Abstract
Background
Cortical electrical stimulation (CES) techniques are practical tools in neurorehabilitation that are currently being used to test models of functional recovery after neurologic injury. However, the mechanisms by which CES has therapeutic effects, are not fully understood.
Objective
In this study, we investigated the effects of CES on unit activity of different neuronal elements in layers of rat primary motor cortex after the offset of stimulation. We evaluated the effects of monopolar CES pulse polarity (anodic-first versus cathodic-first) using various stimulation frequencies and amplitudes on unit activity after stimulation.
Methods
A penetrating single shank silicon microelectrode array enabled us to span the entirety of six layer motor cortex allowing simultaneous electrophysiologic recordings from different depths after monopolar CES. Neural spiking activity before the onset and after the offset of CES was modeled using point processes fit to capture neural spiking dynamics as a function of extrinsic stimuli based on generalized linear model methods.
Results
We found that neurons in lower layers have a higher probability of being excited after anodic CES. Conversely, neurons located in upper cortical layers have a higher probability of being excited after cathodic stimulation. The opposing effects observed following anodic versus cathodic stimulation in upper and lower layers were frequency- and amplitude-dependent.
Conclusions
The data demonstrates that the poststimulus changes in neural activity after manipulation of CES parameters changes according to the location (depth) of the recorded units in rat primary motor cortex. The most effective pulse polarity for eliciting action potentials after stimulation in lower layers was not as effective in upper layers. Likewise, lower amplitudes and frequencies of CES were more effective than higher amplitudes and frequencies for eliciting action potentials. These results have important implications in the context of maximizing efficacy of CES for neurorehabilitation and neuroprosthetic applications.
Keywords: cortical electrical stimulation, anodic, cathodic, penetrating electrode array, rat primary motor cortex
Cortical electrical stimulation (CES) has been used extensively in experimental neuroscience to modulate neuronal or behavioral activity that has led this technique to be considered in neurorehabilitation. In healthy volunteers, cortical stimulation techniques have been shown to affect a wide variety of cortical systems, enhancing the beneficial effects of motor training,1,2 implicit motor learning,3 skilled finger movements,4 probabilistic classification learning,5 working memory,6 and sleep-dependent consolidation of declarative memories.7 Cortical stimulation techniques for use as neuroprosthetics have shown evidence for enhanced recovery from stroke,8 suppression of seizures in epilepsy,9 amelioration of chronic neuropathic pain,10 improved motor symptoms in Parkinson’s disease,11 and reduced depression.12 The mechanisms by which cortical stimulation has therapeutic effects is not fully understood. Because the cortex and the surrounding anatomy have irregular geometries and inhomogeneous and anisotropic electrical properties, the distribution of electric field and current density generated during cortical stimulation cannot be easily predicted. It is also unclear how the distribution of the electric field and current affect the different neuronal elements in the cortex, because cortical neurons vary in shape, size, location, and orientation.13,14 Although there have been several modeling studies on current flow and neuronal polarization in the cortex,14-18 there is little knowledge on the effects of CES in vivo. The therapeutic effects of CES may be improved when the stimulation parameters (polarity, frequency, and amplitude) can be optimized based on knowledge of the neuronal elements to be targeted.
Modeling studies have shown that although neuronal elements perpendicular to the electrode surface are preferentially excited by anodic stimulation, cathodic stimulation excites those with a direction component parallel to its surface.14,15 In this study, we investigated these effects after the offset of stimulation on unit activity of different neuronal elements in rat primary motor cortex by changing the stimulation pulse polarity in various frequencies and amplitudes. A penetrating single shank silicon microelectrode array enabled us to span the entirety of six layer cortex allowing simultaneous electrophysiologic recordings from different depths19 after monopolar CES. Neural spiking activity before the onset and after the offset of CES was modeled using point processes fit to the neural spike trains based on generalized linear model methods.20 The models characterized the differences in spiking propensity to capture temporal variations in neural spiking after the covariation of CES parameters. Our results agree with previous modeling studies and show an increase in unit activity after anodic stimulation and a decrease in unit activity after cathodic stimulation in lower layers (V and VI) units after the offset of stimulation. The opposite poststimulus effect was seen for the units in upper cortical layers (I and II). These opposing effects observed after anodic versus cathodic stimulation in upper and lower layers were frequency and amplitude dependent.
Materials and Methods
Subjects
Six normal male rats (Charles River Laboratories, Wilmington, MA) were implanted with two bone screws used to deliver (source) monopolar CES to primary motor cortex as well as a much larger current return (sink). A microscale penetrating electrode array (NeuroNexus Technologies, Ann Arbor, MI) consisting of 16-electrodes spaced 100 μm apart (Figure 1A) was implanted to span the entirety of six-layer motor cortex.19 This electrode array was implanted to record extracellular action potentials from the forelimb representation of primary motor cortex and was angled toward the cortical stimulation electrode such that extracellular action potentials had a higher probability of being affected by the cortical stimulation (Figure 1B).
Figure 1.
A, Horizontal schematic of the rat skull showing the locations of craniotomy, implanted CES screws and penetrating probe. The current sink was shorted to a much larger bone screw (depicted). Rostral is to the right. B, Conceptual cross-section in the saggital plane of the implanted probe enlarged from the gray box in (A) (rostral is to the right). The penetrating electrode was angled toward the cortical stimulation electrode such that recorded extracellular action potentials had a high probability of being affected by the cortical stimulation (electrodes are on the side of the silicon shank that faces the current source). C, Pulse shapes: Constant current CES was delivered in two configurations, cathodic-first or anodic-first consisting of pulse trains. Pulses consisted of square leading phase (100 μs), followed by an exponentially decaying second phase to balance charge. The pulse width of the leading phase was fixed at 100 μs and the length of the trailing phase varied according to leading phase current to balance the charge. D, Time intervals Ii,a(t) or Ii,f(t) for i=1,2,3,4,5 with respect to stimulation time. Stimulation starts at time 0 and end at 1000 milliseconds. i=1 corresponds to 500 milliseconds before the onset of the stimulation and i={2,3,4,5} correspond to the four 500-millisecond time intervals after the offset of the stimulation.
Electrophysiologic recordings and stimulation protocol
Animals were placed in a faraday cage where all signals could be routed through a commutator to a wireless stimulation device (Northstar Neuroscience, Shoreline, WA) and multichannel neural signal amplifier (MNAP, Plexon Inc, Dallas, TX). For spike recordings, the signals were filtered with a passband of 150-8000 Hz, further amplified and sampled at 40 kHz. Unit spiking activity was sorted offline from each channel using Offline Sorter software (Plexon Inc).
Constant current CES was delivered in two configurations, cathodic first or anodic first consisting of pulse trains at frequencies of 25, 50, 100, 250, or 500 Hz. Pulses consisted of square leading phase (100 μs), followed by an exponentially decaying second phase to balance charge of length dependent on the amplitude of the leading phase (Figure 1C). Before starting the stimulation, a movement inducing current (MIC) was determined for each frequency. MICs were determined as the weakest current passed through the cortical electrode that caused a forced movement in 50% of test pulses.8,21 The average measured MIC for anodic first and cathodic first was 2.33 ± 0.21 and 2.44 ± 0.21 mA, respectively. Once MICs were determined, 25, 50, and 75% of MIC were calculated. Recording sessions proceeded as follows: an entire session consisted of either cathodic-first or anodic-first stimulation pulses. A random combination (without replacement) of stimulation frequency and percentage of MIC current was chosen to be presented to the animal in 25 repetitions of 1 second of stimulation, followed by 4 seconds of recording. It is important to point out that for each frequency of stimulation, there were different numbers of pulses in the 1 second duration of stimulation.
Microlesioning and histology
On completion of the experiment, electrolytic lesions were made, followed by histologic analysis to determine the electrode site locations within the different cortical layers.22 In all cases, electrodes extracted from the brain were intact and were kept attached to the skull/headcap (Figure 2A and B). The 100-μm coronal slices were stained with a standard cresyl-violet (Nissl) staining method (Figure 2C-E). Exact stereotaxic positions of lesion marks and probe tracts were identified by coregistering histologic images to the estimated probe locations from the images of the intact electrode arrays.22
Figure 2.
A and B, Images of explanted skulls: The angle and depth of the probe was estimated using these images. Individual electrodes can be seen along the silicon shank in (B). C-E, Images taken of histology from one rat. Three lesion marks are shown at different depths where the electrode was implanted in this series of serial slide sections. F, Depth distribution of the units in each of the layer categories used in the analyses. There were 19 units in layers I and II (205-359 μm). There were 19 units in layers III and IV (502-698 μm). There were 69 units located in layers V and VI (900-1230 μm).
Point process model
A point process model was formulated to relate the spiking propensity of each unit to factors associated with the stimulation parameters (frequency and amplitude) in the time intervals before the onset and after the offset of stimulation. Point process models have been shown to be useful in characterizing neural spiking activity.20,23,24 Conventional methods for analyzing the spiking activity of neurons are based on linear or nonlinear regression methods.25-27 Although these methods have played an important role in characterizing the spiking properties in many neural systems, they could not be used to fully address several issues. First, neural spike trains form a sequence of discrete events or point process time series.20,28 Standard linear or nonlinear regression methods are not designed for the analysis of discrete events and point process observations. To model spike trains with conventional regression methods, the data are usually smoothed or binned that alters the stochastic structure of the data and therefore the inferences made from their analysis. Second, model goodness-of-fit assessment method should also be appropriate for the discrete nature of neural spike trains.20 To avoid the potential problems associated with these methods, we used a point process likelihood framework to analyze the spiking activity in our dataset. The point process framework provides a flexible, computationally efficient approach for maximum likelihood estimation, goodness-offit assessment, model selection, and neural coding.20 A point process is a set of discrete events that occur in continuous time (eg, the number of neuronal spikes in a given time interval) and is characterized entirely by the conditional intensity function (CIF), which is a history-dependent generalization of the rate function for the Poisson process. It provides a canonical representation of the stochastic properties of a neural spike train.20 In this study, the time interval (0,T) denotes the observation interval and 0 < t1 < t2… < tn ≤ T are a set of spike time measurements. If N(t) is the number of spikes in (0, t), then the conditional intensity function is defined for any t∈(0,T) as:
| (1) |
Ht is the history of the spiking activity and that of any covariates up to time t. Consequently, equation (1) defines the probability of a spike in any small time interval (t, t + Δ) as follows:
| (2) |
When Δ is small, equations (2) is roughly the spiking propensity at any time t. To analyze the spiking propensity of the neurons, we define the CIF as a function of stimulation frequency f∈{1,2,3,4,5} that corresponds to 25, 50, 100, 250, and 500 Hz and stimulation amplitude a∈{1,2,3,4} that corresponds to 25, 50, 75, and 100% MIC. Time was divided into five intervals of 500 milliseconds with variable i, where i = 1 corresponds to the 500 milliseconds before the onset of stimulation and i = {2,3,4,5} correspond to (0-500), (500-1000), (1000-1500), and (1500-2000) millisecond time intervals after the offset of the stimulation (Figure 1D).
| (3) |
The following quantities are computed from data: Ii,a(t) is equal to 1 for the time interval i and stimulation amplitude a and is equal to 0 otherwise. Ii,f(t) is equal to 1 for the time interval i and stimulation frequency f and is equal to 0 otherwise (Figure 1D). Separate models were built for the anodic-first and cathodic-first stimulation sessions.
The model can be fit to the neural spike trains using general linear model (GLM) methods.17 The GLM is an extension of the multiple linear regression model in which the variable being predicted, in this case spike times, need not be Gaussian.20 GLM provides an efficient computational scheme for model parameter estimation and a likelihood framework for conducting statistical inferences based on the estimated model.23,24 The maximum-likelihood estimates and confidence intervals of θ were computed for each neuron using the glmfit.m function in MATLAB.
The model parameter vector θ = {αi,a, βi,f} was fitted to the data, where {αi,a} measured the spiking probability for different amplitudes and {βi,f} measured the spiking probability for different frequencies. The {α1,a, β1,f} parameters measured the spiking probability 500 milliseconds before the onset of stimulation and {α2-5,a, β2-5,f} parameters measured the effects of CES in (0-500), (500-1000), (1000-1500), and (1500-2000) millisecond time intervals after the offset of stimulation on the spiking probability, respectively. Therefore, the difference between {α2-5,a, β2-5,f} and {α1,a, β1,f} can capture the effects of different stimulation parameters on spiking probability at different time intervals after the offset of stimulation. To compare these effects across all units, the model parameters for anodic-first and cathodic-first stimulation models ({αi,a} and {βi,f }) were scaled to have values between −1 and 1 using the following equations:
| (4) |
and
| (5) |
The differences of these scaled parameters for all the four time intervals defined in the model were calculated:
| (6) |
| (7) |
Statistical analysis
To estimate the effect of various parameters in different depths of motor cortex, the neuron populations were divided into three groups. Group 1 is defined as layers I and II (0-400 μm), group 2 as layers III and IV (400-750 μm), and group 3 as layers V and VI (750-1800 μm) below the cortical surface. The distribution of the units over depth for each group can be seen in Figure 2F. The depth of these layers in motor cortex is reported in reference 29 and verified through histology.
The effects of stimulation parameters were divided between amplitude (Dj,a) and frequency (Dj,f) model parameters. A 2 × 4 × 3 × 4 analysis of variance (ANOVA) was constructed in SPSS Statistics 18 (SPSS, Inc, Chicago, IL) for Dj,a factors: pulse polarity (anodic-first versus cathodic-first), percent MIC (25, 50, 75, 100%), depth (layers I, II, III, IV, V, and VI), and time intervals (0-500, 500-1000, 1000-1500, 1500-2000 milliseconds after the offset of the stimulation). Likewise, a 2 × 5 × 3 × 4 ANOVA was constructed for Dj,f factors: pulse polarity (anodic-first versus cathodic-first), frequency (25, 50, 100, 250, 500 Hz), depth (layers I, II, III, IV, V, and VI), and time intervals (0-500, 500-1000, 1000-1500, 1500-2000 milliseconds after the offset of the stimulation). Based on significant interactions effects (P < .05, eg, pulse polarity × frequency × layer) further ANOVA were constructed given that there were only two pulse polarities (eg, 5 × 3 × 4 of factors frequency × depth × time for each pulse polarity) such that post hoc tests could be performed to determine the significance of each factor (eg, anodic pulse polarity in upper layers × frequency).
Results
We recorded from 110 units across six rats. For the both anodic-first and cathodic-first stimulation data, 107 models passed the goodness-of-fit assessment based on the 95% confidence bounds (Supplementary Figure 1).
The effects of CES parameters on the neuron firing rates over time were investigated in four 500-millisecond intervals after the offset of stimulation (Figure 1D). Within the first 500 milliseconds after CES, unit activity was significantly different from the following three 500-millisecond intervals (P < .01), which are described in the following sections:
CES pulse polarity
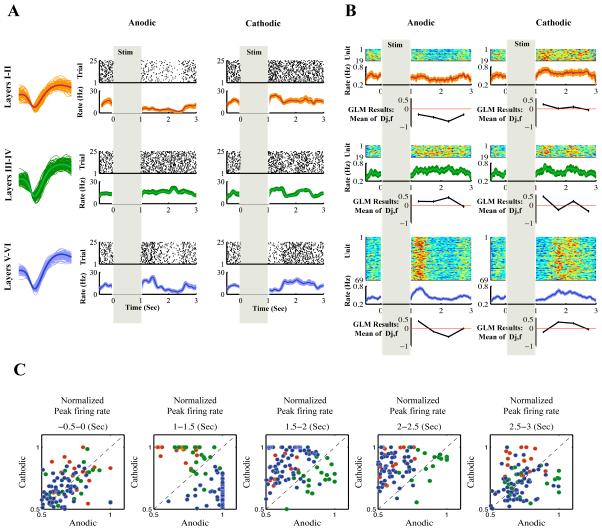
Our modeling results showed that anodic-first and cathodic-first stimulation pulses had a significantly different poststimulus effect on neural spiking activity (P < .01). In Figure 3, we show an example waveform, raster plots, poststimulus time histogram (PSTH) of a unit, the normalized PSTH of all units, the population average PSTH, and generalized linear modeling results to compare them across anodic-first versus cathodic-first stimulation. Our results demonstrated that the units located in lower layers (V and VI) of rat motor cortex have a significantly higher probability of being excited after anodic stimulation with respect to cathodic stimulation (P < .01) in the first 500 milliseconds (Figure 3A and B, lower panel). However, in this interval, units located at the upper cortical layers (I and II) had a significantly higher probability of being excited after cathodic stimulation in comparison to anodic stimulation (P < .005) (Figure 3A and B, upper panel). After the offset of stimulation, an increase in unit activity after anodic stimulation and a decrease after cathodic stimulation was seen in lower layer (V and VI) units (P < .01) (Figure 3A and B, lower panel). The opposite effect was seen for the units in upper cortical layers (I and II) (P < .01) (Figure 3A and B, upper panel). Both anodic and cathodic stimulation caused an increase (P < .01) in activity of the units in layers III and IV (Figure 3A and B, middle panel) after the offset of stimulation.
Figure 3.
The effects of anodic-first versus cathodic-first stimulation regardless of stimulation frequency and amplitude. A, In each layer category an example waveform of a unit, raster plots and poststimulus time histogram (PSTH) of the represented unit for both anodic-first and cathodic-first stimulation are shown. Each dot in the raster represents a single spike event. Each row of the raster represents a separate trial. Vertical shaded bars represent the 1-second stimulus pulse train in which no unit activity was recorded. The PSTH is the average firing rate of the unit in spike events per second in 10 ms bins. Shaded area is standard error. These examples demonstrate the varying effects of stimulation polarity on unit activity in a range of cortical layers. B, In each layer category, the top plot shows the normalized PSTH of all units in false color. Each row represents a single unit. Each column is the unit firing rate in 10 ms bins normalized to the maximum firing rate of each unit (hot colors represent high firing rates, cold colors represent low firing rates). The middle plots show the population average PSTH as the normalized mean firing rate (shaded area is standard error). The bottom plots show the generalized linear model (GLM) results which is the mean estimate of the normalized change in unit activity by the model (mean of Dj,f: equation 7) regardless of stimulation frequency and amplitude. Data points are the mean of the normalized change of unit activity (increase, positive deviation; decrease, negative deviation) from baseline in 500-millisecond intervals after the offset of stimulation. C, Scatter plots of peak firing rate of units following anodic versus cathodic stimulation in five time intervals of −0.5-0, 1-1.5, 1.5-2, 2-2.5, and 2.5-3 seconds. The stimulation starts at time 0 and ends at 1 second. Three layer categories (I and II, III and IV, V and VI) are shown with red, green, and blue dots, respectively, each dot representing the normalized firing rate of a single unit. The data in this figure demonstrate an increase in unit activity after anodic stimulation and a decrease in unit activity after cathodic stimulation in lower layers (V and VI, blue) in the first 500 milliseconds after the offset of stimulation. The opposite effect can be seen for the upper layers (I and II, red).
CES frequency
When varying the frequency of stimulation, our modeling results showed that the opposing poststimulus effects observed after anodic versus cathodic stimulation in upper and lower layers were frequency-dependent (Figure 4)(P < .01). In addition, our results suggest that varying the stimulation frequency induces changes in the firing pattern of the units. The modeling response demonstrate that the probability of neurons firing increased (P < .05) after low-frequency stimulation (<100 Hz) that decreased (P < .05) as we increased the stimulation frequency (> 100 Hz) (Figure 4B). Figure 4A, shows a raster plot and corresponding PSTH of a single unit for each layer category at frequencies of 50 Hz (<100 Hz) and 500 Hz (> 100 Hz) after anodic-first and cathodic-first stimulation. Comparing the poststimulus effects of these frequencies on Figure 4A, across upper and lower layer units, show the opposite effects of anodic versus cathodic stimulation as well as the effects of changing the frequency.
Figure 4.
Effects of the frequency of stimulation on unit activity in layered cortex regardless of stimulation amplitude. A, A raster plot (top) and corresponding PSTH (bottom) is shown for each layer category of a single unit for frequencies of 50 Hz (left column) and 500 Hz (right column) after anodic-first (light shading) and cathodic-first (dark shading) stimulation. Each row of the raster represents a single trial and each dot in the raster represents a single spike event. The PSTH is the mean spike rate across trials in 10 ms bins. Color-coding is the same as Figure 3. B, GLM frequency results (Mean of Dj,f: equation 7) of units in each layer category after anodic-first and cathodic-first CES. Data points are the mean of the normalized change of unit activity (increase, positive deviation; decrease, negative deviation) from baseline in the first 500-millisecond interval after the offset of different frequencies of stimulation. The error bars indicate 95% confidence intervals. Lines are used only for clarity and do not indicate continuous processes. C, Comparison of the GLM results for each stimulation frequency after anodic-first and cathodic-first stimulation. Data points are the mean of the normalized change of unit activity from baseline in the first 500-millisecond interval after the offset of different frequencies of stimulation regardless of stimulation amplitude. The error bars indicate 95% confidence intervals. Lines are only used for clarity and do not indicate continuous processes. The opposing effects observed after anodic versus cathodic stimulation in upper and lower layers are frequency dependent. Stimulation pulses at lower frequencies caused an increase in activity compared with higher frequencies that caused a decrease in activity.
By increasing the frequency of CES in layers I and II, we see a decrease in firing rate after cathodic stimulation (P < .05). In contrast, the decrease in activity observed after anodic stimulation is the same for all frequencies of CES up to 250 Hz in these layers. Increasing the frequency from 250 to 500 Hz causes a further significant decrease from other frequencies (P < .01).
The probability of neuron firing increased after low-frequency stimulation and decreased as we increased the stimulation frequency that we have seen in layers I and II also held true for neurons in layers V and VI (although with opposite pulse polarity) (P < .01). However, we do not see this trend in neurons located in layers III and IV, especially for the cathodic stimulation. Both anodic and cathodic stimulation cause an increase (P < .01) in activity in the units located in these layers (except for 500 Hz, anodic). As we increase the stimulation frequency, the increase in activity by these units remains consistent and we do not see a significant change affected by this parameter (P > .2).
CES amplitude
Opposing poststimulus effects on unit activity were observed after anodic versus cathodic stimulation in upper and lower layers that were also amplitude-dependent (Figure 5) (P < .01). Varying the stimulation amplitude induces changes in the firing pattern of the units as can be seen in the GLM amplitude results (Mean of Dj,a: equation 6) for each layer category after anodic-first and cathodic-first CES (Figure 5). Our results demonstrate that increasing the amplitude of stimulation from 25 to 50% and 75% MIC, caused a significant reduction in the depressive poststimulus effects of anodic-first pulses (P < .01) and a significant increase in the excitatory poststimulus effects of cathodic-first pulses (P < .01) (Figure 5B). However, increasing the amplitude of stimulation from 50% and 75% MIC to 100% showed a decrease in both excitatory and inhibitory effects observed (P < .01) (Figure 5B).
Figure 5.
Effects of changing the amplitude of stimulation on unit activity in layered cortex regardless of stimulation frequency. A, GLM amplitude results (Mean of Dj,a: equation 6) for each layer category after anodic-first and cathodic-first CES. Data points are the mean of the normalized change of unit activity (increase, positive deviation; decrease, negative deviation) from baseline in the first 500-millisecond interval after the offset of different amplitudes. The error bars indicate 95% confidence intervals. Lines are used only for clarity and do not indicate continuous processes. B, Comparison of the GLM results for each stimulation amplitude after anodic-first and cathodic-first stimulation regardless of stimulation frequency. Data points are the mean of the normalized change of unit activity from baseline in the first 500-millisecond interval after the offset of different amplitudes of CES. The error bars indicate 95% confidence intervals. Lines are only used for clarity and do not indicate continuous processes. Opposing effects on unit firing rate observed after anodic and cathodic stimulation in upper and lower layers are amplitude dependent.
Short-term temporal effects of CES
The effects of CES parameters on the neuron firing rates over the first 500 milliseconds after CES, was significantly different from the following three 500-millisecond intervals (P < .01), which were described in the previous sections. The effects observed in the 500-1000-millisecond and 1000-1500-millisecond time interval showed no significant differences (P = .299). We performed a t test of the null hypothesis that data in the 1500-2000 milliseconds after the offset of stimulation are a random sample from a normal distribution with mean 0 and unknown variance, against the alternative that the mean is not 0. The null hypothesis was rejected for cathodic-first stimulation (P < 001) and was accepted for anodic-first stimulation (P = .48). This indicates that the effects of cathodic-first CES on unit activity lasts at least 2 seconds after the offset of stimulation although the effects of anodic-first CES lasts up to 1.5 seconds after the offset of stimulation. To investigate the poststimulus effects of CES for cathodic stimulation after 2 seconds after the offset of stimulation, we have done further analysis. We have calculated the model parameters for 2000-2500-millisecond time interval after the offset of stimulation and performed the null hypothesis on them (see Supplementary materials). The results of our analysis revealed that the poststimulus effects of cathodic stimulation last up to 2 seconds after the offset of stimulation.
These excitatory effects observed after the stimulation in our results were always followed by a decrease in activity that was observed 500 to 1500 milliseconds after the stimulation (P < .01). In addition, our results demonstrated that the inhibitory effects observed in layers V and VI were followed by excitatory effects (P < .01).
Discussion
In this study, we have investigated the poststimulus effects of CES pulse polarity of various frequencies and amplitudes on neural activity. It is important to point out that in stimulation studies, recording is generally difficult for the duration of the stimulus pulse train because of the substantial stimulation artifacts that corrupt the data and/or saturate the recording electronics.30-32 Therefore, we were only able to have a high-quality recording after the offset of stimulation and report the poststimulus effects observed. It has been shown that neuronal electrical stimulation results in two stages of conduction of the stimulation pulse.33-37 These investigations have reported that effects observed directly after the stimulation are followed by a delayed series of stimulation-induced changes. Previous studies suggest that these indirect activities arise from retro-grade, synaptic, or electrical transmission.38-43 Because the results presented in this paper are the poststimulus effects after the 1-second train of stimulation pulses, the effects we have reported could be due to both of the above mechanisms and we cannot distinguish the effects of each separately.
Pulse polarity
When an electrical stimulus is applied within the CNS, cells and fibers over an unknown volume of tissue are activated.37 To make accurate inferences about anatomic structures or physiologic mechanisms involved in electrical stimulation, we must know which elements are stimulated. Our results showed a significantly different poststimulus effect on neural spiking activity across different cortical layers after anodic-first and cathodic-first stimulation pulses. Previous clinical, animal, and modeling studies have suggested that neural elements perpendicular to the electrode surface are preferentially excited by anodic stimulation, whereas cathodic stimulation excites those with a direction component parallel to its surface.14,15,44 Previous studies on the effects of extracellular anodic and cathodic currents on cortical cells45-49 have inferred that the differences obtained are, in part, due to the opposing membrane potential changes induced between oppositely directed poles (dendrite and axon) of vertically oriented cells. Accordingly, surface anodal current is believed to hyperpolarize the dendrites whereas depolarizing cell body and axonal portions of neurons. An opposite sequence of depolarizing-hyperpolarizing events is believed to occur during surface cathodal current flow.50 The neurons located in lower layers (V and VI) of rat motor cortex have elements that are primarily perpendicular to the stimulating electrode. The dendritic tree at the distal end of these neurons coalesces into the soma or cell body and the axon is at the proximal end. If an applied electric field is directed from the cortical surface inward, the dendrites are hyperpolarized, and the axon is depolarized, therefore this mode of stimulation is excitatory. In contrast, if the applied electric field is directed outward from the cortical surface, the dendrites are depolarized, and the axon is hyperpolarized, resulting in no excitation.16,51 In addition, it has been reported that the site of excitation is dependent on the polarity of the stimulus, with cathodic stimuli resulting in lower thresholds for electrode positions closer to the axon and anodic stimuli resulting in lower thresholds for electrode positions closer to the cell body and dendrites.52 These previous findings and the configurations of the surface stimulation in our experiments, lead us to hypothesize that neurons in these lower layers have a higher probability of being excited after anodic stimulation originating at the cortical surface. This hypothesis is confirmed by the evidence presented in our analysis and corroborated by modeling studies. Our results showed an increase in unit activity after anodic stimulation and a decrease in unit activity after cathodic stimulation in lower layer (V and VI) units.
As mentioned before, previous modeling studies have suggested that cathodic stimulation excites the neural elements with a direction component parallel to the surface of stimulation.14,15 These studies have showed a positive activating function (an approximation of the effect of stimulation induced potential fields on the membrane voltage of a nerve fiber14) as the result of surface cathodic stimulation in nerve fibers with a directional component parallel to the electrode surface. This positive activating function indicates membrane depolarization and possibly excitation induced by cathodic stimulation in these nerve fibers.14,15 Therefore, it is expected to see the excitatory effects on upper cortical layers after cathodic stimulation in which the neuronal structure is primarily parallel to CES surface. Our results of unit activity after the first 500 milliseconds after the offset of stimulation in upper cortical layers (I and II) agree with these hypotheses. In addition, it has been suggested that horizontally running axons in the outer layers of the cortex are likely inhibited transsynaptically as the result of anodic stimulation that polarizes the pyramidal cells.47 This was also shown in our results in which a decrease in unit activity was observed in the upper cortical layers (I and II) after anodic stimulation.
Both anodic and cathodic stimulation caused an increase in activity of the units in layers III and IV. This may also be attributed to the direction of the neuronal elements in these layers with respect to the stimulating electrode and the high degree of neural connections and neural interactions that causes both pulse polarities to increase unit firing rates after the offset of stimulation. It is important to point out that here we are reporting the poststimulus effects and therefore cannot distinguish between direct and indirect activation of the recorded units.
CES frequency
The opposing poststimulus effects observed after anodic versus cathodic stimulation in upper and lower layers were frequency dependent; our results suggest that varying the stimulation frequency induces changes in the firing pattern of rat primary motor cortex neurons. Previous work has demonstrated frequency-dependent effects on brain activity after electric stimulation. Studies have shown that within a large range of frequencies (at least up to 130 Hz), neuronal fibers can be recruited by the stimulation, depolarized, and hence, excited.53-55 However, for high-frequency stimulation (>130 Hz), it was proposed that some synapses could not depolarize fast enough to follow the stimulus train, resulting in recurrent hyperpolarization that reduces the overall number of action potentials conveyed.53-56 Also, high-frequency stimulation induces a transient depression of excitatory synaptic currents in postsynaptic motor neurons.56 Therefore, it is expected that by increasing the frequency of stimulation to high frequencies (>130 Hz), the probability of neurons firing an action potential decrease. Our results agree with these hypotheses such that the response observed from the recorded units after different frequencies of stimulation demonstrates that the probability of neurons firing after the offset of stimulation, increased after low-frequency stimulation (<100 Hz) that decreased as we increased the stimulation frequency (>100 Hz).
By increasing the frequency of CES in layers I, II, we see a decrease in firing rate after cathodic stimulation. In contrast, the decrease in activity observed after anodic stimulation is the same for all frequencies of CES up to 250 Hz. Increasing the frequency from 250 to 500 Hz causes a further significant decrease from other frequencies. These poststimulus results are probably due to different mechanisms involved in the excitatory and inhibitory effects observed in motor cortex neurons after cortical stimulation.57 The inhibitory effects have been attributed to intracortical58 or corticospinal59 inhibitory mechanisms. The combination of these inhibitory mechanisms in high frequencies of stimulation could cause higher recruitment of inhibition and therefore at really high frequencies (in our case 500 Hz), recurrent hyperpolarization.
The probability of neuron firing increased after low-frequency stimulation and decreased as we increased the stimulation frequency that we have seen in layers I and II also held true for neurons in layers V and VI (although with opposite pulse polarity). However, we do not see this trend in neurons located in layers III and IV, especially for the cathodic stimulation. As shown previously, both anodic and cathodic stimulation cause an increase in activity in the units located in these layers, except for 500 Hz anodic. As we increase the stimulation frequency, the increase in activity by these units remains consistent and we do not see a significant change affected by this parameter. These results suggest that there is a clear high-frequency post-stimulus effect of anodic stimulation in the units in these cortical layers that will need further investigation to understand the mechanisms involved.
CES amplitude
It has been shown that the probability for eliciting an action potential increases with increasing the amplitude of stimulation60 because neuronal compartments polarize linearly with the amplitude of the applied electric field.52,61,62 Although our results support this hypothesis, when we increase the amplitude of stimulation from 25 to 50% and 75% MIC, it does not agree for 100% MIC stimulation. Increasing the amplitude of stimulation from 50 and 75% MIC to 100% showed a decrease in both excitatory and inhibitory poststimulus effects observed. It is important to point out that the cortical architecture contains several excitatory and inhibitory cell types that are complexly interconnected locally as well as over longer distances.63,64 Also, it is hypothesized that the amplitude of stimulation influences the distribution of the electric field induced into the brain.65 This hypothesis and the high interconnectivity of the cortex predict that changing the stimulation amplitude can cause different populations of cell types to become activated as a result of electrical stimulation.66 Previous modeling studies show that the threshold stimuli amplitude is lower for fibers with larger diameters.65 This suggests that for lower current amplitudes (25 and 50% MIC) it is more probable to stimulate the pyramidal cell fibers, whereas for higher amplitudes (75 and 100% MIC), neurons from inhibitory networks are being recruited.37 Because inhibition can be transmitted by electrically (and synaptically) interconnected networks of interneuron,66-68 it is probable that higher amplitudes in CES can activate these networks and cause an inhibitory effect on the activity.
Previous in vitro studies have shown that layer V and VI neuron action potential thresholds are lower than upper layer pyramidal neurons and interneurons.69 This is also shown in our results when comparing the responses of neurons located in layers V and VI with the ones located in layers I and II for 25% MIC. Increasing the amplitude, as mentioned before will recruit other networks of neurons that causes changes in firing rate that do not support this hypothesis.
Short-term temporal effects of CES
A large body of work from intracellular and extracellular recordings in the neocortex (in vitro and in vivo) describe a close correlation of both excitatory and inhibitory effects after electrical stimulation.38-42 Despite some variation across methods and preparations, the common finding was that excitation is evoked at short latencies and was always followed by an inhibitory response.43 This poststimulus effect was also observed in our results in the cases when we observed excitatory effects after the stimulation. These poststimulus excitatory effects were always followed by a decrease in activity that was observed 500 to 1500 milliseconds after the stimulation. The reduction of excitation in the network could be driven by two different mechanisms. First, intrinsic membrane currents could be triggered by the first wave of excitation and lead to a depression of firing rate.43 Prominent candidates for such a mechanism are long-lasting after hyperpolarizations in pyramidal neurons based on calcium or sodium-dependent currents.70,71 Second, a decrement of network excitability could be based on short-term depression of excitatory intracortical synapses.72-75 Both mechanisms would reduce excitatory drive from cortical neurons by suppressing firing rates in the population of postsynaptic neurons.43 In addition, our results demonstrate that the poststimulus inhibitory effects observed in layers V and VI were followed by excitatory effects. This can also be described with the mechanisms previously described because the dominant population of neurons are pyramidal cells in these layers.
Implications for transcranial magnetic stimulation and transcranial direct current stimulation
Although the results of this study could be related to epidural and subdual motor cortex stimulation applications, there are important differences between our results relative to the use of conventional, noninvasive cortical stimulation methods. Transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) are two of the most important applications of cortical stimulation in neurorehabilitation given their non-invasiveness.3,5-7,11,12 There are important differences between electric and magnetic stimulation of the brain. First, during magnetic stimulation, the direction of the electric field is approximately tangential (parallel to the surface of the skull). However, during electric stimulation the electric field has both radial and tangential components.51 Therefore, the distribution of the electric field as the result of electric and magnetic stimulation and consequently the excited elements are different. There are also latency differences in surface-recorded electromyographic responses after electric and magnetic stimulation of the brain.76,77 The response latency in the contracting muscle during magnetic stimulation was found to be longer than electrical stimulation. These findings suggest that magnetic stimulation activates neurons transsynaptically, whereas electric stimulation activates them directly.51,78 Because of these differences, we suggest that the results in this paper may not be related to TMS applications.
It is also important to point out that there are major differences between CES and tDCS. Although in tDCS the electrodes attached to the scalp can be used to activate neurons in the brain, the high resistance of the skull shunts most of the current through the scalp.51 In addition, tDCS approaches have a low spatial specificity and induce a modulation in a large cortical area.79 CES, in contrast, permits high spatial specificity to targeted neuronal populations.80 Therefore, because of these differences between our study and tDCS, the spatial distribution of electric fields are likely significantly different.
Considerations
In this article, we used the rat model of CES because the rat’s cortex is well defined63 and often used in neuroprosthetics and neurorehabilitation research,18,21,43,73 Therefore, the rat’s cortex will help us to begin to understand the effects of stimulation in vivo without the confounding factors of sulci and gyri. However, in interpreting and comparing the results of this study for human applications, it is important to keep in mind that after the fold of the gyrus, the orientation of the neurons with respect to the electrode changes.14 Bending axons exhibit abrupt changes in the profile of membrane voltage along the axon at the sites of the bends15,18 and therefore the excitation conditions are altered.14
One of the important choices in designing stimulation paradigms is the choice of the waveform and whether to use voltage or current controlled pulses. We used current-controlled pulses because the effects of current pulse stimulation are more tractable than the effects of voltage pulse stimulation, for which the (current-induced) electric field depends on the complex impedance of the stimulation electrode.31 Another constraint was to use charge-balanced stimulation to avoid injection of net charge and prevent electrode and cell damage.31 To be able to compare the effects of anodic versus cathodic stimulation on different neurons in the motor cortex, we used a monopolar stimulation configuration that used biphasic pulse trains consisting of a square leading phase and a decaying exponential phase to balance charge. A third constraint was the width of the leading phase of the stimulus pulses. In this study, the leading phase pulse width was fixed at 100 μs as this pulse width is typically used for neural stimulation.8,21,43,81 There have been several investigations to optimize the stimulus waveform to maximize the injected charge through the electrode interface while keeping the activation threshold at a minimum.82-84 It has been shown that this optimization varies as a function of the stimulus waveform and stimulating electrode material.82 Further investigation is needed to determine whether alternative waveforms can provide similar results with the same polarity, frequency, and amplitude of stimulation as used in this study.
Conclusion
The goal of this study was to design a set of experiments and test the influence of CES pulse polarity of various frequencies and amplitudes on the imposed volume of rat primary motor cortex. The results of this study suggested that neurons in lower layers have a higher probability of being excited after anodic stimulation. Similarly, neurons located in upper cortical layers have a higher probability of being excited after cathodic stimulation. The opposing effects observed after anodic versus cathodic stimulation in upper and lower layers were frequency and amplitude dependent. In addition, our results demonstrate that the changes in neural activity after manipulation of CES parameters are time dependent according to the location (depth) of the recorded units. It is clear that more quantitative data about intracortical distribution of projections and their target, as well as variation of electrical properties of cortical circuits, are needed before the specific effects observed in the current study can be fully understood, especially in the context of maximizing efficacy of CES for neurorehabilitation and neuroprosthetic applications.
Supplementary Material
Acknowledgment
We give special thanks to G. Gage, T. Marzullo, E. Ionides, and U. Eden for their helpful discussions.
This work was supported in part by grants from Northstar Neuroscience, Inc. (all authors), NIBIB P41 EB002030 (A.Y. and D.R.K.), and NIDCD F32 DC7797 (M.J.L.). D.R.K. has a financial interest in NeuroNexus Technologies.
Footnotes
Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.brs.2010.11.004
Uncited reference
29.
References
- 1.Butefisch CM, Khurana V, Kopylev L, Cohen LG. Enhancing encoding of a motor memory in the primary motor cortex by cortical stimulation. J Neurophysiol. 2004;91:2110. doi: 10.1152/jn.01038.2003. [DOI] [PubMed] [Google Scholar]
- 2.Antal A, Nitsche MA, Kruse W, et al. Direct current stimulation over v5 enhances visuomotor coordination by improving motion perception in humans. J Cogn Neurosci. 2004;16:521–527. doi: 10.1162/089892904323057263. [DOI] [PubMed] [Google Scholar]
- 3.Nitsche MA, Schauenburg A, Lang N, et al. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cognit Neurosci. 2003;15:619–626. doi: 10.1162/089892903321662994. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi M, Ng J, Theoret H, Pascual-Leone A. Modulation of intracortical neuronal circuits in human hand motor area by digit stimulation. Exp Brain Res. 2003;149:1. doi: 10.1007/s00221-002-1329-9. [DOI] [PubMed] [Google Scholar]
- 5.Kincses TZ, Antal A, Nitsche MA, Bartfai O, Paulus W. Facilitation of probabilistic classification learning by transcranial direct current stimulation of the prefrontal cortex in the human. Neuropsychologia. 2004;42:113–117. doi: 10.1016/s0028-3932(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 6.Fregni F, Boggio PS, Nitsche M, et al. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp Brain Res. 2005;166:23. doi: 10.1007/s00221-005-2334-6. [DOI] [PubMed] [Google Scholar]
- 7.Marshall L, Molle M, Hallschmid M, Born J. Transcranial direct current stimulation during sleep improves declarative memory. J Neuroscience. 2004;24:9985. doi: 10.1523/JNEUROSCI.2725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown JA, Lutsep HL, Weinand M, Cramer SC. Clinical studies–motor cortex stimulation for the enhancement of recovery from stroke: a prospective, multicenter safety study. Neurosurg. 2006;58:464. doi: 10.1227/01.NEU.0000197100.63931.04. [DOI] [PubMed] [Google Scholar]
- 9.Kinoshita M, Ikeda A, Matsuhashi M, et al. Electric cortical stimulation suppresses epileptic and background activities in neocortical epilepsy and mesial temporal lobe epilepsy. Clin Neurophysiol. 2005;116:1291. doi: 10.1016/j.clinph.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Ativanichayaphong T, He JW, Hagains CE, Peng YB, Chiao JC. A combined wireless neural stimulating and recording system for study of pain processing. J Neurosci Methods. 2008;170:25–34. doi: 10.1016/j.jneumeth.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Lefaucheur JP. Repetitive transcranial magnetic stimulation (rTMS): insights into the treatment of Parkinson’s disease by cortical stimulation. Clin Neurophysiol. 2006;36:125–133. doi: 10.1016/j.neucli.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald PB, Huntsman S, Gunewardene R, Kulkarni J, Daskalakis ZJ. A randomized trial of low-frequency right-prefrontal-cortex transcranial magnetic stimulation as augmentation in treatment-resistant major depression. Int J Neuropsychopharmacol. 2006;9:655–666. doi: 10.1017/S1461145706007176. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen JP, Lefaucheur JP, Keravel Y. Motor cortex stimulation. In: Simpson B, editor. Electrical stimulation and the relief of pain: pain research and clinical management. Elsevier Science; St. Louis: 2003. [Google Scholar]
- 14.Manola L, Holsheimer J, Veltink P, Buitenweg JR. Anodal vs cathodal stimulation of motor cortex: a modeling study. Clin Neurophysiol. 2007;118:464. doi: 10.1016/j.clinph.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Wongsarnpigoon A, Grill WM. Computational modeling of epidural cortical stimulation. J Neural Engineer. 2008;5:443–454. doi: 10.1088/1741-2560/5/4/009. [DOI] [PubMed] [Google Scholar]
- 16.Tranchina D, Nicholson C. A model for the polarization of neurons by extrinsically applied electric-fields. Biophysic J. 1986;50:1139–1156. doi: 10.1016/S0006-3495(86)83558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amassian VE, Eberle L, Maccabee PJ, Cracco RQ. Modeling magnetic coil excitation of human cerebral-cortex with a peripheralnerve immersed in a brain-shaped volume conductor—the significance of fiber bending in excitation. Electroencephalogr Clin Neurophysiol. 1992;85:291–301. doi: 10.1016/0168-5597(92)90105-k. [DOI] [PubMed] [Google Scholar]
- 18.Iles JF. Simple models of stimulation of neurones in the brain by electric fields. Prog Biophysic Molecular Biol. 2005;87:17–31. doi: 10.1016/j.pbiomolbio.2004.06.002. [DOI] [PubMed] [Google Scholar]; Electroencephalogr Clin Neurophysiol. 1992;85:291–301. doi: 10.1016/0168-5597(92)90105-k. [DOI] [PubMed] [Google Scholar]
- 19.Kipke D, Vetter R, Williams J, Hetke J. Silicon-substrate intracortical microelectrode arrays for long-term recording of neuronal spike activity in cerebral cortex. IEEE Transactions Neural Systems Rehabilitation Engineering. 2003;11:151. doi: 10.1109/TNSRE.2003.814443. [DOI] [PubMed] [Google Scholar]
- 20.Truccolo W, Eden UT, Fellows MR, Donoghue JP, Brown EN. A point process framework for relating neural spiking activity to spiking history, neural ensemble, and extrinsic covariate effects. J Neurophysiol. 2005;93:1074–1089. doi: 10.1152/jn.00697.2004. [DOI] [PubMed] [Google Scholar]
- 21.Teskey GC, Flynn C, Goertzen CD, Monfils MH, Young NA. Cortical stimulation improves skilled forelimb use following a focal ischemic infarct in the rat. Neurolog Res. 2003;25:794. doi: 10.1179/016164103771953871. [DOI] [PubMed] [Google Scholar]
- 22.Parikh H, Marzullo TC, Kipke DR. Lower layers in the motor cortex are more effective targets for penetrating microelectrodes in cortical prostheses. J Neural Eng. 2009;6:026004. doi: 10.1088/1741-2560/6/2/026004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown EN, Barbieri R, Eden UT, Frank LM. Likelihood methods for neural spike train data analysis. In: Feng J, editor. Computational neuroscience: a comprehensive approach. CRC; London: 2004. pp. 253–286. [Google Scholar]
- 24.Brown EN, Barbieri R, Ventura V, Kass RE, Frank LM. The time-rescaling theorem and its application to neural spike train data analysis. Neural Comput. 2002;14:325–346. doi: 10.1162/08997660252741149. [DOI] [PubMed] [Google Scholar]
- 25.Ashe J. Movement parameters and neural activity in motor cortex and area-5. Cereb Cortex. 1994;4:590–600. doi: 10.1093/cercor/4.6.590. [DOI] [PubMed] [Google Scholar]
- 26.Fu QG. Temporal encoding of movement kinematics in the discharge of primate primary motor and premotor neurons. J Neurophysiol. 1995;73:836. doi: 10.1152/jn.1995.73.2.836. [DOI] [PubMed] [Google Scholar]
- 27.Luczak A. Multivariate receptive field mapping in marmoset auditory cortex. J Neurosci Methods. 2004;136:77. doi: 10.1016/j.jneumeth.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 28.Brillinger DR. Maximum-likelihood analysis of spike trains of interacting nerve-cells. Biol Cybern. 1988;59:189–200. doi: 10.1007/BF00318010. [DOI] [PubMed] [Google Scholar]
- 29.Skoglund T, Pascher R, Berthold C. The existence of a layer IV in the rat motor cortex. Cereb Cortex. 1997;7:178–180. doi: 10.1093/cercor/7.2.178. [DOI] [PubMed] [Google Scholar]
- 30.Wagenaar DA, Potter SM. Real-time multi-channel stimulus artifact suppression by local curve fitting. J Neurosci Methods. 2002;120:113. doi: 10.1016/s0165-0270(02)00149-8. [DOI] [PubMed] [Google Scholar]
- 31.Wagenaar DA. Effective parameters for stimulation of dissociated cultures using multi-electrode arrays. J Neurosci Methods. 2004;138:27. doi: 10.1016/j.jneumeth.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Grumet AE, Wyatt JL, Rizzo JF. Multi-electrode stimulation and recording in the isolated retina. J Neurosci Methods. 2000;101:31. doi: 10.1016/s0165-0270(00)00246-6. [DOI] [PubMed] [Google Scholar]
- 33.Kombos T, Suss O. Neurophysiological basis of direct cortical stimulation and applied neuroanatomy of the motor cortex: a review. Neurosurg Focus. 2009;27:E3. doi: 10.3171/2009.8.FOCUS09141. [DOI] [PubMed] [Google Scholar]
- 34.Parsons LM, Fox PT, Downs JH, Glass T, Hirsch TB. Use of implicit motor imagery for visual shape discrimination as revealed by PET. Nature. 1995;375:54. doi: 10.1038/375054a0. [DOI] [PubMed] [Google Scholar]
- 35.Patton HD, Amassian VE. Single-unit and multiple-unit analysis of cortical stage of pyramidal tract activation. J Neurophysiol. 1954;17:345–363. doi: 10.1152/jn.1954.17.4.345. [DOI] [PubMed] [Google Scholar]
- 36.Phillips CG, Porter R. Unifocal and bifocal stimulation of motor cortex. J Physiol. 1962;162:532–538. doi: 10.1113/jphysiol.1962.sp006948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ranck JB., Jr. Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res. 1985;98:417–440. doi: 10.1016/0006-8993(75)90364-9. [DOI] [PubMed] [Google Scholar]
- 38.Butovas S. Effects of electrically coupled inhibitory networks on local neuronal responses to intracortical microstimulation. J Neurophysiol. 2006;96:1227. doi: 10.1152/jn.01170.2005. [DOI] [PubMed] [Google Scholar]
- 39.Berman N, Douglas RJ, Martin K, Whitteridge D. Mechanisms of inhibition in cat visual cortex. J Physiol. 1991;440:697–722. doi: 10.1113/jphysiol.1991.sp018731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li CL. the inhibitory effect of stimulation of a thalamic nucleus on neuronal activity in the motor cortex. J Physiol. 1956;133:40–53. doi: 10.1113/jphysiol.1956.sp005565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chagnacamitai Y, Connors BW. Synchronized excitation and inhibition driven by intrinsically bursting neurons in neocortex. J Neurophysiol. 1989;62:1149–1162. doi: 10.1152/jn.1989.62.5.1149. [DOI] [PubMed] [Google Scholar]
- 42.Porter JT, Johnson CK, Agmon A. Diverse types of interneurons generate thalamus-evoked feed forward inhibition in the mouse barrel cortex. J Neurosci. 2001;21:2699. doi: 10.1523/JNEUROSCI.21-08-02699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butovas S, Schwarz C. Spatiotemporal effects of microstimulation in rat neocortex: a parametric study using multielectrode recordings. J Neurophysiol. 2003;90:3024. doi: 10.1152/jn.00245.2003. [DOI] [PubMed] [Google Scholar]
- 44.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–640. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bishop GH, O’Leary JL. The effects of polarizing currents on cell potentials and their significance in the interpretation of central nervous system activity. Electroencephalogr Clin Neurophysiol. 1950;2:401–416. doi: 10.1016/0013-4694(50)90077-0. [DOI] [PubMed] [Google Scholar]
- 46.Creutzfeldt O, Fromm GH, Kapp H. Influence of transcortical D-C current on cortical neuronal activity. Exp Neurol. 1962;5:436–452. doi: 10.1016/0014-4886(62)90056-0. [DOI] [PubMed] [Google Scholar]
- 47.Hern JEC, Porter R, Landgren S, Phillips CG. Selective excitation of corticofugal neurons by surface-anodal stimulation of baboons motor cortex. J Physiol. 1962;161:73–90. doi: 10.1113/jphysiol.1962.sp006874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Landau WM, Bishop GH, Clare MH. Analysis of form distribution of evoked cortical potentials under influence of polarizing currents. J Neurophysiol. 1964;27:788–813. doi: 10.1152/jn.1964.27.5.788. [DOI] [PubMed] [Google Scholar]
- 49.Libet B, Gerard RW. Steady potential fields and neurone activity. J Neurophysiol. 1941;4:438–494. [Google Scholar]
- 50.Gorman ALF. Differential patterns of activation of pyramidal system elicited by surface anodal and cathodal cortical stimulation. J Neurophysiol. 1966;29:547. doi: 10.1152/jn.1966.29.4.547. [DOI] [PubMed] [Google Scholar]
- 51.Basser PJ, Roth BJ. New currents in electrical stimulation of excitable tissues. Ann Rev Biomed Engineer. 2000;2:377–397. doi: 10.1146/annurev.bioeng.2.1.377. [DOI] [PubMed] [Google Scholar]
- 52.McIntyre CC, Grill WM. Electrophysiology—excitation of central nervous system neurons by nonuniform electric fields. Biophy J. 1999;76:878. doi: 10.1016/S0006-3495(99)77251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Priori A, Lefaucheur JP. Chronic epidural motor cortical stimulation for movement disorders. Lancet Neurol. 2007;6:279–286. doi: 10.1016/S1474-4422(07)70056-X. [DOI] [PubMed] [Google Scholar]
- 54.Garcia L, D’Alessandro G, Bioulac B, Hammond C. High-frequency stimulation in Parkinson’s disease: more or less? Trend Neurosci. 2005;28:209–216. doi: 10.1016/j.tins.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 55.Beurrier C, Bioulac B, Audin J, Hammond C. High-frequency stimulation produces a transient blockade of voltage-gated currents in subthalamic neurons. J Neurophysiol. 2001;85:1351. doi: 10.1152/jn.2001.85.4.1351. [DOI] [PubMed] [Google Scholar]
- 56.Anderson W, Kudela P, Cho J, Bergey G, Franaszczuk P. Studies of stimulus parameters for seizure disruption using neural network simulations. Biol Cybern. 2007;97:173–194. doi: 10.1007/s00422-007-0166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Triggs WJ. Motor inhibition and excitation are independent effects of magnetic cortical stimulation. Ann Neurol. 1992;32:345. doi: 10.1002/ana.410320307. [DOI] [PubMed] [Google Scholar]
- 58.Fuhr P, Agostino R, Hallett M. Spinal motor-neuron excitability during the silent period after cortical stimulation. Electroencephalogr Clin Neurophysiol. 1991;81:257–262. doi: 10.1016/0168-5597(91)90011-l. [DOI] [PubMed] [Google Scholar]
- 59.Mills KR. Excitatory and inhibitory effects on human spinal motoneurones from magnetic brain-stimulation. Neurosci Lett. 1988;94:297–302. doi: 10.1016/0304-3940(88)90034-1. [DOI] [PubMed] [Google Scholar]
- 60.Baker LL, McNeal DR, Benton L, Bowman BR, Waters RL. Neuro muscular electrical stimulation. 4th ed. Canada: 2000. [Google Scholar]
- 61.Tranchina D, Nicholson C. A model for the polarization of neurons by extrinsically applied electric fields. Biophys J. 1986;50:1139–1156. doi: 10.1016/S0006-3495(86)83558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Plonsey R, Altman KW. Electrical-stimulation of excitable cells—a model approach. Proc IEEE. 1988;76:1122–1129. [Google Scholar]
- 63.Braitenberg V, Schuz A. Anatomy of the cortex. Springer-Verlag; New York: 1991. [Google Scholar]
- 64.Peters A, Jones EG. Classification of cortical neurons. In: Peters A, Jones EG, editors. Cerebral cortex. Plenum Press; New York: 1984. [Google Scholar]
- 65.Manola L, Roelofsen BH, Holsheimer J, Marani E, Geelen J. Modelling motor cortex stimulation for chronic pain control: electrical potential field, activating functions and responses of simple nerve fiber models. Med Biol Engineer Comput. 2005;43:335–344. doi: 10.1007/BF02345810. [DOI] [PubMed] [Google Scholar]
- 66.Nowak LG, Bullier J. Axons, but not cell bodies, are activated by electrical stimulation in cortical gray matter: II, evidence from selective inactivation of cell bodies and axon initial segments. Exp Brain Res. 1998;118:489. doi: 10.1007/s002210050305. [DOI] [PubMed] [Google Scholar]
- 67.Galarreta M, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature. 1999;402:72–75. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- 68.Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled Inhibitory neurons in neocortex. Nature. 1999;402:75. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- 69.Radman T, Ramos RL, Brumberg JC, Bikson M. Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain Stimulation. 2009;2:215–228. doi: 10.1016/j.brs.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schwindt PC. Long-lasting reduction of excitability by a sodium-dependent potassium current in cat neocortical neurons. J Neurophysiol. 1989;61:233–244. doi: 10.1152/jn.1989.61.2.233. [DOI] [PubMed] [Google Scholar]
- 71.Schwindt PC, Spain WJ, Foehring RC, Chubb MC, Crill WE. Slow conductances in neurons from cat sensorimotor cortex in-vitro and their role in slow excitability changes. J Neurophysiol. 1988;59:450–467. doi: 10.1152/jn.1988.59.2.450. [DOI] [PubMed] [Google Scholar]
- 72.Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science. 1997;275:220. doi: 10.1126/science.275.5297.221. [DOI] [PubMed] [Google Scholar]
- 73.Thomson AM, Deuchars J, West DC. Large, deep layer pyramid-pyramid single axon epsps in slices of rat motor cortex display paired pulse and frequency dependent depression, mediated presynaptically and self-facilitation, mediated postsynaptically. J Neurophysiol. 1993;70:2354. doi: 10.1152/jn.1993.70.6.2354. [DOI] [PubMed] [Google Scholar]
- 74.Tsodyks MV, Markram H. The neural code between neocortical pyramidal neurons depends on neurotransmitter release probability (Correction) Proc Natl Acad Sci U S A. 1997;94:5495. doi: 10.1073/pnas.94.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harvey RL, Nudo RJ. Cortical brain stimulation: a potential therapeutic agent for upper limb motor recovery following stroke. Top Stroke Rehabil. 2007;14:54–67. doi: 10.1310/tsr1406-54. [DOI] [PubMed] [Google Scholar]
- 76.Rothwell J, Burke D, Hicks R, Stephen J. Transcranial electrical stimulation of the motor cortex in man: further evidence for the site of activation. J Physiol. 1994;481:243–250. doi: 10.1113/jphysiol.1994.sp020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Day BL, Thompson PD, Dick JP, Nakashima K, Marsden CD. Different sites of action of electrical and magnetic stimulation of the human-brain. Neurosci Lett. 1987;75:101–106. doi: 10.1016/0304-3940(87)90083-8. [DOI] [PubMed] [Google Scholar]
- 78.Edgley SA, Eyre JA, Lemon RN, Miller S. Excitation of the corticospinal tract by electromagnetic and electrical stimulation of the scalp in the macaque monkey. J Physiol. 1990;425:301–320. doi: 10.1113/jphysiol.1990.sp018104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alappat JJ. Motor cortex stimulation for chronic pain: systematic review and meta-analysis of the literature. Neurology. 2009;72:577. doi: 10.1212/01.wnl.0000344169.51931.e5. [DOI] [PubMed] [Google Scholar]
- 80.Cherney LR, Erickson RK, Small SL. Epidural cortical stimulation as adjunctive treatment for non-fluent aphasia: preliminary findings. J Neurol Neurosurg Psychiatry. 81:1014–1021. doi: 10.1136/jnnp.2009.184036. [DOI] [PubMed] [Google Scholar]
- 81.Bossetti CA, Birdno MJ, Grill WM. Analysis of the quasi-static approximation for calculating potentials generated by neural stimulation. J Neural Engineer. 2008;5:44–53. doi: 10.1088/1741-2560/5/1/005. [DOI] [PubMed] [Google Scholar]
- 82.Sahin M, Tie Y. Non-rectangular waveforms for neural stimulation with practical electrodes. J Neural Engineer. 2007;4:227–233. doi: 10.1088/1741-2560/4/3/008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blair HA. On the intensity-time relations for stimulation by electric currents: I, the Rockefeller University Press. Rockefeller University Press; New York: 1932. [Google Scholar]
- 84.Ayers GM, Aronson SW, Geddes LA. Comparison of the ability of the lapicque and exponential strength-duration curves to fit experimentally obtained perception threshold data. Austr Phys Engineer Sci Med. 1986;9:111–116. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.