Abstract
The structural stability of a cyclically distending elastic artery and the healthy functioning of vascular smooth muscle cells (SMCs) within are maintained by the presence of an intact elastic matrix and its principal protein, elastin. The accelerated degradation of the elastic matrix, which occurs in several vascular diseases, coupled with the poor ability of adult SMCs to regenerate lost elastin, can therefore adversely impact vascular homeostasis. Similarly, efforts to tissue engineer elastic matrix structures are constrained by our inability to induce adult cells to synthesize tropoelastin precursors and to crosslink them into architectural mimics of native elastic matrices, especially within engineered constructs where SMCs/fibroblasts primarily deposit collagen in abundance. In this study, we have shown that transforming growth factor-beta1 (TGF-β1) and hyaluronan oligomers (HA-o) synergistically enhance elastic matrix deposition by adult rat aortic SMCs (RASMCs) seeded within nonelastogenic, statically loaded three-dimensional gels, composed of nonelastogenic type-I collagen. While there was no substantial increase in production of tropoelastin within experimental cases compared to the nonadditive control cultures over 3 weeks, we observed significant increases in matrix elastin deposition; soluble matrix elastin in constructs that received the lowest doses of TGF-β1 with respective doses of HA-o, and insoluble matrix at the highest doses that corresponded with elevated lysyl-oxidase protein quantities. However, despite elastogenic induction, overall matrix yields remained poor in all experimental cases. At all provided doses, the factors reduced the production of matrix metalloproteinases (MMP)-9, especially the active enzyme, though MMP-2 levels were lowered only in constructs cultured with the higher doses of TGF-β1. Immuno-fluorescence showed elastic fibers within the collagen constructs to be discontinuous, except at the edges of the constructs. Von Kossa staining revealed no calcific deposits in any of the cases. This study confirms the benefits of utilizing TGF-β1 and HA-o in inducing matrix elastin synthesis by adult RASMCs over nonadditive controls, within a collagenous environment, that is not inherently conducive to elastogenesis.
Introduction
The normal functioning of various soft connective tissues is maintained by the presence of an intact network of elastic fibers within their extracellular matrix (ECM). Within elastic arteries, such as the aorta, the elastic fibers, composed primarily of elastin protein, account for ∼50% of the dry tissue weight.1 While the elastic matrix is responsible for providing vessels the necessary recoil and compliance to accommodate blood flow, intact elastic fibers also regulate vascular smooth muscle cell (SMC) behavior through mechano-transduction,2 particularly during morphogenesis and disease progression.3 Once injured, the elastic matrix is not repaired due to (a) poor elastin precursor (tropoelastin) synthesis by adult cells, (b) inefficient recruitment and crosslinking of tropoelastin into an elastic matrix, and (c) further organization into elastic fibers.4 The failure to reinstate a healthy matrix, when damaged by injury or disease, or when congenitally malformed or absent, can therefore severely compromise tissue homeostasis. This is one of the reasons why synthetic graft replacements (e.g., ePTFE) for such diseased segments, though capable of reinstating vessel elasticity and compliance, are unable to provide biologic stimuli to restore healthy vascular cell phenotype and tissue homeostasis.
Alternative strategies to tissue engineer elastic vessel replacements using autologous adult vascular SMCs seeded on biodegradable scaffolds, natural or synthetic, have been challenged by poor elastogenicity of these cell types, and lack of knowledge of materials and methods to biomimetically replicate and enhance tropoelastin synthesis, recruitment, crosslinking, and matrix assembly.4 The problem of poor elastic matrix deposition is especially severe in cellular microenvironments within such tissue-engineered constructs invariably rich in regenerated collagen, which switch SMCs to a less synthetic, and even less elastogenic phenotype.5
Of potential benefit to overcoming the poor elastogenicity of adult vascular cells, our laboratory previously determined the synergistic benefits of hyaluronan oligomer (HA-o) and transforming growth factor-beta1 (TGF-β1) to elastin precursor synthesis, elastic matrix deposition, and fiber formation by cultured adult rat and human SMCs.6,7 We also showed that TGF-β1 increases expression levels of mRNA for tropoelastin, and that of the matrix crosslinking enzyme, lysyl oxidase (LOX), while suppressing the activity of matrix degrading matrix metalloproteinases (MMPs).7,8 Negatively charged HA is also thought to coacervate tropoelastin molecules for more efficient localized crosslinking and organization of these precursors into mature fibers, much like the role of glycosaminoglycans (GAGs) in a developing aorta.9,10 Our prior data also suggest that oligomeric forms of HA, specifically 4- and 6-mers enhance elastin mRNA expression as well. The presence of collagenous matrix is centric to replicating vascular tissue architecture and mechanics,11 and vascular cells, regardless of the choice of scaffolds, robustly synthesize collagen.4,12 It is therefore imperative to examine the impact of a pre-existing collagenous microenvironment on the ability of the cells to synthesize fibrous elastic matrix on their own and also their response to provided elastogenic factors.5 Toward utilizing the above factors to enable and enhance elastic matrix deposition within collagenous tissue, in the present study, we investigate the effect of these factors at different dose combinations within statically loaded three-dimensional (3D) cellularized collagen constructs.
Materials and Methods
Isolation and culture of rat aortic SMCs
Abdominal aortae were harvested from adult Sprague-Dawley rats according to animal protocols approved by the Medical University of South Carolina, where this work was performed. After scraping out the intima and the adventitia, the medial layers of the aortae were minced into 1–2-mm-long pieces, incubated first in Dulbecco's modified Eagle's medium (DMEM)-F12 (Gibco-Invitrogen, Carlsbad, CA) containing 20% v/v fetal bovine serum (PAA Scientific, Ontario, Canada), 1% v/v Penstrep (Thermo Scientific, Rockford, IL), and 175 U/mL collagenase II (Worthington Biochemicals, Lakewood, NJ) for 1 h at 37°C. The pieces were then incubated in a second digestion mixture containing the above solution supplemented with 0.25 mg/mL of elastase III (Worthington Biochemicals) for 45 min. The digestate was finally centrifuged at 200 g for 5 min, and the pellet re-constituted and cultured in DMEM-F12 containing 20% v/v serum and 1% v/v Penstrep. Primary rat aortic SMCs (RASMCs) thus obtained were cultured and passaged in T-75 flasks. Cells of low passage (P3-P5) were used to seed the collagen constructs.
Fabrication of cellularized collagen constructs
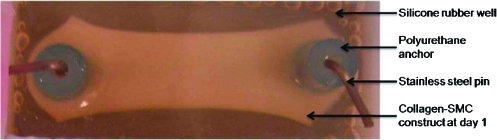
The inherent nature of cell-seeded collagen to shrink and form a compacted gel was utilized to fabricate 3D constructs. Collagen-cell mixtures were aliquoted within rectangular (3.8×2×1.5 mm) silicone rubber (Smooth-On, Inc., Easton, PA) wells cast in 100-mm-diameter glass petri-dishes.13,14 The molds/dishes were steam-sterilized at 121°C for 30 min. To obtain directed compaction of the collagen gels under static tension, polyurethane tubes anchored with stainless steel pins (both sterilized in 1 N HCl for 20 min and rinsed in sterile phosphate-buffered saline) were anchored to the ends of each well.
Acid-solubilized type I collagen (BD Biosciences, Bedford, MA) was mixed with 5×DMEM/F12 and 0.1 N NaOH and added drop wise to titrate the mixture to physiological pH. RASMCs (passages 3–5) were trypsinized, centrifuged, and re-suspended in medium and added to the above solution to obtain a final concentration of 2 mg/mL collagen, 1×106 cells/mL, 20% v/v serum, and 1% v/v Penstrep in DMEM-F12. All mixing was performed on ice to prevent premature polymerization of collagen when physiologic pH was attained. Equal volumes of this collagen-SMC mixture were then aliquoted into each well and cultured in a CO2 incubator at 37°C. Within a few hours, the collagen–cell mixture started to gel and anchor around the polyurethane end holders. These holders prevented longitudinal gel contraction, but enabled contraction in a direction perpendicular to the long axis of the wells (Fig. 1).
FIG. 1.
Statically loaded rat aortic smooth muscle cell-seeded three-dimensional collagen gels. Gels, shown at day 1 of culture, were cast within silicone wells and anchored to polyurethane end-holders. Color images available online at www.liebertonline.com/tea
A sterile cocktail of HA-o and TGF-β1 (Peprotech, Rocky Hill, NJ) was exogenously supplemented in the growth medium, at six different dose combinations (0.1, 1, and 10 ng/mL of TGF-β1, each with 0.2 and 2 μg/mL of HA-o). Since this is a pioneering study utilizing a combination of HA-o and TGF-β1 for elastic matrix synthesis by SMCs within 3D cultures, there is lack of literature indicating appropriate doses for such a model. We have therefore extrapolated the dose range for the current study from our outcomes in two-dimensional (2D) cultures, and utilized a range to include concentrations of an order of magnitude lower and higher than that used in 2D cultures to evaluate optimum conditions.7,15,16 HA oligomer mixtures used in this study primarily consisted of 4-mers (∼75%) with 6-mers and 8-mers forming the balance. They were prepared by digesting high-molecular-weight HA (1.5×106 Da; Genzyme Biosurgery, Cambridge, MA) with testicular hyaluronidase (40 U/mg at 37°C; Worthington Biochemicals) as we have described previously.17 The constructs were cultured for 21 days and spent medium changed every 2 days. Spent medium from each well was pooled with aliquots previously removed from the same wells, and frozen at −20°C until biochemically analyzed at the end of the culture period.
Construct compaction
All constructs were photographed at regular intervals using a digital camera and their central widths measured using ImageJ (NIH, Bethesda, MD) imaging software. The extent of contraction (compaction ratio) of each construct was measured as a percentage difference in width of the well and the central width of the construct at day 21. At least three measurements were made on each construct.
DNA assay for cell quantification
The DNA content of RASMCs within the collagen constructs was measured at 21 days of culture to estimate increases to cell number relative to that at seeding. Briefly, the gels were detached from the holders and digested in 10 mg/mL proteinase-K (Gibco-Invitrogen) at 65°C for 10 h until the matrix structure was completely solubilized. The enzyme was inactivated by boiling the solution for 10 min. The digested solution was then sonicated for 3 min on ice to lyse cells, and DNA content was measured using a fluorometric assay based on use of a Hoescht dye. Cell numbers were calculated assuming 6 pg of DNA/cell.18 Results were represented in terms of cell proliferation ratio (cell count at 21 days/initial seeding density) and as a fold-change in this ratio compared to control.
Fastin assay for elastin content
Total elastin synthesized by RASMCs was measured using a commercially available Fastin assay kit (Accurate Scientific and Chemical Corporation, Westbury, NY). Three distinct elastin fractions were assayed for, namely, (a) tropoelastin, the soluble precursor of elastin synthesized by cells, present within medium fractions, (b) the less crosslinked, alkali-soluble matrix elastin, and (c) the highly crosslinked, alkali-insoluble matrix elastin. Volume fractions from the pooled culture medium were directly used to measure tropoelastin. To measure matrix elastin, the constructs were first treated with 0.1 N NaOH at 95°C for 1 h and centrifuged. The resulting supernatant consisted of alkali-soluble matrix elastin, whereas the pellet comprised of alkali-insoluble matrix elastin. The soluble matrix fraction was neutralized with an equal volume of 6 N HCl at 110°C for 16 h, dried overnight, reconstituted in water, and quantified using the kit. Since the Fastin kit measures only soluble α-elastin (∼60 kDa), the insoluble matrix pellet was first solubilized with 0.25 M oxalic acid at 95°C for 1 h, and then filter-centrifuged with 10 kDa cut-off membranes (Millipore, Billerica, MA). The solubilized matrix elastin retained above the filters was then quantified with the assay kit. Tropoelastin and total matrix (alkali-soluble + alkali-insoluble) thus measured were normalized to the cell count within respective constructs. The matrix yield (i.e., the ratio of matrix elastin/(tropoelastin + matrix elastin)×100) was calculated.
Western blotting for LOX and MMPs-2 and -9
Western blotting was performed to semi-quantitatively compare the amounts of LOX enzyme protein and of the elastolytic MMPs-2 and -9, synthesized by RASMCs within constructs subjected to different treatment conditions. At day 21, harvested constructs were ground into powder in liquid nitrogen, extracted in RIPA buffer containing protease inhibitors (Invitrogen, Carlsbad, CA), and then assayed for total protein content using a BCA assay kit (Thermo Scientific).19 Volumes equivalent to 3 μg of protein were loaded under reduced conditions into 4%–12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel for LOX analysis and 10% SDS-PAGE gel for MMP analysis, along with a prestained molecular weight ladder (Invitrogen). The gels were then transferred wet onto polyvinylidene fluoride membranes. After this, the membranes were blocked in TBST (Tris-buffered saline with 0.01% v/v Tween-20, pH 7.4) containing 5% w/v milk for an hour, immunostained using polyclonal antibodies (1:1000 for 1.5 h) against LOX (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), MMP-2 (Abcam, Cambridge, MA), MMP-9 (Millipore), and HRP-conjugated secondary antibody (1:10,000 for 1 h) (GE Healthcare Biosciences, Pittsburgh, PA), and detected using an ECL-plus chemiluminogen detection kit (GE Healthcare Biosciences). The blotted membranes were then stripped and re-probed for β-actin loading control (Abcam). Bands were quantified using ImageJ software (NIH), expressed as relative density units (RDU), and normalized to control. Analysis was performed on bands generated from 3 blots per condition.
Gelatin zymography for detection of MMPs-2 and -9
Volumes containing 5 μg protein from the digested constructs were loaded per lane of 10% gelatin zymograms (Invitrogen) and run alongside a prestained protein ladder for 2 h at 90 V. The gels were then washed in 2.5% v/v Triton X-100 for 30 min to remove SDS, following which they were incubated overnight in a substrate buffer to activate MMPs. The gels were then stained in Coomassie Brilliant Blue solution for 45 min and de-stained for 1.5 h until clear bands were visible against a blue background.19 The band intensities were quantified using ImageJ and expressed as RDU values of the experimental cases normalized to RDU values of control cultures. Analysis was performed on the bands generated from three gels per condition.
Observation of elastic matrix and mineralization
At 21 days of culture, the constructs were fixed in 4% w/v formalin and embedded in paraffin. Tissue sections (5 μm thick) were then stained for elastin using a Verhoeff Van Gieson (VVG)-based elastic-stain kit (ScyTek Laboratories, Inc., Cache, UT). To observe elastin using immunofluorescence, the sections were incubated in 0.05% w/v Pontamine sky blue (Sigma, St. Louis, MO) for 30 min, mounted using Vectashield containing DAPI (Vector Laboratories, Inc., Burlingame, CA), cover-slipped, and observed with a visible-red (Texas red) fluorescent filter. Pontamine sky blue quenches the auto-fluorescence of elastin and collagen in the visible-green region of the emission spectrum, and transfers the auto-fluorescence of elastin alone to the red region of the spectrum.20 Elastin-specific fluorescence of sections labeled in this manner was confirmed by detecting elastic matrix in separate sections from the same sample with a rabbit anti-rat elastin antibody. Sections were imaged using a fluorescent microscope and alignment of elastic fibers in the constructs were quantified by calculating their angular standard deviation.21 Length and angle of individual fibers were measured using Image Pro software (Bethesda, MD). Elastin deposits with aspect ratio greater than 2 (long axis: short axis) were assumed to be fibers and only these were used for quantification.
The presence/absence of calcific deposits within constructs was ascertained by labeling sections with a Von Kossa–based calcium-staining kit (ScyTek Laboratories Inc.).
Statistical analysis
All quantitative results were analyzed from n=6 independent repeats of each case and reported as mean±standard deviation. Statistical significance between groups was determined using two-way analysis of variance. Results were deemed significant for p-values ≤0.05.
Results
Compaction of constructs
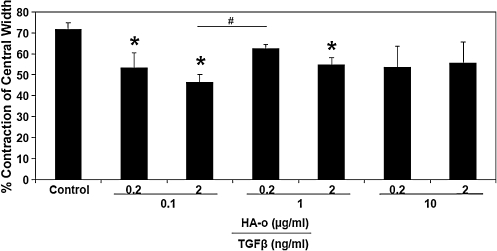
The compaction ratio of constructs cultured with the various dose combinations of TGF-β1 and HA-o was less than that of control constructs cultured without the factors (Fig. 2). Compaction ratios were the lowest in constructs that received one of the two lowest dose combinations, specifically, 0.1 ng/mL TGF-β1 and 2 μg/mL HA-o (46.57%±4.12%, p<0.05).
FIG. 2.
Percentage change in central widths of constructs at day 21 compared to day 0 (n=6 per case). *Significance of differences compared to control cultures, deemed for p<0.05. #Significance of differences in outcomes between treatments paired by horizontal bars, deemed for p<0.05.
Cell quantification
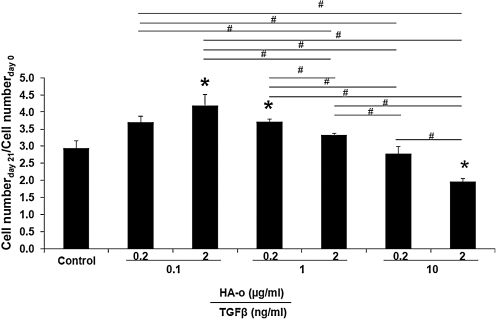
As seen in Figure 3, cell numbers were highest (4.2±0.8-fold increases over 21 days; 1.4±0.1-fold vs. nonfactor treated control) in constructs cultured with the lowest dose combination of TGF-β1 (0.1 ng/mL) and HA-o (2 μg/mL), and lowest (1.96±0.23-fold increase over 21 days; 63%±13% of cell counts in control cultures at day 21) within constructs cultured with the highest dose of factors, that is, TGF-β1 (10 ng/mL) and HA-o (2 μg/mL).
FIG. 3.
Effect of elastogenic factors on cell proliferation ratio. Cell numbers were calculated based on the estimate of 6 pg of DNA per cell, as calculated from DNA assay, at 0 and 21 days postseeding, and their ratios determined and compared to controls (n=6 replicate constructs per treatment). Differences were deemed to be significant from control cultures for p<0.05 (*). #Significance of differences in outcomes between treatments paired by horizontal bars, deemed for p<0.05.
Elastin content
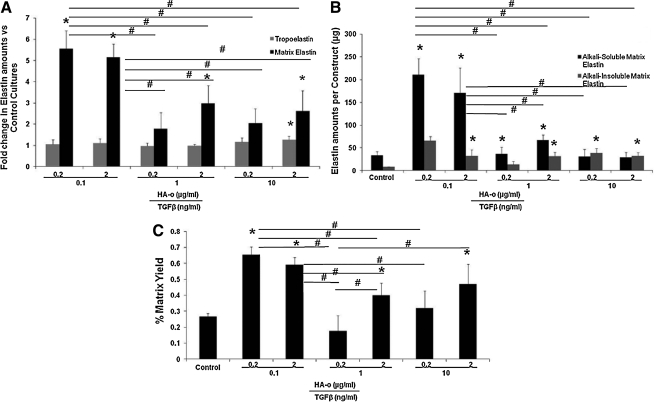
Elastin protein content was normalized to the cell counts in corresponding constructs and these ratios were further normalized to similar ratios determined for factor-treated control constructs. Over 21 days, cells within the control constructs synthesized 4.4±0.5 ng/cell of tropoelastin, 9.4±3.4 pg/cell of matrix elastin, comprising 4.5±1.8 pg/cell of alkali-soluble matrix elastin and 1.5±1.1 pg/cell of alkali-insoluble fraction. Tropoelastin synthesis per cell was unaffected by culture with TGF-β1 and HA-o, except at the highest provided dose combination (10 ng/mL TGF-β1 and 2 μg/mL HA-o) at which significant increases (1.5±0.2-fold vs. control) were noted (Fig. 4A). On the other hand, elastic matrix deposition per cell was significantly greater in most experimental cases compared to control, with greatest increases (5.4±0.8 and 5.3±1.1-folds vs. control) noted in constructs cultured with the lower dose combinations of TGF-β1 (0.1 ng/mL) and HA-o (0.2 or 2 μg/mL) (Fig. 4A). Also noted was that increases in matrix elastin, especially at the lower provided doses of factors, were associated more with increases in the less crosslinked, alkali-soluble matrix elastin fraction than with the more robustly crosslinked, alkali-insoluble fraction; at the highest provided dose combinations, fold increases in both fractions compared to control were similar (Fig. 4B). Matrix yields (Fig. 4C) were greatest in constructs cultured with the lower dose combination of factors, that is, 0.1 ng/mL TGF-β1 with 0.2 μg/mL (0.6%±0.2%) 2 μg/mL of HA-o (0.6%±0.1%) respectively.
FIG. 4.
Effect of TGF-β1 and HA-o on elastin synthesis. (A) Differences in synthesis of tropoelastin and total matrix elastin per construct in samples cultured with HA-o and TGF-β1 relative to control cultures. (B) Effect of factor treatment on synthesis of alkali-soluble matrix elastin and crosslinked, alkali-insoluble matrix elastin. (C) Effect of factor treatment on yields of matrix elastin, that is, the fraction of total elastin amounts produced by cells that is deposited in the matrix. *Differences from controls significant for p<0.05 (n=6 for each case). #Significance of differences in outcomes between treatments paired by horizontal bars, deemed for p<0.05. TGF-β1, transforming growth factor-beta1; HA-o, hyaluronan oligomer.
MMP protein analysis
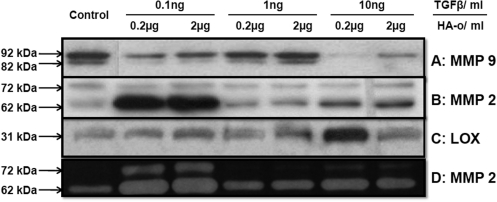
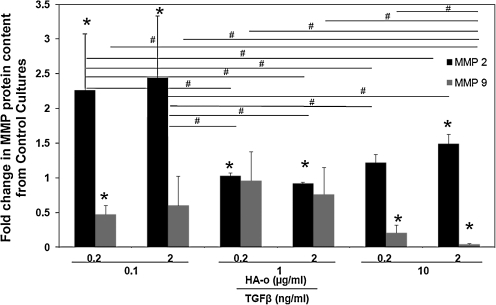
Figure 5 shows representative immunoblots of MMPs-2 and -9 and zymogram of MMP-2. Figure 6 compares MMP-2 and −9 amounts measured within collagen constructs treated with TGF-β1 and HA-o factors, with that measured within untreated control constructs. MMP-2 was detected on the Western blots as two distinct bands corresponding to the zymogen form (∼72 kDa) and active form (∼62 kDa). MMP-9 was also detected in its zymogen (∼92 kDa) and active (∼82 kDa) forms. The greatest increases in total (active and inactive forms) MMP-2 protein amounts over controls were observed within constructs cultured with the lower dose combinations of the factors. In general, MMP-9 synthesis was decreased in factor-treated constructs relative to control, though the decreases were greatest and statistically significant for constructs cultured with the highest TGF-β1 factor (10 ng/mL of TGF-β1 and 0.2 or 2 μg/mL of HA-o) and with the lowest dose combination (0.1 ng/mL TGF-β1 and 0.2 μg/mL HA-o).
FIG. 5.
Effect of factors on enzyme synthesis and active forms. Representative immunoblots of (A) MMP-9 and (B) MMP-2 indicating the active and inactive protein bands for each case, and (C) LOX. (D) Representative gelatin zymogram of MMP-2 indicating the active enzyme band at 62 kDa and zymogen at 72 kDa. LOX, lysyl oxidase; MMP, matrix metalloproteinase.
FIG. 6.
Differences in total protein synthesis of MMP-2 and −9 in experimental cases compared to control, as determined from immunoblotting of the two proteases. *Statistical difference compared to control for p<0.05 (n=3 per case). #Significance of differences in outcomes between treatments paired by horizontal bars, deemed for p<0.05c (n=3 per case).
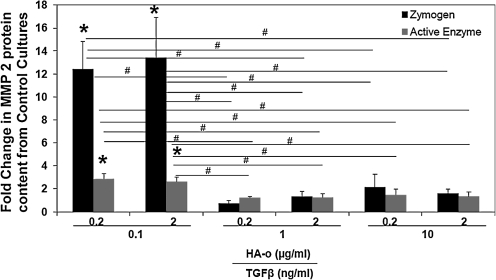
Figure 7 compares active and inactive forms of MMP-2 in factor-treated constructs with that in control constructs. MMP-9 activity is not shown since the bands were absent or too faint to be reliably quantified. In general, MMP-2 zymogen levels were very low, in both control and factor-treated constructs, except those cultured with low doses of HA-o (0.2 or 2 μg/mL) and TGF-β1 (0.1 ng/mL). The quantities of the active form of MMP-2 were also mostly similar between constructs, excepting those cultured with low doses of HA-o (0.2 or 2 μg/mL) and TGF-β1 (0.1 ng/mL), in which significant increases in enzyme activity were measured.
FIG. 7.
Effect of factors on MMP-2 quantities as determined by gelatin zymography. Data represent a fold change in relative density units of zymogram bands in experimental cases compared to control cultures. *Significance in differences compared to control for p<0.05 (n=3 per case). #Significant differences in MMP quantities (p<0.05) between pairs of treatment groups indicated by the horizontal lines.
LOX protein synthesis
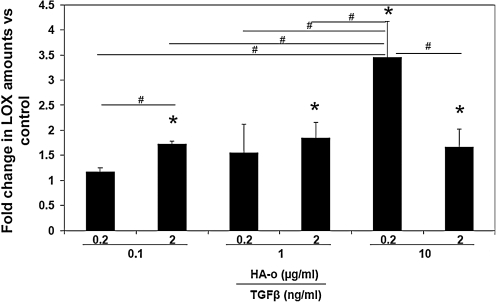
Western blot analysis for LOX enzyme (31 kDa) synthesis within cultured constructs showed elevated levels in all cases compared to control (Fig. 8). The band intensities were quantified from three independent blots for the protein (Fig. 5) in terms of RDU and are represented as fold change from control. LOX protein synthesis was most increased within constructs cultured with 10 ng/mL of TGF-β1 and 0.2 μg/mL of HA-o (3.5±0.5-fold vs. control).
FIG. 8.
Fold change in protein levels of LOX in cultures with TGF-β1 and HA-o factors compared to untreated control cultures, as determined by western blotting. *Differences from control were deemed significant for p<0.05 (n=3 per case). #Significance of differences in outcomes between treatments paired by horizontal bars, deemed for p<0.05.
Observation of elastic matrix
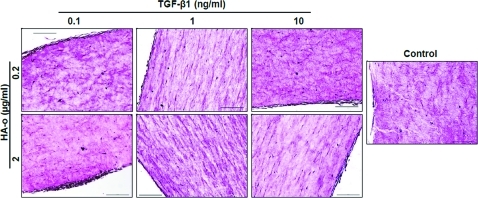
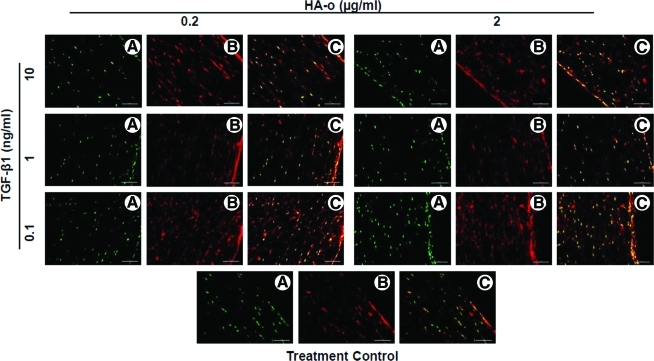
VVG staining for elastin (Fig. 9) and immuno-fluorescence labeling after Pontamine sky-blue quenching (Fig. 10) showed the presence of elastic fibers in all the constructs treated with factors, although due to the limited thickness of the sections, the continuity and total length of individual fibers could not be ascertained. Elastic fibers were more abundant and appeared more complete toward the edges of the constructs.
FIG. 9.
Representative images of 5 μm sections stained for elastin with modified Verhoeff Van Gieson at 20×magnification (scale bar=200 μm). Elastic fibers are stained purple and surrounding collagen is stained pink. Color images available online at www.liebertonline.com/tea
FIG. 10.
Representative fluorescence micrographs of construct sections showing distribution of nuclei in green (A), elastin/elastic fibers in red (B), and the overlay of the two (C). Paraffin-embedded sections were treated with Pontamine sky blue to quench autofluorescence of collagen and shift that of elastin to the red wavelengths. Nuclei were stained with DAPI. Magnification: 20×(scale bar=100 μm). Color images available online at www.liebertonline.com/tea
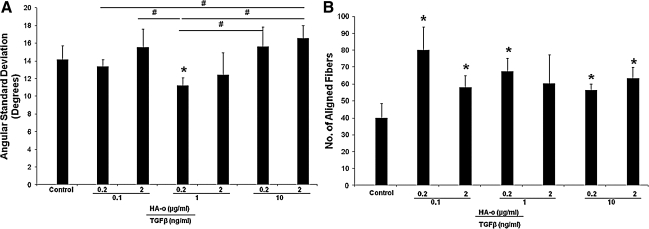
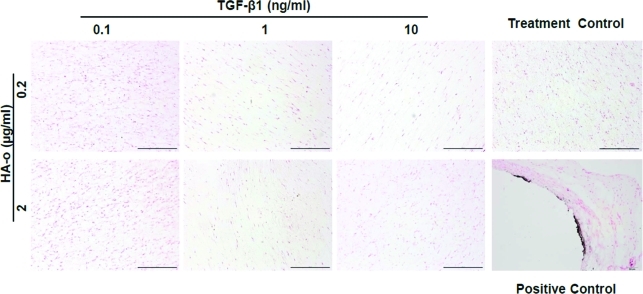
Elastic fiber alignment, calculated in terms of angular standard deviation, did not differ between the various culture conditions (Fig. 11A). However, there were a significantly greater number of elastic fibers (Fig. 11B) present in all cultures that received the elastogenic factors (except cultures that received 1 ng/mL of TGF-β1 and 2 μg/mL of HA-o). Von Kossa staining (Fig. 12) for calcific deposits indicated lack of matrix mineralization in all constructs.
FIG. 11.
Elastic fiber alignment in collagen gel constructs represented by angular standard deviation in degrees (A) and number of aligned fibers (B). *Significance in difference from nonfactor-treated control cultures, deemed for p<0.05. #Significance of differences in outcomes between treatments paired by horizontal bars, deemed for p<0.05.
FIG. 12.
Von Kossa staining for mineral deposits. The absence of any black spots/deposits indicates lack of TGF-β1-induced matrix mineralization in the dose range studied. Aneurysmal rat aortae were stained as positive controls and show the presence of sub-intimal calcific deposits (black). Cell nuclei are stained pink in all cases. Scale bar=500 μm. Color images available online at www.liebertonline.com/tea
Discussion
Attempts at engineering elastic tissue constructs, such as vascular replacements, thus far, have been challenged by poor ability of postneonatal cell types to synthesize tropoelastin and crosslink the precursors into a fibrous matrix.22,23 It is also evident from literature that the extracellular microenvironment critically influences cell phenotype and behavior. In this context, a large body of work attests to the contractile phenotype of SMCs within the intact vessel wall, and the switch to a more synthetic (i.e., proliferative and ECM-generating) phenotype, when these SMCs are isolated and cultured in vitro,5,24 either on tissue culture polystyrene (TCPS), or within scaffolds created from one of the many natural (e.g., fibrin22) or synthetic (e.g., polyglycolic acid [PGA]25) polymers. From the standpoint of elastin regeneration, vascular SMCs in contact with these scaffolds have been found to enhance tropoelastin synthesis. Although the cellular scaffolds may provide the initial framework for cell adherence and proliferation, the expectation is that the scaffold would gradually biodegrade, and cell-synthesized ECM would accumulate and assume primary load-bearing roles. Key among these ECM proteins is collagen, where cells are able to abundantly and preferentially synthesize and deposit, beginning at a fairly early stage in culture. While such collagen fiber deposition is favorable from the standpoint of replicating the 3D collagenous matrix within the aorta, there is also significant and irrefutable evidence that this microenvironment promotes a more quiescent, contractile phenotype among SMCs (compared to other scaffolding materials like PGA and fibrin), which is not particularly conducive to their ability to synthesize elastin and lay down elastic matrix structures.25–29 Thus, efforts to engineer soft elastic tissues, such as vascular tissues, will tremendously benefit from an elastin-regenerative stimulus that can overcome the unfavorable microenvironment for elastin synthesis imposed by a collagenous matrix, standard to most applications.
Previously, our lab established the elastogenic benefits of TGF-β1 and HA-o toward increasing tropoelastin precursor production and crosslinked-matrix deposition by RASMCs.7,8,30 However, these studies were performed on RASMC layers cultured on 2D TCPS surfaces, on which SMCs are known to assume a more proliferative, synthetic phenotype than what they exhibit within intact vessels. To translate the specific benefits of the above-mentioned factors to a culture model more closely evocative of a collagenous 3D matrix microenvironment, naturally present within decellularized vascular tissue-based scaffolds, and that generated by cells seeded on other scaffolds, we studied the effect of the above mentioned factors on adult RASMCs seeded within 3D collagen constructs maintained under static tension.
Relative to untreated control constructs, cell numbers within constructs cultured with HA-o and TGF-β1 were in general slightly greater, with the significant differences being only for the intermediate factor doses. A significant attenuation in the increase in cell count was noted only at the highest provided dose (i.e., 10 ng/mL of TGF-β1 and 2 μg/mL of HA-o). While these results are consistent with observations of other groups, wherein increasing TGF-β1 concentrations was found to lower cell densities,31 the results contrast substantially from our previous observations of a dose-dependent inhibition of SMC proliferation on TCPS surfaces.6 The present results suggest that (a) interaction with the collagenous ECM alters SMC behavior compared to 2D cultures, and (b) such collagen-SMC interaction de-sensitizes the cells to TGF-β1 and HA-o, attested to by a significant decrease in cell number only at the highest provided doses. It is also apparent from our results that similar to our recently published observations in 2D cultures, TGF-β1 effects dominate with a biphasic dose-dependent proliferative response to TGF-β1 at both HA-o doses.32 However, from the standpoint of a tissue engineering application, wherein growth in tissue mass is contingent on increased availability of ECM-generating cells, the HA-o/TGF-β1 dose combination that provides greater impetus to SMC proliferation (i.e., 0.1 ng/mL of TGF-β1 and 0.2 or 2 μg/mL of HA-o) is most desirable.
Within a collagenous 3D matrix, SMCs are likely to switch to a more contractile phenotype characterized by reduced ECM synthesis, which has been previously reported.25–29 Concurrently, our results indicated that within 3D collagen constructs, HA-o and TGF-β1 together had mostly no effects on tropoelastin synthesis. The sole exception was the highest factor dose, which induced a moderate, but significant increase. While this lies in complete contrast with our prior published studies in 2D culture, wherein we noted even the lowest dose combination of factors we test here, to induce multi-fold increase in tropoelastin synthesis compared to nonadditive controls, and the differences could be attributed to a switch in SMC phenotype between 2D and 3D cultures.
HA-o and TGF-β1 in this study appear to influence the process of tropoelastin crosslinking and matrix deposition to a greater extent than tropoelastin synthesis itself; the enhancement of elastic matrix deposition appears to be the predominant effect of the factors, though in the absence of information as to an exact mechanism by which the factors elastogenically induce the cells, this currently remains an unsubstantiated observation. As seen in Figures 4A and B, at both HA-o doses (i.e., 0.2 and 2 μg/mL), a classic biphasic TGF-β1 dose response is seen with regard to elastic matrix deposition, with significant (approximately fivefold) increases noted at the lowest tested TGF-β1 dose (0.1 ng/mL) and much smaller increases noted at higher TGF-β1 doses. Our results also suggest that at higher doses, HA-o reduces this biphasic elastic matrix synthesis response to TGF-β1; especially at higher TGF-β1 doses (i.e., 1 and 10 ng/mL), increasing HA-o dose led to a significant increase in elastic matrix deposition. Matrix elastin increases were primarily associated with enhanced deposition of the less crosslinked alkali-soluble fraction.
In the majority of cases, we found the factors to enhance significantly relative to controls, the synthesis of LOX enzyme, which is believed to play a central role in crosslinking tropoelastin into a mature elastic matrix. This is in agreement with our prior published findings7,33 where we showed HA-o/TGF-β1 to enhance mRNA expression, protein synthesis, and activity of the elastin crosslinking enzyme. However, the most significant increase in LOX synthesis was observed in cell cultures treated with 0.2 μg/mL of HA-o and 10 ng/mL of TGF-β1 (see Fig. 8). Likewise, in comparing ratios of amounts of alkali-insoluble (i.e., highly crosslinked) matrix elastin to amounts of the alkali-soluble fraction (see Fig. 4B), we find that the ratios are far <1, for all cases, especially for cultures treated with the lower doses of the factors, but greater than 1 in the singular case wherein LOX synthesis was most significantly enhanced (i.e., 0.2 μg/mL of HA-o and 10 ng/mL of TGF-β1). We thus hypothesize that the increases in amounts of LOX protein contribute to more efficient crosslinking of elastic matrix into an alkali-insoluble form.
A very interesting observation is that the matrix yield (i.e., mass % of total elastin output crosslinked into a matrix) in control collagen constructs were much lower (∼0.2%) than what we have observed with the same cell type in 2D cultures on noncollagenous substrates (10%–15%). While we have shown a multi-fold increase in matrix yields in the presence of TGF-β1 and HA-o factors, most significantly at the lowest tested doses, the yields remain <1%. One possible reason for the low levels of elastic matrix deposited, which we will confirm in a future study, might be over-production of GAGs such as chondrotin sulfate by the SMCs, which, as studies by Hwang et al.34 and Allison et al.35 have shown, to inhibit elastin synthesis and assembly, and shown to correlate with increased collagen matrix deposition. Regardless, despite the likely LOX-mediated crosslinking of tropoelastin in the presence of HA-o and TGF-β, the poor elastic matrix yields obtained in this study indicate that there is room for improvement in terms of enhancing the deposition of the more robustly crosslinked, and hence the more stable alkali-insoluble elastic matrix fraction.
In the context of enhancing elastin matrix within 3D collagenous constructs, leading to tissue engineering of a clinically sufficient, implantable tissue, it might thus be necessary to seed the scaffolds at much higher cell densities to overcome the poor matrix yield obtained per cell (even when elastogenically induced) or alternatively co-deliver elastin-crosslink-promoting factors (e.g., LOX or LOX-activity enhancing copper nanoparticles), which we have shown to be very useful for the purpose.36,37
MMPs-2 and -9 represent a class of Zn2+-dependent gelatinases that proteolyze matrix proteins, specifically elastin.38 Since intact elastic fibers serve as nucleation sites for organization of new elastic matrix structures, and since net accumulation of elastic matrix is dependent on much attenuated matrix degradation relative to its regeneration, minimizing MMP production/activity is desirable.39 In this context, our study showed that the lowest, but the most elastogenic dose combination of TGF-β1 and HA-o significantly attenuate MMP-9 synthesis, but enhance MMP-2 production by SMCs. At the highest provided dose, also deemed to be elastogenic, but less so, MMP-9 production was negligible and MMP-2 levels were similar to untreated controls. Likewise, active forms of MMP-2 protein were enhanced at the lowest TGF-β1 and HA-o dose combinations, and remained unaffected at higher doses. While it is yet undetermined as to whether the above-mentioned increases in synthesis of MMP-2 protein quantities (both zymogen and active forms) at the lowest tested TGF-β1 and HA-o doses are significant enough on absolute terms to actually compromise the matrix; if it is so, providing MMP-inhibiting culture conditions, or alternatively, providing the higher, non-MMP-enhancing factor dose might be a desirable approach.
Another favorable observation of culturing SMCs within statically loaded collagen gels was the high degree of alignment in the synthesized elastic fibers, as seen in Figure 11A. While the degree of alignment was more or less uniform irrespective of the culture conditions, all cultures that received the elastogenic factors, except those with 1 ng/mL of TGF-β1 and 2 μg/mL of HA-o, consisted of significantly larger number of aligned fibers compared to nonadditive control cultures (Fig. 11B).
TGF-β1, when over-expressed, especially in a sustained manner, can induce SMCs to assume a more osteogenic phenotype, and enhance matrix calcification.15 However, our study did not show any calcification, even in constructs cultured with the highest dose of 10 ng/mL TGF-β1.
Conclusions
This study demonstrates elastogenic induction of RASMCs with HA-o and TGF-β1 factors within nonelastogenic 3D constructs of statically loaded collagen gel constructs. While a much higher dose combination than that shown useful in earlier studies within 2D cultures is necessary to enhance both tropoelastin and matrix synthesis, compared to other studies performed on SMCs in collagen gels, we have demonstrated that synthesis of significant amounts of matrix elastin is possible in the presence of these factors. A major challenge to our ability to obtain the optimum amount of elastin, which engineering of tissue constructs for human use demands, is the rather poor elastic matrix yield (<1%), and requires addressing. Studies on co-delivering elastin-crosslinking LOX or copper nanoparticles with the above factors would address this deficiency. The results are most useful toward addressing a fundamental and widely absent aspect in vascular tissue engineering, that of reinstating an intact, mature, and viable elastic matrix.
Acknowledgments
The authors would like to acknowledge the assistance of Ms. Aimee Phelps at the Medical University of South Carolina for her help with histological processing. This work was supported by funds from the NIH awarded to Ramamurthi, A. (5RO1HL092051-02, RO1HL092051-01S).
Disclosure Statement
No competing financial interests exist.
References
- 1.Rosenbloom J. Abrams W.R. Mecham R. Extracellular matrix 4: the elastic fiber. FASEB J. 1993;7:1208. [PubMed] [Google Scholar]
- 2.Rodgers U.R. Weiss A.S. Cellular interactions with elastin. Pathol Biol. 2005;53:390. doi: 10.1016/j.patbio.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Li D.Y. Brooke B. Davis E.C. Mecham R.P. Sorensen L.K. Boak B.B. Eichwald E. Keating M.T. Elastin is an essential determinant of arterial morphogenesis. Nature. 1998;393:276. doi: 10.1038/30522. [DOI] [PubMed] [Google Scholar]
- 4.Patel A. Fine B. Sandig M. Mequanint K. Elastin biosynthesis: the missing link in tissue-engineered blood vessels. Cardiovasc Res. 2006;71:40. doi: 10.1016/j.cardiores.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 5.Thie M. Schlumberger W. Semich R. Rauterberg J. Robenek H. Aortic smooth muscle cells in collagen lattice culture: effects on ultrastructure, proliferation and collagen synthesis. Eur J Cell Biol. 1991;55:295. [PubMed] [Google Scholar]
- 6.Kothapalli C.R. Gacchina C.E. Ramamurthi A. Utility of hyaluronan oligomers and transforming growth factor-beta1 factors for elastic matrix regeneration by aneurysmal rat aortic smooth muscle cells. Tissue Eng Part A. 2009;15:3247. doi: 10.1089/ten.tea.2008.0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kothapalli C.R. Taylor P.M. Smolenski R.T. Yacoub M.H. Ramamurthi A. Transforming growth factor beta 1 and hyaluronan oligomers synergistically enhance elastin matrix regeneration by vascular smooth muscle cells. Tissue Eng Part A. 2009;15:501. doi: 10.1089/ten.tea.2008.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gacchina C.E. Ramamurthi A. Impact of pre-existing elastic matrix on TGFbeta1 and HA oligomer-induced regenerative elastin repair by rat aortic smooth muscle cells. J Tissue Eng Regen Med. 2011;5:85. doi: 10.1002/term.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu W.J. Vrhovski B. Weiss A.S. Glycosaminoglycans mediate the coacervation of human tropoelastin through dominant charge interactions involving lysine side chains. J Biol Chem. 1999;274:21719. doi: 10.1074/jbc.274.31.21719. [DOI] [PubMed] [Google Scholar]
- 10.Ramamurthi A. Vesely I. Evaluation of the matrix-synthesis potential of crosslinked hyaluronan gels for tissue engineering of aortic heart valves. Biomaterials. 2005;26:999. doi: 10.1016/j.biomaterials.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 11.Huffman M.D. Curci J.A. Moore G. Kerns D.B. Starcher B.C. Thompson R.W. Functional importance of connective tissue repair during the development of experimental abdominal aortic aneurysms. Surgery. 2000;128:429. doi: 10.1067/msy.2000.107379. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell S.L. Niklason L.E. Requirements for growing tissue-engineered vascular grafts. Cardiovasc Pathol. 2003;12:59. doi: 10.1016/s1054-8807(02)00183-7. [DOI] [PubMed] [Google Scholar]
- 13.Shi Y. Vesely I. Fabrication of mitral valve chordae by directed collagen gel shrinkage. Tissue Eng. 2003;9:1233. doi: 10.1089/10763270360728143. [DOI] [PubMed] [Google Scholar]
- 14.Juncosa-Melvin N. Boivin G.P. Galloway M.T. Gooch C. West J.R. Sklenka A.M. Butler D.L. Effects of cell-to-collagen ratio in mesenchymal stem cell-seeded implants on tendon repair biomechanics and histology. Tissue Eng. 2005;11:448. doi: 10.1089/ten.2005.11.448. [DOI] [PubMed] [Google Scholar]
- 15.Simionescu A. Philips K. Vyavahare N. Elastin-derived peptides and TGF-beta1 induce osteogenic responses in smooth muscle cells. Biochem Biophys Res Commun. 2005;334:524. doi: 10.1016/j.bbrc.2005.06.119. [DOI] [PubMed] [Google Scholar]
- 16.Battegay E.J. Raines E.W. Seifert R.A. Bowen-Pope D.F. Ross R. TGF-beta induces bimodal proliferation of connective tissue cells via complex control of an autocrine PDGF loop. Cell. 1990;63:515. doi: 10.1016/0092-8674(90)90448-n. [DOI] [PubMed] [Google Scholar]
- 17.Joddar B. Ramamurthi A. Elastogenic effects of exogenous hyaluronan oligosaccharides on vascular smooth muscle cells. Biomaterials. 2006;27:5698. doi: 10.1016/j.biomaterials.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 18.Labarca C. Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980;102:344. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- 19.Lee J.S. Basalyga D.M. Simionescu A. Isenburg J.C. Simionescu D.T. Vyavahare N.R. Elastin calcification in the rat subdermal model is accompanied by up-regulation of degradative and osteogenic cellular responses. Am J Pathol. 2006;168:490. doi: 10.2353/ajpath.2006.050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loria R.M. Kos W.L. Campbell A.E. Madge G.E. Suppression of aortic elastic tissue autofluorescence for the detection of viral antigen. Histochemistry. 1979;61:151. doi: 10.1007/BF00496527. [DOI] [PubMed] [Google Scholar]
- 21.Bashur C.A. Dahlgren L.A. Goldstein A.S. Effect of fiber diameter and orientation on fibroblast morphology and proliferation on electrospun poly(D,L-lactic-co-glycolic acid) meshes. Biomaterials. 2006;27:5681. doi: 10.1016/j.biomaterials.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Long J.L. Tranquillo R.T. Elastic fiber production in cardiovascular tissue-equivalents. Matrix Biol. 2003;22:339. doi: 10.1016/s0945-053x(03)00052-0. [DOI] [PubMed] [Google Scholar]
- 23.Johnson D.J. Robson P. Hew Y. Keeley F.W. Decreased elastin synthesis in normal development and in long-term aortic organ and cell cultures is related to rapid and selective destabilization of mRNA for elastin. Circ Res. 1995;77:1107. doi: 10.1161/01.res.77.6.1107. [DOI] [PubMed] [Google Scholar]
- 24.Li S. Lao J. Chen B.P. Li Y.S. Zhao Y. Chu J. Chen K.D. Tsou T.C. Peck K. Chien S. Genomic analysis of smooth muscle cells in 3-dimensional collagen matrix. FASEB J. 2003;17:97. doi: 10.1096/fj.02-0256fje. [DOI] [PubMed] [Google Scholar]
- 25.Kim B.S. Nikolovski J. Bonadio J. Smiley E. Mooney D.J. Engineered smooth muscle tissues: regulating cell phenotype with the scaffold. Exp Cell Res. 1999;251:318. doi: 10.1006/excr.1999.4595. [DOI] [PubMed] [Google Scholar]
- 26.Weinberg C.B. Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231:397. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- 27.L'Heureux N. Germain L. Labbe R. Auger F.A. In vitro construction of a human blood vessel from cultured vascular cells: a morphologic study. J Vasc Surg. 1993;17:499. doi: 10.1067/mva.1993.38251. [DOI] [PubMed] [Google Scholar]
- 28.Song J. Rolfe B.E. Hayward I.P. Campbell G.R. Campbell J.H. Effects of collagen gel configuration on behavior of vascular smooth muscle cells in vitro: association with vascular morphogenesis. In Vitro Cell Dev Biol Anim. 2000;36:600. doi: 10.1007/BF02577528. [DOI] [PubMed] [Google Scholar]
- 29.Keire P.A. L'Heureux N. Vernon R.B. Merrilees M.J. Starcher B. Okon E. Dusserre N. McAllister T.N. Wight T.N. Expression of versican isoform V3 in the absence of ascorbate improves elastogenesis in engineered vascular constructs. Tissue Eng Part A. 2010;16:501. doi: 10.1089/ten.tea.2009.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kothapalli C.R. Ramamurthi A. Induced elastin regeneration by chronically activated smooth muscle cells for targeted aneurysm repair. Acta Biomater. 2010;6:170. doi: 10.1016/j.actbio.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grainger D.J. Kemp P.R. Witchell C.M. Weissberg P.L. Metcalfe J.C. Transforming growth factor beta decreases the rate of proliferation of rat vascular smooth muscle cells by extending the G2 phase of the cell cycle and delays the rise in cyclic AMP before entry into M phase. Biochem J. 1994;299(Pt 1):227. doi: 10.1042/bj2990227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gacchina C.E. Deb P.P. Barth J. Ramamurthi A. Elastogenic inductability of smooth muscle cells from a rat model of late-stage abdominal aortic aneurysms. Tissue Eng Part A. 2011;17:1699. doi: 10.1089/ten.tea.2010.0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ibrahim S. Joddar B. Craps M. Ramamurthi A. A surface-tethered model to assess size-specific effects of hyaluronan (HA) on endothelial cells. Biomaterials. 2007;28:825. doi: 10.1016/j.biomaterials.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 34.Hwang J.Y. Johnson P.Y. Braun K.R. Hinek A. Fischer J.W. O'Brien K.D. Starcher B. Clowes A.W. Merrilees M.J. Wight T.N. Retrovirally mediated overexpression of glycosaminoglycan-deficient biglycan in arterial smooth muscle cells induces tropoelastin synthesis and elastic fiber formation in vitro and in neointimae after vascular injury. Am J Pathol. 2008;173:1919. doi: 10.2353/ajpath.2008.070875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allison D.D. Braun K.R. Wight T.N. Grande-Allen K.J. Differential effects of exogenous and endogenous hyaluronan on contraction and strength of collagen gels. Acta Biomater. 2009;5:1019. doi: 10.1016/j.actbio.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 36.Kothapalli C.R. Ramamurthi A. Lysyl oxidase enhances elastin synthesis and matrix formation by vascular smooth muscle cells. J Tissue Eng Regen Med. 2009;3:655. doi: 10.1002/term.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kothapalli C.R. Ramamurthi A. Copper nanoparticle cues for biomimetic cellular assembly of crosslinked elastin fibers. Acta Biomater. 2009;5:541. doi: 10.1016/j.actbio.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keeling W.B. Armstrong P.A. Stone P.A. Bandyk D.F. Shames M.L. An overview of matrix metalloproteinases in the pathogenesis and treatment of abdominal aortic aneurysms. Vasc Endovascular Surg. 2005;39:547. doi: 10.1177/153857440503900601. [DOI] [PubMed] [Google Scholar]
- 39.Galis Z.S. Khatri J.J. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90:251. [PubMed] [Google Scholar]