Abstract
This article focuses on key issues surrounding the needs and application of simulation technologies for technical skills training in otolaryngology. The discussion includes an overview of key topics in training and learning, the application of these issues in simulation environments, and the subsequent applications of these simulation environments to the field of otolaryngology. Examples of past applications are presented, with discussion of how the interplay of cultural changes in surgical training in general, along with the rapid advancements in technology have shaped and influenced their adoption and adaptation. The authors conclude with emerging trends and potential influences advanced simulation and training will have on technical skills training in otolaryngology.
Keywords: Simulation technology, Training simulation, Education, Surgical simulation, Medical simulation, ENT simulation, Continuing education
Introduction
The Need for Surgical Simulation in Otolaryngology
In spite of ever improving less-invasive medical treatment regimens, surgical intervention is still required for many health conditions. As an example, disorders of the temporal bone affect millions of patients in the United States (NIDCD 2010) and many require surgical intervention for resolution. To gain surgical proficiency, trainees must possess comprehension of the complex anatomy and associated pathology of the temporal bone. This knowledge must be integrated with refined microsurgical technical skill. This proficiency requires many hours of deliberate practice and considerable clinical experience. Surgical training requires at least 5 years under current methods at a cost of approximately $80,000 per year per resident (Williams 2009). Conventional temporal bone laboratories with related equipment cost over a million dollars to construct and are expensive to maintain (Welling 2010). Several factors contribute to inefficiencies in traditional training methodologies, adversely impacting the overall cost of healthcare. The barriers to progress include: less time available for teaching and learning, limitations of instructional resources, and perhaps most importantly, the lack of a uniform objective assessment of technical skills.
Less time available for teaching and learning
There are several limiting factors adversely influencing the amount of time available for teaching in our training centers. First, as healthcare costs continue to escalate, financial pressures come to bear on teaching physicians. An increased demand for “clinical efficiency” in both the operating room and outpatient clinics limits the amount of time faculty can devote to actually teaching. Secondly, the limits of duty hours for trainees imposed to reduce fatigue and related errors also limit the amount of time available for hands on learning. This presents a disassociation of the traditional model of “apprentice/master” introduced by Halsted & Osler in the late 1800’s. Furthermore, this reduction leads to the concern for insufficient development of technical skills acquired in the operating room and requires learning and practice outside of patient care.
Limitations of instructional resources
Several limitations of instructional resources are apparent. First, there is a reduced availability of cadaveric material, the previous gold standard for practicing technical maneuvers outside of patient care. Ethical procurement and proper disposal present both necessary and continuous challenges. Sources of human material are no longer available from foreign countries and fewer patients and their families are consenting to donation. Secondly, there is reduced access to faculty expertise, a key instructional resource. Due to financial pressures cited above, faculty have fewer hours available for teaching, especially for critical assessment during formative development that requires considerable time. With financial pressure for more clinical productivity to maintain previous income levels, the numbers of teaching faculty are decreasing. Lastly, of continuing concern is exposure to hazardous materials including pathogens present in cadaveric specimens, e.g., hepatitis B, C, prion derived illness (Scott 2001) and HIV infection, and increased exposure to formalin (NIOSH 2010) that present increased dangers to health. Use of simulation technologies presents the capacity to greatly mitigate these limitations of instructional resources.
Lack of a uniform objective assessment of technical skills
Perhaps most importantly, there is a lack of uniform and objective standardized metrics for use in the assessment of technical skills. Without standardized metrics, uniform formative feedback during training as well as measurement of professional technical proficiency is not being achieved. This has been a direct result of the fact that no methodology previously existed to objectively apply metrics that were valid, reliable, and practical.
Recently it has been asserted that today’s training methodologies are antiquated and that a new balance between patient safety and physician training in necessary (Grantcharov and Resnick, 2008). A more standardized and structured approach to curriculum development, continuous assessment of skills, constructive feedback, and provision for deliberate practice outside of direct patient care are necessary. Elements required in technical skills development include not only facility to practice psychomotor skills but elements that reinforce adequate knowledge of a specific procedure such as relevant anatomy, instrumentation, indications, complications, postoperative management, etc. This reinforcement requires demonstration of a procedure, delineation of the key steps, assurance of comprehension of the key steps, single component mastery followed by entire procedure mastery. Formative continuous and summative assessment of skill is necessary. Simulation technologies provide the mechanism by which this reinforcement can be achieved with efficient use of the expert.
Simulation in Support of Technical Skills Training
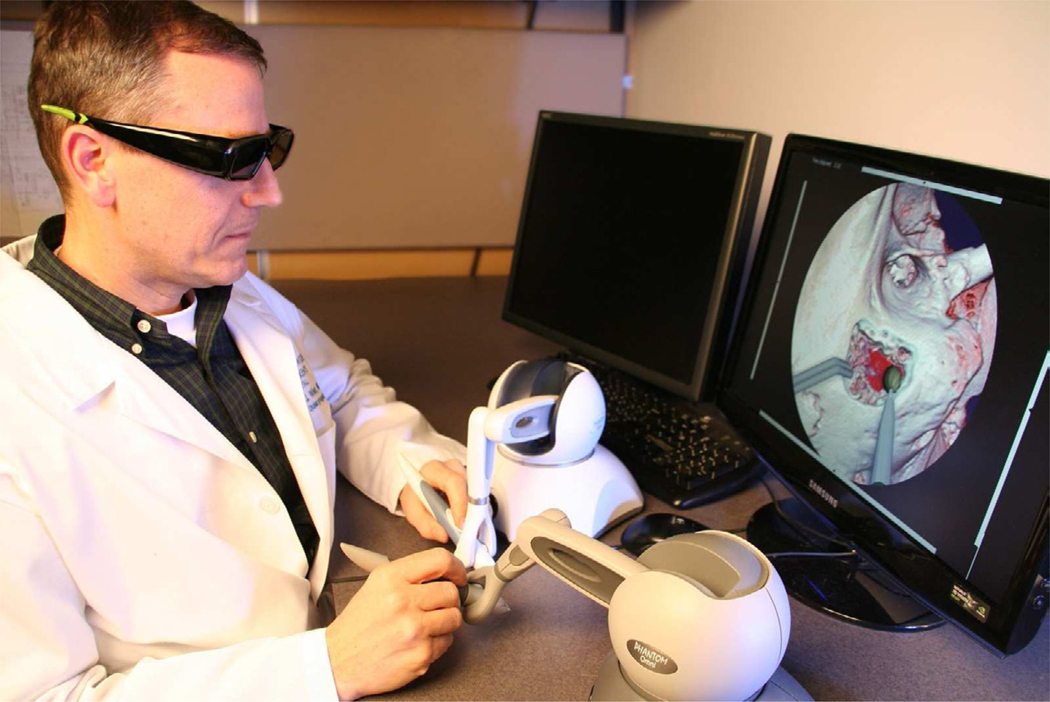
Simulation environments are uniquely suited to allow deliberate practice in a non-threatening environment. (Figure 1) For these systems to be effective they will require the integration of automated expert feedback based on rigorous standards and more than just an environment for the replication of a real world task.
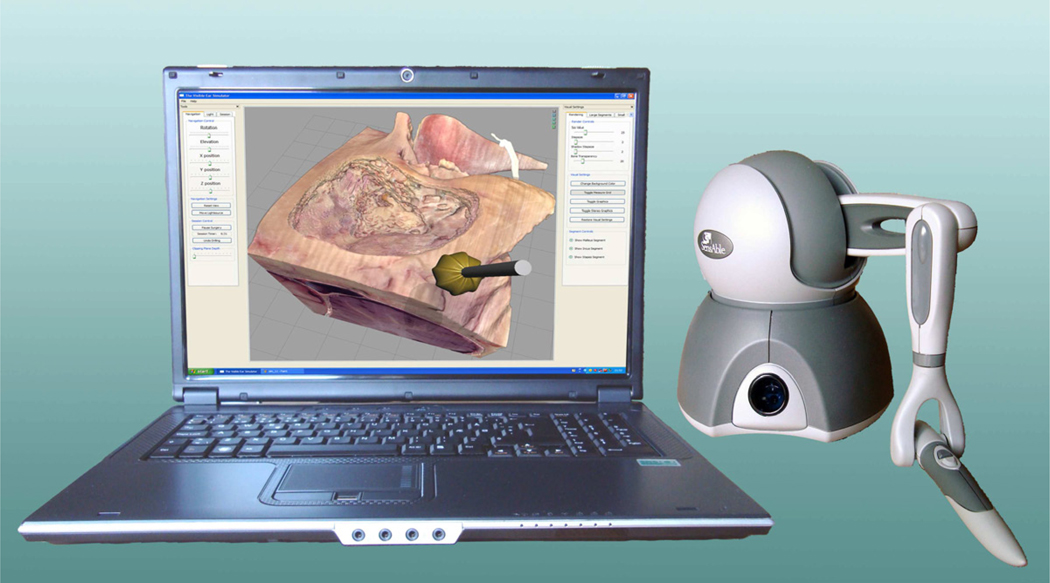
Figure 1.
User in current temporal bone simulator from The Ohio State University. Interface includes stereo visual and dual haptic feedback devices for procedural interaction.
Johnson points out that simulation is particularly suited to provide:
Errors without putting patients at risk, i.e., “error based learning.”
Objective performance measurements and a standardized learning process.
And that a realistic simulation provides “situational context”, with more effective reification of the elements to be learned (Johnson 2007).
In the following pages, we present the issues of applying advanced computing and simulation environments for supporting technical skills training and meeting the proscribed criteria established by Johnson. It should be noted that this discussion excludes other avenues employed for simulation training, including mannequins, box-trainers, etc. The main focus of this chapter is simulation environments that support the development of technical skills needed to successfully execute surgical procedures and that integrate automated standards.
Technical Skills Assessment and Simulation
The need for accurate assessment of skill is paramount for any effective training and maintenance of skill. Two types of assessment are defined and necessary: Formative assessment for improvement and development and summative assessment for evaluation of competency. Formative assessments need to be specific and concrete to suggest actions for improvement (feedback) (Michelson 2008). Summative assessment is data driven and requires statistical rigor/validation in order to make a valid judgment of an individual’s skill level. The goal with respect to simulation is to demonstrate that competency within the simulator translates into competency in the clinical realm. Competency can be assessed in the simulator by objective measures such as time to task, error rate, and economy of movement. For this level of assessment to occur, establishment of standards of competency must be developed. Minimally acceptable performance needs to be determined by a panel of experts. These benchmarks must then be established within the simulation and validated in that context by the experts. The inclusion of expert defined benchmarks helps provide a theoretical link to patient outcomes in the early adaption and adoption of a technical skills training simulator.
Well-designed simulators are posed to provide a paradigm shift in objective assessment of technical skill. Current methods to assess technical competency are seriously flawed and continue to receive little attention from certification bodies (Sidhu et al, 2004). The core competencies defined by the ACGME do not provide an adequate, objective assessment of technical skill. The reasons for this are many. However, the largest roadblock to improving technical skills assessment is the lack of an objective methodology that is reliable, valid and practical. Reliability refers to consistency, repeatability, and dependability of the measures. Validity refers to the concept of a metric actually measuring what it is supposed to measure and can be defined by criterion (correlates with other measures), construct (correlates with level of training), content (reflects content of domain), and face (extent of measure reflecting real life situation). Use of simulators in domains other than otolaryngology has been shown to provide valid, reliable and practical assessment of technical skill (Sidhu et al., 2004). It is imperative that Otolaryngology follows suit in this area of objective assessment.
Technical skills assessment requires a “gold standard” for which other assessment methodologies can be compared. No truly objective, validated assessments are widely available in otolaryngology. Validated assessment tools are beginning to emerge through the use of expert opinions/surveys (Butler and Wiet, 2007, Laeeq et al, 2009, Wan et al. 2010). The otologic experience lags behind that which has been established in sinus surgery (see ES3 below). In the context of otologic surgery, these assessment tools are still cumbersome, ill defined and impractical to administer; often requiring long hours of expert review of individual performances and validation. As a result study sizes are small. With respect to that developed for the sinus surgery simulator studies, most are small and further limited due to the cost of the simulator hardware. Only through large scale, multi-institutional studies can these tools be more standardized, rigorously studied, validated, and accepted.
The establishment of technical skills assessment tools with expert defined metrics integrated into a simulation based training system will be the underpinning for proficiency based training programs. Brydges et al., describe a methodology for developing such a simulation based training program where trainees progress from less to more technically demanding skills and tasks, only after achieving defined criteria (Brydges et al. 2008). This methodology is, in a sense, implicit in the “apprentice/master” training system but not well defined. The current execution has been marked by non-standardized and subjective influences with the ultimate criteria being the completion of a prescribed time in training or “adequate number of procedures”. This current concept of surgical skills training/assessment does not provide objective and valid measures of performance. Simulation based training and assessment provides the means by which valid and objective measures can be instituted and provides a safe environment for surgical trainees to assess their performance rigorously without risk to patients (Tavakol et al. 2008). As previously mentioned, the experience with the ES3, although on a small scale, has demonstrated that this can be accomplished within the Otolaryngology community.
Simulation Systems in Otolaryngology
Since the introduction of simulation in medicine, Otolaryngology has increasingly been involved in promoting its development and validation, most notably in endoscopic sinus surgery and temporal bone surgery. A complete review of these two areas was recently published in Cummings - Otolaryngology Head and Neck Surgery (Fried et al., 2010). The following provides a brief summary of these past developments as well as more current progress.
Endoscopic Sinus Surgery Simulator (ES3)
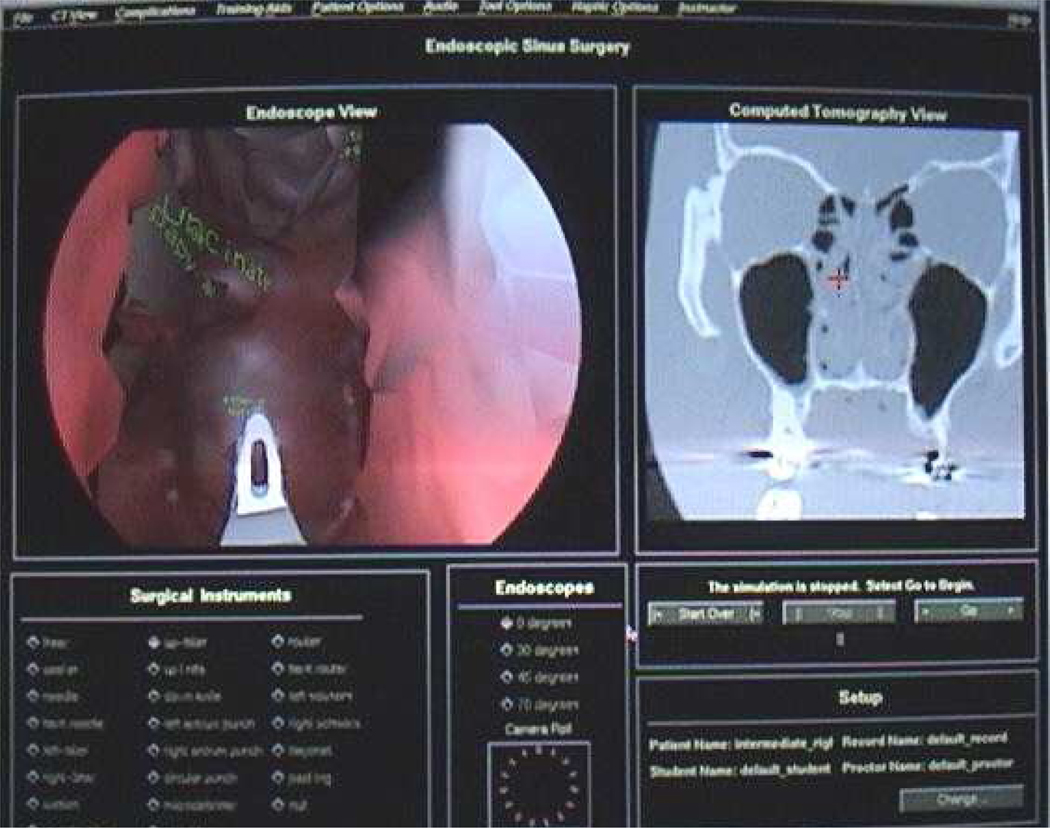
The Endoscopic Sinus Surgery Simulator (ES3) was the first sinus surgery simulator developed and to this day remains a leading system with several validation studies completed (Gallagher 2003). Inspired by aviation training simulators for military aircraft, the ES3 was developed between 1995 and 1998 by Lockheed Martin, in association with the University of Washington, The Ohio State University, and the Ohio Supercomputer Center, with sponsorship from the U.S. Army Medical Research and Materiel Command (Edmond et al. 1997). The ES3 was first utilized in the Army in 1997 and gained popularity among medical students and residents within the armed forces. The simulation system involves CT-derived three-dimensional paranasal sinus anatomy models and interactions with a virtual endoscopic instrument with haptic feedback. The ES3 utilizes an “expert surgical assistant” that interprets multimodal input to provide automated feedback to the user and warnings as critical structures are approached. It also allows the user to query the system regarding relevant anatomy and procedural maneuvers (Billinghurst et al. 1996). Yale University has developed a state-of-the-art curriculum to standardize training on the ES3 regardless of level, available on a compact disc. The ES3 consortium is currently led by Albert Einstein College of Medicine and includes the Agency for Healthcare Research and Quality (funding), Yale University (curriculum development), New York University Medical Center, New York Eye and Ear Infirmary, and Mount Sinai Medical Center (data collection), and the University of Washington – Human Interface Technology Laboratory (web database). See Figure 2.
Figure 2.
ES3 visual interface. Image courtesy of Marvin P. Fried, M.D., Albert Einstein College of Medicine.
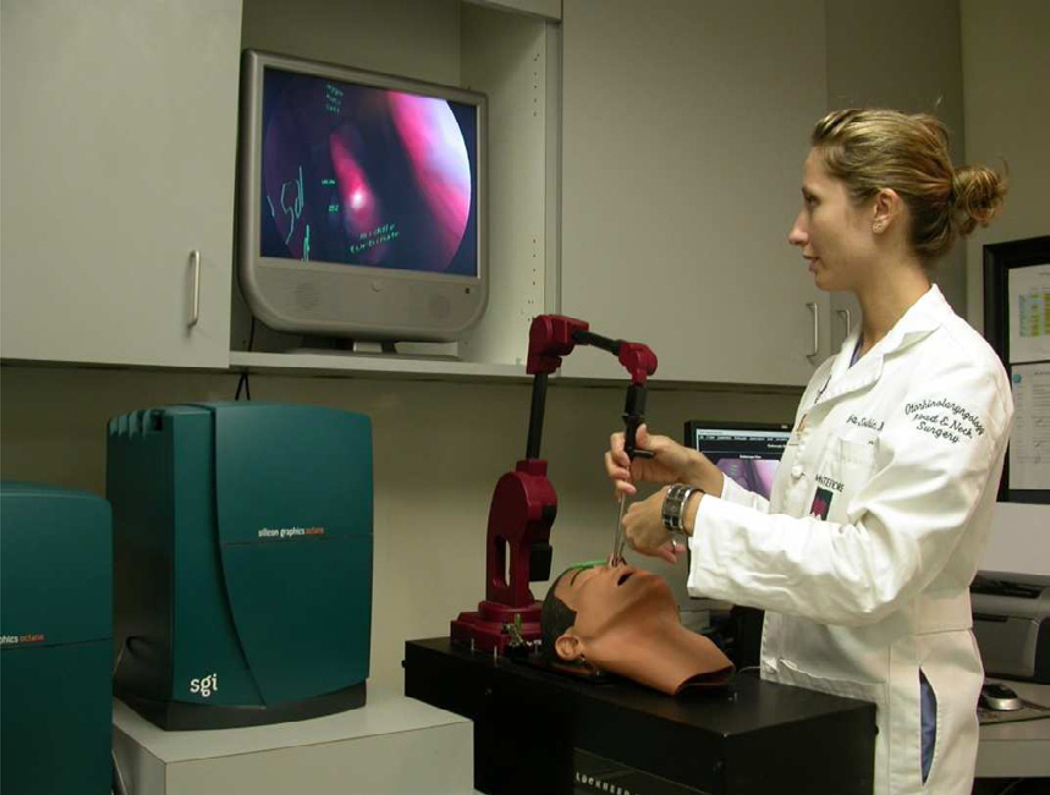
“The ES3 is one of the few virtual reality simulators with a comprehensive validation record” (Fried et al. 2010), a notion that allows it to be claimed as one of the leading ESS simulation systems. An initial study demonstrating construct validity of the ES3 showed significant correlation between performance on the ES3 and performance on other validated tests of innate ability in psychomotor, visuospatial, and perceptual capacities (to parallel the ESS-required skill of two-handed coordination of surgical instruments in a 3D space) (Arora et al. 2005). A second study compared the performance of medical students, otolaryngology residents, and attendings on the ES3. The ES3 was clearly able to distinguish between the three levels in initial trials, with the expert performing at the highest level, followed by residents, then medical students. This study also showed that all groups achieved a remarkably similar plateau score by the tenth trial on the simulator, demonstrating the ability of the ES3 to consistently achieve a standard performance goal in users (Fried et al. 2007). The most recent validation study (“Virtual Reality to Operating Room”, or “VR to OR”), which provides the strongest clinical correlation, evaluates whether training on the ES3 translates to improved performance in actual surgery. This study showed that otolaryngology junior residents who received both conventional sinus surgery training as well as ES3 training (experimental group) performed significantly better than residents who received only conventional training (control group). Improved performance in the experimental group includes significantly shorter operating time, demonstration of higher confidence, better skills in instrument manipulation, and fewer technical errors (Fried et al. 2010). Of interest, the ES3 has also been shown to be an effective tool in training opthalmology residents in endoscopic endonasal dacryocystorhinostomy at the Albert Einstein College of Medicine, effectively extending its use beyond just the field of Otolaryngology (Weiss et al. 2008). The limitations of the ES3 are that it is no longer in production and there are only a handful of systems in existence. Additionally, there has been no update to the underlying technology supporting the system since its inception over a decade ago. Dr. Marvin Fried of Albert Einstein College of Medicine has continued to champion the use of this simulator and as noted above, has been a pioneer in its application. See Figure 3.
Figure 3.
User in ES3 simulator. Image courtesy of Marvin P. Fried, M.D., Albert Einstein College of Medicine.
Other ESS Simulators
While the ES3 remains the primary ESS simulation system in the US, several other ESS simulators are worthy of mention. The University of Hamburg-Eppendorf group in Germany (developers of the VOXEL-MAN TempoSurg simulator) recently developed a new paranasal sinus surgery simulator that is compatible with standard PC hardware. This system utilizes 3D models of human skulls obtained from high-resolution CT images with mucosa and vital relevant organs added manually. It employs a lower-cost haptic feedback device to promote affordability. Learning effects of this simulation have yet to be quantified (Tolsdorff et al. 2010). The Innovation Center for Computer Assisted Surgery group (ICCAS) at the Medical Faculty of the University of Leipzig, Germany is also developing a Virtual Reality Functional Endoscopic Sinus Surgery (VR-FESS) simulator and a transphenoidal pituitary surgery simulator. The ICCAS projects generally place a stronger emphasis on clinical applications (ie, pre-operative planning and intra-operative guidance) rather than training. Another group at Colombia is using “telesimulation” to create a virtual reality tool aiding trainees in resource-limited countries to gain skills to perform FESS. Their project, the Web Environment for Surgery Skills Training on Otolaryngology (WESST-OT), utilizes an internet-based educational cycle that simulates the stages of a real procedure. This system still requires work in its development, but represents a valuable concept of telesimulation to promote distance learning in disadvantaged countries (Navarro et al. 2005). Researchers at Stanford have recently implemented a sinus surgery simulation environment that introduces automatically derived data sets from preoperative, patient-specific imaging (Wired Science 2009).
Simulation of Temporal Bone Surgery
Below, we provide a comprehensive history of the evolution of technologies that have been explored in the otological curriculum.
Traditional media in the otological curriculum
To date, temporal bone surgery has been learned through contemporary media: textbooks and atlases (Schuknecht and Gulya 1986, Glasscock and Shambaugh 1990, Donaldson 1992, Swartz and Harnsberger 1998); illustrations; CDROMS (Brodie 1997, Blevins 1998); models (Golding-Wood 1994, Pettigrew 2002); and cadaver dissections (Nelson 1991, Sando 1986)). Although CDROMs provided a cost-effective solution through the integration of photographs, illustrations, movies, computer graphics and tomographic images, the solutions from interaction remain predetermined and provide limited, if any, task fidelity, i.e., correlation between the training and performance environment. Selections are from a limited number of choices, are schematic in representation, and the results are not unique to the individual.
It is difficult to achieve a consummate comprehension of the subtle spatial relationships required for temporal bone surgery without diligent studies through dissection over a period of four to five years (Nelson 1991, Sando 1996). To facilitate understanding of the intricacies of the regional anatomy of the temporal bone, some authors have presented techniques for tissue preservation and display (Golding-Wood 1994). The resulting displays provide the student with only limited and passive means to study the intricate relationships of structures found in the temporal bone. In addition, the physical limitation of the material, associated risk of infection (HIV, Hepatitis B, C), exposure to formalin, and decreasing availability make this method increasingly problematic. To increase the availability of material and reduce the risks of infection, Pettigrew introduced a plastic model of the otic capsule and middle ear for drilling practice (Pettigrew 2002). These models are structurally realistic and serve as substitute materials for drilling practice. However, plastic models are subject to similar physical limitations as cadaver specimens, i.e., to start over, a new plastic specimen must be used, and provide a limited force correlate, i.e., biofidelity, to actual bone due to the homogeneity of the plastics.
Use of Computer Simulations in Histopathological and Morphological Studies
Three-dimensional reconstructions from computed tomography have been extensively integrated with computer aided design (CAD) techniques to provide a non real-time system for use in the diagnosis and surgical planning of craniofacial disorders (Vannier 1983). Methods for characterizing the morphology and histopathology of the temporal bone soon followed (Sando 1986). Subsequently these methods were combined with computer-aided reconstruction techniques for visualization and morphometric analysis (Takagi 1988, 1989, Nakashima 1993, Yasumura 1993, Fujita 1994, Sakashita 1995, Sando 1996, Rosowski 1996, Hinojosa 1996, Ikui 1997, Sando 1998). Although specimen preparation and integration of the photomicrographs through manual methods was time intensive, the advantage of three-dimensional reconstructions to demonstrate subtle morphological relationships and changes clearly became evident. More recently several groups have used photomicrographs to create elegant data sets of the human temporal bone (Sorensen et al, 2002). Although providing exquisite detail and near natural coloration, these preparations take considerable time and effort, and consequently provide limited variance.
Harada first introduced the concept of exploiting 3D volumetric reconstructions from computed tomography for emulating drilling and exposing the intricate regional anatomy of the temporal bone (Harada 1988). However, owing to the computational overhead of volumetric representation at the time, real-time interactions were unavailable. Subsequently, surface-based (isosurfaces) approaches were predominantly employed for modeling structure to exploit the hardware-accelerated surface rendering techniques that have been developed for other applications such as video gaming. Similar techniques using reconstructions from histological sections to derive iso-surfaces have focused on clarifying the spatial relationships of the regional anatomy (Takagi and Sando 1988, Green 1990, Kuppersmith 1997, Mason 1998). Stereo presentations of surface-based models acquired from the Visible Human Project (VHP-NLM 2010) have been presented for trans-petrosal, retro-sigmoid, and middle fossa approaches to the cerebro-pontine angle (Serra 2002). Albeit surface-based representations of soft tissues and bone structures have been developed, the systems did not provide haptic feedback to the user, and only provided a schematic emulation of dissection and surgical technique. The development of stereo volumetric, physically based simulations have been presented (John 2001, Agus 2002, 2003). These systems did not support real-time viewing and aural simulation. Although volumetric data sets have been integrated, no multi-scale data, i.e., data acquired at multiple scales, have been reported. Agus has extensively explored efforts to present secondary characteristics, such as bleeding, debris formation, and fluid flow simulation. Ray-casting techniques have been developed to provide stereo simulations of cutting and drilling based on reconstructions from computing tomography (Pflesser 2000). Although all investigators present initial enthusiasm from local surgeons and residents, local or extensive multi-institutional studies have not been conducted to validate the efficacy of these systems as compared to traditional methods of training and assessment.
Current Systems
Stanford University
The temporal bone simulation system developed at Stanford University utilizes CT data with hybrid volume and surface-based 3D rendering, as well as a haptic interface providing force feedback and vibration. The haptic interface is networked, allowing a trainee to “feel” forces and maneuvers being applied by an expert in a linked system (Morris et al. 2006). The simulator has demonstrated construct validity with a study showing significantly different global scores received by experienced users compared to novice users performing a mastoidectomy on the simulator (Sewell et al. 2007). A validation study demonstrating translation of the simulator’s beneficial learning effects to improved performance in the operating room is still in process. Stanford’s group places special emphasis on promoting independent learning on the simulator, eliminating the need for constant supervision by an instructor. Sewell et al. (2008) presented specific metrics integrated into the simulator. Implementation of these metrics allowed the simulator to provide instant automated instructional feedback to the user. Integrated metrics include drilling and suctioning techniques, bone removal, facial nerve exposure, and drill forces and velocities.
The Ohio State University
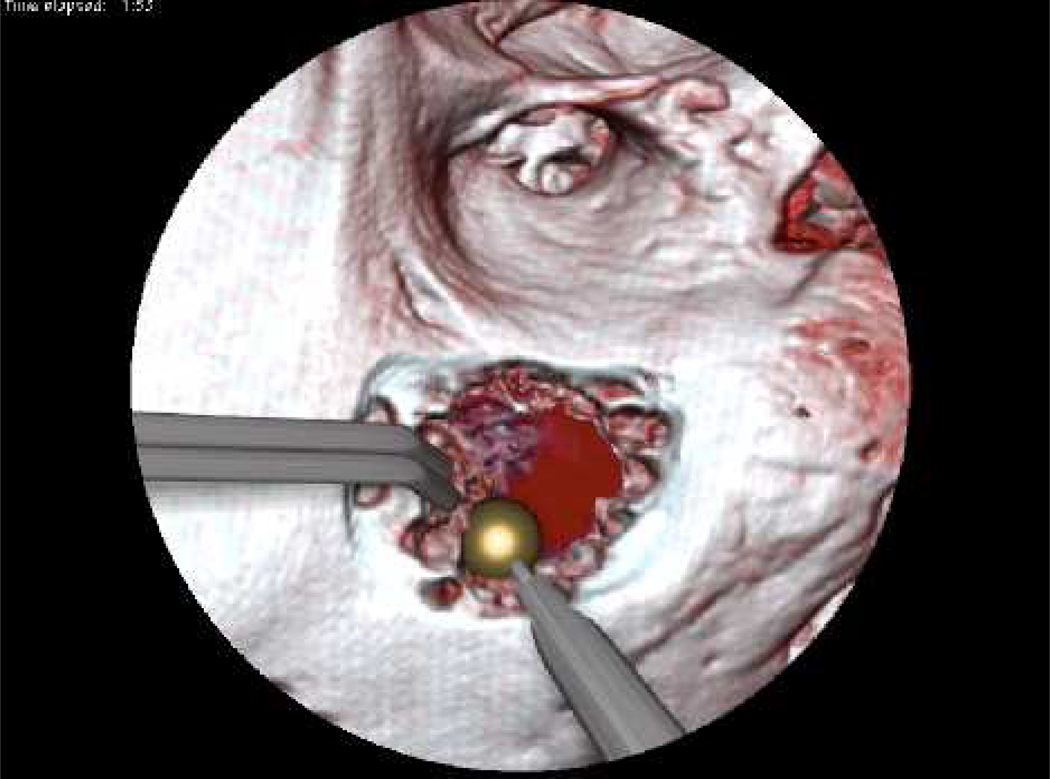
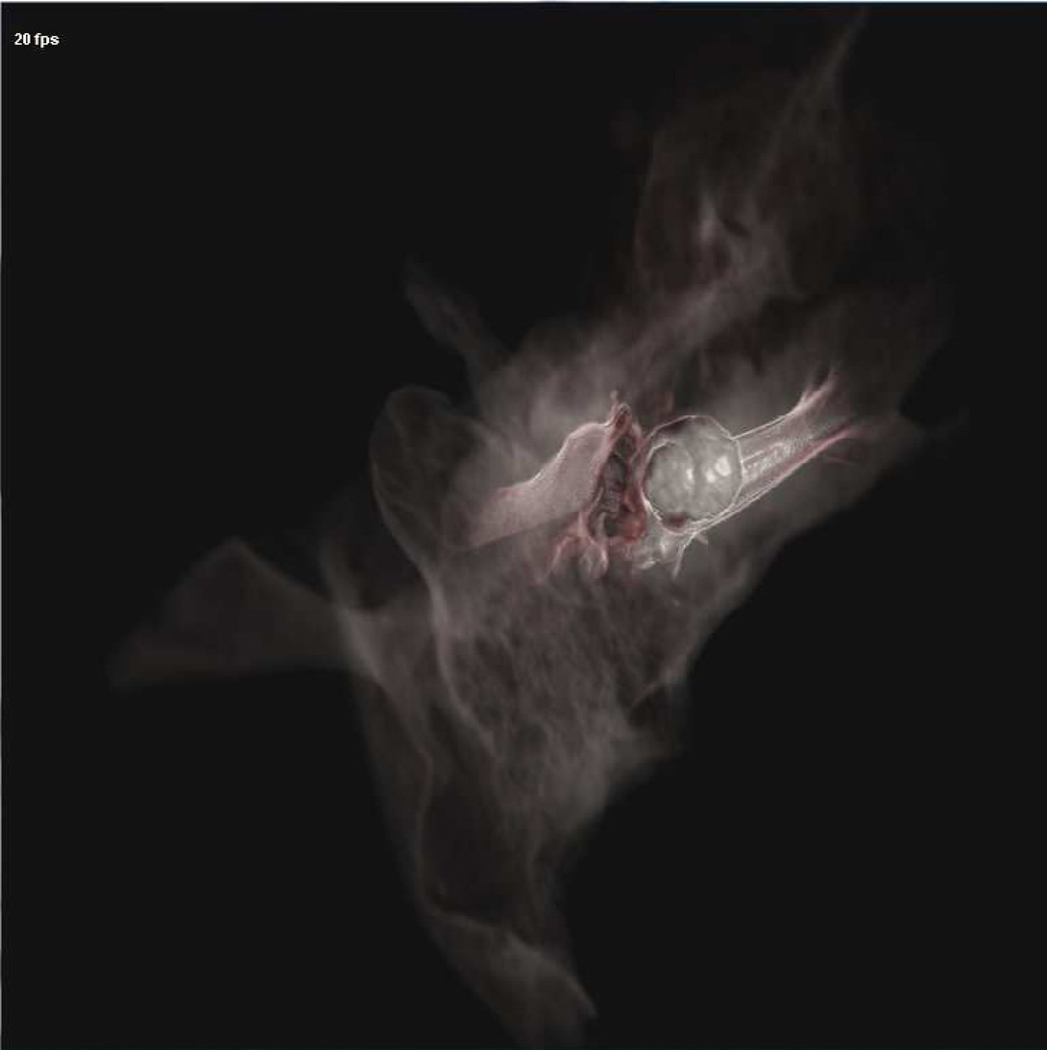
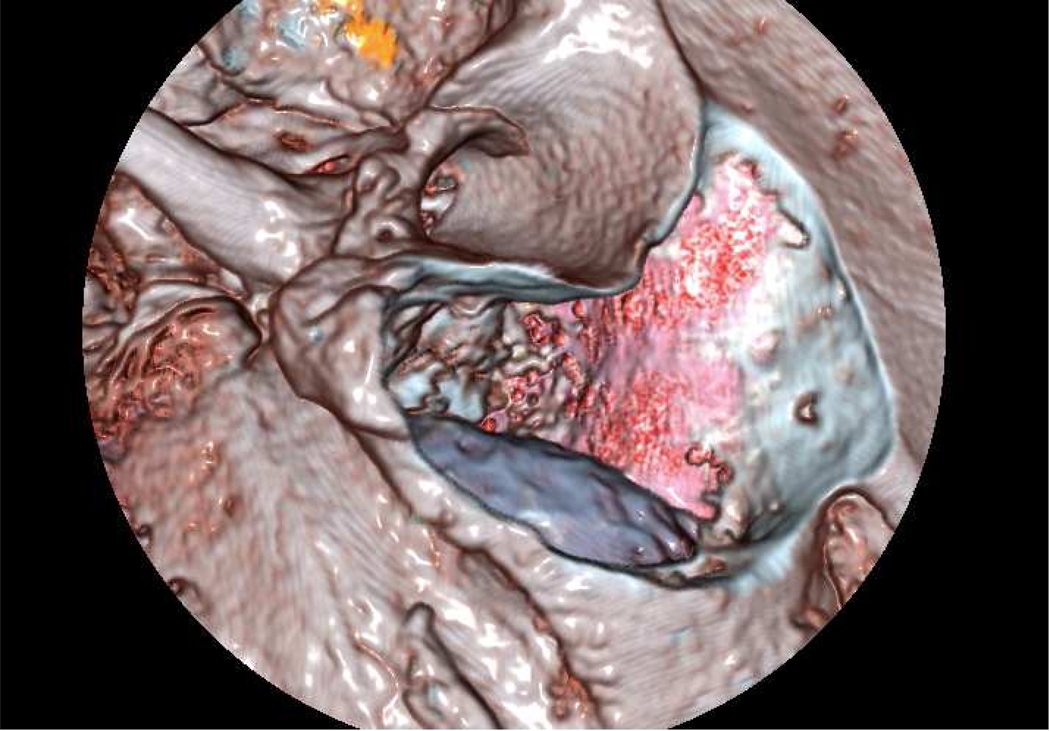
The temporal bone simulator developed at The Ohio State University Medical Center in conjunction with the Ohio Supercomputer Center also uses a hybrid renderer similar to that used in the Stanford system (See Figure 1, 6, 7, and 8). The OSU system is being developed under funding from the National Institutes of Health/National Institute on Deafness and Other Communication Disorders (NIH/NIDCD). The difference in approaches between the two systems centers on how the bone is rendered. The OSU simulator employs direct volume rendering while the Stanford simulator utilizes a surface based approach. The OSU simulator uses three interfaces to create a seamless environment, including a visual component emulating a stereomicroscope, a haptic component with force feedback emulating drilling burrs and irrigation/suction devices, and an aural element producing drill/suction sounds that spatially correlate with drill pressure and speed (Wiet 2006). It also integrates an “intelligent tutor” that highlights relevant anatomic structures within the temporal bone upon the user’s prompt. In an attempt to provide an exceptional contextual realism (Johnson 2007) the OSU system places special emphasis on structural and contextual realism. Recent additions include enhancement of fluid rendering (bleeding effects, meniscus rendering, and refraction) and a shading model to enhance wet surfaces (Kerwin et al. 2009). Preliminary trials show that this simulator has reached the standard of development to be used in otology residency training curriculums (Wiet et al. 2000, 2002; Stredney et al. 2002). Further, the system is currently completing a large-multi-institutional (10 sites, 70 study subjects) trial evaluating performance outcomes of medical students and residents. This study looked at training in the simulator versus cadaveric temporal bone specimens (See Figure 4). Current efforts seek to address identified limitations, which include a seamless hierarchical representation of data acquired at multiple resolutions, a distance field approach for rendering key structures in an intelligent tutor (Kerwin 2010), and automated assessments derived from expert validated metrics (Wan et al 2010).
Figure 6.
View from OSU temporal bone simulator demonstrating interactive fluidic effects of suction/irrigation and bleeding.
Figure 7.
Image of cochlea employing distance field effect to delineate and emphasize associated structures and cavities.
Figure 8.
View from OSU temporal bone simulator showing thinning near tegmen and coloration of underlying sigmoid sinus.
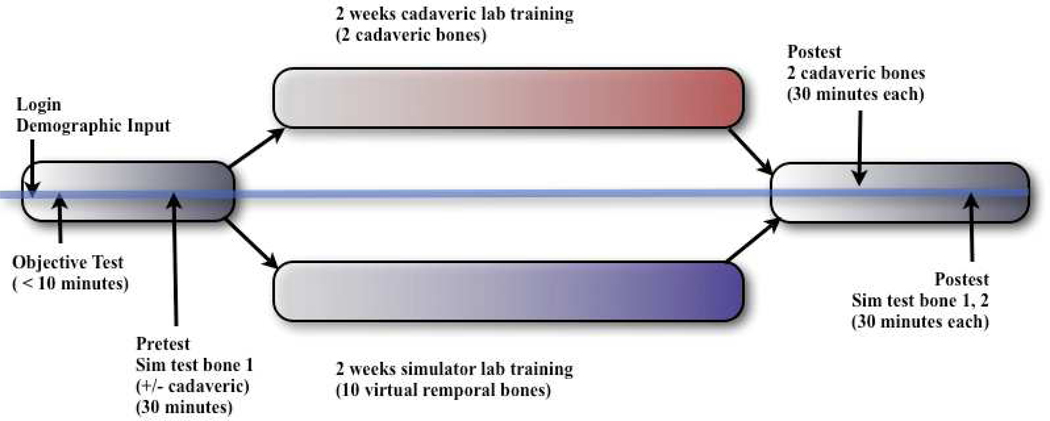
Figure 4.
Diagram of protocol employed in multi-institutional study of OSU temporal bone project.
VOXEL-MAN, University of Hamburg-Eppendorf, Germany
The VOXEL-MAN TempoSurg simulator (Spiggle and Theis, Overath, Germany; www.uke.de/voxel-man) is currently the only commercial temporal bone simulator on the market. Similar to existing temporal bone simulators, VOXEL-MAN uses 3D temporal bone models derived from high resolution CT and a haptic device providing force feedback. Images are displayed stereoscopically through a mirror. The most recent validation study involves 20 otolaryngology physicians of varying seniority who completed a self-evaluation of skill level and subsequently performed a mastoidectomy on the simulator (McDonald et al. 2009). Two expert raters blindly assessed video recordings of their performance and gave an overall impression score and a score for specific domains (i.e. flow of operation, respect for tissues) for added objectivity. Results demonstrated significant positive correlation between participants’ self-ratings of skill level and scores given by the expert raters. There was also significant positive correlation between the overall impression scores and the total score obtained from the specific domains. An earlier validation study involving otolaryngology surgeons at the University of Toronto yielded more ambiguous findings (Zirkle et al. 2007). In that study, videos were recorded of novice and experienced surgeons performing dissection on the simulator or on a cadaveric temporal bone. Expert raters were able to discriminate novice from experienced trainees at a significant level on cadaveric bones only. There was also a trend toward discrimination in the simulator recordings, though this correlation was not significant. The use of expert raters to review performance in these studies was both time consuming and lent itself to subjective biases.
Integrated Environment for the Rehearsal and Planning of Surgical Intervention (IERAPSI) Project, European Union
The IERAPSI Project is led by a consortium of European physicians and technologists and is funded by the European Commission. This group produced a temporal bone simulator that utilizes stereoscopically rendered 3D models of temporal bones derived from CT and a haptic feedback device. The three main utilities of their system include: 1) pre-operation planning using patient-specific data, 2) surgical simulation, and 3) education and training (John et al. 2001; Jackson et al. 2002). The simulator functionalities were divided into “fast” and “slow” subsystems, each responsible for different tasks. Agus et al. utilized this dichotomy to decouple the simulation on a multi-processor PC platform (Agus 2002). In another report detailing the performance of a canal wall-up mastoidectomy on the simulator, it showed strong three-dimensional simulation and dust and color representation during the first part of the procedure, but poor simulation when approaching the deeper portions of the model due to lack of haptic feedback from soft tissue and under-representation of anatomy in the pneumatized bone (Neri et al. 2006).
Other Temporal Bone Surgery Simulators
The Innovation Center for Computer Assisted Surgery (ICCAS) at the University of Leipzig has developed a Patient-Specific High-Resolution Ear Model, the main purpose of which is surgical guidance. They utilize a high-resolution microMR model overlaid onto the patient CT image, transforming the patient image into a high resolution model in a course-to-fine approach. The group is also working on a Model-Guided Mastoid Surgery, which uses a high-resolution patient-specific CT image of the mastoid region to conduct preoperative planning. This program offers applications such as ‘Navigated Control’, where the drill is automatically switched off when approaching risky structures. Again, most of the work from ICCAS focuses on clinical applications such as surgical workflow, which uses simulation to quantify and automate surgical strategies.
Finally, Sorensen has compiled a exquisite high-resolution data set derived from a single cadaveric specimen. This data set provides an unprecedented image resolution. It has been subsequently integrated into a temporal bone simulator (See Figure 5) and is available for free download at http://www.alexandra.dk/ves/index.htm (Sorensen et al, 2009).
Figure 5.
Visible Ear Simulator: Image courtesy of Mads Sorensen, M.D., Rigshospitalet, Copenhagen, Denmark.
Simulation in other Areas of Otolaryngology
The University of Western Ontario recently developed a three-dimensional virtual reality myringotomy simulator using haptic feedback. This system is one of the first VR simulators in outer/middle ear surgery and demonstrated good-to-excellent face validity with high consistency in initial validation trials (Sowerby et al. 2010).
A group at the University of Bern in Switzerland has created a simulator that provides surgical planning for anterior and lateral skull based surgery. CT, MRI, and angiography-derived images of the skull base are overlaid onto the surgical view using the simulator. This system provides a navigation system that is shown to improve surgical outcomes including decreased surgery time and less invasive approaches (Caversaccio et al. 2007).
Efforts have also been made toward developing the Computer Aided Design/Computer Aided Manufacturing (CAD/CAM) technique in mandibular reconstruction surgery. In this approach, the patient’s CT image is transferred into a software application that allows the design of patient-specific plates or titanium membranes for optimal reconstruction. This approach has been shown to improve operative time and accuracy of surgical technique (Clijman et al. 2007; Xu et al. 2007).
Discussion
Clearly, the emulation of the complex interaction of human surgery is daunting. However disparate many of these developments have been, all have presented improvements to various elements. These improvements provide strong evidence of emerging end-to-end comprehensive systems. The following presents a discussion regarding continued and emerging influences and trends for further integration of simulation in the resident curriculum.
Advantages of emerging technology
Emerging technologies, most notably in graphical processing units (GPU’s), provide unprecedented opportunities for advancements in simulation environments. These advancements include the following: (1) Reduced costs in GPU and multi-core technologies serve to promote the dissemination and adoption of simulation technologies. Whereas initial simulations in the mid 1990s required hardware environments in the 100s of thousands of dollars, more complete and complex systems are generally available today for 10s of thousands. This drop in the cost, by an order of magnitude, allows for increased participation by a broader and more diverse community of users, and subsequently can serve to promote the adaptation of simulation use. (2) The increased processor speed and capacity allow for more complex data sets to be loaded into memory. Advances in imaging technologies and integration of data will continue. As imaging technologies become more sophisticated, we will see an increase in the precision that data is acquired, in the types of data acquired at multiple scales, as well as the integration of data from different modalities. These emerging techniques will lead to unprecedented levels of detail in structural representation of the regional anatomy. As these techniques are translated to safe, non-irradiating methods, we will see an increase in the use of simulation environments for precise pre-operative assessment and planning (Leung 2010). (3) Increases in processing capacity will continue to exert positive influences on representation. These advances in processing size and power will lead to unprecedented levels of realism and dynamic interaction. Increased processor capabilities and speed provide new avenues to achieve increasingly realistic representations, providing both more realistic lighting and coloration models as well as methods to modify representations to guide the users focal attention (Kerwin 2010) See Figure 6, 7 & 8. These effects can be amortized by using the same data sets, allowing for depiction of various levels of detail, from schematic through realistic (Kerwin 2009, 2010). This capability allows for the amortization of acquisition costs by expanding the use of the single data set to include introductory training sessions, intermediate training sessions, as well as more complex procedural training at high levels of realism.
In addition, subtle interaction such as tissue deformation (Sessanna 2008, Sparks 2008) and fluidics (Kerwin 2009), previously unavailable are beginning to become achievable (See Figure 6). These improvements in contextual realism open opportunities for more advanced training and interests in pre-operative assessment, including the presentation of representations of adverse consequences. These realistic contextual representations can directly affect the motivation for users. Further studies will help to pursue correlation between the simulation (training) environments, including its depiction of various outcomes measured in the performance
Interfaces
Haptic interfaces need to be further developed, or new methods to provide dexterous interaction need to become available. For instance, cutting (scissoring) and pinching (fine grasping) need to be developed. This would allow for an expanded repertoire of surgical techniques and interaction, and may lead to new interfaces for robotic intervention (Leung 2010). As these simulation interfaces improve, they will become more widely used to for studying surgical innovation, and the pursuit of new operative techniques. Coupled with advances in emulating soft-tissue deformation, these systems will provide unprecedented levels of realism to reach the Johnson’s objective of realism to present a strong situational context.
User Monitoring
With the establishment of objective standards, we foresee improved assessment of proficiency. This includes the deliberate translation of expert assessment into objective automated assessments of proficiency and the use of more objective assessment in a more continuous fashion.
Furthermore, we anticipate increased use of physiological metrics of users. The metrics will be employed to establish levels of attention, motivation, and perseveration during various conditions of difficulty. As simulation increasingly support multiple cases to present a wider variance to the user, systems that integrate and capture physiological metrics will provide sophisticated vehicles to first better characterize shared levels of difficulty, and second, how user’s respond to adverse events encountered in a simulation.
Factors Limiting Progress
In our experience, the most limiting factor adversely effecting progress is the lack of objective standards. Standardized metrics form the basis for an objective tool to evaluate different training modalities, as well as support standardized curriculum development. Both functions are essential not only for proper development of proficiency, but also to more effectively evaluate simulation environments. Furthermore, establishing quantitative objective standards is crucial to promoting a community that employs standardize metrics for proficiency assessment.
A second factor impeding progress is reluctance to participate in formative development studies. It takes considerable time and effort for the iterative development and validation of systems required to emulate the complex environment and interactions of surgery. However, many possible participants expect systems to be further developed and validated, and are unwilling to volunteer time and effort for early development. Yet, in order for development and validation to properly occur, multi-center consortiums and government funding sources must be willing to invest the time and effort and support in formative development to help realize the full potential of emerging systems. Potential study subjects, trainees and experts need time and support for these endeavors. In our experience, many individuals have limited time to participate in developmental research (Wiet et al. 2009). Those that do participate are volunteers with limited, if any, compensation. Furthermore, institutional review boards (IRB’s) stipulate anonymity. In addition, the IRB protocol must assure the prevention of coercion of residents in participation, effort, or study completion. All of the above issues can adversely influence motivation of study subjects and significantly impact study outcomes.
Simulation technology provides the platform for which efficient and objective evaluation of technical skills can be implemented. The development of such systems for uniform, metrics based training and assessment is crucial for surgical training to progress to a new level of competence and accountability. As outlined in the introduction, pressures from both society at large, as well as from within the healthcare community have reached a critical mass that is now forcing change upon the entire training and assessment aspects of surgery. The onus is upon the Otolaryngology community to become involved in development of simulation systems for standardized, metric driven training and technical skills assessment. It is not only in the development of these systems that our responsibility lies, but also in the scientifically rigorous evaluation and iterative refinement as well. The use of simulation technology truly has the potential for paradigm shift from methodologies for training and assessment developed in the 1800s to move forward in a practical and efficient manner.
Acknowledgements
Portions of this research, Validation/Dissemination of Virtual Temporal Bone Dissection, is supported by a grant from the National Institute on Deafness and Other Communication Disorders, of the National Institutes of Health, 1 R01 DC06458-01A1. Thomas Kerwin, BS for help with preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Gregory J. Wiet, Email: Gregory.Wiet@nationwidechildrens.org.
Don Stredney, Email: don@osc.edu.
Dinah Wan, Email: dinah.wan@osumc.edu.
References
- Arora H, Uribe J, Ralph W, et al. Assessment of construct validity of the endoscopic sinus surgery simulator. Arch Otolaryngol Head Neck Surg. 2005;131:217–221. doi: 10.1001/archotol.131.3.217. [DOI] [PubMed] [Google Scholar]
- Agus M, Giachetti A, Gobbetti E, Zanetti G, Zorcolo A, John NW, Stone RJ. Mastoidectomy Simulation with Combined Visual and Haptic Feedback. In: Westwood JD, et al., editors. Proc. MMVR10; Amsterdam: IOS Press; 2002. pp. 17–23. [PubMed] [Google Scholar]
- Agus M, Giachetti A, Gobbetti E, Zanetti G, Zorcolo, Picasso B. A Haptic Model of a Bone-Cutting Burr. In: Westwood JD, et al., editors. Proc. MMVR11; Amsterdam: IOS Press; 2003. pp. 4–10. [PubMed] [Google Scholar]
- Billinghurst M, Savage J, Oppenheimer P, Edmond C. The expert surgical assistant. An intelligent virtual environment with multimodal input. Stud Health Technol Inform. 1996;29:590–607. [PubMed] [Google Scholar]
- Blevins NH, Jackler RK, Gralapp C. CDROM. Mosby-Year Book, Inc.; 1998. Temporal Bone Dissector - The Interactive Otology reference. [Google Scholar]
- Brodie H, Singh T. CDROM. University of California Davis Medical School; 1997. Oct 13, Temporal Bone Anatomy. [Google Scholar]
- Brydges R, Kurahashi A, Brummer V, et al. Developing criteria for proficiency-based training of surgical technical skills using simulation: Changes in performances as a function of training year. J Am Coll Surg. 2008;206:205–211. doi: 10.1016/j.jamcollsurg.2007.07.045. [DOI] [PubMed] [Google Scholar]
- Butler NN, Wiet GJ. Reliability of the Welling Scale (WS1) for rating temporal bone dissection performance. Laryngoscope. 2007 October;117(10):1803–1808. doi: 10.1097/MLG.0b013e31811edd7a. [DOI] [PubMed] [Google Scholar]
- Caversaccio M, Langlotz F, Nolte LP, et al. Impact of a self-developed planning and self-constructed navigation system on skull-base surgery: 10 years of experience. Acta Otolaryngol. 2007;127(4):403–407. doi: 10.1080/00016480601002104. [DOI] [PubMed] [Google Scholar]
- Donaldson JA. Surgical Anatomy of the Temporal Bone. Fourth Ed. New York: Raven Press; 1992. [Google Scholar]
- Clijmans T, Gelaude F, Abeloos J, et al. Integration of application-specificity and automation in computer-aided pre-operative planning of mandibular reconstruction surgery. Int J CARS. 2007;2:S226–S235. [Google Scholar]
- Edmond CV, Heskamp D, Sluis D, Stredney D, et al. ENT endoscopic surgical training simulator. Stud Health Technol Inform. 1997;39:518–528. [PubMed] [Google Scholar]
- Fried MP, Sadoughi B, Weghorst SJ, et al. Construct validity of the endoscopic sinus surgery simulator: II. Assessment of discriminant validity and expert benchmarking. Arch Otolaryngol Head Neck Surg. 2007;133(4):350–357. doi: 10.1001/archotol.133.4.350. [DOI] [PubMed] [Google Scholar]
- Fried MP, Sadoughi B, Gibber MJ, et al. From virtual reality to the operating room: The endoscopic sinus surgery simulator experiment. Otolaryngol Head Neck Surg. 2010;142:202–207. doi: 10.1016/j.otohns.2009.11.023. [DOI] [PubMed] [Google Scholar]
- Fried MP, Wiet GJ, Sadoughi B. Simulation and Haptics in Otlaryngology Training. In: Flint PW, et al., editors. Cummings Otolaryngology – Head and Neck Surgery. 5th Ed. Chapter 4. Philadelphia, PA: Mosby; 2010. pp. 45–51. [Google Scholar]
- Fujita S, Sando I. Postnatal Development of the Vestibular Acqueduct in Relation to the Internal Auditory Canal, Computer-Aided Three-Dimensional Reconstruction and Measurement Study. Ann Otol Rhinol Laryngol. 1994;103:719–722. doi: 10.1177/000348949410300910. [DOI] [PubMed] [Google Scholar]
- Gallagher AG. VR to OR; presentation at MMVR11; Newport Beach, CA. 2003. Jan 24, [Google Scholar]
- Glasscock ME, Shambaugh GE. Surgery of the Ear. Fourth Edition. Philadelphia, PA: WB Saunders Company; 1990. [Google Scholar]
- Golding-Wood DG. Temporal bone dissection for display. J Laryngology and Otology. 1994 January;:3–8. doi: 10.1017/s0022215100125691. [DOI] [PubMed] [Google Scholar]
- Grantcharov TP, Reznick RK. Teaching procedural skills. Brit J Med. 2008;336:1129–1131. doi: 10.1136/bmj.39517.686956.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JD, Jr, Marion MS, Erikson BJ, Robb RA, Hinojosa R. Three-Dimensional Reconstruction of the Temporal Bone. Laryngoscope. 1990 January;:1–4. doi: 10.1288/00005537-199001000-00001. [DOI] [PubMed] [Google Scholar]
- Harada T, Ishii S, Tayama N. Three-dimensional Reconstruction of the Temporal Bone From Histological Sections. Arch Otolaryngol Head Neck Surg. 1988;114:1139–1142. doi: 10.1001/archotol.1988.01860220073027. [DOI] [PubMed] [Google Scholar]
- Hinojosa R, Green D, Brecht K, Robb RA. Otocephalus: Histopathology and Three-Dimensional Reconstruction. Otolaryngology, H&NS. 1996 January;:44–53. doi: 10.1016/S0194-59989670282-6. [DOI] [PubMed] [Google Scholar]
- Ikui A, Sudo M, Sando I, Fujita S. Postnatal Change in Angle Between the Typanic Annulus and Surrounding Structures-Computer-Aided Three-Dimensional Reconstruction Study. Ann Otol Rhinol Laryngol. 1997;106:33–36. doi: 10.1177/000348949710600106. [DOI] [PubMed] [Google Scholar]
- Jackson A, John NW, Thacker NA, et al. Developing a virtual reality environment in petrous bone surgery: A state-of-the-art review. Otology & Neurotology. 2002;23(2):111–121. doi: 10.1097/00129492-200203000-00001. [DOI] [PubMed] [Google Scholar]
- John N, et al. An Integrated Simulator for Surgery of the Petrous Bone. Proc. MMVR9. 2001:218–224. [PubMed] [Google Scholar]
- Johnson E. Surgical simulators and Simulated Surgeons: Reconstituting Medical Practice and Practitioners in Simulations. Soc. Stu. Science. 2007 August;37(4):585–608. doi: 10.1177/0306312706072179. [DOI] [PubMed] [Google Scholar]
- Kerwin T, Shen HW, Stredney D. Enhancing Realism of Wet Surfaces in Temporal Bone Surgical Simulation. IEEE Transactions on Visualization and Computer Graphics. 2009 September/October;15(5):747–758. doi: 10.1109/TVCG.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See: http://www.youtube.com/watch?v=Y_6finmrqao.
- Kerwin T, Hittle B, Shen HW, Stredney D, Wiet GJ. Anatomical Volume Visualization with Weighted Distance Fields; to be presented at The second Eurographics Workshop on Visual Computing for Biology and Medicine (VCBM); 2010. [PMC free article] [PubMed] [Google Scholar]
- See: http://www.youtube.com/watch?v=DViQuoPxycM&feature=channel.
- Kuppersmith RB, Johnston R, Moreau D, Loftin RB, Henkins H. Building a Virtual Reality Temporal Bone Dissection Simulator. In: Morgan KS, et al., editors. Proc. of Medicine Meets Virtual Reality. Amsterdam: IOS Press; 1997. pp. 180–186. [PubMed] [Google Scholar]
- Laeeq K, Bhatti NI, Carey JP, Della Santina CC, Limb CJ, Niparko JK, Minor LM, Francis HW. Pilot Testing of an Assessment Tool for Competency in Mastoidectomy. The Laryngoscope. 2009 December;119:2402–2410. doi: 10.1002/lary.20678. [DOI] [PubMed] [Google Scholar]
- Leung R, Samy RN, Leach JL, Murugappan S, Stredney D, Wiet GJ. Radiographic Anatomy of the Infracochlear Approach to the Petrous Apex for Computer-Assisted Surgery. J Otology & Neurotology. 2010 January 15; doi: 10.1097/MAO.0b013e3181c99524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason TP, Applebaum EL, Rasmussen M, Millman A, Evenhouse R, Panko W. The Virtual Temporal Bone. In: Westwood JD, et al., editors. Proc. of Medicine Meets Virtual Reality. Amsterdamn: IOS Press; 1998. pp. 346–352. [Google Scholar]
- McDonald S, Alderson D, Powles J. Assessment of ENT registrars using a virtual reality mastoid surgery simulator. J. Larngol & Otol. 2009;123:e14. [Google Scholar]
- Michelson JD, Manning L. Competency assessment in simulation-based procedural education. Am J Surg. 2008;196:609–615. doi: 10.1016/j.amjsurg.2007.09.050. [DOI] [PubMed] [Google Scholar]
- Morris D, Sewell C, Barbagli F, et al. Visiohaptic simulation of bone surgery for training and evaluation. IEEE Trabs Comput Graph Appl. 2006;26(6):48–57. doi: 10.1109/mcg.2006.140. [DOI] [PubMed] [Google Scholar]
- Nakashima S, Sando I, Takahashi H, Fujita S. Computer-Aided 3-D Reconstruction and Measurement of the Facial canal and Facial Nerve. Cross-Sectional Area and Diameter: Preliminary Report. Laryngoscope. 1993 October;103:1150–1156. doi: 10.1288/00005537-199310000-00013. [DOI] [PubMed] [Google Scholar]
- Navarro AA, Hernandez CJ, Velez JA, et al. Virtual Surgical Telesimulations in Otolaryngology. In: Westwood JamesD, et al., editors. Proc. Medicine Meets Virtual Reality. Vol. 13. Amsterdam: IOS Press; 2005. [Google Scholar]
- Nelson RA. Temporal Bone Surgical Dissection Mannual. 2nd Edition. Los Angeles, CA: House Ear Institute; 1991. [Google Scholar]
- Neri E, Sellari Franceschini S, Berrettini S, et al. IERAPSI project: Simulation of a can wall-up mastoidectomy. Computer Aided Surgery. 2006;11(2):99–102. doi: 10.3109/10929080600653033. [DOI] [PubMed] [Google Scholar]
- NIDCD. 2010 see: http://www.nidcd.nih.gov/health/statistics/hearing.asp.
- NIOSH. 2010 http://www.cdc.gov/niosh/npg/npgd0294.html.
- Pettigrew AM. 2010 see www.temporal-bone.com/plastic.htm.
- Pflesser B, Leuwer R, Tiede U, Hohne KH. Planning and Rehearsal of Surgical Interventions in the Volume Model. In: Westwood JD, et al., editors. Proc. MMVR9. Amsterdam: IOS Press; 2000. pp. 259–264. [PubMed] [Google Scholar]
- Rosowski JJ, Dobrzenieki AB, Flandermeyer DT. Computer Assisted Three-Dimensuiional Reconstruction of Normal and Pathological Human Ears. Abst. Am. Acad. Of Otolaryngology H&NS. 1996:813. [Google Scholar]
- Rasmussion A, Modegaard J, Sorensen TS. Exploring Parallel Algorithms for Volumetric Mass-Spring-Damper Models in CUDA. Biomedical Simulation. 2008 [Google Scholar]
- Sakashita T, Sando I. Postnatal Development of the Internal Auditory Canal Studied by Computer-Aided Three-Dimensional Reconstruction and Measurement. Ann Otol Rhinol Laryngol. 1995;104:469–475. doi: 10.1177/000348949510400610. [DOI] [PubMed] [Google Scholar]
- Sando I, Takahara T, Doyle JD, Kitajiri M, Okuno H, Coury WJ. A Method for the Histopathological Analysis of the Temporal Bone and the Eustachian Tube and its Accessory Structures. Ann Otol Rhinol Laryngol. 1986;95:267–274. doi: 10.1177/000348948609500311. [DOI] [PubMed] [Google Scholar]
- Sando I, Takahashi T. Stereophotography of Computer-Aided 3-Dimensional reconstructions of the Temporal Bone Structures. Abst. Of Am. Acad. Of Otolaryngology H&NS. 1996:811. doi: 10.1177/019459989210600139. [DOI] [PubMed] [Google Scholar]
- Sando I, Sudo M, Suzuki C. Three-Dimensional Reconstruction and Measurement Study of Human Eustachian Tube Structures: A Hypothesis of Eustacian Tube Function. Ann Otol Rhinol Laryngol. 1998;107:547–554. doi: 10.1177/000348949810700701. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF, Gulya AJ. Anatomy of the Temporal Bone with Surgical Implications. Philadelphia, PA: Lea & Febiger; 1986. [Google Scholar]
- Scott A, De R, Sadek SAA, Garrido MC, Oath MRC, Courtney-Harris RG. J Laryn. & Oto. 5. Vol. 115. Royal Society of Medicine Press; 2001. Temporal bone dissection: a possible route for prion transmission? pp. 374–375. [DOI] [PubMed] [Google Scholar]
- Serra L, Kockro R, Goh LC, Ng H, Lee ECK. The DextroBeam: a stereoscopic presentation system for volumetric medical data. In: Westwood JD, et al., editors. Proc. MMVR10. Amsterdam: IOS Press; 2002. pp. 478–484. [PubMed] [Google Scholar]
- Sessanna D, Stredney D, Hittle B, Lambert D. Simulation of Punch Biopsies: A Case Study. In: Westwood JD, et al., editors. Proc. MMVR16. Amsterdam: IOS Press; 2008. pp. 451–453. [PubMed] [Google Scholar]
- Sewell C, Morris D, Blevins NH, Agrawal S, Dutta S, Barbagli F, Salisbury K. Validating Metrics for a Mastoidectomy Simulator. In: Westwood JD, et al., editors. Proc. MMVR15. Amsterdam: IOS Press; 2007. pp. 421–426. [PubMed] [Google Scholar]
- Sewell C, Morris D, Blevins NH, et al. Providing metrics and performance feedback in a surgical simulator. Computer Aided Surgery. 2008;13(2):62–81. doi: 10.3109/10929080801957712. [DOI] [PubMed] [Google Scholar]
- Sidhu RS, Grober ED, Musselman LJ, et al. Assessing competency in surgery: Where to begin? Surgery. 2004;135(1):6–20. doi: 10.1016/s0039-6060(03)00154-5. [DOI] [PubMed] [Google Scholar]
- Sowerby LJ, Rehal G, Husein M, et al. Development and face validity testing of a three-dimensional myringotomy simulator with haptic feedback. J Otolaryngol Head Neck Surg. 2010;39(2):122–129. [PubMed] [Google Scholar]
- Sparks J, Dupaix RB. Constitutive Modeling of Rate Dependent Stress-Strain Behavior of Human Liver Tissue in Blunt Impact Loading. Annals of Biomedical Engineering. 2008;36:1883–1892. doi: 10.1007/s10439-008-9555-3. [DOI] [PubMed] [Google Scholar]
- Stanford. Stanford Sinus Surgery. 2009 August 10; see: http://www.youtube.com/watch?v=KxpjiVDVo8Y.
- Stredney D, Wiet GJ, Bryan J, Sessanna D, Murakami J, Schmalbrock P, Powell K, Welling DB. Temporal Bone Dissection Simulation – An Update. In: Westwood JD, et al., editors. Proc. MMVR10. Amsterdam: IOS Press; 2002. pp. 507–513. [PubMed] [Google Scholar]
- Swartz JD, Harnsberger HR. Imaging the Temporal Bone. 3rd Edition. New York: Thieme; 1998. [Google Scholar]
- Takagi A, Sando I. Computer-aided three-dimensional reconstruction and measurements of the vestibular end-organs. Otolaryngology – Head and Neck Surgery. 1988;98(3):195–202. doi: 10.1177/019459988809800303. [DOI] [PubMed] [Google Scholar]
- Takagi A, Sando I. Computer –Aided Three-Dimensional Reconstruction: A Method of Measuring Temporal Bone Structures Including the Length of the Cochlea. Ann Otol Rhinol Laryngol. 1989;98:515–522. doi: 10.1177/000348948909800705. [DOI] [PubMed] [Google Scholar]
- Tavakol M, Mohagheghi MA, Dennick R. Assessing the skills of surgical residents using simulation. J Surg Ed. 2008;65(2):77–83. doi: 10.1016/j.jsurg.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Tolsdorff B, Pommert A, Hohne KH, et al. Virtual reality: A new paranasal sinus surgery simulator. The Laryngoscope. 2010;120:420–426. doi: 10.1002/lary.20676. [DOI] [PubMed] [Google Scholar]
- Vanier MW, Marsh JL, Warren JO. Three-dimensional computer Graphics for CranioFacial Surgical Planning and Evaluation. Computer Graphics. 1983 July;17(3):263–273. [Google Scholar]
- VHP-NLM. 2010 see: http://www.nlm.nih.gov/research/visible/visible_human.html.
- Volsky PG, Hughley BB, Peirce SM, et al. Construct validity of a simulator for myringotomy with ventilation tube insertion. Otolaryngol Head Neck Surg. 2009;141:603–608. doi: 10.1016/j.otohns.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Wan D, Wiet GJ, Welling B, et al. Creating a cross-institutional grading scale for temporal bone dissection. Laryngoscope. 2010 doi: 10.1002/lary.20957. Accepted for print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M, Lauer SA, Fried MP, et al. Endoscopic endonasal surgery simulator as a training tool for ophthalmology residents. Ophthal Plast Reconstr Surg. 2008;24(6):460–464. doi: 10.1097/IOP.0b013e31818aaf80. [DOI] [PubMed] [Google Scholar]
- Welling DB. Personal Communication, 2/14/2010. Recently outfitted complete temporal bone laboratory with 12 stations with instructor station, approximately $2 million. 2010 [Google Scholar]
- Williams TE, Santiani B, Thomas A, Ellison EC. The Impending Shortage and the Estimated Cost of Training the Future Surgical Workforce. Ann. Surg. 2009 August 27;:590–597. doi: 10.1097/SLA.0b013e3181b6c90b. [DOI] [PubMed] [Google Scholar]
- Wiet GJ, Bryan J, Dodson E, Sessanna D, Stredney D, Schmalbrock P, Welling B. Virtual Temporal Bone Dissection. In: Westwood, et al., editors. Proc. MMVR8. Amsterdam: IOS Press; 2000. pp. 378–384. [PubMed] [Google Scholar]
- Wiet GJ, Stredney D. Update on surgical simulation: The Ohio State University experience. Otolaryngologic Clinics of North America. 2002 December;35(6):1283–1288. doi: 10.1016/s0030-6665(02)00089-0. viii. [DOI] [PubMed] [Google Scholar]
- Wiet GJ, Stredney D, Kerwin T, Sessanna D. Dissemination/validation of virtual simulation temporal bone dissection: a project update. Assoc. Res. Otolaryngol. Abs. 2006:718. [Google Scholar]
- Wiet GJ, Rastatter J, Bapna S, Packer M, Stredney D, Welling DB. Training Otologic Surgical Skills through Simulation – Moving Towards Validation: A Pilot study and Lessons learned. J. Grad. Med. Ed. 2009;1(1):61–66. doi: 10.4300/01.01.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wired Science. Virtual Reality Could Keep You From Being a Surgical Guinea Pig. 2009 August 10; [Google Scholar]
- Yasumura S, Takahashi H, Sando I, Aoki H, Hirsch BE. Facial Nerve Near the External Auditory Meatus in Man: Computer Reconstruction Study-Preliminary Report. Laryngoscope. 1993 September;103:1043–1047. doi: 10.1288/00005537-199309000-00016. [DOI] [PubMed] [Google Scholar]
- Zirkle M, Taplin MA, Anthony R, Dubrowski A. Objective Assessment of Temporal Bone Drilling Skills. Annals of Otolgy, Rhinology, & Laryngology. 2007;116(11):793–798. doi: 10.1177/000348940711601101. [DOI] [PubMed] [Google Scholar]