Abstract
Background Previous studies on childhood cancer and nuclear power plants (NPPs) produced conflicting results. We used a cohort approach to examine whether residence near NPPs was associated with leukaemia or any childhood cancer in Switzerland.
Methods We computed person-years at risk for children aged 0–15 years born in Switzerland from 1985 to 2009, based on the Swiss censuses 1990 and 2000 and identified cancer cases from the Swiss Childhood Cancer Registry. We geo-coded place of residence at birth and calculated incidence rate ratios (IRRs) with 95% confidence intervals (CIs) comparing the risk of cancer in children born <5 km, 5–10 km and 10–15 km from the nearest NPP with children born >15 km away, using Poisson regression models.
Results We included 2925 children diagnosed with cancer during 21 117 524 person-years of follow-up; 953 (32.6%) had leukaemia. Eight and 12 children diagnosed with leukaemia at ages 0–4 and 0–15 years, and 18 and 31 children diagnosed with any cancer were born <5 km from a NPP. Compared with children born >15 km away, the IRRs (95% CI) for leukaemia in 0–4 and 0–15 year olds were 1.20 (0.60–2.41) and 1.05 (0.60–1.86), respectively. For any cancer, corresponding IRRs were 0.97 (0.61–1.54) and 0.89 (0.63–1.27). There was no evidence of a dose–response relationship with distance (P > 0.30). Results were similar for residence at diagnosis and at birth, and when adjusted for potential confounders. Results from sensitivity analyses were consistent with main results.
Conclusions This nationwide cohort study found little evidence of an association between residence near NPPs and the risk of leukaemia or any childhood cancer.
Keywords: Childhood, cancer, leukaemia, ionizing radiation, nuclear power plants, population based, cancer registry
Introduction
Since Black reported a cluster of children with leukaemia near Sellafield in 1984,1 numerous studies investigated cancer incidence near nuclear power plants (NPP), with conflicting results. Some found an increased risk also at places where NPPs were planned but not built, concluding that factors other than radiation might be responsible.2,3 A recent case–control study from Germany, which reported odds ratios (ORs) of 1.61 for all cancers and 2.19 for leukaemia in 0–4 year olds living <5 km from a NPP, refuelled the debate on a possible link between NPPs and childhood cancer.4,5
Most previous analyses were ecological and may have been affected by exposure misclassification and confounding.6–11 The German case–control study was criticized for potential selection and participation bias.4,5,12,13 A recent Finnish study combining ecological, cohort and case–control analyses was small, with no children living <5 km from NPPs.14 All previous studies analysed residence at diagnosis. Given the higher vulnerability to radiation of fetuses and infants and the latency between radiation exposure and cancer development, residence at birth might be more relevant.15
We investigated the risk of childhood leukaemia and all childhood cancers in the vicinity of Swiss NPPs, using a cohort approach with person-years derived from the Swiss censuses 1990 and 2000 and incident cases from the Swiss Childhood Cancer Registry (SCCR). We analysed distance of residence at birth to the nearest NPP in the main analysis, and distance to residence at diagnosis in a secondary analysis. Outcomes of interest were leukaemia and all cancers diagnosed at ages 0–4 (<5) and 0–15 (<16) years. We adjusted for confounders, included other nuclear installations (research reactors and storage sites), and locations where NPPs were planned but not built, and assessed the robustness of the results in sensitivity analyses.
Methods
Nuclear facilities in Switzerland
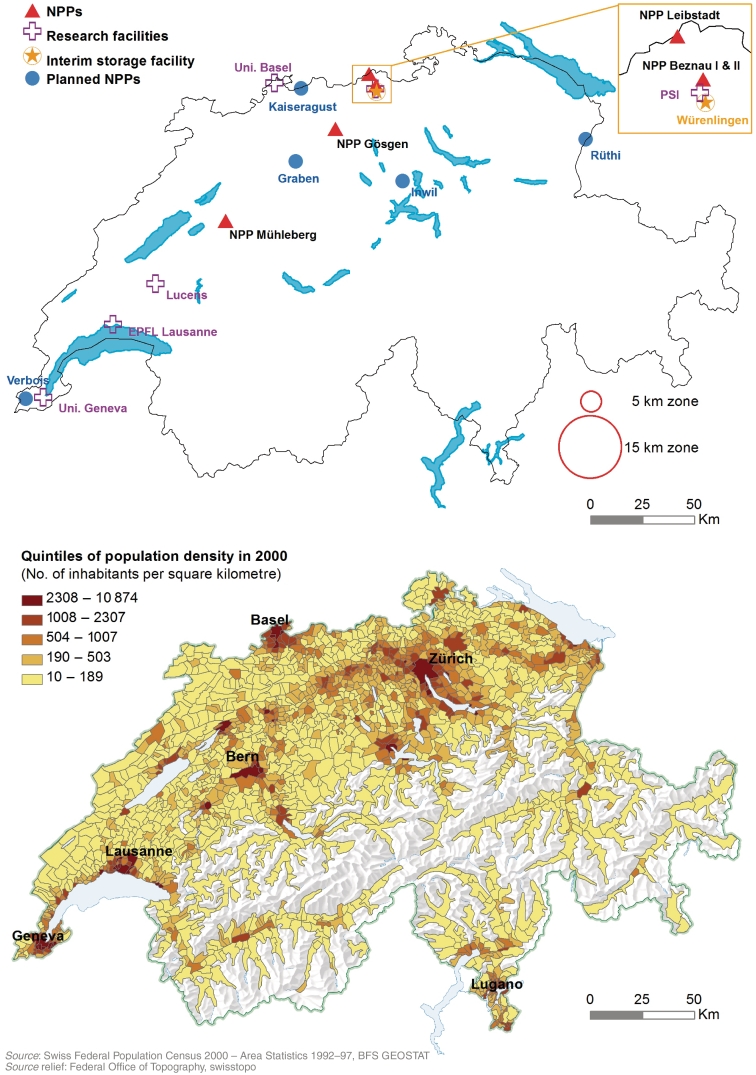
Figure 1 shows the location of Switzerland's five NPPs: Beznau I and II (in operation since 1969 and 1971), Leibstadt (6 km from Beznau, since 1984), Mühleberg (since 1972) and Gösgen (since 1979). Other nuclear facilities include three research reactors (Universities of Lausanne and Basel and Paul Scherrer Institute [PSI] in Villigen, operating since 1983, 1959 and 1957, respectively), a prototype reactor (Lucens, 1968–69, shut down after a partial meltdown) and an interim storage facility in Würenlingen (since 2001). Airborne and liquid radioactive discharges and direct radiation in the vicinity of facilities are monitored.16 Emissions are comparable to those reported from France, Germany and the UK.13 In 2009, the total exposure to ionizing radiation of the Swiss population was estimated at 5.5 mSv/year, including 3.2 mSV from radon, 1.2 mSv from medical and 0.75 mSV from cosmic and terrestrial radiation.16 Among people living in the vicinity of a NPP, a small fraction of the annual exposure (<0.01 mSv/year) was attributable to discharges from the NPP. At five sites (Graben, Inwil, Kaiseraugst, Rüthi, Verbois), NPPs had been planned but not built.
Figure 1.
Maps showing sites of nuclear facilities and population density in Switzerland. Locations of NPPs, research facilities, the interim storage facility and sites where NPPs were planned but never built (upper map) and population density in 2000 in quintiles (lower map)
NPP, nuclear power plant; EPFL, École Polytechnique Fédérale de Lausanne; Uni, University; PSI, Paul Scherrer Institute
Definition of birth and resident cohorts
We analysed two cohorts: a birth cohort and a resident cohort. For both cohorts, person-time at risk was calculated based on the censuses 1990 and 2000 using data from the Swiss National Cohort.17 The birth cohort included all children born in Switzerland between January 1985 and December 2009 and person-time at risk was measured from birth. The resident cohort included all 0–15 year olds residing in Switzerland for any amount of time between January 1985 and December 2009. Person-time at risk was measured from the date the child entered the cohort.
All children diagnosed with cancer 1985–2009 and registered in the population-based Swiss Childhood Cancer Registry (SCCR, www.childhoodcancerregistry.ch)18,19 were eligible. The SCCR includes all children diagnosed with leukaemia, malignant solid tumours or brain tumours classified according to the International Classification of Childhood Cancer (ICCC3).20 Children with Langerhans cell histiocytosis are also included. Completeness of registration is >90% and incidence rates are similar to other countries with national registries.18,19,21
Exposure assessment and geo-coding
Study exposures were the distance of the residence from the nearest nuclear facility at birth (birth cohort, main analysis) or the distance at diagnosis (resident cohort, secondary analysis). We considered (i) distance to the nearest NPP, (ii) smallest distance to any nuclear facility and (iii) distance to nearest planned but not built NPP. Address at diagnosis is collected routinely in the SCCR. For this study, we additionally obtained resident histories back to birth by contacting the communal population registries. Addresses of cancer cases were geo-coded by the Swiss Federal Statistical Office (FSO) using specialized software, or coded manually using the fixpoint data service (FPDS) of the Swiss Federal Office of Topography (http://map.fpds.admin.ch). The census data include geo-codes of almost all residents. Distances were computed using ArcGIS (ArcGIS 9.3; Redlands, CA, USA).
Potential confounders
Informed by the literature,22 we considered the following potential confounders: (i) background ionizing radiation (cosmic, terrestrial, artificial and total radiation); (ii) electromagnetic radiation from radio and TV transmitters (from an area exposure model), multi-track railways and high voltage (≥200 kV) power lines (indexed by distance to nearest installation); (iii) carcinogens related to traffic exhaust (distance to major roads); (iv) agricultural pesticides (distance to the nearest land use plot with fruit trees, vineyards and golf courses); (v) socio-economic status measured at community level based on income, education and job position (Sotomo Index);23 (vi) population mixing and exposure to childhood infections (indexed by average number of children per household in the community and degree of urbanization). We also adjusted for distance to the nearest paediatric cancer centre, which may have affected the probability of registration in the SCCR. Details on data sources and variable definitions are given in Supplementary Appendix 1, available as Supplementary Data at IJE online.
Statistical analysis
We used Poisson regression to estimate incidence rate ratios (IRRs) and 95% CIs, comparing children living <5 km, 5–10 km and 10–15 km from the nearest NPP with those living >15 km away, adjusting for sex, age and calendar year. We calculated the number of cases and person-years at risk attributable to each combination of exposure (four strata), sex, age (five or 16 strata), calendar year (1985–2009, 25 strata) and one potential confounder. Due to small numbers, we only included one confounder at a time. For each cohort, we calculated person-years in 1990 and 2000 using census data and, from these values, person-years before (1985–89), between (1991–99) and after the censuses (2001–09) by linear extra- and interpolation.24 The calculations are described in detail in Supplementary Appendix 1, available as Supplementary Data at IJE online.
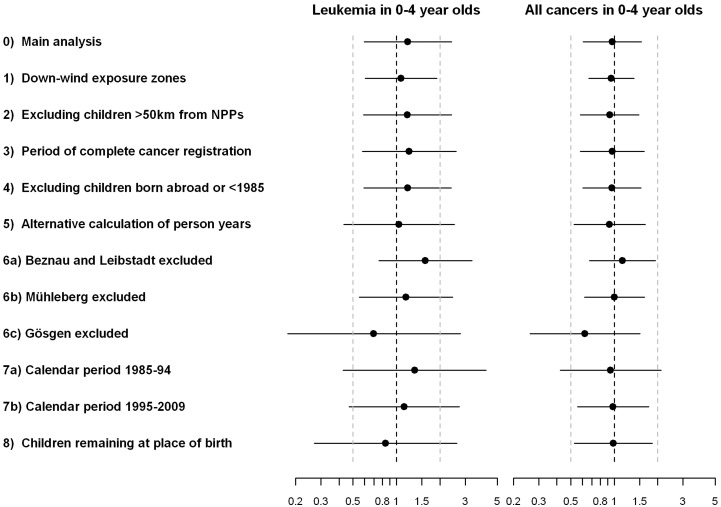
We performed 10 additional analyses, including eight sensitivity analyses (the rationale for these analyses is given in Box 1): (1) accounting for main dispersal directions of airborne emissions from NPPs (see Figure E31 in Supplementary Appendix 2, available as Supplementary Data at IJE online); (2) excluding children living >50 km from a NPP; (3) excluding calendar years 1985–90 and 2009, when registration in the SCCR was less complete; (4) excluding children born abroad or before 1985 from the resident cohort; (5) using an alternative method for calculating person-years (see Supplementary Appendix 1, available as Supplementary Data at IJE online); (6) recalculating distance to nearest NPP by excluding each NPP in turn; (7) stratifying by calendar period (1985–94; 1995–2009); (8) including only children who remained in the community where they were born.
Box 1 Description of sensitivity analyses and additional analyses.
| Analysis | Description | Background and rationale |
|---|---|---|
| Sensitivity analyses | ||
| 1 | Account for airborne emissions by redefining the exposure as living in a zone around a NPP that is equivalent in area to a circle with 5 km radius, but extends to a distance proportional to the average duration of slow winds (<3 m/s) in a given direction.a,b | Stronger associations in this analysis compared with the main analysis would support a causal effect of airborne emissions from NPPs (radionuclides or other). Downwind concentrations of pollutants are inversely related to wind speed.34 We therefore defined an exposure zone based on the direction of slow winds. |
| 2 | Exclude children living >50 km from a NPP. | Concentration of emissions from NPPs decrease rapidly with distance and will be virtually zero at distances >50 km. Regional differences in incidence rates in children living >50 km from NPPs, which are not related to emissions from NPPs, could have introduced bias. |
| 3 | Exclude calendar years 1985–90 and 2009. | Registration in the SCCR was less complete in 1985–90 and 2009. |
| 4 | Exclude children born abroad or before 1985 from the resident cohort. | Differences between the results for the birth and resident cohort might be due to the fact that the birth cohort includes only children born in Switzerland ≥1985, whereas the resident cohort also includes children born <1985 and children who were born abroad. This sensitivity analysis eliminates the difference in the two study populations. |
| 5 | Use an alternative method to calculate person-years.a | The main analysis calculates person-years for a particular age group and calendar year based on the number of children of the same age in the two census years. The alternative method uses the number children belonging to the same cohort in the census years instead. |
| 6 | Recalculate distance to nearest NPP by excluding each NPP in turn. | To investigate whether results were influenced by the characteristics of a particular NPP. Beznau I & II and Leibstadt are excluded together because of their proximity to each other. |
| 7 | Stratify the analysis by calendar period (1985–94 and 1995–2009). | All NPPs were in operation 1985–2009. Differences in results between the earlier and later periods might indicate confounding by unknown time-varying factors, or changes in emissions over time. |
| 8 | Include only children who remained in the same community since birth.b | Families with children at greater risk of developing cancer may have preferentially relocated closer or further away from NPPs as the children grew older. This could have affected results when using addresses at diagnosis, compared with addresses at birth. |
| Additional analyses | ||
| 9 | Use 1/(distance in km) as a continuous exposure variable. | To avoid the loss of information caused by categorising the exposure variable. According to an atmospheric dispersion model, air concentrations of radionuclides are approximately proportional to (distance in km)−1.4.34 We chose (distance in km)−1 for comparability with previous studies.4,5 |
| 10 | Perform a direct comparison of distances to nearest NPPs between cases and the population at risk using two-sample t-tests and the Mann–Whitney U-test. We used addresses at birth of cases born in 1988–92 and 1998–2002 and addresses of infants in the 1990 and 2000 census populations. Only distances <50 km were included.b | This analysis avoids computation of person-years; rather it uses the directly observed distances of the population at risk in the census years. It also avoids categorising the exposure variable as in analysis (9), but unlike (9), no assumptions about the relationship between distance to NPPs and cancer incidence are made. |
aFurther details given in Supplementary Appendix 1, available as Supplementary Data at IJE online.
bFurther details given in Supplementary Appendix 2, available as Supplementary Data at IJE online.
KEY MESSAGES.
This large-scale study examined the association between childhood cancer and residence near nuclear power plants at birth and at diagnosis.
The study found little evidence of an association, either for residence at birth or residence at diagnosis, but the number of exposed cases was small and confidence intervals wide.
Results remained consistent after adjustment for potential confounders and in a number of sensitivity analyses.
A major strength of this study was the nationwide cohort approach, leaving little room for selection bias.
Finally, we performed two analyses using distance to nearest NPPs as a continuous rather than a categorical variable (Box 1): (9) we used 1/(distance in km) as the exposure measure; (10) we compared distances to nearest NPPs between cases and the population at risk using two-sample t-tests and the Mann–Whitney U-test (see Supplementary Appendix 2, available as Supplementary Data at IJE online). The latter approach is related to the case-specular method in case–control research.25 Stata version 11.0 (StataCorp, College Station, TX, USA) was used for all analyses.
Results
Selection of childhood cancer cases and geo-coding
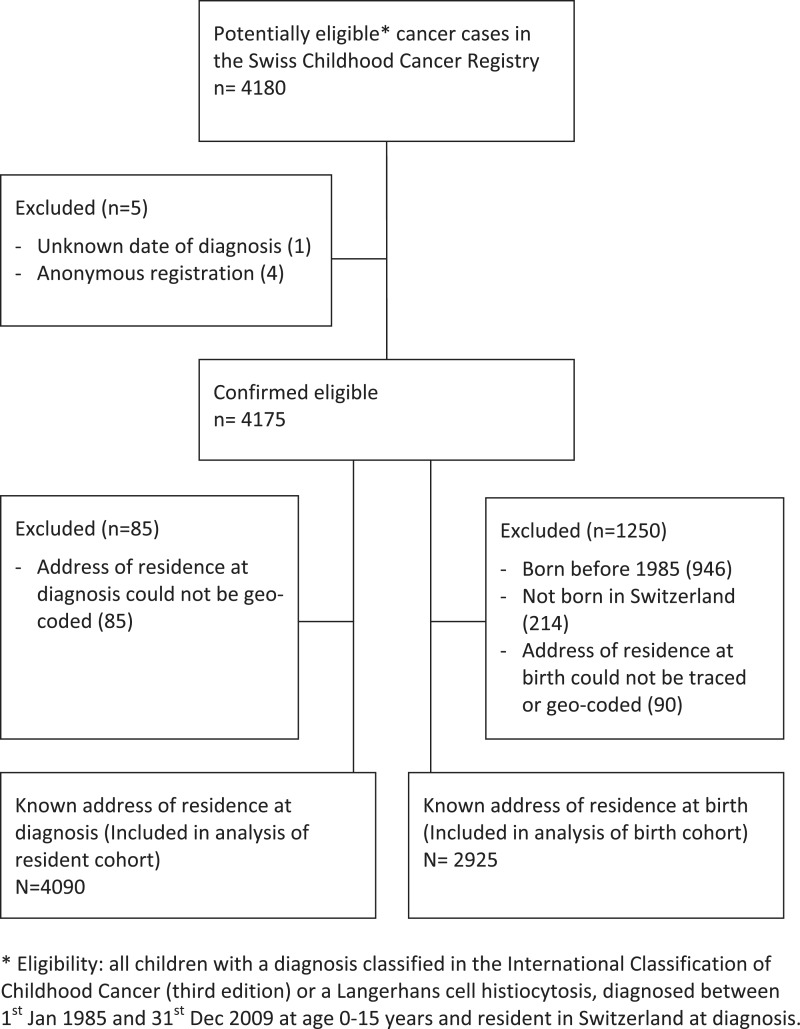
Geo-coded addresses were available for 2925 children diagnosed in the birth cohort and for 4090 cases in the resident cohort (Figure 2). Precision was <50 m for 95% of geo-codes. Among cases in the birth cohort, 1976 (67.6%) had not moved between birth and diagnosis, 648 (22.2%) had moved once, 191 (6.5%) twice and 110 (3.8%) three times or more. Of the 1 240 198 and 1 333 538 children aged 0–15 years registered in the censuses 1990 and 2000, 1 191 536 (96.1%) and 1 301 465 (97.6%), respectively, had geo-coded addresses.
Figure 2.
Flow chart of selection of children with childhood cancer
Characteristics of childhood cancer cases
Among the 2925 cancer cases in the birth cohort, 953 (32.6%) had been diagnosed with leukaemia, 303 (10.4%) with lymphoma and 594 (20.3%) with a neoplasm of the central nervous system (Table 1). The distribution of diagnoses was similar in the 4090 children included in the resident cohort, and comparable to the SCCR in general.18,19 The 1250 and 85 children excluded from birth and resident cohorts, respectively, had higher proportions of lymphomas and bone tumours as they were, on average, older at diagnosis (Table 1).
Table 1.
Characteristics of childhood cancer cases included in birth and resident cohorts
| Birth cohort n (%) |
Resident cohort n (%) |
|||||
|---|---|---|---|---|---|---|
| Included |
Excluded | Included |
Excluded | |||
| 0–4 years | 0–15 years | 0–15 years | 0–4 years | 0–15 years | 0–15 years | |
| 1618 (100.0) | 2925 (100.0) | 1250 (100.0) | 1830 (100.0) | 4090 (100.0) | 85 (100.0) | |
| Diagnosis (ICCC3)20 | ||||||
| I Leukaemias, myeloproliferative diseases and myelodysplastic diseases | 573 (35.4) | 953 (32.6) | 413 (33.0) | 680 (37.2) | 1345 (32.9) | 21 (24.7) |
| II Lymphomas and reticuloendothelial neoplasms | 72 (4.4) | 303 (10.4) | 242 (19.4) | 88 (4.8) | 530 (13.0) | 15 (17.6) |
| III CNS and miscellaneous intracranial and intraspinal neoplasms | 252 (15.6) | 594 (20.3) | 228 (18.2) | 273 (14.9) | 796 (19.5) | 26 (30.6) |
| IV Neuroblastoma and other peripheral nervous cell tumours | 227 (14.0) | 242 (8.3) | 33 (2.6) | 245 (13.4) | 274 (6.7) | 1 (1.2) |
| V Retinoblastoma | 88 (5.4) | 92 (3.1) | 10 (0.8) | 93 (5.1) | 101 (2.5) | 1 (1.2) |
| VI Renal tumours | 143 (8.8) | 182 (6.2) | 52 (4.2) | 166 (9.1) | 232 (5.7) | 2 (2.4) |
| VII Hepatic tumours | 28 (1.7) | 37 (1.3) | 7 (0.6) | 28 (1.5) | 43 (1.1) | 1 (1.2) |
| VIII Malignant bone tumours | 11 (0.7) | 115 (3.9) | 83 (6.6) | 13 (0.7) | 195 (4.8) | 3 (3.5) |
| IX Soft tissue and other extra-osseous sarcomas | 96 (5.9) | 184 (6.3) | 77 (6.2) | 106 (5.8) | 255 (6.2) | 6 (7.1) |
| X Germ cell tumours, trophoblastic tumours and neoplasms of gonads | 38 (2.3) | 68 (2.3) | 41 (3.3) | 40 (2.2) | 104 (2.5) | 5 (5.9) |
| XI and XII Other malignant epithelial neoplasms and malignant melanomas or unspecified malignant neoplasms | 13 (0.8) | 44 (1.5) | 27 (2.2) | 14 (0.8) | 70 (1.7) | 1 (1.2) |
| Langerhans cell histiocytosis | 77 (4.8) | 111 (3.8) | 37 (3.0) | 84 (4.6) | 145 (3.5) | 3 (3.5) |
| Gender | ||||||
| Male | 894 (55.3) | 1621 (55.4) | 711 (56.9) | 1007 (55.0) | 2278 (55.7) | 54 (63.5) |
| Female | 724 (44.7) | 1304 (44.6) | 539 (43.1) | 823 (45.0) | 1812 (44.3) | 31 (36.5) |
| Median age at diagnosis in years (interquartile range) | 2.2 (1.0–3.4) | 4.3 (2.0–8.6) | 10.4 (6.0–13.5) | 2.3 (1.2–3.5) | 5.8 (2.6–11.2) | 7.6 (3.5–12.3) |
ICCC3, International Classification of Childhood Cancer, third edition; CNS, central nervous system.
Incidence of childhood cancer in birth and resident cohorts
Observation time in the birth cohort totalled 21 117 524 person-years. The incidence per 100 000 person-years in children aged 0–15 years was 4.51 (95% CI 4.24–4.81) for leukaemia and 13.85 (13.36–14.36) for all cancers. The corresponding figures for the resident cohort were 31 279 898 person-years at risk, 4.30 (95% CI 4.08–4.54) and 13.08 (95% CI 12.68–13.48) per 100 000 person-years. Table E1 in Supplementary Appendix 2 presents incidence rates for leukaemia and all cancers by sex, age and calendar year. Incidence was higher in boys than in girls, higher in younger children and slightly higher in recent years.
Incidence by distance to nuclear facilities
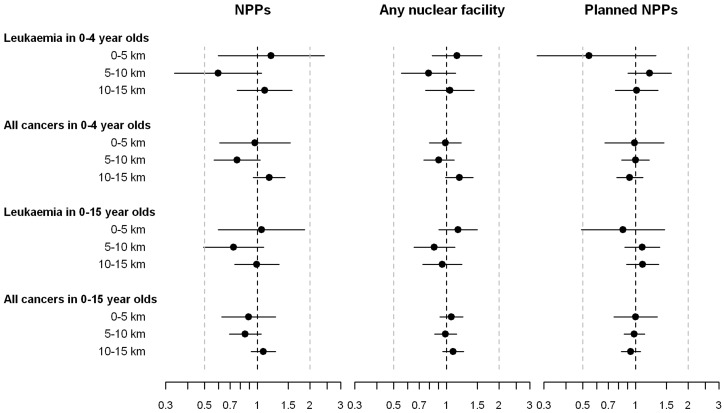
In the birth cohort, 8 and 12 children diagnosed with leukaemia at ages 0–4 and 0–15 years, respectively, and 18 and 31 children diagnosed with any cancer lived <5 km from a NPP at birth (Table 2, and Figure 3). IRRs (95% CI) for leukaemia diagnosed in 0–4 and 0–15 year olds, comparing children in this inner circle with children living >15 km away, were 1.20 (0.60–2.41) and 1.05 (0.60–1.86), respectively. The corresponding IRRs for any cancer were 0.97 (0.61–1.54) and 0.89 (0.63–1.27). In the 5–10 km zone, IRRs tended to be <1 for all outcomes; in the 10–15 km zone they were close to 1 for 0–15 year olds and slightly >1 in 0–4 year olds. A similar variation of IRRs around 1 was observed for distance to any nuclear facility and for distance to sites of NPPs that were planned but never built (Table 2 and Figure 3). In all analyses, the 95% CIs included 1 and there was little evidence of a dose–response relationship (P-value from test for linear trend ≥0.3).
Table 2.
Incidence of childhood cancer in birth cohort according to distance to nuclear power plant, any nuclear facility and NPPs that were planned but never built
| Age 0–4 years |
Age 0–15 years |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cases | PY at risk in 100 years | IR per 100 000 PY | IRR (95% CI)a | Cases | PY at risk in 100 years | IR per 100 000 PY | IRR (95% CI)a | |
| Distance to nearest NPP (km) | ||||||||
| Leukaemias | ||||||||
| 0–5 | 8 | 1000 | 8.00 | 1.20 (0.60–2.41) | 12 | 2548 | 4.71 | 1.05 (0.60–1.86) |
| 5–10 | 12 | 3011 | 3.99 | 0.60 (0.34–1.06) | 25 | 7631 | 3.28 | 0.73 (0.49–1.09) |
| 10–15 | 31 | 4194 | 7.39 | 1.10 (0.77–1.58) | 47 | 10487 | 4.48 | 0.99 (0.74–1.33) |
| >15 | 522 | 77 375 | 6.75 | 1 | 869 | 190 509 | 4.56 | 1 |
| All cancers | ||||||||
| 0–5 | 18 | 1000 | 17.99 | 0.97 (0.61–1.54) | 31 | 2548 | 12.17 | 0.89 (0.63–1.27) |
| 5–10 | 43 | 3011 | 14.28 | 0.77 (0.57–1.04) | 89 | 7631 | 11.66 | 0.85 (0.69–1.05) |
| 10–15 | 92 | 4194 | 21.94 | 1.17 (0.95–1.44) | 156 | 10 487 | 14.88 | 1.08 (0.92–1.27) |
| >15 | 1465 | 77 375 | 18.93 | 1 | 2649 | 190 509 | 13.91 | 1 |
| Distance to nearest nuclear facility (NPP, research, or storage) (km) | ||||||||
| Leukaemias | ||||||||
| 0–5 | 39 | 5034 | 7.75 | 1.15 (0.83–1.59) | 65 | 12 184 | 5.34 | 1.16 (0.90–1.50) |
| 5–10 | 32 | 6013 | 5.32 | 0.79 (0.55–1.13) | 57 | 14 624 | 3.90 | 0.85 (0.65–1.12) |
| 10–15 | 41 | 5858 | 7.00 | 1.05 (0.76–1.44) | 62 | 14 547 | 4.26 | 0.95 (0.73–1.22) |
| >15 | 461 | 68 674 | 6.71 | 1 | 769 | 169 821 | 4.53 | 1 |
| All cancers | ||||||||
| 0–5 | 94 | 5034 | 18.67 | 0.98 (0.80–1.21) | 180 | 12 184 | 14.77 | 1.07 (0.92–1.24) |
| 5–10 | 103 | 6013 | 17.13 | 0.90 (0.74–1.11) | 200 | 14 624 | 13.68 | 0.99 (0.86–1.14) |
| 10–15 | 130 | 5858 | 22.19 | 1.18 (0.99–1.42) | 217 | 14 547 | 14.92 | 1.09 (0.95–1.25) |
| >15 | 1291 | 68 674 | 18.80 | 1 | 2328 | 169 821 | 13.71 | 1 |
| Distance to nearest planned NPP (km) | ||||||||
| Leukaemias | ||||||||
| 0–5 | 5 | 1387 | 3.60 | 0.54 (0.22–1.31) | 13 | 3443 | 3.78 | 0.85 (0.49–1.47) |
| 5–10 | 53 | 6627 | 8.00 | 1.20 (0.90–1.60) | 79 | 16 102 | 4.91 | 1.09 (0.87–1.38) |
| 10–15 | 55 | 8126 | 6.77 | 1.01 (0.77–1.34) | 97 | 19 557 | 4.96 | 1.10 (0.89–1.36) |
| >15 | 460 | 69 439 | 6.62 | 1 | 764 | 172 073 | 4.44 | 1 |
| All cancers | ||||||||
| 0–5 | 26 | 1387 | 18.74 | 0.98 (0.67–1.45) | 48 | 3443 | 13.94 | 1.00 (0.75–1.33) |
| 5–10 | 127 | 6627 | 19.16 | 1.00 (0.83–1.20) | 223 | 16 102 | 13.85 | 0.98 (0.86–1.13) |
| 10–15 | 145 | 8126 | 17.84 | 0.93 (0.78–1.10) | 260 | 19 557 | 13.30 | 0.94 (0.83–1.07) |
| >15 | 1320 | 69 439 | 19.01 | 1 | 2394 | 172 073 | 13.91 | 1 |
aAdjusted for sex, age and calendar year at diagnosis.
PY, person-year; IR, incidence rate; IRR, incidence rate ratio; NPP, nuclear power plant.
Figure 3.
Results for birth cohort. Incidence rate ratios adjusted for sex, age and year at diagnosis and 95% CIs comparing children living in the inner 5 km, 5–10 km and 10–15 km zones with children outside the 15 km zone. Results for nuclear power plants (NPPs); any nuclear facility including NPPs, research and storage facilities; and sites of planned but not built NPPs are shown
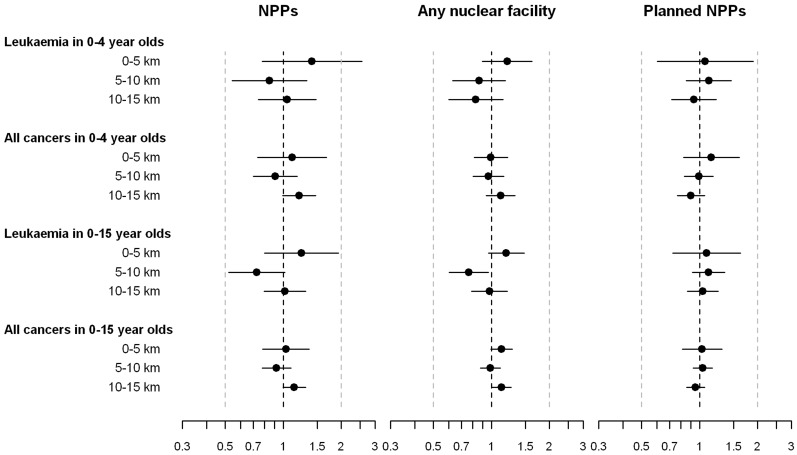
In the resident cohort, 11 and 20 children in age groups 0–4 and 0–15 years, respectively, lived within 5 km of a NPP when diagnosed with leukaemia, and 23 and 50 children, respectively, were diagnosed with any cancer (Table 3 and Figure 4). Compared with children living >15 km away, IRRs for children in the 0–5 km zone were >1 for leukaemia in 0–4 and 0–15 year olds: 1.41 (95% CI 0.78–2.55) and 1.24 (95% CI 0.80–1.94). Corresponding IRR for any cancer were 1.11 (95% CI 0.74–1.67) and 1.03 (0.78–1.36). For the 5–10 km zone, IRRs were <1 for both outcomes and age groups. In the analyses including any nuclear facility, IRRs were again >1 in the 5 km zone for leukaemia in 0–4 and 0–15 year olds (Table 3 and Figure 4). IRRs by distance to planned but not built NPPs were close to 1 (Table 3, and Figure 4). Again, in all analyses the 95% CIs included 1 and P-values for a linear trend across distance categories were ≥0.1.
Table 3.
Incidence of childhood cancer in resident cohort according to distance to nuclear power plant, any nuclear facility and NPPs that were planned but never built
| Age 0–4 years |
Age 0–15 years |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cases | PY at risk in 100 years | IR per 100 000 PY | IRR (95% CI)a | Cases | PY at risk in 100 years | IR per 100 000 PY | IRR (95% CI)a | |
| Distance to nearest NPP (km) | ||||||||
| Leukaemias | ||||||||
| 0–5 | 11 | 1094 | 10.06 | 1.41 (0.78–2.55) | 20 | 3784 | 5.29 | 1.24 (0.80–1.94) |
| 5–10 | 20 | 3297 | 6.07 | 0.85 (0.54–1.32) | 35 | 11 255 | 3.11 | 0.73 (0.52–1.02) |
| 10–15 | 34 | 4542 | 7.49 | 1.05 (0.74–1.48) | 66 | 15 039 | 4.39 | 1.02 (0.80–1.31) |
| >15 | 615 | 85 579 | 7.19 | 1 | 1224 | 282 721 | 4.33 | 1 |
| All cancers | ||||||||
| 0–5 | 23 | 1094 | 21.03 | 1.11 (0.74–1.67) | 50 | 3784 | 13.21 | 1.03 (0.78–1.36) |
| 5–10 | 57 | 3297 | 17.29 | 0.91 (0.70–1.18) | 133 | 11 255 | 11.82 | 0.92 (0.77–1.10) |
| 10–15 | 105 | 4542 | 23.12 | 1.21 (0.99–1.47) | 222 | 15 039 | 14.76 | 1.14 (0.99–1.30) |
| >15 | 1645 | 85 579 | 19.22 | 1 | 3685 | 282 721 | 13.03 | 1 |
| Distance to nearest nuclear facility (NPP, research, or storage) (km) | ||||||||
| Leukaemias | ||||||||
| 0–5 | 47 | 5389 | 8.72 | 1.21 (0.90–1.62) | 90 | 17 244 | 5.22 | 1.19 (0.96–1.48) |
| 5–10 | 42 | 6687 | 6.28 | 0.86 (0.63–1.18) | 74 | 22 367 | 3.31 | 0.76 (0.60–0.97) |
| 10–15 | 39 | 6480 | 6.02 | 0.83 (0.60–1.15) | 91 | 21 545 | 4.22 | 0.98 (0.79–1.21) |
| >15 | 552 | 75 955 | 7.27 | 1 | 1090 | 251 643 | 4.33 | 1 |
| All cancers | ||||||||
| 0–5 | 104 | 5389 | 19.30 | 0.99 (0.81–1.21) | 254 | 17 244 | 14.73 | 1.13 (0.99–1.28) |
| 5–10 | 125 | 6687 | 18.69 | 0.96 (0.80–1.16) | 286 | 22 367 | 12.79 | 0.99 (0.87–1.11) |
| 10–15 | 139 | 6480 | 21.45 | 1.12 (0.94–1.33) | 312 | 21 545 | 14.48 | 1.13 (1.00–1.26) |
| >15 | 1462 | 75 955 | 19.25 | 1 | 3238 | 251 643 | 12.87 | 1 |
| Distance to nearest planned NPP (km) | ||||||||
| Leukaemias | ||||||||
| 0–5 | 12 | 1563 | 7.68 | 1.07 (0.60–1.89) | 24 | 5222 | 4.60 | 1.09 (0.72–1.63) |
| 5–10 | 58 | 7270 | 7.98 | 1.11 (0.85–1.46) | 112 | 23 555 | 4.76 | 1.11 (0.92–1.35) |
| 10–15 | 60 | 8950 | 6.70 | 0.93 (0.71–1.22) | 130 | 29 322 | 4.43 | 1.04 (0.87–1.24) |
| >15 | 550 | 76 728 | 7.17 | 1 | 1079 | 254 700 | 4.24 | 1 |
| All cancers | ||||||||
| 0–5 | 35 | 1563 | 22.39 | 1.15 (0.82–1.61) | 70 | 5222 | 13.40 | 1.03 (0.81–1.31) |
| 5–10 | 141 | 7270 | 19.39 | 0.99 (0.83–1.18) | 323 | 23 555 | 13.71 | 1.04 (0.93–1.16) |
| 10–15 | 159 | 8950 | 17.77 | 0.90 (0.77–1.06) | 370 | 29 322 | 12.62 | 0.95 (0.86–1.06) |
| >15 | 1495 | 76 728 | 19.48 | 1 | 3327 | 254 700 | 13.06 | 1 |
aAdjusted for sex, age and calendar year at diagnosis.
PY, person-year; IR, incidence rate; IRR, incidence rate ratio; NPP, nuclear power plant.
Figure 4.
Results for resident cohort. Incidence rate ratios adjusted for sex, age and year at diagnosis and 95% CIs comparing children living in the inner 5 km, 5–10 km and 10–15 km zones with children outside the 15 km zone. Results for nuclear power plants (NPPs); any nuclear facility including NPPs, research and storage facilities; and sites of planned but not built NPPs are shown
Adjustment for confounders and sensitivity analyses
Results were closely similar when adjusting, one at a time, for potential confounders (Figures E1–E30 of Supplementary Appendix 2, available as Supplementary Data at IJE online). As shown in Figure 5, the results from the sensitivity analyses were closely similar to the main analysis (detailed results are shown in Figures E31–E48 of Supplementary Appendix 2, available as Supplementary Data at IJE online). In the analyses using 1/(distance in km), estimated IRRs were close to 1 for the birth cohort and >1 for the resident cohort, with 95% CIs including 1 (Figure E49 in Supplementary Appendix 2, available as Supplementary Data at IJE online). The results of the direct comparison of distances to nearest NPP between childhood cancer cases and the population at risk are shown in Table E2 in Supplementary Appendix 2, available as Supplementary Data at IJE online. Among infants living <50 km from a NPP, cases born in 1988–92 and diagnosed with leukaemia at 0–4 years lived on average 28.70 km (SD 11.53 km) from a NPP at birth compared with 27.62 km (11.85 km) for the population at risk in 1990 (P-value Mann–Whitney test: 0.38). Results were similar for other outcomes. Differences were found in 2000 for any cancer in 0–4 years, with cases living slightly closer to NPPs (P = 0.01). However, histograms show that cases were mainly overrepresented at intermediate rather than small distances from NPPs (Figures E50–51 in Supplementary Appendix 2, available as Supplementary Data at IJE online).
Figure 5.
Comparison of results of main and sensitivity analyses. Incidence rate ratios adjusted for sex, age and year at diagnosis and 95% CIs comparing children living <5 km with children living >15 km from a nuclear power plant. Results are shown for the birth cohort and are adjusted for sex, age and year at diagnosis. Analyses numbered as in Box 1
Discussion
This nationwide census-based cohort study found little evidence of an association between residence near NPPs and the risk of leukaemia or any childhood cancer, either for residence at birth or at diagnosis. In particular, there was no evidence for a dose–response over distance categories, results were not changed by adjustment for confounders and remained robust in numerous sensitivity analyses designed to evaluate potential biases related to the study population, modelling of person-years or definition of exposure. However, due to the small number of cases, statistical power was limited and we cannot exclude a moderately increased or reduced incidence in the 0–5 km zone, particularly for leukaemia in children aged 0–4 years.
Methodological considerations
A major strength of this study is the nationwide cohort approach, leaving little room for selection bias. Only two other studies used a cohort approach14,24 and one of them did not study the 5 km zone around NPPs.14 The German case–control study was criticized for control selection bias, with controls selected retrospectively from community records and community response rates varying by distance to NPPs.4,5 By using census data to compute person-years and a national cancer registry to obtain incident cases, we avoided participation bias. There was no evidence that variation in the completeness of the SCCR biased our results: findings were similar when we adjusted for distance to the nearest paediatric cancer centre, excluded children living further than 50 km from any NPP or excluded calendar years with poorer registration coverage. As all Swiss NPPs were built in populated regions near large paediatric cancer centres (Bern, Aarau, Zurich) any bias would likely have strengthened rather than attenuated associations.
Our main analysis was based on residence at birth: humans are particularly sensitive to ionizing radiation during intrauterine development and infancy;15 there is a latency between exposure and development of cancer;15 and young families tend to move. Therefore, studies based on residence at diagnosis might suffer from exposure misclassification, and address at birth will be a better proxy for the place of residence during pregnancy. Whereas most previous studies were ecological using community mid-points to determine the distance to the nearest NPP, our study used precise (<50 m in most cases) residential locations, thus allowing the calculation of exact distances. Finally, we adjusted for many potential confounders, including background ionizing radiation,26 non-ionizing radiation,27 traffic-related carcinogens28 and proxies for population mixing.29
The main weakness of our study is the relatively small sample size, particularly of 0–4 year olds living close to NPPs, resulting in large CIs around estimated IRRs. Second, we could not adjust for some potential individual or family level confounders, such as health behaviours, diet, infections, medical radiation or medications. However, with the exception of high dose radiation treatment, these exposures are all weak risk factors (if at all) and thus unlikely to be strong confounders.22 Another methodological limitation of our and other studies14,24 was the need to compute person-years from census data. We accounted for non-linear population fluctuations at the aggregate level, but internal migration was only accounted for by linear inter-/extrapolation of person-years between and around census years. Finally, the birth cohort did not include cases who had emigrated before diagnosis. However, data from the Swiss National Cohort show that only 2% (4%) of <1 year olds in the 1990 census had emigrated in the first 4 (15) years of life, making emigration an unlikely source of bias.
Our results in context with the literature
Most previous analyses, summarized in recent reviews,13,30,31 were ecological, using routine data to calculate standardized mortality or incidence ratios. Studies of local clusters near single NPPs have been performed for 198 different sites in 10 countries. Among these, only three clear clusters emerged (Seascale near Sellafield, Dounreay, Kruemmel), two relating to nuclear processing rather than power generating stations. A meta-analysis of 136 single nuclear facilities concluded that there was evidence of an excess leukaemia risk near NPPs.30 However, the methodology of this study has been criticized.32 A total of 25 studies from eight countries analysed leukaemia risk in the vicinity of several NPPs.31 Overall, these show no consistent evidence for an increased risk of childhood leukaemia near NPPs. Few, however, have specifically investigated leukaemia in under-fives living close (<5 km) to NPPs.4,9,13,24,33,34 Among these, the German case–control study, the only other study using exact distances, found elevated ORs for leukaemia (OR 2.19, lower one-sided confidence limit 1.51) and all cancers (OR 1.61, lower one-sided confidence limit 1.26).4,5 The Committee on Medical Aspects of Radiation in the Environment (COMARE) recently analysed the data for children aged 0–4 years when diagnosed with leukaemia or non-Hodgkin lymphoma 1969–2004.13 The analysis was based on electoral wards and postcode zones near 13 NPPs in the UK and showed no evidence for an increased risk in the 5-km perimeter: the relative risk was 1.01 (compared with the national average), with relatively narrow 95% CI (0.70–1.47). Interestingly, the analysis of six possible locations for NPPs, where no installation was constructed, showed an increased risk: 1.72 (95% CI 1.12–2.52), for reasons that are not understood.13 A similar approach in France found no evidence for increased IRRs in the 5 km zone (IRR 0.96, 95% CI 0.31–2.24).31
Population-attributable risks and potential causes
Assuming the association is causal and the point estimate from the analysis of the birth cohort (IRR 1.20) reflects the true underlying effect for leukaemia, 1.3 (0.2%) of the 573 leukaemia cases observed during the 25-year study period in 0–4 year olds would be attributable to living <5 km from a NPP. If we assume that the upper confidence limit (IRR 2.41) reflects the true underlying effect, 4.7 cases (0.8%) would be attributable to living <5 km from a NPP. Repeating the analysis for all cancers, using the upper confidence limit (IRR 1.54), 6.3 cases (0.4% of 1618 cases) would be attributable to living near a NPP. Exposure to radioactivity set free by the nuclear facilities is unlikely to explain an excess in cancer risk in their vicinity. The NPPs are responsible for <1/500 of the total radiation received yearly by people living near NPPs; the main sources are natural radiation and medical examinations.16 Other hypotheses that could explain a link between leukaemia and NPPs include paternal exposure to radiation before the child's conception35 or an infectious agent linked to population mixing near NPP sites.31
Conclusions
In summary, this nationwide cohort study, adjusting for confounders and using exact distances from residence at birth and diagnosis to the nearest NPPs, found little evidence for an association between the risk of childhood cancer and living near NPPs.
Supplementary Data
Supplementary Data are available at IJE online.
Funding
This study was supported by the Swiss Federal Office of Public Health (BAG 08.001616) and the Swiss Cancer League (KLS 02224-03-2008).
Supplementary Material
Acknowledgements
We thank the members of the scientific Advisory Board for thoughtful advice on the study design and critical review of the results: Maria Blettner, François Bochud, Paolo Boffetta, Sander Greenland, Andreas Hirt, Charles Stiller and Jan Vandenbroucke. We are grateful to the following individuals for their vital support in obtaining data: Werner Zeller and Sybille Estier from the Swiss Federal Office of Health, Benno Bucher from the Swiss Federal Nuclear Safety Inspectorate (ENSI), René Vogt from the Federal Office of Communications (BAKOM), Raffael Bovier from the Federal Office of Topography (Swisstopo), Thomas Schlegel from the Federal Office of Meteorology and Climatology (MeteoSwiss), Urs Huber from the Federal Inspectorate for Heavy Current Installations (ESTI), Hubert Gerhardinger from the Institute of Geography, University of Bern and Adrian Spoerri from the Institute of Social and Preventive Medicine, University of Bern. Finally, we thank Anke Huss for advice in the early stages of the project and Aysel Güler for her great help with the geo-coding of addresses.
Swiss Paediatric Oncology Group (SPOG): R. Angst, Aarau; M. Paulussen, Basel; T. Kuehne, Basel; P. Brazzola, Bellinzona; A. Hirt, K. Leibundgut, Bern; A. H. Ozsahin, Geneva; M. Beck Popovic, Lausanne; NX. von der Weid, Lausanne; L. Nobile Buetti, Locarno; J. Rischewski, Lucerne; U. Caflisch, Lucerne; J. Greiner, St. Gallen; H. Hengartner, St. Gallen; M. Grotzer, Zürich; F. Niggli, Zürich.
Swiss National Cohort Study Group: F. Gutzwiller (Chairman of the Executive Board), M. Bopp and D. Faeh, Zurich; M. Egger (Chairman of the Scientific Board), K. Clough-Gorr, K. Schmidlin, A. Spoerri, M. Sturdy (Data Manager), and M. Zwahlen, Bern; N. Künzli, Basel; F. Paccaud, Lausanne; and M. Oris, Geneva.
Conflict of interest: None declared.
References
- 1.Black D. Investigation of the Possible Increased Incidence of Cancer in West Cumbria; Report of the Independent Advisory Group. London: HMSO; 1984. [Google Scholar]
- 2.Michaelis J, Keller B, Haaf G, Kaatsch P. Incidence of childhood malignancies in the vicinity of west German nuclear power plants. Cancer Causes Control. 1992;3:255–63. doi: 10.1007/BF00124259. [DOI] [PubMed] [Google Scholar]
- 3.Cook-Mozaffari P, Darby S, Doll R. Cancer near potential sites of nuclear installations. Lancet. 1989;2:1145–47. doi: 10.1016/s0140-6736(89)91500-6. [DOI] [PubMed] [Google Scholar]
- 4.Spix C, Schmiedel S, Kaatsch P, Schulze-Rath R, Blettner M. Case-control study on childhood cancer in the vicinity of nuclear power plants in Germany 1980-2003. Eur J Cancer. 2008;44:275–84. doi: 10.1016/j.ejca.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 5.Kaatsch P, Spix C, Schulze-Rath R, Schmiedel S, Blettner M. Leukaemia in young children living in the vicinity of German nuclear power plants. Int J Cancer. 2008;122:721–26. doi: 10.1002/ijc.23330. [DOI] [PubMed] [Google Scholar]
- 6.Evrard AS, Hemon D, Morin A, et al. Childhood leukaemia incidence around French nuclear installations using geographic zoning based on gaseous discharge dose estimates. Br J Cancer. 2006;94:1342–47. doi: 10.1038/sj.bjc.6603111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White-Koning ML, Hemon D, Laurier D, et al. Incidence of childhood leukaemia in the vicinity of nuclear sites in France, 1990-1998. Br J Cancer. 2004;91:916–22. doi: 10.1038/sj.bjc.6602068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Committee on Medical Aspects of Radiation in the Environment (COMARE) The Incidence of Childhood Cancer Around Nuclear Installations in Great Britain. London: Health Protection Agency; 2005. Tenth report. [PubMed] [Google Scholar]
- 9.Kaatsch P, Kaletsch U, Meinert R, Michaelis J. An extended study on childhood malignancies in the vicinity of German nuclear power plants. Cancer Causes Control. 1998;9:529–33. doi: 10.1023/a:1008883530341. [DOI] [PubMed] [Google Scholar]
- 10.Iwasaki T, Nishizawa K, Murata M. Leukaemia and lymphoma mortality in the vicinity of nuclear power stations in Japan, 1973-1987. J Radiol Prot. 1995;15:271–88. [Google Scholar]
- 11.Jablon S, Hrubec Z, Boice JD., Jr Cancer in populations living near nuclear facilities. A survey of mortality nationwide and incidence in two states. JAMA. 1991;265:1403–08. [PubMed] [Google Scholar]
- 12.Little J, McLaughlin J, Miller A. Leukaemia in young children living in the vicinity of nuclear power plants. Int J Cancer. 2008;122:x–xi. doi: 10.1002/ijc.23347. [DOI] [PubMed] [Google Scholar]
- 13.Committee on Medical Aspects of Radiation in the Environment (COMARE) Further Consideration of the Incidence of Childhood Leukaemia Around Nuclear Power Plants in Great Britain. London: Health Protection Agency; 2011. Fourteenth report. [Google Scholar]
- 14.Heinavaara S, Toikkanen S, Pasanen K, Verkasalo PK, Kurttio P, Auvinen A. Cancer incidence in the vicinity of Finnish nuclear power plants: an emphasis on childhood leukemia. Cancer Causes Control. 2010;21:587–95. doi: 10.1007/s10552-009-9488-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pierce DA, Shimizu Y, Preston DL, Vaeth M, Mabuchi K. Studies of the mortality of atomic bomb survivors. Report 12, Part I. Cancer: 1950-1990. Radiat Res. 1996;146:1–27. [PubMed] [Google Scholar]
- 16.OFSP. Radioprotection et surveillance de la radioactivité en Suisse: résultats 2009 [Radioprotection and surveillance of radioactivity in Switzerland: results 2009] Bern: Office Fédéral de la Santé Publique; 2009. [Google Scholar]
- 17.Bopp M, Spoerri A, Zwahlen M, et al. Cohort Profile: the Swiss National Cohort—a longitudinal study of 6.8 million people. Int J Epidemiol. 2009;38:379–84. doi: 10.1093/ije/dyn042. [DOI] [PubMed] [Google Scholar]
- 18.Michel G, von der Weid NX, Zwahlen M, Redmond S, Strippoli MPF, Kuehni CE. Incidence of childhood cancer in Switzerland: the Swiss childhood cancer registry. Pediatr Blood Cancer. 2008;50:46–51. doi: 10.1002/pbc.21129. [DOI] [PubMed] [Google Scholar]
- 19.Michel G, von der Weid NX, Zwahlen M, Adam M, Rebholz CE, Kuehni CE. The Swiss Childhood Cancer Registry: rationale, organisation and results for the years 2001-2005. Swiss Med Wkly. 2007;137:502–09. doi: 10.4414/smw.2007.11875. [DOI] [PubMed] [Google Scholar]
- 20.Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International Classification of Childhood Cancer, 3rd edn. Cancer. 2005;103:1457–67. doi: 10.1002/cncr.20910. [DOI] [PubMed] [Google Scholar]
- 21.Adam M, von der Weid NX, Michel G, et al. Access to specialized pediatric cancer care in Switzerland. Pediatr Blood Cancer. 2010;54:721–27. doi: 10.1002/pbc.22426. [DOI] [PubMed] [Google Scholar]
- 22. Matthes R, Ziegelberger G (eds). Risk Factors for Childhood Leukaemia, Special Issue: Radiation Protection Dosimetry, Vol. 132 No. 2. Oxford: Oxford University Press, 2008.
- 23.Hermann M, Heye C, Leuthold H GIUZ. Soziokulturelle unterschiede in der Schweiz: vier indizes zu räumlichen disparitäten, 1990–2000 [Socio-cultural differences in Switzerland: four indices of spatial disparities, 1990–2000] Neuchâtel: Swiss Federal Statistical Office; 2005. [Google Scholar]
- 24.Bithell JF, Keegan TJ, Kroll ME, Murphy MF, Vincent TJ. Childhood leukaemia near British nuclear installations: methodological issues and recent results. Radiat Prot Dosimetry. 2008;132:191–97. doi: 10.1093/rpd/ncn254. [DOI] [PubMed] [Google Scholar]
- 25.Ebi KL, Zaffanella LE, Greenland S. Application of the case-specular method to two studies of wire codes and childhood cancers. Epidemiology. 1999;10:398–404. doi: 10.1097/00001648-199907000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Raaschou-Nielsen O. Indoor radon and childhood leukaemia. Radiat Prot Dosimetry. 2008;132:175–81. doi: 10.1093/rpd/ncn288. [DOI] [PubMed] [Google Scholar]
- 27.Schuz J, Ahlbom A. Exposure to electromagnetic fields and the risk of childhood leukaemia: a review. Radiat Prot Dosimetry. 2008;132:202–11. doi: 10.1093/rpd/ncn270. [DOI] [PubMed] [Google Scholar]
- 28.Raaschou-Nielsen O, Reynolds P. Air pollution and childhood cancer: a review of the epidemiological literature. Int J Cancer. 2006;118:2920–29. doi: 10.1002/ijc.21787. [DOI] [PubMed] [Google Scholar]
- 29.Law GR. Host, family and community proxies for infections potentially associated with leukaemia. Radiat Prot Dosimetry. 2008;132:267–72. doi: 10.1093/rpd/ncn263. [DOI] [PubMed] [Google Scholar]
- 30.Baker PJ, Hoel DG. Meta-analysis of standardized incidence and mortality rates of childhood leukaemia in proximity to nuclear facilities. Eur J Cancer Care. 2007;16:355–63. doi: 10.1111/j.1365-2354.2007.00679.x. [DOI] [PubMed] [Google Scholar]
- 31.Laurier D, Jacob S, Bernier MO, et al. Epidemiological studies of leukaemia in children and young adults around nuclear facilities: a critical review. Radiat Prot Dosimetry. 2008;132:182–90. doi: 10.1093/rpd/ncn262. [DOI] [PubMed] [Google Scholar]
- 32.Spix C, Blettner M, Re: Baker PJ, Hoel DG. (2007) European Journal of Cancer Care 16, 355-363. Meta-analysis of standardized incidence and mortality rates of childhood leukaemia in proximity to nuclear facilities. Eur J Cancer Care. 2009;18:429–30. doi: 10.1111/j.1365-2354.2008.01027.x. [DOI] [PubMed] [Google Scholar]
- 33.Laurier D, Hemon D, Clavel J. Childhood leukaemia incidence below the age of 5 years near French nuclear power plants. J Radiol Prot. 2008;28:401–03. doi: 10.1088/0952-4746/28/3/N01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaatsch P, Spix C, Jung I, Blettner M. Childhood leukemia in the vicinity of nuclear power plants in Germany. Dtsch Arztebl Int. 2008;105:725–32. doi: 10.3238/arztebl.2008.0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Draper G. Preconception exposures to potential germ-cell mutagens. Radiat Prot Dosimetry. 2008;132:241–45. doi: 10.1093/rpd/ncn256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.