Abstract
The expression and function of nicotinic receptor subunits (nAChRs) in the inner ear before the onset of hearing is not well understood. We investigated the mRNA expression of the α9 and α10 nAChR subunits in sensory hair cells of the embryonic and postnatal rat inner ear. We mapped their spatial and temporal expression in cochlear and vestibular hair cells using qPCR, [35S] labeled cRNA in situ hybridization, and α-bungarotoxin (α-Bgt) to label the presumptive membrane-bound receptor on cochlear hair cells. The results suggest that (1) the mRNA expression of the α9 subunit precedes expression of the α10 subunit in both cochlear and vestibular hair cells; (2) the mRNA expression of both the α9 and α10 subunits occurs earlier in the vestibular system than in the cochlea; (3) the mRNA expression of both subunits is required for the assembled receptor complexes, and (4) the presumptive assembled receptor, at least in the cochlea, is associated with synapse formation and the onset of function.
Keywords: Inner Ear; Hair Cells; Synaptogenesis; Olivocochlear; In Situ Hybridization, qPCR; α-Bungarotoxin
The descending pathways to the cochlea originate in the superior olivary complex. The axons of olivocochlear neurons make axosomatic synapses on outer hair cells (OHCs) and also transiently synapse on inner hair cells (IHCs) in early postnatal development (Simmons, 2002). Acetylcholine (ACh) is the principal neurotransmitter at those synapses, where its effects are mediated by the α9/α10 receptor of the nicotinic acetylcholine receptor (nAChR) gene family (Elgoyhen et al., 1994, Morley et al., 1998, Elgoyhen et al., 2001, Gomez-Casati et al., 2005, Gomez-Casati et al., 2009). The vesicular acetylcholine transporter (VAChT) and the high-affinity choline transporter are present presynaptically at about the time of synaptogenesis (Simmons et al., 1998, Simmons, 2002, Bergeron et al., 2005), suggesting that these are classic cholinergic synapses. The α9 and α10 subunits are also expressed in vestibular hair cells (Hiel et al., 1996, Elgoyhen et al., 2001) where they likely form functional receptors (Holt et al., 2003).
Although we have a good understanding of the physiology, pharmacology, expression pattern, and function of the nAChR formed by the α9 and α10 subunits in mature cochlear OHCs, questions remain about possible functions of the subunits in the embryonic cochlea. Additionally, nothing is known about their role in the development of the vestibular peripheral organs. In this study we mapped the spatial and temporal expression of the α9 and α10 subunits in the vestibular periphery and compared it to our previous results in the cochlea. The results of these experiments provide important baseline data for studying the role of the α9 and α10 subunits in the developing cochlea and vestibular peripheral organs. We also mapped the distribution of the presumptive assembled receptor in cochlear ha ir cells using fluorescently labeled α-bungarotoxin (α-Bgt). Our results suggest that α9 and/or α10 subunits may have functions that precede efferent innervation, but support the hypothesis that the assembled receptor mediating cholinergic neurotransmission is inserted into the membrane of IHCs and OHCs at about the time of efferent synaptogenesis.
Experimental Procedures
Animals and tissue preparation
Sprague-Dawley albino rats varying in age from embryonic day 16 (E16) to young adult (6 weeks) were anesthetized with near-lethal IP injections of sodium pentobarbital (Nembutal, 100 mg/kg) or by hypothermia (only animals less than 1 postnatal week). The day of birth (E21.5) represented postnatal day 0 (P0). We also bred transgenic mice overexpressing the α9 gene [Tg(Chrna9-EGFP)1Jnz] that were obtained from J. Zuo (Zuo et al., 1999). All procedures used for this study followed NIH guidelines and were approved by the respective institutional animal care committees at Boys Town National Research Hospital, Washington University, and UCLA.
qPCR
Total RNA was extracted and DNase treated with RNeasy Lipid Tissue Mini Kit (Cat#74804, Qiagen). The integrity of the RNA of all samples was assessed by gel electrophoresis and by equivalent expression of the housekeeping gene in all samples.
First strand synthesis was performed using the RETROscript Kit (Cat#AM1710, Applied Biosystems) using 2 µg of RNA template. Quantitative RT-PCR was carried out on an ABI 7000 real-time PCR system (Applied Biosystems) using SYBR Green PCR Core Reagents (Cat# 4309159, Applied Biosystems). RNA was pooled from a minimum of five animals for each age. All samples were tested in triplicate, and average CT values were calculated and normalized to the housekeeping gene β-Actin. The average CT for the beta actin gene was 18.78 ± 0.862 (S.D). There was no difference between the auditory and vestibular tissue samples and there was no change with age in either tissue. Each of sample’s standard deviation was calculated. Comparisons between samples were made for fold change estimation using the comparative Ct method. The sequences of primers used in this study were β-actin (5’primer: ATGGAATCCTGTGGCATCCA; 3’primer: CGCTCAGGAGGAGCAATGAT); α9 (5’ primer: TGGCAGTGAGGGTGTTTTGAG; 3’primer: AAAAGATCGCTGAACAATTTCTGA); α10 (5’ primer: GTCCTACATCGCTCCTGAAGACTT; 3’primer: GCCACTGGTCTCAGAGCACTT).
In situ hybridization
A full-length (1441 bp) α9 nAChR cRNA probe was transcribed from cDNA received as a gift from Elgoyen and has previously been described (Elgoyhen et al., 1994). In our experiments, the cDNA was transcribed using 35S-UTP (DuPont-NEN; sp. act. > 1200 µCi/mmol) and the MaxiScript kit (Ambion), and purified on Bio-Spin chromatography columns (Bio-Rad). The probe was diluted in hybridization buffer to approximately 10 million dpm/ml and 180 µl of probe solution was used on each slide, as previously described. Controls included brain sections as negative controls, a 35S-sense α9 cRNA, a α7 antisense cRNA, and the radiolabeled α9 antisense probe absorbed with 20X unlabeled α9. No signal was observed over hair cell regions in these sections.
For the α10 nAChR in situ hybridization probe, first strand cDNA synthesis was accomplished and the α10 cDNA sequence was used to design primer pairs for PCR. Forward and reverse oligonucleotide primers were synthesized to the 1200–1643 bp region of the α10 nAChR cDNA and used to amplify a 444-bp fragment, as previously described (Morley and Simmons, 1998). Briefly, primers were designed to a unique sequence of α10. Specific cDNA was amplified by a touchdown PCR procedure as described above. The PCR product was separated by agarose gel electrophoresis and detected by ethidium bromide staining. The resulting band was eluted from the gel and the α10 sequence confirmed. The α10 cDNA was inserted into pGemT-Easy (Promega) and 35S-labeled antisense and sense riboprobes were transcribed with SP6 and T7, respectively, using the Ambion Maxi Transcription Kit per the manufacturer’s instructions. The incorporation of 35S (specific activity >1200 µCi/mmol; Perkin-Elmer Life Science) was determined. The probes were diluted in hybridization buffer (Sigma-Aldrich Chemical Co., St. Louis, MO) for storage at −20°C, and further diluted to approximately 106 dpm/ml before use.
Following anesthesia, animals were fixed by immersion in 4% paraformaldehyde buffered with 0.1 M sodium phosphate (pH 7.4) for 7 days (embryonic tissues only), by perfusion with 4% paraformaldehyde buffered with 0.1 M sodium phosphate (pH 7.4), or by perfusion with an acid/base fixation as previously described (Simmons and Morley, 1998, Morley and Simmons, 2002). After fixation, the tissue was decalcified (Decalcifier F, Baxter Scientific) for up to 5 days, rinsed, and cryopreserved for 3–5 days in buffered 30% sucrose. Tissue from developing (younger than P15) rats was sectioned without removing from the skull. After P15, the cochlea and brain were removed from the skull and cut separately. Tissue was sectioned at 16 µm in a Reichert cryostat and freeze-thaw mounted onto glass slides, dried at 56° C for one-hour, and then stored desiccated at −70° C. Slides were removed from the freezer and dried for 30 min on a warming plate.
The slides were then processed using an in situ hybridization procedure previously reported (Morley et al., 1998). Briefly, the slides were rinsed in appropriate solutions, dehydrated, in a series of ethanols, and air-dried under vacuum. The probes were diluted in hybridization buffer, as described above, and applied using cover glass to the tissue sections. Hybridization was done at 56° C in a humidified environment for 16 h (overnight). Tissue sections were then treated with RNase and formamide. Finally, the slides were dehydrated in 50% ethanol, air-dried and dipped in Kodak NTB3 emulsion. Nine days later, the slides were developed, counterstained, and coverslipped.
α-Bungarotoxin labeling
We used three (3) to five (5) animals per age for these α-Bgt labeling experiments. Nicotinic acetylcholine receptors were labeled by incubating freshly dissected organs for 1 – 2 hours with 50 µM α-Bgt in Hank’s Buffered Saline Solution (HBSS). Organs were rinsed for 10 min in α-Bgt-free HBSS at 4°C. To take advantage of the intrinsic radial and longitudinal gradients of the cochlea, entire organ of Corti spirals were labeled in vitro with α-Bgt. After a prescribed incubation period, the preparations are immediately fixed (2 – 4% paraformaldehyde in 0.1 M PBS at 4°C), and processed for immunocytochemical identification of specific antigenic markers. Using confocal microscopy, a minimum of three regions were sampled in an X–Y box at least 51 × 51 µm (roughly 6–8 hair cells across). We identify α-Bgt puncta or clusters and analyze basolateral position, relative size of puncta, and colocalization of any efferent fiber label with 3D rendering and deconvolution software (Volocity, Improvision/PerkinElmer). All hair cells (defined by hair cell marker, position and/or phalloidin labeling) were imaged initially at low magnifications and then cell position/location maps created.
From these maps each hair cell was imaged at high magnification and high gain to determine the presence of α-Bgt-labeled nAChRs. Typically, we used three different fluorescent labels. We used a fluorescent α-Bgt conjugate to label nicotinic receptor individual puncta and clusters (typically with a 488 nm excitation wavelength), and immunocytochemical labeling of hair cell body markers (typically with a 546 or 596 nm wavelength), and cholinergic fiber markers (at 647 nm wavelength).
Specificity of labeling was determined using unlabeled α-Bgt and a high affinity peptide (HAP) known to competitively block α-Bgt binding to the nAChR subunit in diaphragm skeletal muscle (Schrattenholz et al., 1998, Harel et al., 2001, Kasher et al., 2001).
Immunocytochemical studies
For immunocytochemical experiments, ears were prepared as sensory organ whole mount preparations. Whole organ preparations were fixed in 2 – 4% paraformaldehyde, permeabilized in 0.5 – 4% Triton X-100 in PBS to enhance antisera penetration, and incubated in a blocking solution consisting of 10% normal serum and 1% bovine serum albumin (BSA) in PBS to reduce non-specific labeling.
Hair cells were identified with primary antisera a gainst either myosin VI (rabbit anti-myosin VI, 1:500 dilution, Proteus) or α-parvalbumin (mouse anti-Parvalbumin, 1:1000 dilution; Sigma; clone PARV-19) and efferent terminals were labeled with primary antisera against vesicle acetylcholine transferase (VAChT; antirabbit, 1:1000; Chemicon). Primary antibodies were diluted in the appropriate blocking solution and applied to tissues overnight at 4°C. Alexa 488 goat anti-rabbit secondary antibody (Molecular Probes, Eugene, OR, USA), Alexa 647 goat anti-mouse (Molecular Probes), or Cy3 donkey anti-mouse (Chemicon) secondary antibodies (1:500 dilution, 2 hr RT).
Cochlear hair cells were also labeled with phalloidin at the same spectral wavelength as the hair cell marker to label the hair bundles and cuticular plates. Organs were then mounted onto glass slides and coverslipped using Mount Quick Aqueous (Fisher). The specificity of primary antisera was confirmed in experiments using secondary antisera in the absence of primary antibody (data not shown). For organ preparations, measurements are taken from five to seven preparations (approximately 20 – 30 hair cells per dissociated preparation).
Confocal microscopy
Isolated regions of the cochlear spiral were sequentially scanned at high (500–1000×) magnification, exciting the green (488 nm), red (543 nm) and far-red (637 nm) channels using a laser scanning confocal microscope (Zeiss LSM 510 or Bio-Rad Radiance 2000, Thornton, NY, USA). Fluorescent emissions were separated with appropriate blocking and emission filters, scanned at slow (50 lines/s) scan speeds for high resolution, and independently detected with 8-bit or 12-bit accuracy by photomultiplier tubes using, if necessary, accumulation to increase signal and reduce noise. Three-dimensional images of serially reconstructed image stacks from the confocal microscope were rendered using Volocity software (v4; Improvision, PerkinElmer, Shelton, CT). Whole mount images were captured in the X–Y plane and digitally rotated and viewed in the X–Z plane. Z-projections of image stacks were routinely performed. Single and projected images were exported to Canvas (vX, ACD Systems, Canada) or Photoshop (Adobe Systems, USA) and image quality (brightness/contrast or histogram levels) adjusted to maximize signal and minimize background.
Analysis of colocalization in 3-demensional pixel volumes (voxels) and co-association (adjacent voxels) was performed on VAChT and α-Bgt labeling with Volocity software using 3D image stacks obtained from the confocal microscope. Image stacks were captured sequentially to minimize any crosstalk between channels. Threshold values were determined for each channel to minimize signal background. Laser power and gain settings were limited to the level just below pixel saturation detection on the VAChT and α-Bgt channels. After generating a colocalization map, the colocalization voxels are displayed, highlighted, merged to a summed (projected) image, and counted. The Pearson correlation coefficient and Mander’s overlap coefficients (Mx and My) were calculated for the VAChT and α-Bgt labeling channels according to previously published methods (Manders et al., 1993, Osman et al., 2008, Adler and Parmryd, 2010). Once α-Bgt labeled puncta or clusters were defined as ROIs from our colocalization analysis, these ROIs were assessed and counted for co-association with VAChT-ir labeling. Co-association was defined as voxels from one channel physically adjacent (contacting) voxels from another channel.
Results
α9 and α10 mRNA expression in the inner ear using RT-PCR
How neuronal nicotinic synapses acquire nAChRs at cholinergic synapses in the inner ear is not well understood. A recent study suggests that a rapsyn-mediated signaling cascade similar to the NMJ may be involved in neuronal nicotinic synapse formation and demonstrated an early expression of rapsyn in the embryonic inner ear as well as punctate localization of rapsyn immunoreactivity in vestibular hair cells before cochlear hair cells (Osman et al., 2008). Our previous studies (Simmons and Morley, 1998, Morley and Simmons, 2002) suggested that α9 and α10 nAChR subunits were expressed as early as E18 in the cochlea, but vestibular expression patterns were not studied.
In order to examine nicotinic receptor subunit expression in both cochlear and vestibular organs, we used real time RT-PCR to quantify the expression of the α9 and α10 subunits from rat inner ears at ages E16 through P10 using (Figure 1). Expression of the α9 subunit was detected in the inner ear at E16, the earliest age measured. Expression was highest between P0 and P2, with a subsequent increase at P10 (Figure 1A). In vestibular organs, α9 expression was also detected at E16, with the highest expression between P0 and P2 and later at P10. In both the cochlea and vestibule, expression at P6 was lower than at earlier and subsequent developmental ages.
Figure 1. α9 and α9α10 nAChR subunit expression in the cochlea and vestibular peripheral organ during embryonic and postnatal development quantified with real time PCR.
(A) Expression of the α9 subunit is highest at P0–2 and P10 in both the cochlea and vestibule. Expression is also transiently downregulated at P6 in both tissues. (B) The expression of the α10 subunit is highest at P10 in both the cochlea and vestibule. Expression is transiently downregulated at P6 in both tissues.
Expression for the α10 subunit had a somewhat different expression pattern from α9 suggesting that the two genes are differentially regulated. In the cochlea, α10 expression could not be detected at E16 (Figure 1B). The highest expression was at P0 – P2 and P10. Vestibular organs had α10 expression at E16 and E18. Postnatally, vestibular organs had much higher α10 expression than in cochlear tissues at P0 and P2. Both vestibular and cochlear organs also had high expression levels at P10. In both the cochlea and vestibule, expression was lowest at P6. These data are consistent with our previous studies of cRNA transcript labeling over IHCs and OHCs, where we found higher α9 and α10 expression over IHCs around P0 and over OHCs around P10 (Simmons and Morley, 1998, Morley and Simmons, 2002).
α9 and α10 expression in the inner ear using In Situ hybridization
Although real time PCR provides quantitative expression of a subunit, it is limited in defining the specific cells expressing a gene. The specific cell types expressing α9 and α10 were therefore investigated using in situ hybridization. As shown in Figure 2, the data confirm our previous work in the cochlea and further suggest vestibular hair cells express both α9 and α10 receptor subunits before cochlear hair cells. Embryonic day 18 was the earliest age studied for in situ hybridization studies. Figure 2A shows that the densest α9 labeling was over the Greater Epithelial Ridge (GER), an embryonic structure that gives rise to IHCs and is innervated by immature efferent axons (Bruce et al., 2000), as similarly described in our previous study (Simmons and Morley, 1998). After E18, α9 labeling decreased over the GER and increased over IHCs and OHCs (Figure 2E), as previously reported. Expression of the α9 subunit in OHCs was restricted to middle basal regions of the cochlear spiral at E21. This restricted expression pattern contrasted with vestibular organs where α9 labeling was observed directly over hair cells in all vestibular organs at E18 and E21 (Figures 2B, 2F).
Figure 2. α9 and α10 nAChR subunit expression was localized in the cochlea and vestibular periphery of the rat at E18 and E21 using radiolabeled in situ hybridization.
(A) cRNA probes specific to the α9 and α10 nAChR subunits were prepared as described in the text. Using mid-modiolar sections, expression of α9 at E18 was detected in the in the cochlea over the Greater Epithelial Ridge (GER). (B) Expression of α9 at E18 was detected over vestibular hair cells in the cristae. (C) No transcript for α10 was detected in the organ of Corti at E18. (D) Transcript for α10 was detected over vestibular hair cells at E18. (E) At E21, α9 transcript was detected over cochlear IHCs and OHCs. (F) At E21, α9 transcript was detected over hair cells in the utricle. (G) Transcript for α10 was detected over cochlear hair cells at E21. (H) Transcript for α10 was detected over vestibular hair cells in the saccule at E21. Stippled outline demarcates hair cells. Scale bars represent 40 µm and are the same in panels A, B; C, D; E, F; and G, H.
In contrast to α9 expression, the expression of α10 occurred at a later embryonic age in the cochlea. At E18, specific α10 labeling along the cochlear spiral was absent (Figure 2C). Also, unlike α9 subunit transcript labeling, we confirmed a lack of labeling for the α10 subunit over the GER (Figure 2C). At E21, we observed an increase in α10 labeling over both IHCs and OHCs (Figure 2H). However, similar to α9 labeling, the α10 subunit was expressed over most, if not all, vestibular hair cells in these embryonic ages. Robust labeling was observed in the E18 cristae (Figure 2D) and in the E21 saccule (Figure 2H). The robust vestibular α10 expression is consistent with the real time PCR data suggesting that α10 expression occurs in vestibular hair cells before cochlear hair cells.
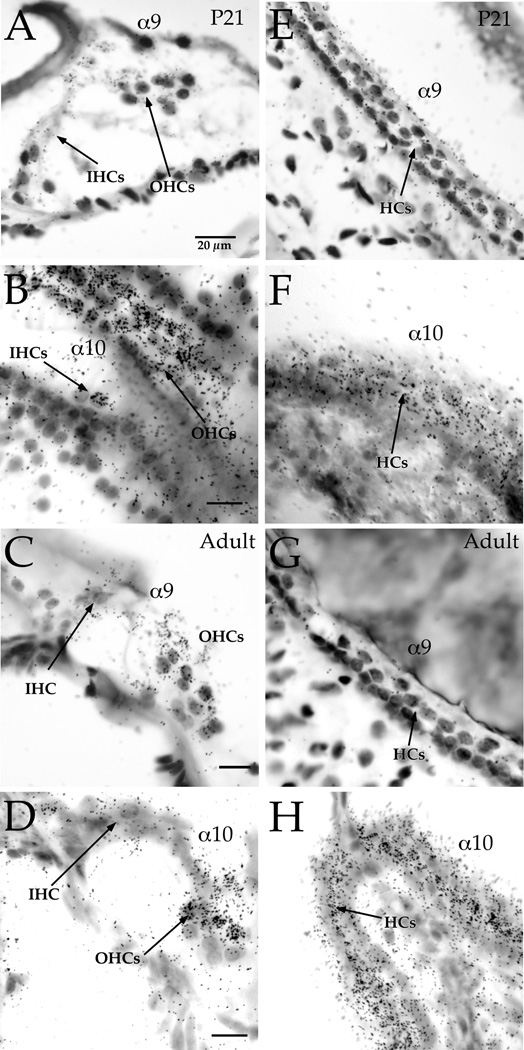
As we previously reported, α9 and α10 subunit expression detected with in situ hybridization was high during the late postnatal period (around P10) in the cochlea, at about the onset of hearing (Simmons and Morley, 1998, Morley and Simmons, 2002). In this study we analyzed cRNA transcript labeling patterns in cochlear and vestibular hair cells at P21 and in the mature adult (Figure 3). At P21, IHCs showed a decline in α9 and α10 transcript expression relative to OHCs (Figures 3A, 3B). By P30, α9 transcript labeling had noticeably declined further in the IHCs whereas α10 transcript labeling remained strong in OHCs (Figures 3C, 3D). In all vestibular organs, transcript expression for α9 also noticeably declined over hair cells at P21 and at adult ages (Figures 3E, 3G). However, α10 expression remained relatively uniform over most, if not all vestibular hair cells, albeit at somewhat reduced levels from younger ages (Figures 3F, 3H). We did not observe any evidence for a differential distribution of nAChR subunit expression in type I versus type II hair cells.
Figure 3. α9 and α10 nAChR subunit expression w as localized in the cochlea and vestibular periphery of mature (P21 and adult) rats using radiolabeled in situ hybridization.
cRNA probes specific to the α9 and α10 nAChR subunits were prepared as described in the text. Expression of α9 in the cochlea was observed over OHCs (A, C) and over vestibular hair cells (E, G) in cross sections of P21 and adult rats. Expression of α10 in the cochlea was observed over OHCs (B, D) and over vestibular hair cells (F, H). Panel B is a longitudinal section showing a row of IHCs and 3 rows of OHCs. Expression of α9 in IHCs is greatly reduced by P21 (A, C) and α10 expression is also reduced (B, D). Scale bars represent 20 µm and are the same for A,E; B,F; C,G; and D,H.
α-Bungarotoxin labeling in the cochlea
α-Bungarotoxin (α-Bgt) is a specific ligand that labels membrane bound nAChRs. Although α9 and α10 mRNA may be present in hair cells, these receptor subunits may not be effectively translated into an appropriately processed protein sequence or inserted into the hair cell membrane. Using an in vitro preparation of isolated organ of Corti spirals, we localized nicotinic receptors on the hair cell membrane with α-Bgt labeling from E18 – P10. Although vestibular hair cells did not show any α-Bgt labeling between E18 and P10, cochlear hair cells demonstrated α-Bgt labeling patterns reminiscent of longitudinal and radial gradients previously described for efferent innervation and subunit expression (Simmons and Morley, 1998). At all embryonic ages studied, robust α-Bgt labeling was observed on skeletal muscle but no labeling was observed on hair cells in the embryonic inner ear (Figure 4A).
Figure 4. α-Bgt labeling of embryonic (E) and postnatal (P) hair cells from isolated organ of Corti preparations at different embryonic and postnatal ages.
Longitudinal image views from freshly isolated cochlear spirals were labeled vitally with labeled α-Bgt (488 nm, green) and then fixed prior to labeling with phalloidin (546 nm, red), either parvalbumin or myosin VI (also 546 nm, red), and VAChT (647 nm, light purple) (A–C). Diaphragm skeletal muscle was used as a control for each age (Insert in A). Similar α-Bgt puncta (546 nm, red) were observed below the hair cells (α 9-GFP, 488 nm, green) of mice overexpressing the α9 gene. (D). The scale bars represent 10 µm.
The first appearance of α-Bgt labeling on isolated cochlear spirals occurred after E21. In the P0 organ of Corti, α-Bgt puncta were observed exclusively on IHCs and were restricted to spiral regions taken from middle basal regions of the cochlea (Figure 4B). No α-Bgt labeling was observed on OHCs nor on IHCs in more apical or basal locations along the cochlear spiral. In middle regions of the cochlear spiral, single IHCs had α-Bgt puncta located near their basal pole and along the lateral surfaces of the hair cell. α-Bgt labeled puncta were never found above the reticular lamina. At the earliest postnatal ages (P0), we counted any where from 0 to five α-Bgt puncta on individual IHCs in confocal reconstructions of the organ of Corti. We did not find any clear modiolar or pillar side bias to the location of puncta. α-Bgt clusters were also localized on IHCs in the cochlea at P2 (Figure 4C) in the FVB transgenic mouse that overexpresses the α9 subunit (Zuo et al., 1999). The FVB transgenic mouse model did not show a noticeable increase in α-Bgt labeling compared to the rat cochlea at an equivalent age. The developmental patterns of the P2 mouse should be equivalent to the P0 rat (Simmons, 2002).
High gain, high-resolution confocal microscopy and iterative deconvolution were used to resolve further α-Bgt-labeled clusters and punctate VAChT-ir labeling in whole preparations of the isolated organ of Corti from P2 to P10. Cholinergic terminals were marked by the presence of the vesicular acetylcholine choline transporter (VAChT), a protein that transports acetylcholine into presynaptic vesicles. Using immunohistochemical techniques, we found a heavy innervation of VAChT-immunoreactive (VAChT-ir) terminals below IHCs. In the P2 rat organ of Corti, VAChT-ir was observed below IHCs in both basal (Figure 5A) and apical (Figure 5C) cochlear regions. These apical and basal cochlear regions in the P2 organ of Corti contained no α-Bgt labeling. Only in middle regions of the same spiral, IHCs demonstrated clustered α-Bgt-labeled puncta and VAChT-ir puncta as shown in Figure 5B. Individual IHCs in the upper middle turn had as many as 10 individual α-Bgt clusters. The size of α-Bgt-labeled clusters typically ranged from 0.1 to 1.1 µm in diameter (mean 0.3±0.3, n=375). In upper middle turn regions, one or two α-Bgt-labeled clusters were seen on OHCs. Additionally, upper middle turn regions contained substantial VAChT-ir innervation below IHCs.
Figure 5. α-Bgt labeling in different regions along the cochlear spiral at P2.
VAChT-immunoreactive (VAChT-ir, 647 nm, light blue) labeling on or below IHCs (546 nm, red) was observed in both basal (A) and apical (C) cochlear regions and precedes α-Bgt clustering (488 nm, green). In middle regions of the same spiral, IHCs exhibited α-Bgt-labeled clusters (B). The scale bars represent 10 µm.
In the P6 organ of Corti, α-Bgt puncta were found on both IHCs and OHCs in apical and basal cochlear regions. At this age, α-Bgt puncta were observed in all three OHC rows and appeared as larger puncta or clusters, mostly but not exclusively below the level of the OHC nucleus. Figure 6 identifies a middle turn cochlear region where we found a heavy VAChT-ir innervation of terminals below IHCs. Few, if any, of those IHC terminals appeared to overlap α-Bgt-labeled puncta (Figure 6). Additionally on OHCs, there was little correlation between the location of VAChT-ir terminals and α-Bgt labeled clusters.
Figure 6. α-Bgt and VAChT-ir labeling at P6.
Selected single images were taken from a confocal stack through the organ of Corti located in upper middle regions of the cochlear spiral. α-Bgt-labeled clusters (488 nm, green) and VAChT-ir (647 nm, light blue) were found on IHCs (red) and OHCs (red). The top-most image (A) shows a majority of the VAChT-ir and α-Bgt clusters present on IHCs. As one proceeds through the stack (B, C, D), α-Bgt clusters appear on the first and second rows of OHCs. The scale bars represent 10 µm.
From P2 – P10, we observed a gradual shift from α-Bgt labeling localized mostly on IHCs to α-Bgt labeling localized on both IHCs and OHCs, and an increasing co-association between α-Bgt labeling and VAChT-ir. In P8 and P10 cochleae, middle regions along the cochlear spiral showed an increase in the complexity of the α-Bgt-labeling. During this latter age period, the size of α-Bgt-labeled clusters was very similar to their size at P2. Typically, α-Bgt clusters were 0.1 to 0.8 µm in diameter (mean 0.4±0.3, n=287), however the frequency of the clusters decreased along the basal surface of the hair cells. At P8 and P10, α-Bgt clusters were noticeably more closely associated with VAChT-ir. Although the majority of VAChT-ir label was not associated with α-Bgt labeling, it was the case that nearly all α-Bgt clusters were associated with VAChT-ir labeling. Both IHCs and OHCs had α-Bgt clusters juxtaposed with VAChT-ir labeling. As can also be seen in Figure 7, the juxtaposition of α-Bgt labeling and VAChT-ir occurs without any significant indication of mixing green (Bgt) and red (VAChT) channels.
Figure 7. α-Bgt and VAChT-ir labeling at P10.
(A) A projection image of a confocal series taken through the organ of Corti is shown for a lower middle turn location of the cochlear spiral. α-Bgt clusters (green) and VAChT-ir (red) are found on IHCs (blue) and OHCs (blue). Saturation in all channels was minimized during acquisition and post-processing. The stippled boxed regions in panel (A) of a single α-Bgt cluster on an IHC (B) and OHC (C) were digitally zoomed for high magnification views. The scale bar in (A) represents 10 µm. The scale bar in (B) and (C) represents 1 µm.
In order to determine whether the localized puncta are overlapping (colocalized) versus adjacent, we examined the degree of overlap in the P2 and P10 cochlea using the Pearson’s correlation coefficient (PCC) and Mander’s colocalization coefficient (M) (Adler and Parmryd). A PCC value near +1 suggests perfect correlation, −1 perfect negative correlation and 0 no correlation of overlapping intensities between two channels. The PCCs for α-Bgt overlapping with VAChT-ir were 0.166 for 18 α-Bgt labeled clusters at P2 and 0.041 for 37 α-Bgt labeled clusters at P10. The Manders colocalization coefficients (MBgt and MVAChT) give the fraction of fluorescent intensities within pixels that co-occur. At P2, MBgt was 0.469 for the fraction of α-Bgt signal with overlapping VAChT-ir and MVAChT was 0.445 for the fraction of VAChT-ir with overlapping α-Bgt signal. At P10, MBgt was 0.783 and MVAChT was 0.548. Thus, although the Mander’s colocalization coefficients suggested that there was increasing overlap between α-Bgt labeling and VAChT-ir from P2 to P10, the very low PCCs at both ages indicated that there was little, if any, correlation between the overlapping signals. Thus as Figures 7A, 7B indicate, α-Bgt labeling and VAChT-ir are closely associated but not substantially colocalized.
We also determined whether the association between α-Bgt clusters and VAChT-ir changes at P2 and at P10. For this analysis, counts of the number of α-Bgt clusters juxtaposed (touching) VAChT-ir labeled terminals were made at two different ages. In the P2 cochlea, overall about 11% of α-Bgt puncta was associated with VAChT-ir whereas in the P10 cochlea, about 22% α-Bgt labeling was associated with VAChT-ir. However as indicated by Table 1, the percentages within more restricted cochlear locations were more revealing. The upper middle base (the region just before the apical spiral) had 21% association between α-Bgt-VAChT labeling by P2. The juxtaposition of α-Bgt and VAChT-ir increased to 38% by P10. At P2 and P10, the lower middle base (outside of the basal hook region) had 12% co-associated α-Bgt-VAChT labeling, respectively. In both P2 and P10 animals, none of the α-Bgt-labeling demonstrated co-association with VAChT-ir in the apex.
TABLE 1.
| Postnatal Age | αBgt labeling associated with VAChT-ir labeling | |||
|---|---|---|---|---|
| Middle Base | Upper Base | Apex | Sum | |
| P2 | 12/103 (12%) | 6/28 (21%) | 0/34 (0) | 18/165 (11%) |
| P10 | 12/23 (52%) | 25/65 (38%) | 0/80 (0) | 37/168 (22%) |
A higher degree of association between α-Bgt clusters and VAChT-ir occurs as the extent of VAChT-ir decreases. For example, at P2 there was little juxtaposition between VAChT-ir terminals and α-Bgt-labeling although there was extensive VAChT-ir. A plot of the number of VAChT-ir terminals and α-Bgt puncta is shown in Figure 8A for a single P2 cochlea. The highest number of α-Bgt puncta and VAChT-ir terminals occurs mostly below IHCs from the middle regions of the cochlear spiral. The number of α-Bgt puncta and VAChT-ir terminals follow similar trends along the P2 cochlear spiral. VAChT-ir and α-Bgt labeling ratios in the P2 cochlea varied between 12:1 in apical cochlear locations, 6:1 in upper middle cochlear locations and 2:1 in lower basal cochlear locations. At P10 we observe a greater juxtaposition between VAChT-ir terminals and α-Bgt clusters; however, the VAChT-ir was less extensive than in similar regions at P2. Figure 8B shows that the number of α-Bgt-labeled puncta was highest in upper middle and apical portions of the cochlea, but VAChT-ir was lowest in apical region. Apical locations had a more α-Bgt puncta than VAChT-ir terminals. Labeling ratios were near 8:1, 4:1, and 0.13, respectively in middle, upper middle and apical cochlear regions.
Figure 8. Relationship between α-Bgt labeling and VAChT-ir at P2 and P10.
All puncta profiles were digitized from confocal stacks. Counts of α- Bgt (488 nm) and VAChT (647 nm) were done using Volocity morphometric software. (A) Plot of the number of α-Bgt puncta and VAChT puncta at P2 from 5 different regions apex to base. (B) Plot of the number of α- Bgt puncta and VAChT puncta at P10 from two different basal regions and the apex.
Discussion
The present study further demonstrates the differential regulation of α9 and α10 subunits in the inner ear. Although hair cells in vestibular organs express α9 and α10 subunits before cochlear hair cells, cell surface receptors could only be detected on cochlear hair cells following the arrival of cholinergic terminals during the early postnatal period in rats. Importantly, this work supports the hypothesis that functional cell surface expression may require not only the expression of both nicotinic subunits, but also the presence of cholinergic terminals.
There is little doubt that the functional axosomatic cholinergic receptor expressed in mature OHCs, and transiently on IHCs, consists of a receptor assembled from the α9 and α10 subunits of the nAChR gene family. The evidence includes electrophysiological recordings from OHCs and early postnatal IHCs and the nearly identical pharmacology and physiology when the two subunits are expressed in oocytes (Elgoyhen et al., 2001, Katz et al., 2004, Plazas et al., 2005, Vetter et al., 2007). The expression of the receptor is obligatory for normal synaptogenesis (Vetter et al., 1999, Vetter et al., 2007, Murthy et al., 2009a, Murthy et al., 2009b). Our data expand these observations by providing evidence supporting the hypothesis that the assembled receptor (as detected by α-Bgt labeling) is inserted into the membrane of IHCs and OHCs after the arrival of efferent axons expressing VAChT. The developmental pattern observed with cell surface expression correlates well with previous electrophysiological studies that show functional nAChRs as early as P3 in the rat (Katz et al., 2004). Taken together, these data provide evidence that nAChRs appear to cluster on hair cells to make functional receptors during the period of efferent synaptogenesis.
Understanding the role of nicotinic acetylcholine receptor subunits during efferent synaptogenesis necessitates that we know the spatiotemporal pattern of nAChRs in hair cells at different developmental ages. Using similar methods as were used for heterologous cell expression (Osman et al., 2008), we localized nAChRs on the hair cell surface membrane using fluorescent conjugated α-Bgt from animals at ages E18 – P10. In the cochlea during this period, α-Bgt labeling followed similar longitudinal and radial gradients as previously described for efferent innervation and subunit expression (Simmons et al., 2011). Cochlear hair cells did not demonstrate any α-Bgt labeling until after birth, and vestibular hair cells did not show any α-Bgt labeling at least through P10. This labeling pattern contrasted with diaphragm skeletal muscle, where robust α-Bgt labeling was found at all embryonic and postnatal ages examined.
Given that the density of nAChRs at the embryonic neuromuscular junction is extremely high, the lack of α-Bgt labeling on hair cells represents either a lack of assembled receptors, or more likely, that the density of label is below the level of detection (Osman et al., 2008). In contrast with the muscle nAChR, in which is α-Bgt binding is irreversible (Chang and Lee, 1963), α9-containing nAChRs are reversibly blocked by this toxin (Elgoyhen et al., 1994, Elgoyhen et al., 2001). Such reversible binding would most likely decrease the sensitivity of α-Bgt labeling on hair cells compared to muscle nAChRs.
Initially, none of the α-Bgt label overlaps substantially with cholinergic efferent terminals, as labeled by VAChT-ir. Over the course of development, however, there is an increasing association between α-Bgt labeling with cholinergic terminals. At least in the cochlea, we conclude that efferent innervation and the expression of the α10 nAChR subunit occur prior to the development of α-Bgt labeled clusters, and that the initial spatial location of efferent terminals is uncorrelated with the initial formation of α-Bgt labeled clusters. A current hypothesis is that efferent activity is necessary for the assembly of postsynaptic receptors (Katz et al., 2004). Although our data do not rule out this hypothesis, we further hypothesize that the presence of efferent terminals may be important for the postsynaptic clustering of the nAChR at the hair cell neural pole.
The absence of α-Bgt clusters prior to α10 expression in either IHCs or OHCs could indicate that α10 subunits are obligatory for the formation of nicotinic receptor clusters. The available data suggest that most α9 expression in hair cells occurs embryonically before efferent synaptogenesis, whereas α10 expression occurs only after α9 expression. Our current study confirms previous work that indicated the α10 subunit is expressed at the time of birth in both IHCs and OHCs (Elgoyhen et al., 2001, Morley and Simmons, 2002). We show α9 expression but not α10 expression as early as E16 in the cochlea. Our results are consistent with our earlier data showing α10 subunit expression peaks immediately following birth for IHCs and around P10 for OHCs (Morley and Simmons, 2002). Although we demonstrate that both α9 and α10 expression occur prior to birth in the vestibule, only a much lower level of α10 expression can be detected before birth in the cochlea. At least in the cochlea, α-Bgt clusters appear only after the onset of α10 expression and the presence of efferent terminals. The demonstration that efferent terminals are present prior to α-Bgt clusters suggests that efferent innervation has a role in α-Bgt cluster formation as discussed above. The formation of α-Bgt clusters is temporally consistent with the appearance of ACh-induced Ca2+ currents in IHCs (Katz et al., 2004).
In the developing inner ear, little is known about the relationship between cholinergic neurotransmission and postsynaptic responses, especially Ca2+ signaling cascades that are activated by the release of ACh by efferent axons. We first hypothesized that medial OC efferent terminals made transient α9α10 nAChR-mediated synapses with IHCs (Simmons et al., 1996a, Simmons et al., 1996b, Simmons et al., 1998, Morley and Simmons, 2002). Several studies have now shown that transient synaptic connections below IHCs give rise to an early inward cation current (Ca2+) followed by an outward current (K+) (Glowatzki and Fuchs, 2000, Katz et al., 2004, Gomez-Casati et al., 2005, Gomez-Casati et al., 2009, Zorrilla de San Martin et al., 2010). Whether this early Ca2+ entry plays an important role during development is not known. Interestingly, targeted deletion of either α9 or α10 subunits leads to changes in efferent innervation, but no known effect on hair cell physiology (Vetter et al., 1999, Vetter et al., 2007).
In recent studies of heterologous cell expression, transfection of α9 and α10 subunit cDNAs, along with other accessory proteins, caused an increase in α-Bgt labeling of cell surface nAChRs (Osman et al., 2008). In that study, we demonstrated an early expression of rapsyn in the embryonic inner ear as well as punctate localization of rapsyn immunoreactivity in vestibular hair cells before cochlear hair cells. If rapsyn and muscle specific kinase (MuSK) play a role in cholinergic synaptogenesis within the inner ear, then the earlier localization of rapsyn could suggest an earlier expression of α9 and α10 in vestibular hair cells before cochlear hair cells. Indeed, we found evidence that vestibular hair cells express nAChR subunits much earlier than cochlear hair cells suggesting that nAChRs may play an early role in vestibular development. However, we were unable to find any α-Bgt labeling on vestibular hair cells at least through P10. The data in the present study suggests that vestibular hair cells begin to express nAChR subunits earlier than cochlear hair cells and raise questions as to the factors that influence cell surface receptor expression and localization in hair cells. If cell surface expression of nAChRs requires the presence of efferent terminals, then the lack of any observable α-Bgt labeling is consistent with studies that show cholinergic innervation of vestibular organs is significantly delayed in the rodent (Dememes and Broca, 1998, Dememes et al., 2001).
Our data also indicate that α-Bgt puncta probably do not localize with cholinergic terminals at birth, but become progressively associated with α-Bgt-labeled clusters in both IHCs and OHCs. The data are consistent with the hypothesis that both the α9 and α10 subunits are required for the initial clustering of the receptor in hair cells. Peak mRNA expression and the labeling of α-Bgt under hair cells coincides with peak ACh sensitivity found in IHCs at P7 – P9 (Katz et al., 2004) and about the time that cholinergic response of OHCs switches to an outward current at PN12 (Dulon and Lenoir, 1996), suggesting that the receptors are transcriptionally regulated. Although equivalent evidence is not available for hair cells in the vestibular organs, previous studies show expression of nicotinic subunits in vestibular hair cells (Hiel et al., 1998), and, at least in frog saccular hair cells, demonstrate a physiology and pharmacology consistent with an α 9α10 nAChR (Holt et al., 2003).
The embryonic expression of the α9 and α10 subunits in the cochlea and vestibule may have functional significance not associated with synapses. The α9 and α10 subunits are members of “Subfamily I” of the nAChR gene family, which is comprised of the α7– α10 subunits (Le Novere and Changeux, 1995). Non-synaptic roles for several members the nAChR gene family are well-established (Steffl et al., 2006, Resende and Adhikari, 2009, Colomer et al., 2010), where ACh may or may not be the endogenous agonist. Of particular interest is a recent report (Scheffer et al., 2007) that the transcription factor Atoh1 directly activates transcription of the α1 nAChR gene by binding to two E boxes on the proximal promoter of CHRNA1. The authors propose that CHRNA1 is the first transcriptional target of Atoh1 in the inner ear. The putative receptor would be a combination of the α1 and γ subunits, which they observed was downregulated at P6 – P7 using Affymetrix GeneChip microarray analysis (Scheffer et al., 2007). A similar decline in expression is observed in our study for the α9 and α10 subunits in both the cochlea and vestibule. Although the α9 and α10 subunits were first identified and characterized in cochlear hair cells, recent studies have revealed their important presence in peripheral tissues where they are related to cell proliferation (Koval et al., 2011). The relationship of the α9 subunit to regulators of the epithelial cell cycle in other tissues suggests the possibility that in embryonic development the cochlea and vestibule, α9 and/or α10 subunits may be involved with the control of hair cell epithelial cell growth.
Conclusions
Knowledge of the expression and cell surface localization pattern of nAChRs in hair cells at different developmental ages is essential to understanding the role of the receptor subunits during efferent synaptogenesis. α9 and α10 nAChR subunits show differential expression patterns among inner and outer hair cells as well as between cochlear and vestibular hair cells. The data reported in this study are consistent with the following developmental sequence: first α9 subunits are expressed, followed by efferent innervation and the expression of α10 subunits, and then the appearance of nAChR clusters on the cell surface. This report suggests that cholinergic innervation and the expression of the α10 nAChR subunit are necessary for the clustering of nicotinic acetylcholine receptors on hair cells.
Highlights.
α9 and α10 nicotinic receptor subunits first appear in vestibular hair cells.
α9 and α10 subunits have higher expression at birth and at postnatal day 10.
Bungarotoxin labeling is found first on inner hair cells at postnatal day 0.
α9/α10 receptors are on hair cells after α10 expression and efferent innervation.
Acknowledgments
This publication was made possible by NIH Grants DC004086 (DDS), DC006907 (BJM) and NIDCD P30 core center grants DC004665 and DC004662. This research was also supported by the Deafness Research Foundation, National Organization for Hearing Research, the American Hearing Research Foundation, and the State of Nebraska (BJM). The authors thank Ms. Xiaona Huang, Mr. Steve Gum, , Ms. Jennifer Spice, and Ms. Aubrey Hornak for their excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler J, Parmryd I. Quantifying colocalization by correlation: the Pearson correlation coefficient is superior to the Mander's overlap coefficient. Cytometry A. 2010;77:733–742. doi: 10.1002/cyto.a.20896. [DOI] [PubMed] [Google Scholar]
- Bergeron AL, Schrader A, Yang D, Osman AA, Simmons DD. The final stage of cholinergic differentiation occurs below inner hair cells during development of the rodent cochlea. JARO Journal of the Association for Research in Otolaryngology. 2005;6:401–415. doi: 10.1007/s10162-005-0018-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomer C, Olivos-Ore LA, Vincent A, McIntosh JM, Artalejo AR, Guerineau NC. Functional characterization of alpha9-containing cholinergic nicotinic receptors in the rat adrenal medulla: implication in stress-induced functional plasticity. J Neurosci. 2010;30:6732–6742. doi: 10.1523/JNEUROSCI.4997-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dememes D, Broca C. Calcitonin gene-related peptide immunoreactivity in the rat efferent vestibular system during development. Brain Res Dev Brain Res. 1998;108:59–67. doi: 10.1016/s0165-3806(98)00030-3. [DOI] [PubMed] [Google Scholar]
- Dememes D, Dechesne CJ, Venteo S, Gaven F, Raymond J. Development of the rat efferent vestibular system on the ground and in microgravity. Brain Res Dev Brain Res. 2001;128:35–44. doi: 10.1016/s0165-3806(01)00146-8. [DOI] [PubMed] [Google Scholar]
- Dulon D, Lenoir M. Cholinergic responses in developing outer hair cells of the rat cochlea. Eur J Neurosci. 1996;8:1945–1952. doi: 10.1111/j.1460-9568.1996.tb01338.x. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S. Alpha 9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell. 1994;79:705–715. doi: 10.1016/0092-8674(94)90555-x. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Vetter DE, Katz E, Rothlin CV, Heinemann SF, Boulter J. alpha10: a determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc Natl Acad Sci U S A. 2001;98:3501–3506. doi: 10.1073/pnas.051622798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA. Cholinergic synaptic inhibition of inner hair cells in the neonatal mammalian cochlea. Science. 2000;288:2366–2368. doi: 10.1126/science.288.5475.2366. [DOI] [PubMed] [Google Scholar]
- Gomez-Casati ME, Fuchs PA, Elgoyhen AB, Katz E. Biophysical and pharmacological characterization of nicotinic cholinergic receptors in rat cochlear inner hair cells. J Physiol. 2005;566:103–118. doi: 10.1113/jphysiol.2005.087155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Casati ME, Wedemeyer C, Taranda J, Lipovsek M, Dalamon V, Elgoyhen AB, Katz E. Electrical properties and functional expression of ionic channels in cochlear inner hair cells of mice lacking the alpha10 nicotinic cholinergic receptor subunit. J Assoc Res Otolaryngol. 2009;10:221–232. doi: 10.1007/s10162-009-0164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel M, Kasher R, Nicolas A, Guss JM, Balass M, Fridkin M, Smit AB, Brejc K, Sixma TK, Katchalski-Katzir E, Sussman JL, Fuchs S. The binding site of acetylcholine receptor as visualized in the X-Ray structure of a complex between alpha-bungarotoxin and a mimotope peptide. Neuron. 2001;32:265–275. doi: 10.1016/s0896-6273(01)00461-5. [DOI] [PubMed] [Google Scholar]
- Hiel H, Elgoyhen AB, Drescher DG, Morley BJ. Expression of nicotinic acetylcholine receptor mRNA in the adult rat peripheral vestibular system. Brain Res. 1996;738:347–352. doi: 10.1016/s0006-8993(96)01046-3. [DOI] [PubMed] [Google Scholar]
- Holt JC, Lioudyno M, Guth PS. A pharmacologically distinct nicotinic ACh receptor is found in a subset of frog semicircular canal hair cells. J Neurophysiol. 2003;90:1526–1536. doi: 10.1152/jn.00273.2002. [DOI] [PubMed] [Google Scholar]
- Kasher R, Balass M, Scherf T, Fridkin M, Fuchs S, Katchalski-Katzir E. Design and synthesis of peptides that bind alpha-bungarotoxin with high affinity. Chem Biol. 2001;8:147–155. doi: 10.1016/s1074-5521(00)90063-2. [DOI] [PubMed] [Google Scholar]
- Katz E, Elgoyhen AB, Gomez-Casati ME, Knipper M, Vetter DE, Fuchs PA, Glowatzki E. Developmental regulation of nicotinic synapses on cochlear inner hair cells. J Neurosci. 2004;24:7814–7820. doi: 10.1523/JNEUROSCI.2102-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koval L, Lykhmus O, Zhmak M, Khruschov A, Tsetlin V, Magrini E, Viola A, Chernyavsky A, Qian J, Grando S, Komisarenko S, Skok M. Differential involvement of alpha4beta2, alpha7 and alpha9alpha10 nicotinic acetylcholine receptors in B lymphocyte activation in vitro. Int J Biochem Cell Biol. 2011;43:516–524. doi: 10.1016/j.biocel.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Le Novere N, Changeux JP. Molecular evolution of the nicotinic acetylcholine receptor: an example of multigene family in excitable cells. J Mol Evol. 1995;40:155–172. doi: 10.1007/BF00167110. [DOI] [PubMed] [Google Scholar]
- Manders EMM, Verbeek FJ, Aten A. Measurement of co-localization of objects in dual-colour confocal images. Journal of Microscopy. 1993;169:375–382. doi: 10.1111/j.1365-2818.1993.tb03313.x. [DOI] [PubMed] [Google Scholar]
- Morley BJ, Li HS, Hiel H, Drescher DG, Elgoyhen AB. Identification of the subunits of the nicotinic cholinergic receptors in the rat coch lea using RT-PCR and in situ hybridization. Brain Res Mol Brain Res. 1998;53:78–87. doi: 10.1016/s0169-328x(97)00272-6. [DOI] [PubMed] [Google Scholar]
- Morley BJ, Simmons DD. Developmental mRNA expression of the alpha10 nicotinic acetylcholine receptor subunit in the rat cochlea. Brain Res Dev Brain Res. 2002;139:87–96. doi: 10.1016/s0165-3806(02)00514-x. [DOI] [PubMed] [Google Scholar]
- Murthy V, Maison SF, Taranda J, Haque N, Bond CT, Elgoyhen AB, Adelman JP, Liberman MC, Vetter DE. SK2 channels are required for function and long-term survival of efferent synapses on mammalian outer hair cells. Mol Cell Neurosci. 2009a;40:39–49. doi: 10.1016/j.mcn.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy V, Taranda J, Elgoyhen AB, Vetter DE. Activity of nAChRs containing alpha9 subunits modulates synapse stabilization via bidirectional signaling programs. Dev Neurobiol. 2009b;69:931–949. doi: 10.1002/dneu.20753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman AA, Schrader AD, Hawkes AJ, Akil O, Bergeron A, Lustig LR, Simmons DD. Muscle-like nicotinic receptor accessory molecules in sensory hair cells of the inner ear. Molecular and Cellular Neuroscience. 2008;38:153–169. doi: 10.1016/j.mcn.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plazas PV, Katz E, Gomez-Casati ME, Bouzat C, Elgoyhen AB. Stoichiometry of the alpha9alpha10 nicotinic cholinergic receptor. J Neurosci. 2005;25:10905–10912. doi: 10.1523/JNEUROSCI.3805-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resende RR, Adhikari A. Cholinergic receptor pathways involved in apoptosis, cell proliferation and neuronal differentiation. Cell Commun Signal. 2009;7:20. doi: 10.1186/1478-811X-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer D, Sage C, Plazas PV, Huang M, Wedemeyer C, Zhang DS, Chen ZY, Elgoyhen AB, Corey DP, Pingault V. The alpha1 subunit of nicotinic acetylcholine receptors in the inner ear: transcriptional regulation by ATOH1 and co-expression with the gamma subunit in hair cells. J Neurochem. 2007;103:2651–2664. doi: 10.1111/j.1471-4159.2007.04980.x. [DOI] [PubMed] [Google Scholar]
- Schrattenholz A, Pfeiffer S, Pejovic V, Rudolph R, Godovac-Zimmermann J, Maelicke A. Expression and renaturation of the N-terminal extracellular domain of torpedo nicotinic acetylcholine receptor alpha-subunit. J Biol Chem. 1998;273:32393–32399. doi: 10.1074/jbc.273.49.32393. [DOI] [PubMed] [Google Scholar]
- Simmons D, Duncan J, Caprona DCd, Fritzsch B. Development of the Inner Ear Efferent System. In: Ryugo DK, et al., editors. Auditory and vestibular efferents. New York: Springer; 2011. pp. 187–216. [Google Scholar]
- Simmons DD. Development of the inner ear efferent system across vertebrate species. Journal of Neurobiology. 2002;53:228–250. doi: 10.1002/neu.10130. [DOI] [PubMed] [Google Scholar]
- Simmons DD, Bertolotto C, Kim J, Raji-Kubba J, Mansdorf N. Choline acetyltransferase expression during a putative developmental waiting period. Journal of Comparative Neurology. 1998;397:281–295. [PubMed] [Google Scholar]
- Simmons DD, Mansdorf NB, Kim JH. Olivocochlear innervation of inner and outer hair cells during postnatal maturation: Evidence for a waiting period. Journal of Comparative Neurology. 1996a;370:551–562. doi: 10.1002/(SICI)1096-9861(19960708)370:4<551::AID-CNE10>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Simmons DD, Morley BJ. Differential expression of the alpha9 nicotinic acetylcholine receptor subunit in neonatal and adult cochlear hair cells. Molecular Brain Research. 1998;56:287–292. doi: 10.1016/s0169-328x(98)00056-4. [DOI] [PubMed] [Google Scholar]
- Simmons DD, Moulding HD, Zee D. Olivocochlear innervation of inner and outer hair cells during postnatal maturation: An immunocytochemical study. Developmental Brain Research. 1996b;95:213–226. doi: 10.1016/0165-3806(96)00084-3. [DOI] [PubMed] [Google Scholar]
- Steffl M, Schweiger M, Wessler I, Kunz L, Mayerhofer A, Amselgruber WM. Non - neuronal acetylcholine and choline acetyltransferase in oviductal epithelial cells of cyclic and pregnant pigs. Anat Embryol (Berl) 2006;211:685–690. doi: 10.1007/s00429-006-0132-y. [DOI] [PubMed] [Google Scholar]
- Vetter DE, Katz E, Maison SF, Taranda J, Turcan S, Ballestero J, Liberman MC, Elgoyhen AB, Boulter J. The alpha10 nicotinic acetylcholine receptor subunit is required for normal synaptic function and integrity of the olivocochlear system. Proc Natl Acad Sci U S A. 2007;104:20594–20599. doi: 10.1073/pnas.0708545105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter DE, Liberman MC, Mann J, Barhanin J, Boulter J, Brown MC, Saffiote-Kolman J, Heinemann SF, Elgoyhen AB. Role of alpha9 nicotinic ACh receptor subunits in the development and function of cochlear efferent innervation. Neuron. 1999;23:93–103. doi: 10.1016/s0896-6273(00)80756-4. [DOI] [PubMed] [Google Scholar]
- Zorrilla de San Martin J, Pyott S, Ballestero J, Katz E. Ca(2+) and Ca(2+)-activated K(+) channels that support and modulate transmitter release at the olivocochlear efferent-inner hair cell synapse. J Neurosci. 2010;30:12157–12167. doi: 10.1523/JNEUROSCI.2541-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J, Treadaway J, Buckner TW, Fritzsch B. Visualization of alpha9 acetylcholine receptor expression in hair cells of transgenic mice containing a modified bacterial artificial chromosome. Proc Natl Acad Sci U S A. 1999;96:14100–14105. doi: 10.1073/pnas.96.24.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]