9. [DOI] [PubMed] [Google Scholar]
- Tang B, Dutt K, Papale L, Rusconi R, Shankar A, Hunter J, Tufik S, Yu FH, Catterall WA, Mantegazza M, Goldin AL, Escayg A. A BAC transgenic mouse model reveals neuron subtype-specific effects of a Generalized Epilepsy with Febrile Seizures Plus (GEFS+) mutation. Neurobiol Dis. 2009;35:91–102. doi: 10.1016/j.nbd.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thavendiranathan P, Chow C, Cunnane S, McIntyre Burnham W. The effect of the 'classic' ketogenic diet on animal seizure models. Brain Res. 2003;959:206–213. doi: 10.1016/s0006-8993(02)03744-7. [DOI] [PubMed] [Google Scholar]
- Thio LL, Erbayat-Altay E, Rensing N, Yamada KA. Leptin contributes to slower weight gain in juvenile rodents on a ketogenic diet. Pediatr Res. 2006;60:413–417. doi: 10.1203/01.pdr.0000238244.54610.27. [DOI] [PubMed] [Google Scholar]
- Wang ZJ, Bergqvist C, Hunter JV, Jin D, Wang DJ, Wehrli S, Zimmerman RA. In vivo measurement of brain metabolites using two-dimensional double-quantum MR spectroscopy--exploration of GABA levels in a ketogenic diet. Magn Reson Med. 2003;49:615–619. doi: 10.1002/mrm.10429. [DOI] [PubMed] [Google Scholar]
- Yu FH, Mantegazza M, Westenbroek RE, Robbins CA, Kalume F, Burton KA, Spain WJ, McKnight GS, Scheuer T, Catterall WA. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat Neurosci. 2006;9:1142–1149. doi: 10.1038/nn1754. [DOI] [PubMed] [Google Scholar]
- Yudkoff M, Daikhin Y, Nissim I, Grunstein R. Effects of ketone bodies on astrocyte amino acid metabolism. J Neurochem. 1997;69:682–692. doi: 10.1046/j.1471-4159.1997.69020682.x. [DOI] [PubMed] [Google Scholar]
- Yudkoff M, Daikhin Y, Nissim I, Horyn O, Lazarow A, Luhovyy B, Wehrli S. Response of brain amino acid metabolism to ketosis. Neurochem Int. 2005;47:119–128. doi: 10.1016/j.neuint.2005.04.014. [DOI] [PubMed] [Google Scholar]