Abstract
Pulmonary fibrosis refers to a group of lung diseases characterized by inflammation, fibroblast proliferation, and excessive collagen deposition. Although the mechanisms underlying pulmonary fibrosis are poorly understood, current evidence suggests that epithelial injury contributes to the development of fibrosis. Regenerative medicine approaches using extracellular matrix (ECM) scaffolds have been shown to promote site-specific tissue remodeling. This led to the hypothesis that particulate ECM would promote normal tissue repair and attenuate bleomycin-induced pulmonary fibrosis. C57BL/6 mice were treated intratracheally with bleomycin or saline with or without a particulate form of ECM scaffold from porcine urinary bladder matrix (UBM-ECM) or enzymatically digested UBM-ECM. Mice were sacrificed 5 and 14 days after exposure. Compared to control mice, bleomycin-exposed mice had similar increases in inflammation in the bronchoalveolar lavage fluid regardless of UBM-ECM treatment. However, 14 days after exposure, lung histology and collagen levels revealed that mice treated with bleomycin and the particulate or digested UBM-ECM had negligible fibrosis, whereas mice given only bleomycin had marked fibrosis. Administration of the particulate UBM-ECM 24 h after bleomycin exposure also significantly protected against pulmonary injury. In vitro epithelial cell migration and wound healing assays revealed that particulate UBM-ECM promoted epithelial cell chemotaxis and migration. This suggests that promotion of epithelial wound repair may be one mechanism in which UBM-ECM limits pulmonary fibrosis.
Introduction
Idiopathic pulmonary fibrosis (IPF) is a debilitating disease characterized by inflammation, fibroblast proliferation, and excessive extracellular matrix (ECM) deposition in the lung. The pathogenesis of pulmonary fibrosis has been studied widely in animal models. The most widely used model to study pulmonary fibrosis in rodents is the bleomycin model.1,2 While the mechanisms for injury are still not fully understood, it appears that the pathology begins with epithelial damage followed by activation of alveolar macrophages and infiltration of the lung tissue by circulating inflammatory cells that release cytokines such as tumor necrosis factor-α and interleukin-1β.3–5 Ultimately, this proinflammatory environment leads to the recruitment and proliferation of fibroblasts/myofibroblasts that produce transforming growth factor-β6–10 and deposit large quantities of collagenous, fibrotic tissue. Currently, pulmonary transplantation is the only viable treatment option for patients with IPF.1,2 New molecular therapies for pulmonary fibrosis have been suggested and are under investigation, but there is still a need for alternatives for treatment of IPF.
Biologic scaffolds composed of mammalian ECM have been shown to promote site-specific remodeling of musculoskeletal, cardiovascular, urogenital, and dermal tissues. The mechanisms by which these ECM scaffolds promote tissue remodeling are not fully understood, but appear to include the presentation of a three-dimensional microenvironment supportive of cell growth and migration that transmits biochemical and mechanical cues to the cells,11,12 rapid degradation with subsequent release of small peptide fragments that possess innate bioactivity (e.g., chemotaxis for progenitor cells and antibacterial behavior),13–17 and modulation of the host immune response.18–20
ECM scaffolds have recently received attention for treatment of airway injury in several preclinical models. Recent studies have shown that ECM scaffold materials can prevent air leakage into the pleural cavity when used as a primary treatment or when used as reinforcement for a surgical staple line after partial lung resection.21–23 Repair of the lung with urinary bladder matrix (UBM) scaffold showed moderately dense well-organized collagenous tissue formation at the site of resection without evidence of inflammation, necrosis, or scarring in the lung.23 In addition, UBM has recently been shown to promote the formation of site-appropriate pseudostratified, columnar, ciliated epithelium when used for patch tracheoplasty in a canine model.24 Due to its ability to promote site-specific tissue remodeling in the airway, it was hypothesized that UBM could attenuate bleomycin-induced pulmonary fibrosis, which is the focus of the present study. Further, the results for an intact, lyophilized UBM powder were compared to an enzymatically digested form of UBM that would simulate the release of bioactive degradation products from the intact UBM, thereby allowing an understanding of whether the intact morphology of the UBM or the degradation products are more important to the prevention of pulmonary fibrosis.
Materials and Methods
Preparation of UBM-ECM
The preparation of UBM has been previously described.11 Porcine urinary bladders were harvested from market-weight pigs (∼110–130 kg) immediately after sacrifice at an abattoir and transported to the lab on ice. The urothelial layer of the bladders was removed by soaking the material in 1 M saline. The tunica serosa, tunica muscularis externa, tunica submucosa, and most of the muscularis mucosa were mechanically delaminated from the bladder tissue. The remaining basement membrane of the tunica epithelialis mucosa and the subjacent tunica propria were collectively termed UBM. UBM was decellularized and disinfected by immersion in 0.1% (v/v) peracetic acid, 4% (v/v) ethanol, and 96% (v/v) deionized water (diH2O) (pH ∼2.5) for 2 h at room temperature. The material was then washed twice for 15 min with phosphate-buffered saline (pH 7.4) and twice for 15 min with diH2O.11
After the UBM was decellularized and disinfected, the scaffold was lyophilized and chopped into small sheets. The chopped material was then fed through a rotary knife mill. A #60 screen was used to restrict the collected powder size to <250 μm. The powder was then sifted through stainless steel mesh on a Sonic Sifter to <75 μm. For all studies, the particulate material was terminally sterilized with 2 MRad γ-irradiation before use in vivo.25
Particulate UBM was also added to 1 mg/mL pepsin (Sigma) in 0.01 M HCl for a final concentration of 10 mg/mL UBM suspension. The suspension was mixed on a stir plate at room temperature for ∼48 h until no visible pieces of UBM remained. Pepsin buffer control samples were prepared by mixing the pepsin digestion buffer (1 mg/mL pepsin in 0.01 M HCl) at room temperature for 48 h.
Animals
All animal experiments were reviewed and approved by the University of Pittsburgh Institutional Animal Care and Use Committee. All treatments were done intratracheally as previously described.26 Ten-week-old male C57BL/6 mice (unless otherwise noted) were intratracheally instilled with 0.07 units of bleomycin sulfate (Hospira, Inc) with or without 280 μg particulate UBM or digested UBM to determine the effect of UBM on the development of fibrosis. Control mice were treated with 0.9% saline vehicle with and without 280 μg of particulate or digested UBM. Mice were euthanized either 5 or 14 days after exposure. In a separate experiment, 16-week-old male C57BL/6 wild-type mice were intratracheally treated with bleomycin or saline vehicle and then given particulate UBM or saline intratracheally 24 h later. These mice were euthanized 14 days after the initial bleomycin or saline treatment. For both experiments, bronchoalveolar lavage fluid (BALF) was obtained by the intratracheal instillation and recovery of 0.8 mL of 0.9% saline as previously described.26 Lungs from mice 14 days after exposure were inflation fixed with 10% buffered formalin and paraffin embedded for histological analysis of fibrosis.
Bronchoalveolar lavage fluid
Total protein was determined by use of the Coomassie Plus Protein Assay Reagent (Pierce). Total white blood cell counts were obtained with a Beckman Z1 Coulter particle counter (Beckman Coulter). To obtain a differential count, BALF samples were adhered to glass slides with a cytospin, stained with DiffQuik, and the numbers of macrophages, neutrophils, lymphocytes, and eosinophils were counted under a microscope (at least 200 cells). The remaining BALF was centrifuged at 200×g and supernatants were stored at −70°C. Protein concentration in undiluted BALF was measured as previously described.27
Histology and fibrosis scoring
Standard hematoxylin and eosin staining was performed on 5-μm-thick lung sections as previously described.26 Hematoxylin and eosin-stained sections were scored as previously described28,29 by a pathologist (T.D.O.) who was blinded to sample groups. Individual fields were examined with a light microscope at ×200 magnification. Briefly, every field in the entire lung was scored, starting peripherally. To be counted, each field had to contain alveolar tissue in >50% of the field. Scoring in each field was based on the percentage of alveolar tissue with interstitial fibrosis according to the following scale: 0=no fibrosis, 1=up to 25%, 2=25%–50%, 3=50%–75%, 4=75%–100%. The pathological index score was then reported as a ratio of the sum of all of the scores divided by the total number of fields counted for each sample.
Picrosirius red collagen staining
Slides stained with picrosirius red were viewed on a Nikon E600 microscope (Nikon) with Nuance digital camera (CRi). Images were captured using Nuance Imaging System software. At ×400 magnification, three random images of the lung parenchyma were captured and saved. Analysis of the red areas of the slide was completed using ImageJ (NIH) and the “Threshold Colour” plug-in (courtesy of Gabriel Landini).30 Images were loaded and colors were isolated by using the hue histogram filter available in “Threshold Colour.” Images were subjected to a threshold such that each nonwhite pixel was turned black and each white pixel remained white. Then, the number of black pixels in each image was used to calculate the percentage of the image area that corresponded to a certain color. The average percentage was then calculated for the saline, saline + particulate UBM, bleomycin, and bleomycin + particulate UBM groups.
Toxicity assay
A549 human epithelial cells (ATCC) were cultured in F12K media supplemented with 10% fetal bovine serum (FBS). A549 cells were seeded on 96-well plates (5000 cells/well). Cells were serum starved for 4 h before treatment with either 0.02 units of bleomycin, or bleomycin with various amount of particulate and digested UBM as indicated in serum-free media. Twenty-four hours after treatment, cell viability was measured using CellTiter 96 AQ Nonradioactive Assay according to manufacturer's instructions (Promega).
Chemotaxis assay
Responses of epithelial cells to UBM degradation products were quantitatively evaluated utilizing the Neuro Probe 48-well microchemotaxis chamber (Neuro Probe). A549 cells were serum-starved for 14–17 h before experimentation. Based upon pilot studies to determine the appropriate filter pore size, 5 μm polycarbonate chemotaxis filters (Neuro Probe, PFB5) were coated equally on both sides (by immersion) with 0.05 mg/mL rat tail collagen I (BD Biosciences) and allowed to dry before chamber assembly. About 27.5 μL of F12K media (Cellgro, 10–025-CV), F12K media supplemented with 10% FBS, 0.5 mg/mL UBM digest, 0.1 mg/mL UBM digest, 0.5 mg/mL Pepsin digest, and 0.1 mg/mL Pepsin digest was added to the bottom chamber wells. The filter was placed over the bottom chamber, and the apparatus was assembled according to the manufacturer's instructions. Approximately 30,000 cells were then added to each upper chamber well of the apparatus, and the chamber was incubated for 4 h at 37°C in a humidified atmosphere in 95% air:5% CO2. Cells remaining on the topside of the membrane (i.e., nonmigrated cells) were removed, and then cells on the bottom side of the membrane (i.e., migrated cells) were stained with Diff Quik (Dade AG). The filter was then mounted with Vectashield containing DAPI (Vector Laboratories, H-1200) and fluorescent images of each conditioned well were captured at 10×magnification using a Nuance multispectral imaging system and Nikon microscope. Each experimental condition was tested in quadruplicate in three independent experiments. The average number of migrated cells in each experiment was normalized to the positive control (10% FBS) by calculating the average percentage of migrated cells as a percentage of the positive control for each condition.
Wound healing assay
A549 cells cultured in F12K media (Cellgro 10–025-CV) supplemented with 10% FBS were seeded and grown to confluence in a six-well plate. Cells were serum starved in nonsupplemented F12K media overnight before initiation of the experiment. Straight wounds were created as previously described31,32 by scratching vertically with a p-200 micropipette tip. The wounds were then washed and treated with the following experimental conditions: 0.075 U/mL bleomycin; 0.075 U/mL bleomycin +0.5 mg/mL particulate UBM; 0.075 U/mL bleomycin +0.5 mg/mL UBM digest; 0.075 U/mL bleomycin +0.5 mg/mL pepsin digest; and 0.5 mg/mL UBM digest. Images were captured at 0 and 24 h after addition of treatments. Using ImageJ, average wound widths in pixels were obtained for each well at each time point.
Statistical analyses
Data were analyzed using GraphPad Prism 5 (Graphpad Software Inc.). The significance of all quantitative data was assessed using a one-way analysis of variance with Tukey's post-test except for the delayed administration animal study in which a two-way analysis of variance with a Bonferroni post-test was used. All values are means (±standard error of the mean). A p-value <0.05 was considered to be statistically significant.
Results
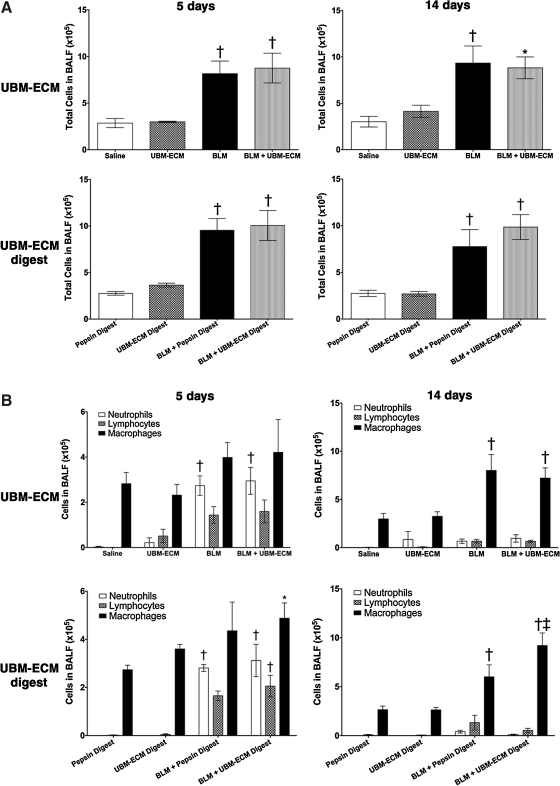
Wild-type C57BL/6 mice were treated with bleomycin or saline vehicle with and without particulate UBM or digested UBM and sacrificed 5 and 14 days after exposure. There was an increase in the number of leukocytes within the BALF at both 5 and 14 days after bleomycin exposure regardless of UBM treatment (Fig. 1A). The BALF differentials showed a significant increase in macrophages and modest increases in neutrophils and lymphocytes in the BALF of mice treated with bleomycin and bleomycin with either particulate or digested UBM when compared to controls at both 5 and 14 days after intratracheal instillation (Fig. 1B).
FIG. 1.
Treatment with ECM does not prevent bleomycin-induced leukocyte accumulation. Wild-type mice were administered bleomycin or saline (vehicle) with and without particulate UBM-ECM or digested UBM-ECM and euthanized 5 and 14 days after intratracheal instillation. All bleomycin-treated mice regardless of UBM-ECM co-administration had increased total cells in their BALF (A) and specifically significantly more macrophages and modest increases in neutrophils and lymphocytes when compared to controls (B). *p<0.05 compared to vehicle control (saline or pepsin digest), †p<0.05 compared to ECM control (UBM-ECM or UBM-ECM Digest) and vehicle control, ‡p<0.01 compared to BLM + Pepsin Digest; n=4–7/treatment/timepoint. ECM, extracellular matrix; UBM, urinary bladder matrix; BALF, bronchoalveolar lavage fluid.
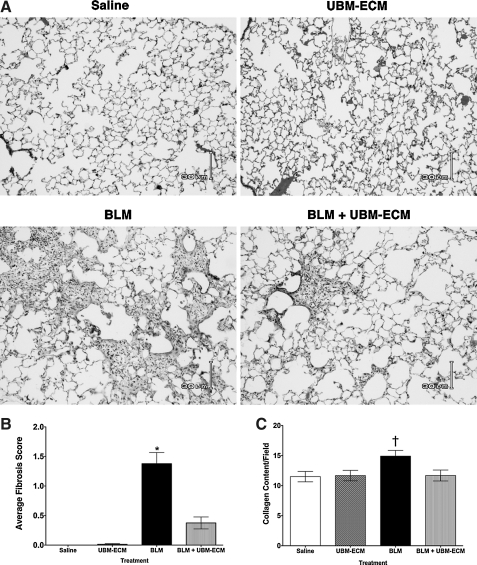
Although UBM treatment did not affect the inflammatory response to bleomycin, histological analyses of the lungs 14 days after treatment showed a significant reduction in fibrosis in the lungs of mice that were co-administered particulate UBM with bleomycin when compared to the mice that received bleomycin alone (Fig. 2A). These differences in fibrosis were quantified through histological scoring (Fig. 2B) and further confirmed by picrosirius red staining, another measure of collagen deposition in the lungs (Fig. 2C). Administration of UBM alone did not cause any adverse effects and the lungs were pathologically identical to the saline controls (Fig. 2A). Similar protective effects were seen for mice given UBM digest and bleomycin when compared to mice that only received bleomycin (data not illustrated).
FIG. 2.
UBM-ECM prevents bleomycin-induced fibrosis. Wild-type mice were euthanized 14 days after intratracheal administration of bleomycin or saline vehicle with or without particulate UBM-ECM. (A) Histological analyses of the lungs revealed that UBM-ECM limited pulmonary fibrosis. (B) Average histology score was determined for each group. (C) Fibrosis was also assessed by determining the amount of collagen deposition in the lungs using picosirius red staining and is reported as the area of collagen content per total area of the field. Similar results were seen with co-administration of a digested form of UBM-ECM. *p<0.05 when compared to all other treatment groups and †p<0.05 when compared to saline control; n=4–7/treatment group.
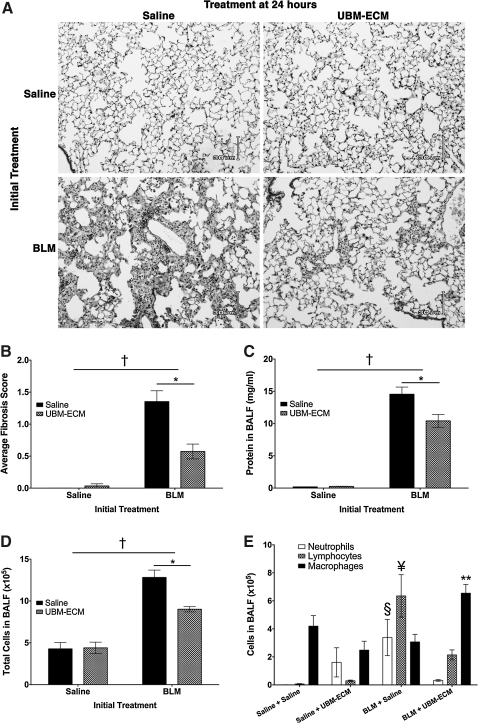
In addition to co-administration, particulate UBM was also administered after bleomycin exposure to assess its ability to limit the development of fibrosis after the initial injury has occurred. To investigate this, 16-week-old male C57BL/6 wild-type mice were intratracheally treated with bleomycin or saline vehicle and then given UBM or saline intratracheally 24 h later. Based on histology of the lungs, bleomycin-treated mice that received UBM 24 h after injury developed significantly less fibrosis 14 days later when compared to bleomycin-treated mice that received saline control (Fig. 3A). Histological scoring also revealed that UBM treatment 1 day after bleomycin exposure markedly limited pulmonary fibrosis in mice (Fig. 3B). In addition to fibrosis, bleomycin-treated mice that received UBM also had less protein (Fig. 3C) and lower levels of inflammatory cells (Fig. 3D) in their BALF than bleomycin-treated mice that received saline. Cell differentials of the BALF showed that bleomycin-treated mice that received UBM have significantly less neutrophils and lymphocytes, but significantly more macrophages than bleomycin-treated mice that received saline (Fig. 3E). Although delayed administration of UBM significantly decreased bleomycin injury in the lung, saline-treated mice that received UBM 24 h later had small focal areas of lymphocytic inflammation and interstitial thickening present in their lungs (not illustrated) and some neutrophils present in their airspaces (Fig. 3E).
FIG. 3.
Administration of UBM-ECM 1 day after bleomycin exposure attenuates pulmonary injury. Wild-type mice were intratracheally treated with bleomycin or saline control and 24 h later were given either particulate UBM-ECM or saline via intratracheal instillation. Mice were euthanized 14 days after bleomycin exposure to assess injury in the lungs. Histological analyses of the lungs by microscopy (A) and by average histologic scoring of fibrosis (B) revealed that UBM-ECM limited pulmonary fibrosis when administered after bleomycin exposure. In addition, subsequent treatment with UBM-ECM after bleomycin exposure also limited the levels of protein (C) and total cells (D) present in the BALF when compared to bleomycin-treated mice that were given saline. Differential counts of the BALF cells were conducted and there were significantly more lymphocytes and neutrophils, whereas less macrophages in the bleomycin-treated mice that received saline when compared to bleomycin-treated mice that received UBM-ECM (E). *p<0.05 between treatment groups, †p<0.05 shows interaction, p<0.05 when comparing the cell type indicated between the following groups: **Saline + UBM-ECM and BLM + Saline, §Saline + Saline and BLM + UBM-ECM, ¥all other treatment groups; n=4–5/treatment group.
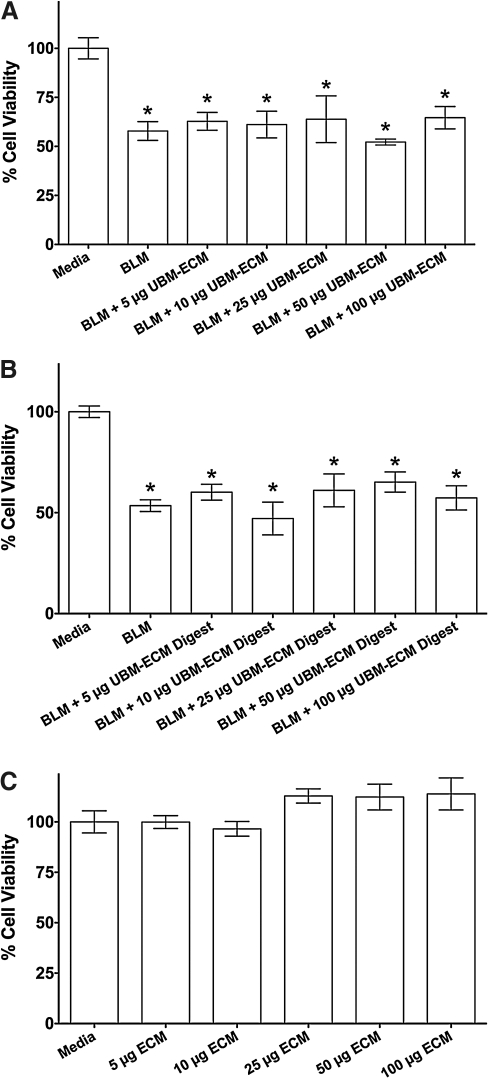
A549 cells were treated with bleomycin and different doses of UBM or digested UBM to verify that the presence of the ECM scaffold material does not alter the toxicity of the bleomycin. These results showed that reduction in cell viability caused by bleomycin was not significantly altered due to bleomycin treatment simultaneous with UBM (Fig. 4A) or UBM digest (Fig. 4B). Treatment with 5, 10, 25, 50, or 100 μg of UBM, UBM digest, or pepsin digest alone did not affect cell viability (representative graph of all ECM components is shown in Fig. 4C). Similar results were also seen using the MH-S murine alveolar macrophage cell line (ATCC; data not illustrated).
FIG. 4.
ECM treatment does not affect bleomycin toxicity. A549 cells (5000 cells/well) were incubated with 0.02 units of bleomycin (BLM), BLM with indicated amounts of particulate UBM-ECM (A) or digested UBM-ECM (B), or ECM (particulate or digested UBM-ECM) without BLM (C) for 24 h. Cell viability was reported as the percentage of viable cells when compared to media control (mean±SEM). *p<0.05 when compared to media control. SEM, standard error of the mean.
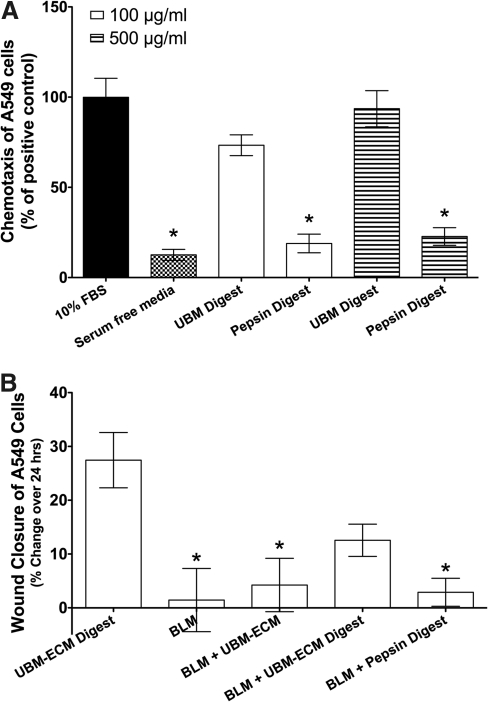
Migration of serum-starved A549 cells toward the digested form of UBM approached the migration promoted by the positive control, 10% FBS (Fig. 5A). The digested UBM at 500 μg/mL concentration showed 94%±35% of the cell migration observed for the positive control, whereas 100 μg/mL concentration showed 73%±20% of the cell migration observed for the positive control. The relative cell migration toward the pepsin digest controls were 23%±17% and 19%±18% for 500 and 100 μg/mL concentration, respectively. The migration toward both concentrations of pepsin digest was similar to the F12K serum free media (13%±11%). At both concentrations, the UBM digest showed significantly improved migration compared to the pepsin digest control (Fig. 5A).
FIG. 5.
UBM-ECM promotes chemotaxis of epithelial cells and stimulates re-epithelialization. (A) Chemotaxis of A549 cells (30,000 cells/well) was evaluated in response to F12K media with 10% FBS, serum-free F12K media, 100 and 500 μg/mL UBM-ECM digest, or control pepsin digest. The average percentage of migrated cells for each condition was normalized to the average percentage of cells that migrated toward the media with 10% FBS in each experiment (mean±SEM). *p<0.05 when compared to 10% FBS. (B) A549 cell monolayers were wounded and exposed to UBM-ECM digest, bleomycin (BLM), BLM with particulate or digested UBM-ECM, or BLM with pepsin digest control. Wound widths were measured in triplicate for each treatment for three independent experiments. Results were reported as the percentage of change in wound width over 24 h (mean±SEM). *p<0.05 when compared to UBM-ECM digest control. FBS, fetal bovine serum.
Using a wound healing assay, serum-starved A549 cells were treated with particulate UBM or UBM digest in the presence of bleomycin to evaluate the ability of ECM to improve wound repair. UBM digest treatment promoted wound closure in the presence of bleomycin, whereas the pepsin digest control did not affect wound width (Fig. 5B). Particulate UBM treatment showed a trend toward improved wound closure, but did not significantly reduce wound width in the presence of bleomycin.
Discussion
UBM scaffold material prevented bleomycin-induced pulmonary fibrosis regardless of the form of the material (particulate or digested). UBM treatment significantly reduced the histologic presentation of fibrosis, such that there was no significant difference between the histologic appearance of the lungs in animals treated with bleomycin and UBM as compared to those treated with the saline alone. More impressively, UBM treatment significantly prevented the development of bleomycin injury even when administered 1 day after bleomycin exposure in mice. Less than 1% of bleomycin intratracheally instilled in the lungs of mice is present 24 h after injection.33 Thus, UBM can attenuate the severity of fibrosis even when administered after the bleomycin injury has occurred. Therefore, these novel findings demonstrate the immense potential for the use of UBM as a novel therapy for pulmonary fibrosis.
It is clear that the attenuation of fibrosis in response to UBM was not due to neutralization of the bleomycin when co-administered with these compounds as cell culture studies showed that UBM did not prevent cell death. Further, animals treated with bleomycin and ECM products had the same increase in the number of inflammatory cells and similar cellular composition in the BALF as animals treated with bleomycin alone. These results suggest that UBM limited bleomycin-induced fibrogenesis and did not do so by interfering with the direct injurious effects of bleomycin.
The digested form of UBM had the same protective effect as the lyophilized particulate form in vivo. This suggests that the composition of the UBM and its degradation products may be more important to the repair process than the specific ultrastructure of the particulate form of UBM.25 UBM is known to possess basement membrane structure that is preserved through the process of making a powder.11 The presence of a basement membrane structure has been shown to promote healing in various organs, including the lungs, by providing guidance for re-epithelialization and separating the epithelium from the interstitial connective tissue.34 Chemotaxis and wound healing assays performed in the present study showed that the digested form of UBM could promote migration of airway epithelial cells and also could stimulate wound healing in the presence of bleomycin. It is possible that small peptides from the degradation of laminin and collagen IV in the basement membrane increase the migration of epithelial cells.
Many studies have noted that degradation of the UBM is an important component of the host response that leads to site-appropriate tissue remodeling. Previous studies have shown that UBM degradation products recruit progenitor cells to the site of remodeling,13,15,35 promote angiogenesis,17 and provide bacteriostasis.14 On the other hand, studies have found that the oxidative fragmentation of ECM components, such as heparan sulfate, collagen, syndecan-1, and hyaluronan, influences the development of fibrosis.31,36–38 As the UBM scaffold material is a mixture of ECM proteins and other substances, such as growth factors,39 it is likely that this complex composition of ECM promotes healing that outweighs the detrimental effects of the proteoglycans and glycosoaminoglycans degradation products in these ECM-based materials.
Although this study clearly illustrates that UBM attenuates bleomycin-induced pulmonary fibrosis, the mechanism by which it does so is still unclear. Current evidence is presented to suggest that the UBM plays a role in epithelial repair, but additional studies are warranted to fully understand this phenomenon. The current work also shows that treatment of UBM with or after bleomycin showed an elevated number of immune cells, predominantly macrophages. In previous studies in which UBM scaffolds were used to bridge a defect in the rat abdominal wall, site-specific tissue repair was associated with a predominately M2 macrophage phenotype during the first month after surgery.18–20 Within the lung, the M2 macrophage phenotype has been reported to be associated with the onset of fibrosis.40,41 Therefore, it is likely important to fully characterize the immune response to UBM in this model as it could provide evidence that the ability for the UBM to modulate the immune response may be tissue dependent. The phenotype of the macrophages could also play a role in the matrix metalloproteases that are present within the lung to degrade the instilled particulate ECM and to participate in remodeling of the lung parenchyma.
Finally, UBM was selected for investigation because it contains a basement membrane component and has been shown to be beneficial for other airway applications. Organ-specific ECM derived from the trachea and lung has recently been explored for airway repair.42–48 In the lung models, the acellular lung matrix with its three-dimensional architecture and composition largely intact promoted site-specific cell differentiation and retained similar mechanical characteristics.42,45–47 It is possible that an airway-derived ECM may be even more effective for prevention of fibrosis than a heterotopic ECM source due to the organ-specific signaling molecules that would be present within the ECM.
Conclusion
The present study showed that ECM from porcine urinary bladder can protect against pulmonary fibrosis in a bleomycin model, and that the composition and degradation of the scaffold is likely more important that the ultrastructure of the intact scaffold in guiding this response. These findings also strongly suggest that one mechanism by which UBM prevents fibrosis is through release of bioactive degradation products that promote epithelial cell chemotaxis and re-epithelialization. Future studies will investigate whether UBM scaffolds also play a role in modulation of the immune response. In addition, future studies will elucidate whether UBM can also attenuate the fibrotic response to chronic environmental irritants, such as asbestos and silica, which would suggest that it may have broader therapeutic use in different forms of pulmonary fibrosis.
Acknowledgments
The authors would like to acknowledge Kristen Agnew for participating in the animal care for the studies described. This study was funded by NIH R01 HL63700-09 (T.D.O.), AHA #0715279U (M.L.M.), and Commonwealth of Pennsylvania (T.W.G.).
Disclosure Statement
No competing financial interests exist. T.W.G. serves on the Scientific Advisory Board for ACell, Inc.
References
- 1.Meltzer E.B. Noble P.W. Idiopathic pulmonary fibrosis. Orphanet J Rare Dis. 2008;3:8. doi: 10.1186/1750-1172-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moeller A. Ask K. Warburton D. Gauldie J. Kolb M. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int J Biochem Cell Biol. 2008;40:362. doi: 10.1016/j.biocel.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piguet P.F. Collart M.A. Grau G.E. Sappino A.P. Vassalli P. Requirement of tumour necrosis factor for development of silica-induced pulmonary fibrosis. Nature. 1990;344:245. doi: 10.1038/344245a0. [DOI] [PubMed] [Google Scholar]
- 4.Piguet P.F. Kaufman S. Barazzone C. Muller M. Ryffel B. Eugster H.P. Resistance of TNF/LT alpha double deficient mice to bleomycin-induced fibrosis. Int J Exp Pathol. 1997;78:43. doi: 10.1046/j.1365-2613.1997.d01-240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y. Lee T.C. Guillemin B. Yu M.C. Rom W.N. Enhanced IL-1 beta and tumor necrosis factor-alpha release and messenger RNA expression in macrophages from idiopathic pulmonary fibrosis or after asbestos exposure. J Immunol. 1993;150:4188. [PubMed] [Google Scholar]
- 6.Broekelmann T.J. Limper A.H. Colby T.V. McDonald J.A. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci USA. 1991;88:6642. doi: 10.1073/pnas.88.15.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giri S.N. Hyde D.M. Hollinger M.A. Effect of antibody to transforming growth factor beta on bleomycin induced accumulation of lung collagen in mice. Thorax. 1993;48:959. doi: 10.1136/thx.48.10.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khalil N. Bereznay O. Sporn M. Greenberg A.H. Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J Exp Med. 1989;170:727. doi: 10.1084/jem.170.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korfhagen T.R. Swantz R.J. Wert S.E. McCarty J.M. Kerlakian C.B. Glasser S.W., et al. Respiratory epithelial cell expression of human transforming growth factor-alpha induces lung fibrosis in transgenic mice. J Clin Invest. 1994;93:1691. doi: 10.1172/JCI117152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santana A. Saxena B. Noble N.A. Gold L.I. Marshall B.C. Increased expression of transforming growth factor beta isoforms (beta 1, beta 2, beta 3) in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 1995;13:34. doi: 10.1165/ajrcmb.13.1.7541221. [DOI] [PubMed] [Google Scholar]
- 11.Brown B. Lindberg K. Reing J. Stolz D.B. Badylak S.F. The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Eng. 2006;12:519. doi: 10.1089/ten.2006.12.519. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert T.W. Stewart-Akers A.M. Sydeski J. Nguyen T.D. Badylak S.F. Woo S.L.-Y. Gene expression by fibroblasts seeded on small intestinal submucosa and subjected to cyclic stretching. Tissue Eng. 2007;13:1313. doi: 10.1089/ten.2006.0318. [DOI] [PubMed] [Google Scholar]
- 13.Beattie A.J. Gilbert T.W. Guyot J.P. Yates A.J. Badylak S.F. Chemoattraction of progenitor cells by remodeling extracellular matrix scaffolds. Tissue Eng Part A. 2009;15:1119. doi: 10.1089/ten.tea.2008.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brennan E.P. Reing J. Chew D. Myers-Irvin J.M. Young E.J. Badylak S.F. Antibacterial activity within degradation products of biological scaffolds composed of extracellular matrix. Tissue Eng. 2006;12:2949. doi: 10.1089/ten.2006.12.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brennan E.P. Tang X.H. Stewart-Akers A.M. Gudas L.J. Badylak S.F. Chemoattractant activity of degradation products of fetal and adult skin extracellular matrix for keratinocyte progenitor cells. J Tissue Eng Regen Med. 2008;2:491. doi: 10.1002/term.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilbert T.W. Stewart-Akers A.M. Simmons-Byrd A. Badylak S.F. Degradation and remodeling of small intestinal submucosa in canine Achilles tendon repair. J Bone Joint Surg Am. 2007;89:621. doi: 10.2106/JBJS.E.00742. [DOI] [PubMed] [Google Scholar]
- 17.Li F. Li W. Johnson S. Ingram D. Yoder M. Badylak S.F. Low-molecular-weight peptides derived from extracellular matrix as chemoattractants for primary endothelial cells. Endothelium. 2004;11:199. doi: 10.1080/10623320490512390. [DOI] [PubMed] [Google Scholar]
- 18.Badylak S.F. Gilbert T.W. Immune response to biologic scaffold materials. Semin Immunol. 2008;20:109. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Badylak S.F. Valentin J.E. Ravindra A.K. McCabe G.P. Stewart-Akers A.M. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A. 2008;14:1835. doi: 10.1089/ten.tea.2007.0264. [DOI] [PubMed] [Google Scholar]
- 20.Brown B.N. Valentin J.E. Stewart-Akers A.M. McCabe G.P. Badylak S.F. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30:1482. doi: 10.1016/j.biomaterials.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Downey D.M. Harre J.G. Pratt J.W. Functional comparison of staple line reinforcements in lung resection. Ann Thorac Surg. 2006;82:1880. doi: 10.1016/j.athoracsur.2006.02.079. [DOI] [PubMed] [Google Scholar]
- 22.Downey D.M. Michel M. Harre J.G. Pratt J.W. Functional assessment of a new staple line reinforcement in lung resection. J Surg Res. 2006;131:49. doi: 10.1016/j.jss.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert T.W. Nieponice A. Spievack A.R. Holcomb J. Gilbert S. Badylak S.F. Repair of the thoracic wall with an extracellular matrix scaffold in a canine model. J Surg Res. 2008;147:61. doi: 10.1016/j.jss.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert T.W. Gilbert S. Madden M. Reynolds S.D. Badylak S.F. Morphologic assessment of extracellular matrix scaffolds for patch tracheoplasty in a canine model. Ann Thorac Surg. 2008;86:967. doi: 10.1016/j.athoracsur.2008.04.071. discussion 73–74. [DOI] [PubMed] [Google Scholar]
- 25.Gilbert T.W. Stolz D.B. Biancaniello F. Simmons-Byrd A. Badylak S.F. Production and characterization of ECM powder: implications for tissue engineering applications. Biomaterials. 2005;26:1431. doi: 10.1016/j.biomaterials.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 26.Fattman C.L. Chu C.T. Kulich S.M. Enghild J.J. Oury T.D. Altered expression of extracellular superoxide dismutase in mouse lung after bleomycin treatment. Free Radic Biol Med. 2001;31:1198. doi: 10.1016/s0891-5849(01)00699-2. [DOI] [PubMed] [Google Scholar]
- 27.Ramsgaard L. Englert J.M. Tobolewski J. Tomai L. Fattman C.L. Leme A.S., et al. The role of the receptor for advanced glycation end-products in a murine model of silicosis. PLoS One. 2010;5:e9604. doi: 10.1371/journal.pone.0009604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Englert J.M. Hanford L.E. Kaminski N. Tobolewski J.M. Tan R.J. Fattman C.L., et al. A role for the receptor for advanced glycation end products in idiopathic pulmonary fibrosis. Am J Pathol. 2008;172:583. doi: 10.2353/ajpath.2008.070569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fattman C.L. Tan R.J. Tobolewski J.M. Oury T.D. Increased sensitivity to asbestos-induced lung injury in mice lacking extracellular superoxide dismutase. Free Radic Biol Med. 2006;40:601. doi: 10.1016/j.freeradbiomed.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilbert T.W. Agrawal V. Gilbert M.R. Povirk K.M. Badylak S.F. Rosen C.A. Liver-derived extracellular matrix as a biologic scaffold for acute vocal fold repair in a canine model. Laryngoscope. 2009;119:1856. doi: 10.1002/lary.20575. [DOI] [PubMed] [Google Scholar]
- 31.Kliment C.R. Englert J.M. Gochuico B.R. Yu G. Kaminski N. Rosas I., et al. Oxidative stress alters syndecan-1 distribution in lungs with pulmonary fibrosis. J Biol Chem. 2009;284:3537. doi: 10.1074/jbc.M807001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang C.C. Park A.Y. Guan J.L. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 33.Lazo J.S. Pham E.T. Pulmonary fate of 3H bleomycin A2 in mice. J Pharmacol Exp Ther. 1984;228:13. [PubMed] [Google Scholar]
- 34.Vracko R. Basal lamina scaffold-anatomy and significance for maintenance of orderly tissue structure. Am J Pathol. 1974;77:314. [PMC free article] [PubMed] [Google Scholar]
- 35.Reing J.E. Zhang L. Myers-Irvin J. Cordero K.E. Freytes D.O. Heber-Katz E., et al. Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Eng Part A. 2009;15:605. doi: 10.1089/ten.tea.2007.0425. [DOI] [PubMed] [Google Scholar]
- 36.Gao F. Koenitzer J.R. Tobolewski J.M. Jiang D. Liang J. Noble P.W., et al. Extracellular superoxide dismutase inhibits inflammation by preventing oxidative fragmentation of hyaluronan. J Biol Chem. 2008;283:6058. doi: 10.1074/jbc.M709273200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kliment C.R. Tobolewski J.M. Manni M.L. Tan R.J. Enghild J. Oury T.D. Extracellular superoxide dismutase protects against matrix degradation of heparan sulfate in the lung. Antioxid Redox Signal. 2008;10:261. doi: 10.1089/ars.2007.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petersen S.V. Oury T.D. Ostergaard L. Valnickova Z. Wegrzyn J. Thogersen I.B., et al. Extracellular superoxide dismutase (EC-SOD) binds to type i collagen and protects against oxidative fragmentation. J Biol Chem. 2004;279:13705. doi: 10.1074/jbc.M310217200. [DOI] [PubMed] [Google Scholar]
- 39.Badylak S.F. Freytes D.O. Gilbert T.W. Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater. 2009;5:1. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 40.Hancock A. Armstrong L. Gama R. Millar A. Production of interleukin 13 by alveolar macrophages from normal and fibrotic lung. Am J Respir Cell Mol Biol. 1998;18:60. doi: 10.1165/ajrcmb.18.1.2627. [DOI] [PubMed] [Google Scholar]
- 41.Murray L.A. Rosada R. Moreira A.P. Joshi A. Kramer M.S. Hesson D.P., et al. Serum amyloid P therapeutically attenuates murine bleomycin-induced pulmonary fibrosis via its effects on macrophages. PLoS One. 2010;5:e9683. doi: 10.1371/journal.pone.0009683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cortiella J. Niles J. Cantu A. Brettler A. Pham A. Vargas G., et al. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng Part A. 2010;16:2565. doi: 10.1089/ten.tea.2009.0730. [DOI] [PubMed] [Google Scholar]
- 43.Jungebluth P. Go T. Asnaghi A. Bellini S. Martorell J. Calore C., et al. Structural and morphologic evaluation of a novel detergent-enzymatic tissue-engineered tracheal tubular matrix. J Thorac Cardiovasc Surg. 2009;138:586. doi: 10.1016/j.jtcvs.2008.09.085. [DOI] [PubMed] [Google Scholar]
- 44.Macchiarini P. Jungebluth P. Go T. Asnaghi M.A. Rees L.E. Cogan T.A., et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 45.Ott H.C. Clippinger B. Conrad C. Schuetz C. Pomerantseva I. Ikonomou L., et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 46.Petersen T.H. Calle E.A. Zhao L. Lee E.J. Gui L. Raredon M.B., et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Price A.P. England K.A. Matson A.M. Blazar B.R. Panoskaltsis-Mortari A. Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded. Tissue Eng Part A. 2010;16:2581. doi: 10.1089/ten.tea.2009.0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Remlinger N.T. Czajka C.A. Juhas M.E. Vorp D.A. Stolz D.B. Badylak S.F., et al. Hydrated xenogeneic decellularized tracheal matrix as a scaffold for tracheal reconstruction. Biomaterials. 2010;31:3520. doi: 10.1016/j.biomaterials.2010.01.067. [DOI] [PubMed] [Google Scholar]