Abstract
The extracellular matrix (ECM) plays important roles in influencing cellular behavior such as attachment, differentiation, and proliferation. However, in conventional culture and tissue engineering strategies, single proteins are frequently utilized, which do not mimic the complex extracellular microenvironment seen in vivo. In this study we report a method to decellularize brain tissue using detergents. This decellularized brain matrix is rich in glycosaminoglycans and contains collagen I, collagen III, collagen IV, collagen V, collagen VI, perlecan, and laminin. By further processing the material into a liquid form, the brain matrix can be used as a cell culture coating. Neurons derived from human induced pluripotent stem cells plated on the brain matrix express neuronal markers and assume neuronal morphology. Additionally, the same material can potentially be used as a scaffold for tissue engineering as it reassembles upon injection in vivo to form a gel. Thus, our work demonstrates the ability to use decellularized brain ECM for cell culture and tissue engineering applications.
Introduction
The extracellular microenvironment has been demonstrated to provide important cues for cellular behavior such as cell migration, differentiation, and maturation.1–5 The extracellular matrix (ECM) is composed of a complex variety of proteins and polysaccharides, and despite its complexity in vivo, many studies use purified proteins for cell culture coatings or tissue engineering scaffolds. While many components of the ECM are similar, each tissue contains a unique composition of these proteins and polysaccharides.6,7 Recently, the use of decellularized matrices for cell culture coatings have been explored for a variety of tissues such as skin, fat, pericardium, heart, skeletal muscle, and liver.8–12 These matrices are able to provide a better mimic of native ECM, and have revealed tissue-specific effects on cellular behavior,10 including increased maturation of progenitor cell types.8 To date, no such studies have been performed utilizing decellularized brain ECM.
Brain ECM contains relatively small amounts of fibrous proteins such as collagen or fibronectin, but also includes large amounts of glycosaminoglycans (GAGs) and proteoglycans.13 It has been shown that neural outgrowth and survival is highly sensitive to surface composition.14,15 Recently, several groups attempted to recapitulate the physical, chemical, and biological properties of brain tissue in culture by using hydrogels of hyaluronic acid, collagen, and laminin to provide proteins and GAGs for neural stem cell growth16 or by studying neural cell response to collagen, fibronectin, and GAGs.17 While these materials are an approximation of the in vivo microenvironment, they do not fully mimic the complex native ECM of the brain.
Similarly, a variety of scaffolds have been used in vivo to treat traumatic brain injury and other neurological disorders in small animal models. These scaffolds attempt to regenerate or replace damaged brain tissue by providing a platform for neurite growth and axonal alignment. To develop a scaffold for tissue engineering, one approach is to mimic the structure and/or components of the native ECM. Hyaluronic acid,18,19 collagen,20 polycarbonate,20 polycaprolactone,21 and poly(glycolic acid) meshes and gels22 have been studied as scaffolds in brain lesions in animal models to provide three-dimensional constructs for cellular repopulation or as cell delivery vehicles. Most of these scaffolds need to be implanted requiring major surgery. However, injectable scaffolds of synthetic particles,23 methylcellulose,24 and a combination of fibronectin/collagen I25 have been developed, which would allow for minimally invasive delivery. To date, no scaffolds, which contain the appropriate tissue-specific ECM biochemical cues, have been developed for the brain.
Here, we report a method to decellularize brain ECM and process the isolated matrix into a form that can be used for cell culture coatings, thereby providing a more in vivo–like microenvironment for neural cell culture. Additionally, we tested the feasibility of using the solubilized brain matrix as an injectable scaffold for tissue engineering applications.
Materials and Methods
Decellularization of porcine brains
Brains were removed from female Yorkshire pigs (∼30–45 kg), which were anesthetized with ketamine (25 mg/kg) and xylazine (2 mg/kg) followed by euthanasia with Pentobarbital (90 mg/kg). Brains were cut into halves; a small sample was removed for comparison studies, and then decellularized by stirring in 400 mL of 0.1% wt/vol of sodium dodecyl sulfate (SDS) in phosphate-buffered saline (PBS) with 1% penicillin/streptomycin. The supernatant containing the cellular remnants was decanted every 24 h, and refilled to the start volume for 3–4 days until the tissue was decellularized. The slurry was separated into 50 mL conical centrifuge tubes and centrifuged at 10,000 rpm for 5 min. To rinse the brain ECM, the conicals were decanted and refilled with deionized water, shaken, and centrifuged. This process was repeated between 10 and 12 times to remove residual SDS. A sample of decellularized brain ECM was frozen in Tissue Tek O.C.T. freezing medium for histological analysis, and the remaining brain ECM was lyophilized and stored at −80°C until further use.
Brain matrix solubilization
Prior to cell culture, the brain matrix was solubilized by enzymatic digestion using previously modified protocols.8,26,27 Porcine pepsin (Sigma) was dissolved in 0.1 M hydrochloric acid at 1 mg/mL and then sterile filtered through a 0.22 μm filter. The pepsin was added to the brain matrix at a ratio of 20:1 for a final concentration of 20 mg/mL for the brain matrix, and then stirred at ∼70 rpm for 48 h.
Characterization of brain matrix material
Fresh frozen decellularized brain ECM was sectioned into 10 μm slices and then stained using hematoxylin and eosin (H&E) to confirm the absence of cells. To quantify DNA removal, a DNeasy assay (Qiagen) was performed on lyophilized native brain tissue and decellularized brain ECM in triplicate according to manufacturer's instructions. In brief, samples were digested using the provided proteinase K, and DNA was separated using a filter trap. After several washes and purification steps, DNA was reconstituted in deionized water and estimated using absorbance readings at 260 nm on a Synergy H4 microplate reader (Biotek). Samples were normalized to original dry starting weight. To determine sulfated GAG content, the Blyscan assay (Biocolor) was used as per manufacturer's instructions. Samples were run in triplicate and averaged. Rat tail collagen served as a negative control for the GAG content determination. Tandem mass spectrometry (MS/MS) was utilized to more fully characterize the protein content of the brain matrix. Matrix samples were digested using pepsin or trypsin (FASP Protein Digestion Kit, Protein Discovery) and analyzed by liquid chromatography (LC)-MS/MS with electrospray ionization. A QSTAR-Elite hybrid mass spectrometer (AB/MDS Sciex) was interfaced to a nanoscale reversed-phase high-pressure liquid chromatograph (Tempo) using a 10 cm-180 ID glass capillary packed with 5-μm C18 Zorbax™ beads (Agilent). The buffer compositions were as follows. Buffer A was composed of 98% H2O, 2% acetonitrile (ACN), 0.2% formic acid, and 0.005% trifluoroacetic acid (TFA); buffer B was composed of 100% ACN, 0.2% formic acid, and 0.005% TFA. Peptides were eluted from the C-18 column into the mass spectrometer using a linear gradient of 5–60% buffer B over 60 min at 400 μL/min. LC-MS/MS data were acquired in a data-dependent fashion by selecting the four most intense peaks with charge state of 2–4 that exceeds 20 counts, with exclusion of former target ions set to “360 s” and the mass tolerance for exclusion set to 100 ppm. Time-of-flight MS were acquired at m/z 400–1600 Da for 1 s with 12 time bins to sum. MS/MS data were acquired from m/z 50–2000 Da by using “enhance all” and 24 time bins to sum, dynamic background subtract, automatic collision energy, and automatic MS/MS accumulation with the fragment intensity multiplier set to 6 and maximum accumulation set to 2 s before returning to the survey scan. Peptide identifications were made using paragon algorithm executed in Protein Pilot 2.0 (Life Technologies). Proteins were labeled based on at least one identified peptide with the confidence of above 95% for that peptide identification.
Generation, culture, and differentiation of human induced pluripotent stem cells
Adult human skin biopsies were obtained from healthy volunteers at the Alzheimer's Disease Research Center at the University of California, San Diego. Informed consent procedures were approved by the UCSD IRB and obtained from patients prior to biopsy. Adult human dermal fibroblasts were transduced with retroviruses encoding Oct3/4, Klf4, Sox2, and c-Myc according to previously published protocols.28 An SDIA (stromal cell–derived inducing activity) with PA6 method was used to induce induced pluripotent stem cell (iPSC) to neuroectoderm as described by Zeng et al.29 Neural progenitor cells (NPCs) were prepared from day-12 SDIA induction. NPCs were propagated in DMEM/F12, 1XN2, and 1XB27 medium containing 20 ng/mL FGF-2. After NPCs were differentiated for 3 weeks, neurons were sorted with cell-surface antibodies by FACSAria (BD Biosciences) using markers recently developed,28 namely CD184−, CD44−, CD15low, and CD24+.
Culture of human iPSC-derived neurons
Ninety-six-well imaging plates (BD Biosciences) were coated with 20 μg/mL polyornithine (PO) at 37°C overnight. PO was utilized as it is a substrate that has been used extensively with neural cell culture with and without30,31 other ECM proteins, and has demonstrated to increase additional protein adsorption. Next day, the wells were rinsed twice with sterile DI water to remove unattached PO. Plates were coated with the liquid brain matrix diluted to 1 mg/mL using 0.1 M acetic acid for 1 h at 37°C and rinsed twice with sterile PBS prior to use. Matrigel was diluted to 0.25 mg/mL in DMEM/F12 and incubated for the same period of time. Total protein adsorption content was measured using a micro BCA assay (Pierce) as per manufacturer's instructions. The protein adsorption was determined by subtraction of protein content in the coating solutions pre- and postcoating. Water was used as a control. Sorted neurons were plated at 0.625 million cells per cm2 in neuron differentiation media. Media was changed every 3 days. Cells were fixed and processed at 7 or 14 days.
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde. After blocking with 3% bovine serum albumin and permeabilizing with 0.3% Triton, cells were labeled with primary antibodies, followed by incubation using secondary antibodies. The following antibodies were used: rabbit anti-GABA 1:200 (Sigma), mouse anti-β-III-tubulin 1:1000 (Covance), rabbit anti-synapsin (Millipore) 1:2500, mouse anti-MAP2a,b 1:500 (Sigma), goat anti-mouse IgG Alexa 488 1:1000, and goat anti-rabbit IgG Alexa 568 1:1000 (Invitrogen). Images were taken from a Nikon Axiophot. Synapsin expression was quantified by measuring area of fluorescence using ImageJ. Additionally, to measure dendritic branching, a low density of cells were transfected with pBOS.eGFP32,33 using Lipofectamine (Invitrogen), as per manufacturer's instructions. Cells were fixed on day 8 for dendrite analysis. Images were taken from a Nikon Axiophot and primary and secondary branching was counted in ImageJ.28,34
In vivo scaffold feasibility
All animal procedures were performed in accordance with the guidelines established by the Committee on Animal Research at the University of California, San Diego, and the American Association for Accreditation of Laboratory Animal Care. Brain matrix was brought to a physiological pH and salt concentration through the addition of sodium hydroxide and 10× PBS, diluted to 16 and 12 mg/mL using 1× PBS, and kept on ice prior to use. Female C57 mice were anesthetized with isoflurane and kept on heating pads whereupon 100 μL of brain ECM was injected through a 27-gauge needle subcutaneously into the dorsal region. Twenty minutes postinjection the mice received an overdose of sodium pentobarbital, the site of injection was excised, and gels were fresh frozen in Tissue Tek O.C.T. for histological analysis or prepared for scanning electron microscopy (SEM).
Characterization of in vivo–injected brain matrix gels
Frozen brain matrix gels were sectioned to 10 μm slices, and stained using H&E to verify the presence and structure of the gel. Additionally, samples were prepared for SEM analysis by fixation with 2.5% glutaraldehyde for 2 h, followed by dehydration in a series of ethanol rinses (30–100%). For comparison, Matrigel and native porcine brain tissue were also prepared for SEM analysis through fixation and dehydration. Samples were critical point dried and coated with iridium using an Emitech K575× sputter coater. Electron microscope images were taken using a Phillips XL30 Environmental SEM Field Emission microscope.
Immunofluorescent staining was used to identify proteins retained in the brain matrix, and compared with native brain tissue. Sections of native brain tissue and of brain matrix gels were fixed with acetone and blocked with staining buffer (2% goat serum with 0.3% Triton X-100 in PBS). The following primary antibodies were used: collagen I, collagen III, collagen IV, and laminin (1:100; Abcam) and goat anti-rabbit IgG Alexa 568 1:200 (Invitrogen). Only primary or secondary antibodies were used as controls to confirm positive staining. Images were taken using a Carl Zeiss Observer D1.
Statistical analysis
All data are presented as mean±standard error of the mean. All assays were performed in triplicate and the results averaged.
Results
Decellularization of brain ECM
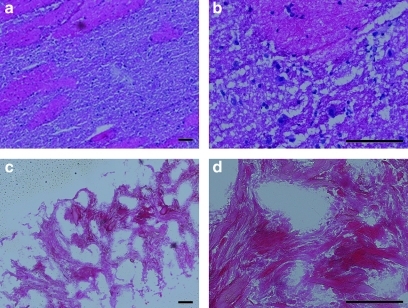
The brain matrix material was derived through decellularization of porcine brains using SDS detergent buffered in PBS with antibiotics. Over time, the brain matrix turned white, an indication of cellular removal. This was similar over multiple isolations; however, it should be noted that the timing of solution exchange is critical. The brain matrix material was collected, rinsed, frozen, sectioned, and stained with H&E to confirm cellular removal. The absence of intact nuclei in histological sections indicated removal of cellular content when compared with native brain tissue (Fig. 1). The DNeasy assay was used to quantify the DNA removal and it was found that there was a clearance ratio of 95.7% with 0.13±0.02 μg DNA content/mg lyophilized native brain tissue and 0.0069±0.001 μg DNA content/mg in the postprocessed brain matrix. A colorimetric Blyscan assay was used to determine sulfated GAG content. The brain matrix material contained 34.7±0.17 μg sulfated GAG/mg dry weight, and this value was comparable over multiple isolations. No sulfated GAGs were found in collagen control. Through mass spectrometry analysis, various ECM proteins and proteoglycans were identified: collagen I, collagen V, collagen IV, collagen VI, laminin, and perlecan. However, it should be noted that mass spectrometry is not an all-inclusive technique and other proteins may not have been identified.
FIG. 1.
Hematoxylin and eosin–stained sections of porcine brain matrix (a, b) compared with decellularized brain matrix (c, d). Scale bars are 100 μm. Note the absence of cells in the decellularized matrix, as compared with native tissue.
In vitro culture of iPSC-derived neurons on brain matrix
To test brain matrix as a cell culture coating, decellularized brain matrix was turned into a liquid form using enzymatic digestion with pepsin. After visual confirmation that the material was formed into a liquid as indicated by the lack of particles in solution, brain matrix was diluted using acetic acid. Imaging plates were coated overnight with PO and then rinsed and incubated with solubilized brain matrix or Matrigel. The difference in protein concentration in solution pre- versus postadsorption was calculated to be 46.1±3.26 μg/mL for Matrigel and 18.9±5.25 μg/mL for the brain matrix, demonstrating adsorption of both material to the PO-coated wells. As the coatings were performed on a 96-well plate, this translates to roughly 15.4±1.08 μg/cm2 for Matrigel and 6.29±1.75 μg/cm2 for the brain matrix. Water incubated on the PO coatings was used as a negative control.
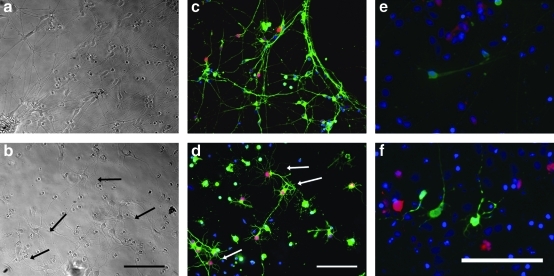
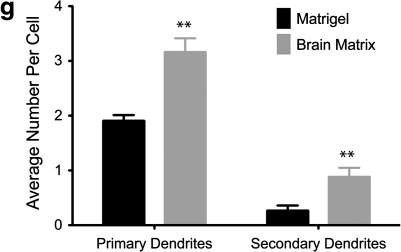
Neurons derived from iPSC were sorted by FACS, and then cultured on Matrigel, a standard coating for neurons differentiated from human embryonic or iPSCs,35 and compared with neurons cultured on the decellularized brain matrix. The brain matrix was able to maintain mature neurons, as the iPSC neurons were able to attach, extend dendritic processes, and were positively stained for the neuronal marker βIII-tubulin (Fig. 2a–d). Some neurons cultured on the brain matrix exhibited a distinct morphology with highly complex dendritic processes. These highly arborized dendrites were primarily observed on the brain matrix–coated wells, and not on the Matrigel substrates. To quantify the dendritic branching at 1 week, a low concentration of cells was transfected with eGFP using Lipofectamine. The number of primary dendrites that come directly from the cell body, and secondary dendrites that branch from a primary dendrite were quantified. The neurons cultured on the brain matrix coatings had significantly higher dendritic primary and secondary processes when compared with Matrigel-plated neurons (Fig. 2e–g).
FIG. 2.
The brain matrix coating (b, d, f) is able to support neurons derived from induced pluripotent stem cells. Highly branched neurons were identified on the brain matrix, which were not seen in the Matrigel coated wells (a, c, e). Cells were stained (c, d) using β-III-tubulin, a neuronal marker (green), and GABA-ergic neurons were identified through staining for GABA (red). Arrows identify highly branched neurons on the brain matrix coating. eGFP-transfected cells (e, f) were assessed for primary and secondary dendritic branching at day 8. Quantification of primary dendrites and secondary dendrites indicate statistically significant dendritic formation on the brain matrix coatings when compared with Matrigel coatings (g). Scale bar at 100 μm. **p<0.01.
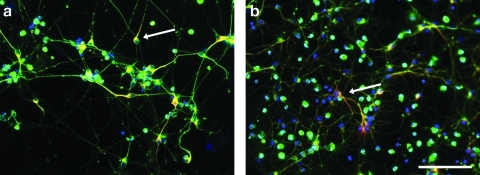
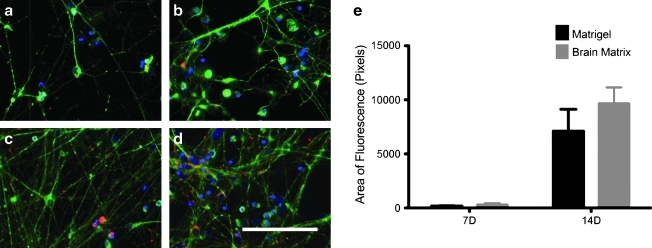
The neurons were able to mature on the brain matrix, as neurons cultured for 2 weeks polarized and formed dendrites as suggested by MAP2 staining of dendrites, and lack of MAP2 staining of axons (Fig. 3). Additionally, synapsin, which is a phosphoprotein localized to synapses, increased in expression from 1-week cultures to 2-week cultures, indicating maturation of the neurons (Fig. 4). At 1 week, area of synapsin was measured to be 178.5±29.4 pixels on Matrigel and 324.0±145.6 pixels on brain matrix. At 2 weeks, synapsin was measured as 7096.6±2962.7 pixels on Matrigel and 9687.5±1454.2 pixels on the brain matrix. Synapsin expression as measured through immunofluorescence increased over time in both groups with a trend toward increased synapsin expression on the brain matrix, although this was not significant.
FIG. 3.
Neurons cultured on Matrigel (a) and brain matrix coatings (b) are able to polarize as evidenced by MAP2 localization (red) at 1 week. Arrows identify MAP2 positive cells. Neurons were costained with β-III-tubulin, a general neuronal marker (green). Scale bar at 100 μm.
FIG. 4.
Synapsin levels increased in both the Matrigel coated (a, c) and brain matrix coated (b, d) wells over time, where synapsin (red) is costained with β-III-tubulin, a general neuronal marker (green). One-week cultures (a, b) demonstrate less synapsin than at 2 weeks (c, d). Scale bar at 100 μm. Quantification of synapsin expression as area per field of view is demonstrated (e), and while there is no significance between the coatings, there is a trend of increased synapsin expression on the brain matrix at 2 weeks.
Brain matrix scaffold generation in vivo
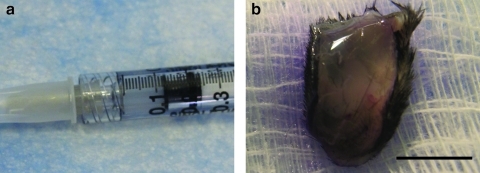
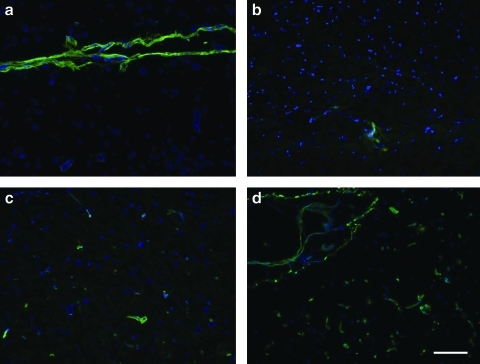
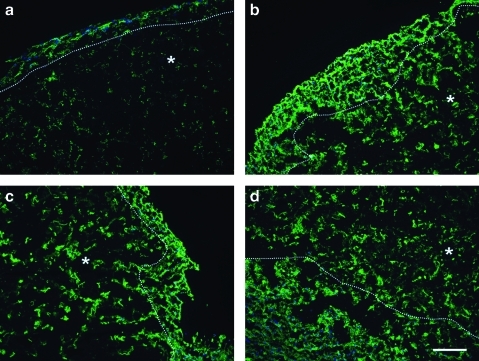
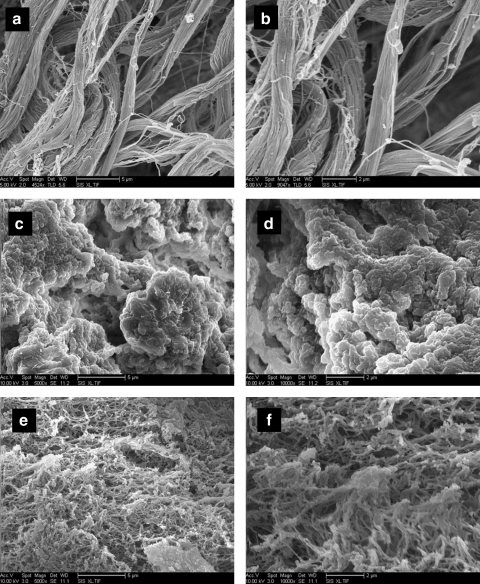
The brain matrix material was able to form a gel in vivo when brought to a physiological pH and injected subcutaneously into C57 mice. Solubilized brain matrix remained liquid on ice or at room temperature, but was able to form a gel upon injection at 16 and 12 mg/mL (Fig. 5). The gel was identified as a large bolus underneath the skin and did not move when gently pressed after 20 min postinjection. Immunohistochemistry of sections of the native brain tissue contained collagen I, collagen III, collagen, and laminin (Fig. 6). These ECM proteins were also identified in the brain matrix hydrogel after injection, which did not contain visible amounts of DNA after injection (Fig. 7). Control sections demonstrated negative staining. To view the structure of the gels, SEM analysis demonstrated that the brain matrix was composed of a series of nanofibrous structures (Fig. 8). Many of the nanofibers appeared to assemble and form larger microscale fibrils within the gel. For comparison, Matrigel gels and native porcine brain tissue were also imaged using SEM. Matrigel is also able to form a fibrous structure after gelation, but did not form large self-assembled microscale fibers as compared with the brain matrix.
FIG. 5.
Brain matrix material was loaded into a syringe (a) and injected subcutaneously, whereupon the injected material self-assembles into a gel (b). Scale bar at 5 mm.
FIG. 6.
Immunohistochemistry of native brain tissue sections for extracellular matrix proteins. Collagen I (a), collagen III (b), collagen IV (c), and laminin (d) were observed in the native brain tissue. Scale bar at 100 μm.
FIG. 7.
Immunohistochemistry of injected brain matrix gels demonstrates retention of extracellular matrix proteins, matrix gel denoted by ‘*’. Collagen I (a), collagen III (b), collagen IV (c), and laminin (d) were observed. Scale bar at 100 μm.
FIG. 8.
SEM analysis was performed on brain matrix gels (a, b), native brain tissue (c, d), or Matrigel gels (e, f). SEM reveals that brain matrix gels formed after subcutaneous injection in vivo are composed of a network of nanofibers and microfibers. Matrigel gels are also composed of a meshwork of fibers. Scale bars 5 μm (a, c, e) and 2 μm (b, d, f). SEM, scanning electron microscopy.
Discussion
Extracellular cues play a role in many aspects of neural development in vivo36–38 as well an in vitro.38,39 The substrate chosen for in vitro neuron studies is thus highly important because phenotypic, electrophysiological, and molecular changes have been identified in neural cells when cultured on various proteins or synthetic coatings.38–40 Indeed, cell–matrix interactions of neurons derived from human embryonic stem cells were seen to strongly affect differentiation, as neurons demonstrated increased differentiation on laminin-rich substrates, and higher expansion and neurite outgrowth in a dose-dependent manner.41 As the culture substrate has demonstrated effects on morphology, differentiation, and function of neurons, the development of a complex tissue-matched culture substrate may be beneficial for in vitro assays and neural growth.
The native ECM is a complex combination of proteins and polysaccharides that play an important role in cellular behavior such as attachment, proliferation, and differentiation. Current cell culture methods and tissue engineering scaffolds conventionally use purified proteins and do not mimic the complexity of the brain extracellular microenvironment. Combinations of purified proteins have been shown to improve cell proliferation and differentiation, which indicates that complex coatings are beneficial, and thus there has been a shift toward more complex materials.42,43 As there are limitless potential combinations, using a naturally derived matrix may be more physiologically relevant. A variety of tissues have been decellularized and used as cell culture coatings to provide a closer mimic to the in vivo microenvironment.8–10 These coatings have shown tissue-specific effects on cellular behavior, and in some instances increased maturation when compared with conventional substrates.8,10
In this study, we isolated and solubilized ECM from porcine brain using a detergent decellularization method and processed it to a liquid form using enzymatic digestion. We used SDS detergents to remove cellular content, and though SDS has been shown to denature ECM proteins,44 others have reported that SDS decellularization was milder than other techniques such as those using Triton X-100 and trypsin.45,46 Our initial trials attempting to decellularize brain with other detergents such as sodium deoxycholate and Triton X-100 did not remove as much cellular content. We found many differences in developing a decellularization protocol for brain compared with other tissues, as the brain ECM is very weak and the tissue fell apart readily, leading to difficulties in rinsing and recovering the brain matrix. Through this method we were able to isolate brain ECM, but could not maintain the original structure of the brain. However, the isolated ECM was able to be processed into a cell culture coating and an injectable scaffold. The matrix that was retained in our method was rinsed and processed, and while there were no visible nuclei present in the H&E sections, not all nuclear content was fully removed from the brain matrix material. The remaining DNA content was, however, less than that reported with other decellularization techniques.10
Overall, brain ECM is composed mostly of GAGs and proteoglycans with relatively small amounts of fibrous proteins such as collagen and fibronectin. Our results indicated that the decellularized material contains protein components that are found in the native brain ECM, and also retains sulfated GAGs. Multiple collagen isoforms and laminin as well as the proteoglycan perlecan were retained postprocessing, though there may be other components that were not identified. The retention of laminin may prove to be important, as laminin has been shown to increase neurite expansion, survival and outgrowth for neurons,40,41 as well as retinal explant attachment and axonal outgrowth.47 The decellularization process was able to retain sulfated GAGs, despite the use of SDS, and was found to have one of the highest GAG contents when compared with other reported tissues that have been decellularized.8–10,27,44 GAGs have been shown to have an effect on cell behavior, either alone or through association with other molecules.48 While our decellularization protocol likely reduces protein and GAG content relative to native tissue, we are still able to retain many of the components while removing over 95% of DNA. The presence of the proteoglycans and GAGs may be an important mediator of the cellular behavior seen in our studies. While there is minor DNA content remaining in our brain matrix, a recent study demonstrated that several commercially available decellularized ECM scaffolds, although contained measureable amounts of DNA, could still be used successfully in the clinic.49 This may indicate that there is a threshold of DNA levels to avoid a negative immune response, or that the detergents may disrupt the structure of DNA so that the immune response will not be triggered.
The brain matrix can be used as an in vitro coating and it was shown to support the culture and maturation of neurons. iPSC neurons were studied, as these cells have the potential to be an autologous source for neuronal formation. Neurons expressed synapsin, a protein marker that identifies formation of synapses and maturation of the neurons.50 Synapsin expression increased over time in culture, demonstrating that the neurons matured on the brain matrix coating. Interestingly, extensive dendritic processes were observed on the decellularized brain matrix, supporting complex arborization, but were not seen on Matrigel. Branching complexity has been theorized to have an important effect on the electrophysiology of dendritic neurons,51 though mechanisms controlling dendritic architecture are not fully known. It should be noted that the PO coating was important for this cell type, as the iPSC-derived neurons cultured on Matrigel or brain matrix coatings alone resulted in lower attachment.
While there are no clinically used materials for brain tissue reconstruction yet,52 several naturally derived and synthetic materials have been studied as scaffolds in small animal models. None of these scaffolds offer the complexity of native brain ECM, and require major surgery for implantation. Injectable scaffolds23–25 have been developed for minimally invasive delivery, but again do not recapitulate the composition of the native microenvironment. Thus, we tested proof-of-concept for utilizing our solubilized decellularized brain ECM as an injectable tissue engineering scaffold. While the brain matrix remains liquid at room temperature, when brought to physiological pH and injected subcutaneously, the material self-assembles into a gel in vivo. However, the brain matrix material was unable to form a gel in vitro, despite the fact that it was able to self-assemble upon injection. SEM analysis shows that the structure of the injectable scaffold is different from native brain tissue, but is porous and fibrous which may be suitable for endogenous cell infiltration. The advantage of using an injectable hydrogel is that it will allow minimally invasive delivery of the material, injection into multiple sites, and could be tailored to fit the size of the brain lesion. As the material has been decellularized and ECM proteins are largely conserved across species, a negative immune response would not be expected. In fact, many decellularized ECM scaffolds have already been FDA approved for use in the clinic.49 Though only injected subcutaneously, this preliminary work supports the potential for the use of this material as a tissue-matched scaffold, and for minimally invasive therapy. In addition to potential use as an acellular scaffold, the brain matrix may also have the potential to be used as a cell delivery vehicle given our in vitro results. However, long-term in vivo studies will be critical to assess in vivo biocompatibility, degradation time, and cell infiltration and survival. With this work, we demonstrate a method to decellularize and isolate brain ECM, the development of cell culture coatings derived from brain ECM, and in vivo feasibility of a brain matrix scaffold for tissue engineering applications.
Conclusions
We demonstrate that brain tissue can be decellularized but retain ECM protein components and GAGs. The brain matrix may be used as a cell culture platform and has neural biocompatibility as human iPSC-derived neurons are able to grow and mature on the brain matrix. The brain matrix is also able to self-assemble and gel in vivo forming a nanofibrous scaffold, demonstrating feasibility as an injectable, brain-specific tissue engineering scaffold.
Acknowledgments
This research is funded in part by the National Institutes of Health (NIH) Director's New Innovator Award Program (Karen L. Christman, DP2OD004309), which is part of the NIH Roadmap for Medical Research, and by a CIRM comprehensive grant (Lawrence S.B. Goldstein, RC1-00116-1). Lawrence S.B. Goldstein is an Investigator of the Howard Hughes Medical Institute. Jessica A. DeQuach would like to acknowledge the California Institute for Regenerative Medicine (CIRM) under the UCSD Interdisciplinary Stem Cell Research and Training Program for a pre-doctoral fellowship (TG-01154). Shauna H. Yuan was funded by CIRM as a clinical fellow during part of this work (TG-01154). The authors would also like to acknowledge Carolina Rogers for her assistance with harvesting porcine specimens, Sonya Seif-Naraghi for her aid with mass spectroscopy, Vaibhav Bajaj and Anthony Monteforte for sectioning tissue samples, Grace Woodruff for her help with the FACS sorting, Mason Shaner who aided with microscopy, and Cheryl Herrera who helped with tissue culture.
Disclosure Statement
No competing financial interests exist.
References
- 1.Bosnakovski D. Mizuno M. Kim G. Takagi S. Okumura M. Fujinaga T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol Bioeng. 2006;93:1152. doi: 10.1002/bit.20828. [DOI] [PubMed] [Google Scholar]
- 2.Chastain S.R. Kundu A.K. Dhar S. Calvert J.W. Putnam A.J. Adhesion of mesenchymal stem cells to polymer scaffolds occurs via distinct ECM ligands and controls their osteogenic differentiation. J Biomed Mater Res A. 2006;78:73. doi: 10.1002/jbm.a.30686. [DOI] [PubMed] [Google Scholar]
- 3.Koochekpour S. Merzak A. Pilkington G.J. Extracellular matrix proteins inhibit proliferation, upregulate migration and induce morphological changes in human glioma cell lines. Eur J Cancer. 1995;31A:375. doi: 10.1016/0959-8049(94)00476-l. [DOI] [PubMed] [Google Scholar]
- 4.Simon-Assmann P. Kedinger M. De Arcangelis A. Rousseau V. Simo P. Extracellular matrix components in intestinal development. Experientia. 1995;51:883. doi: 10.1007/BF01921739. [DOI] [PubMed] [Google Scholar]
- 5.Williams C.M. Engler A.J. Slone R.D. Galante L.L. Schwarzbauer J.E. Fibronectin expression modulates mammary epithelial cell proliferation during acinar differentiation. Cancer Res. 2008;68:3185. doi: 10.1158/0008-5472.CAN-07-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lutolf M.P. Hubbell J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 7.Uriel S. Labay E. Francis-Sedlak M. Moya M.L. Weichselbaum R.R. Ervin N., et al. Extraction and assembly of tissue-derived gels for cell culture and tissue engineering. Tissue Eng Part C Methods. 2009;15:309. doi: 10.1089/ten.tec.2008.0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeQuach J.A. Mezzano V. Miglani A. Lange S. Keller G.M. Sheikh F., et al. Simple and high yielding method for preparing tissue specific extracellular matrix coatings for cell culture. PLoS One. 2010;5:e13039. doi: 10.1371/journal.pone.0013039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stern M.M. Myers R.L. Hammam N. Stern K.A. Eberli D. Kritchevsky S.B., et al. The influence of extracellular matrix derived from skeletal muscle tissue on the proliferation and differentiation of myogenic progenitor cells ex vivo. Biomaterials. 2009;30:2393. doi: 10.1016/j.biomaterials.2008.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y. He Y. Bharadwaj S. Hammam N. Carnagey K. Myers R., et al. Tissue-specific extracellular matrix coatings for the promotion of cell proliferation and maintenance of cell phenotype. Biomaterials. 2009;30:4021. doi: 10.1016/j.biomaterials.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young D.A. Ibrahim D.O. Hu D. Christman K.L. Injectable hydrogel scaffold from decellularized human lipoaspirate. Acta Biomater. 2010;7:1040. doi: 10.1016/j.actbio.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seif-Naraghi S.B. Salvatore M.A. Schup-Magoffin P.J. Hu D.P. Christman K.L. Design and characterization of an injectable pericardial matrix gel: a potentially autologous scaffold for cardiac tissue engineering. Tissue Eng Part A. 2010;16:2017. doi: 10.1089/ten.tea.2009.0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Novak U. Kaye A.H. Extracellular matrix and the brain: components and function. J Clin Neurosci. 2000;7:280. doi: 10.1054/jocn.1999.0212. [DOI] [PubMed] [Google Scholar]
- 14.Stenger D.A. Pike C.J. Hickman J.J. Cotman C.W. Surface determinants of neuronal survival and growth on self-assembled monolayers in culture. Brain Res. 1993;630:136. doi: 10.1016/0006-8993(93)90651-3. [DOI] [PubMed] [Google Scholar]
- 15.Ruoslahti E. Brain extracellular matrix. Glycobiology. 1996;6:489. doi: 10.1093/glycob/6.5.489. [DOI] [PubMed] [Google Scholar]
- 16.Suri S. Schmidt C.E. Cell-laden hydrogel constructs of hyaluronic acid, collagen, and laminin for neural tissue engineering. Tissue Eng Part A. 2010;16:1703. doi: 10.1089/ten.tea.2009.0381. [DOI] [PubMed] [Google Scholar]
- 17.Carbonetto S. Gruver M.M. Turner D.C. Nerve fiber growth in culture on fibronectin, collagen, and glycosaminoglycan substrates. J Neurosci. 1983;3:2324. doi: 10.1523/JNEUROSCI.03-11-02324.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang T.-W. Spector M. Development of hyaluronic acid-based scaffolds for brain tissue engineering. Acta Biomater. 2009;5:2371. doi: 10.1016/j.actbio.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 19.Cui F.Z. Tian W.M. Hou S.P. Xu Q.Y. Lee I.-S. Hyaluronic acid hydrogel immobilized with RGD peptides for brain tissue engineering. J Mater Sci Mater Med. 2006;17:1393. doi: 10.1007/s10856-006-0615-7. [DOI] [PubMed] [Google Scholar]
- 20.Eagle K.S. Chalmers G.R. Clary D.O. Gage F.H. Axonal regeneration and limited functional recovery following hippocampal deafferentation. J Comp Neurol. 1995;363:377. doi: 10.1002/cne.903630304. [DOI] [PubMed] [Google Scholar]
- 21.Wong D.Y. Krebsbach P.H. Hollister S.J. Brain cortex regeneration affected by scaffold architectures. J Neurosurg. 2008;109:715. doi: 10.3171/JNS/2008/109/10/0715. [DOI] [PubMed] [Google Scholar]
- 22.Park K.I. Teng Y.D. Snyder E.Y. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nat Biotechnol. 2002;20:1111–1117. doi: 10.1038/nbt751. [DOI] [PubMed] [Google Scholar]
- 23.Mahoney M.J. Saltzman W.M. Transplantation of brain cells assembled around a programmable synthetic microenvironment. Nat Biotechnol. 2001;19:934. doi: 10.1038/nbt1001-934. [DOI] [PubMed] [Google Scholar]
- 24.Tate M. Shear D. Hoffman S. Stein D. Biocompatibility of methylcellulose-based constructs designed for intracerebral gelation following experimental traumatic brain injury. Biomaterials. 2001;22:1113–1123. doi: 10.1016/s0142-9612(00)00348-3. [DOI] [PubMed] [Google Scholar]
- 25.Tate M. Shear D. Hoffman S. Fibronectin promotes survival and migration of primary neural stem cells transplanted into the traumatically injured mouse brain. Cell Transplant. 2002;11:283–295. [PubMed] [Google Scholar]
- 26.Freytes D.O. Martin J. Velankar S.S. Lee A.S. Badylak S.F. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials. 2008;29:1630. doi: 10.1016/j.biomaterials.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Singelyn J.M. DeQuach J.A. Seif-Naraghi S.B. Littlefield R.B. Schup-Magoffin P.J. Christman K.L. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 2009;30:5409. doi: 10.1016/j.biomaterials.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan S.H. Martin J. Elia J. Flippin J. Paramban R.I. Hefferan M. Vidal J.G. Mu Y. Killian R.L. Israel M.A. Emre N. Marsala S. Marsala M. Gage F.H. Goldstein L.S.B. Carson C.T. Cell surface marker signatures for the isolation of neural stem cells, glia and neurons derived from human pluripotent stem cells. PLoS ONE. 6(3):e17540. doi: 10.1371/journal.pone.0017540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng X. Cai J. Chen J. Luo Y. You Z.-B. Fotter E., et al. Dopaminergic differentiation of human embryonic stem cells. Stem Cells. 2004;22:925. doi: 10.1634/stemcells.22-6-925. [DOI] [PubMed] [Google Scholar]
- 30.Ye Z.-C. Sontheimer H. Modulation of glial glutamate transport through cell interactions with the extracellular matrix. Int J Dev Neurosci. 2002;20:209. doi: 10.1016/s0736-5748(02)00048-5. [DOI] [PubMed] [Google Scholar]
- 31.Ang L.C. Bhaumick B. Munoz D.G. Sass J. Juurlink B.H. Effects of astrocytes, insulin and insulin-like growth factor I on the survival of motoneurons in vitro. J Neurol Sci. 1992;109:168. doi: 10.1016/0022-510x(92)90164-g. [DOI] [PubMed] [Google Scholar]
- 32.Mizushima S. Nagata S. pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. 1990;18:5322. doi: 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan S.H. Qiu Z. Ghosh A. TOX3 regulates calcium-dependent transcription in neurons. Proc Natl Acad Sci U S A. 2009;106:2909. doi: 10.1073/pnas.0805555106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Griffin G.D. Flanagan-Cato L.M. Sex differences in the dendritic arbor of hypothalamic ventromedial nucleus neurons. Physiol Behav. 2009;97:151. doi: 10.1016/j.physbeh.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian L. Saltzman W.M. Improving the expansion and neuronal differentiation of mesenchymal stem cells through culture surface modification. Biomaterials. 2004;25:1331. doi: 10.1016/j.biomaterials.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 36.Czyz J. Wobus A. Embryonic stem cell differentiation: the role of extracellular factors. Differentiation. 2001;68:167. doi: 10.1046/j.1432-0436.2001.680404.x. [DOI] [PubMed] [Google Scholar]
- 37.Venstrom K.A. Reichardt L.F. Extracellular matrix. 2: role of extracellular matrix molecules and their receptors in the nervous system. FASEB J. 1993;7:996. doi: 10.1096/fasebj.7.11.8370483. [DOI] [PubMed] [Google Scholar]
- 38.Pavlov I. Lauri S. Taira T. Rauvala H. The role of ECM molecules in activity-dependent synaptic development and plasticity. Birth Defects Res C Embryo Today. 2004;72:12. doi: 10.1002/bdrc.20001. [DOI] [PubMed] [Google Scholar]
- 39.Goetz A.K. Scheffler B. Chen H-X. Wang S. Suslov O. Xiang H., et al. Temporally restricted substrate interactions direct fate and specification of neural precursors derived from embryonic stem cells. Proc Natl Acad Sci USA. 2006;103:11063. doi: 10.1073/pnas.0510926103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edgar D. Timpl R. Thoenen H. The heparin-binding domain of laminin is responsible for its effects on neurite outgrowth and neuronal survival. EMBO J. 1984;3:1463. doi: 10.1002/j.1460-2075.1984.tb01997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma W. Tavakoli T. Derby E. Serebryakova Y. Rao M.S. Mattson M.P. Cell-extracellular matrix interactions regulate neural differentiation of human embryonic stem cells. BMC Dev Biol. 2008;8:90. doi: 10.1186/1471-213X-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brafman D.A. Shah K.D. Fellner T. Chien S. Willert K. Defining long-term maintenance conditions of human embryonic stem cells with arrayed cellular microenvironment technology. Stem Cells Dev. 2009;18:1141. doi: 10.1089/scd.2008.0410. [DOI] [PubMed] [Google Scholar]
- 43.Flaim C.J. Teng D. Chien S. Bhatia S.N. Combinatorial signaling microenvironments for studying stem cell fate. Stem Cells Dev. 2008;17:29. doi: 10.1089/scd.2007.0085. [DOI] [PubMed] [Google Scholar]
- 44.Gilbert T.W. Sellaro T.L. Badylak S.F. Decellularization of tissues and organs. Biomaterials. 2006;27:3675. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 45.Grauss R.W. Hazekamp M.G. van Vliet S. Gittenberger-de Groot A.C. DeRuiter M.C. Decellularization of rat aortic valve allografts reduces leaflet destruction and extracellular matrix remodeling. J Thorac Cardiovasc Surg. 2003;126:2003. doi: 10.1016/s0022-5223(03)00956-5. [DOI] [PubMed] [Google Scholar]
- 46.Lumpkins S.B. Pierre N. McFetridge P.S. A mechanical evaluation of three decellularization methods in the design of a xenogeneic scaffold for tissue engineering the temporomandibular joint disc. Acta Biomater. 2008;4:808. doi: 10.1016/j.actbio.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 47.Smalheiser N.R. Crain S.M. Reid L.M. Laminin as a substrate for retinal axons in vitro. Brain Res. 1984;314:136. doi: 10.1016/0165-3806(84)90184-6. [DOI] [PubMed] [Google Scholar]
- 48.Ushakova G.A. Nikonenko I.R. Nikonenko A.G. Skibo G.G. Extracellular matrix heparin induces alteration of the cell adhesion during brain development. Neurochem Int. 2002;40:277. doi: 10.1016/s0197-0186(01)00028-6. [DOI] [PubMed] [Google Scholar]
- 49.Gilbert T.W. Freund J.M. Badylak S.F. Quantification of DNA in biologic scaffold materials. J Surg Res. 2009;152:135. doi: 10.1016/j.jss.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin H.J. O'Shaughnessy T.J. Kelly J. Ma W. Neural stem cell differentiation in a cell-collagen-bioreactor culture system. Brain Res Dev Brain Res. 2004;153:163. doi: 10.1016/j.devbrainres.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 51.Rall W. Electrophysiology of a dendritic neuron model. Biophys J. 1962;2:145. doi: 10.1016/s0006-3495(62)86953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woerly S. Petrov P. Sykova E. Roitbak T. Simonova Z. Harvey A.R. Neural tissue formation within porous hydrogels implanted in brain and spinal cord lesions: ultrastructural, immunohistochemical, and diffusion studies. Tissue Eng. 1999;5:467. doi: 10.1089/ten.1999.5.467. [DOI] [PubMed] [Google Scholar]