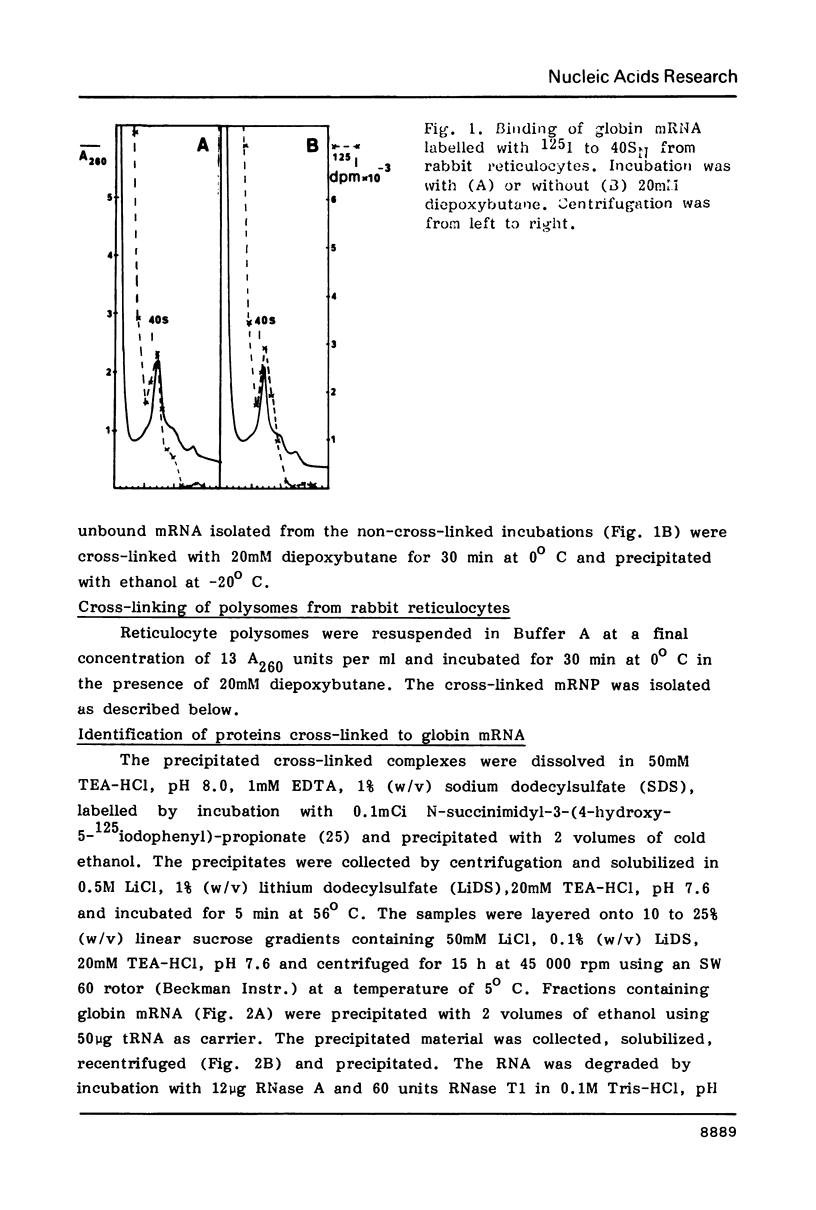
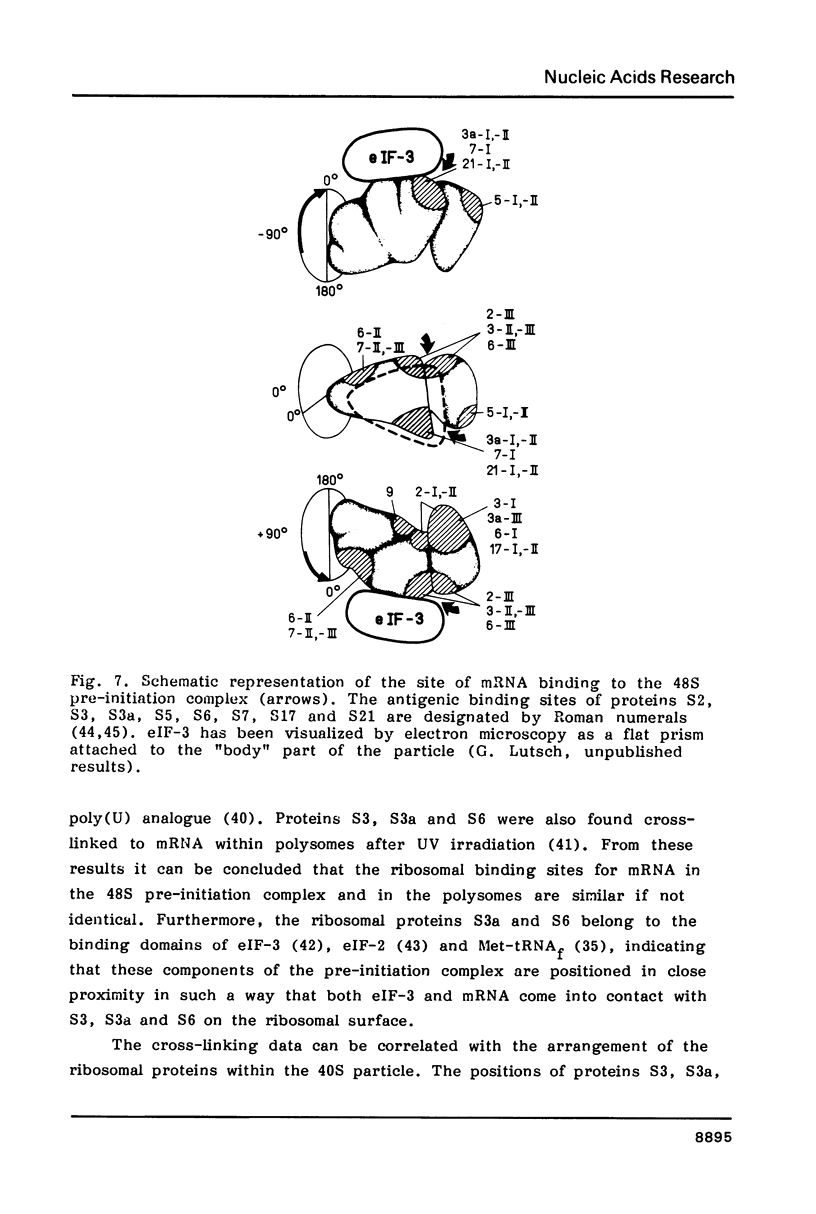
Abstract
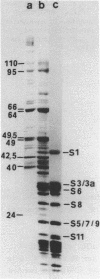
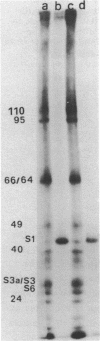
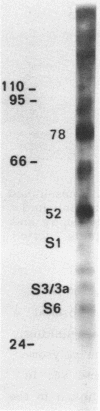
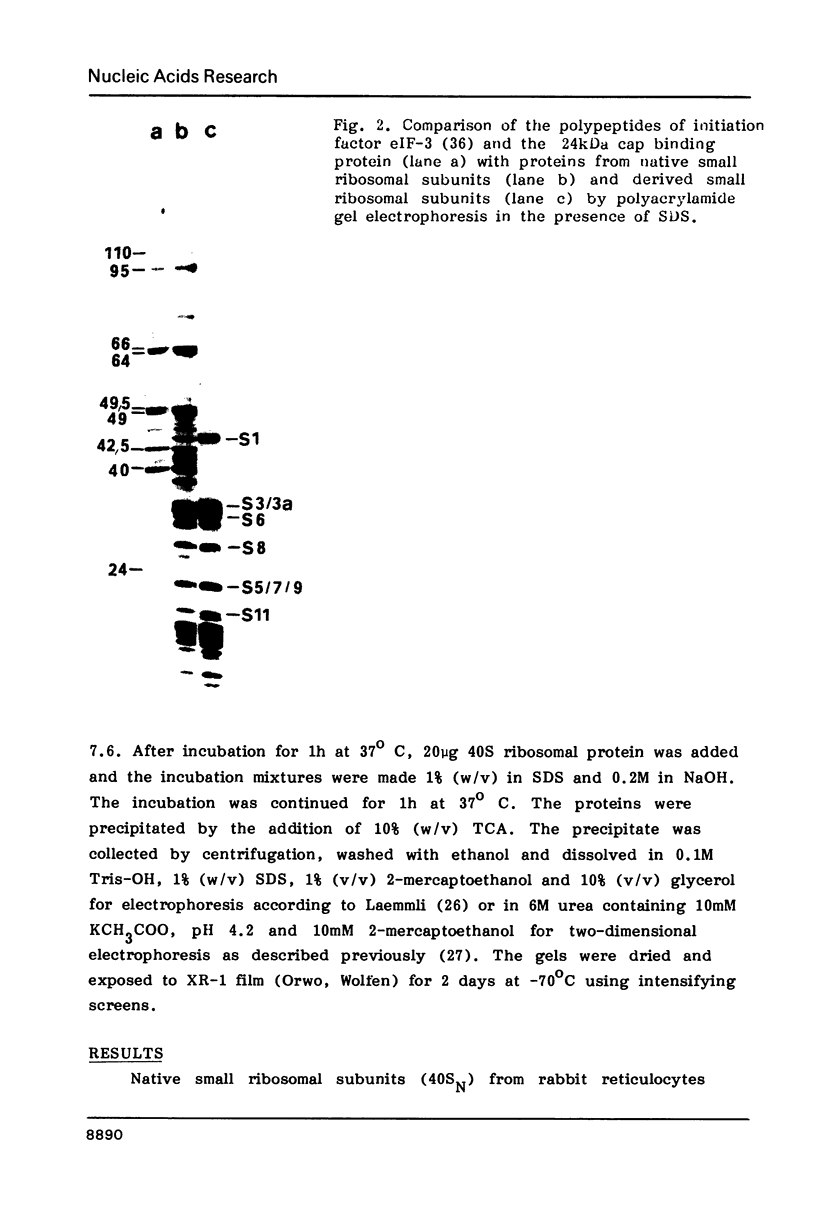
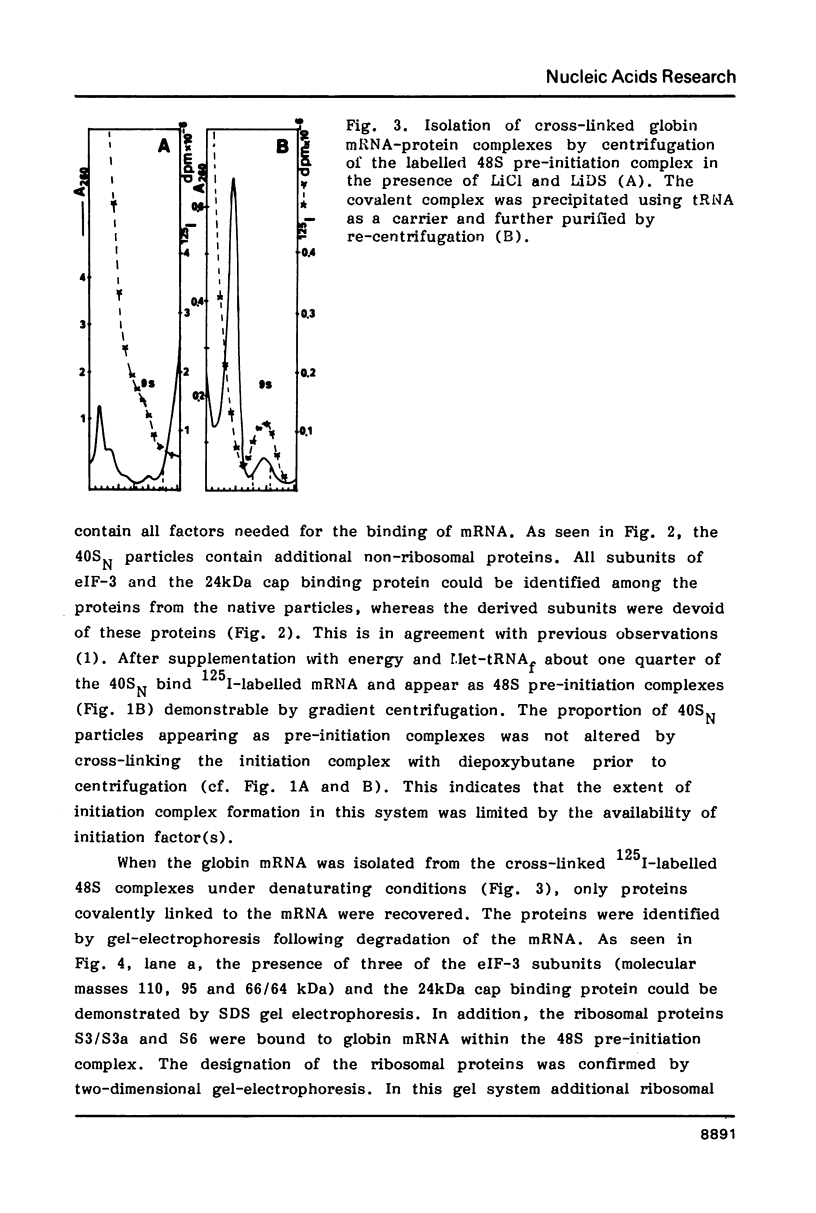
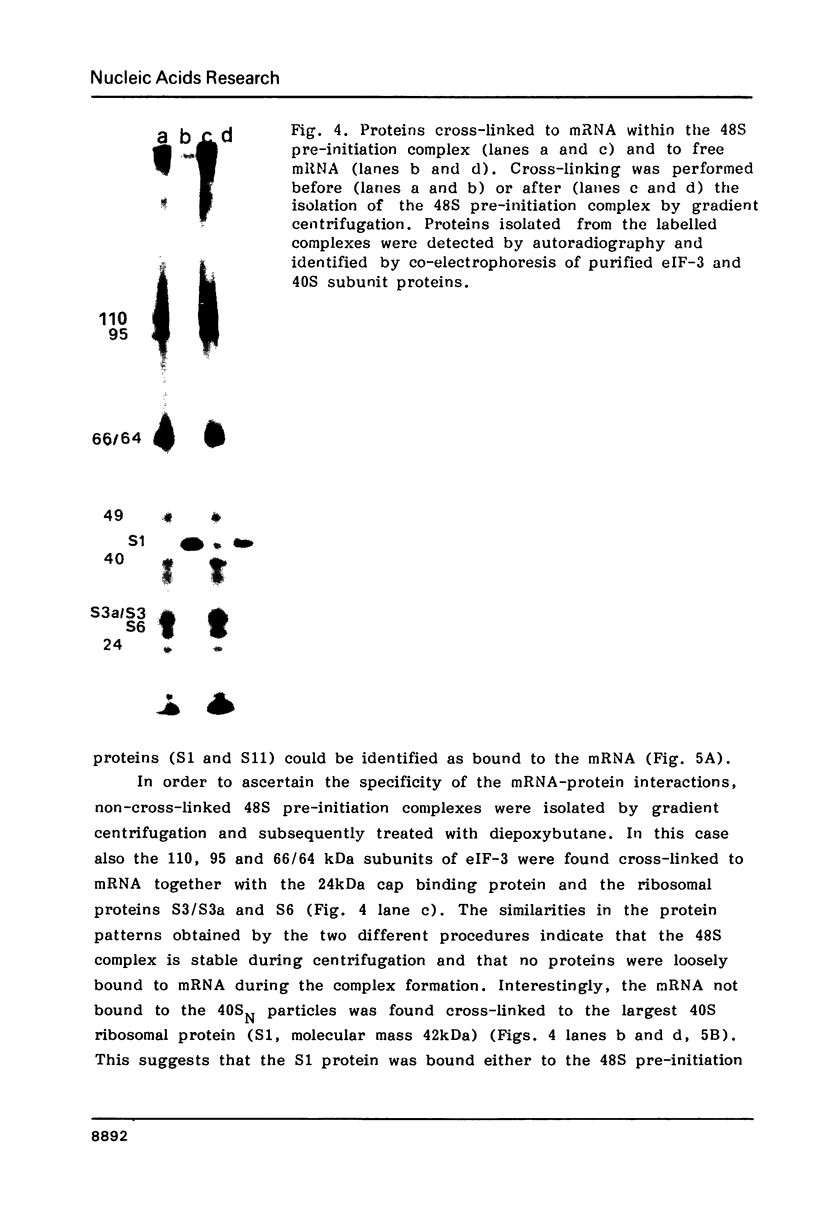
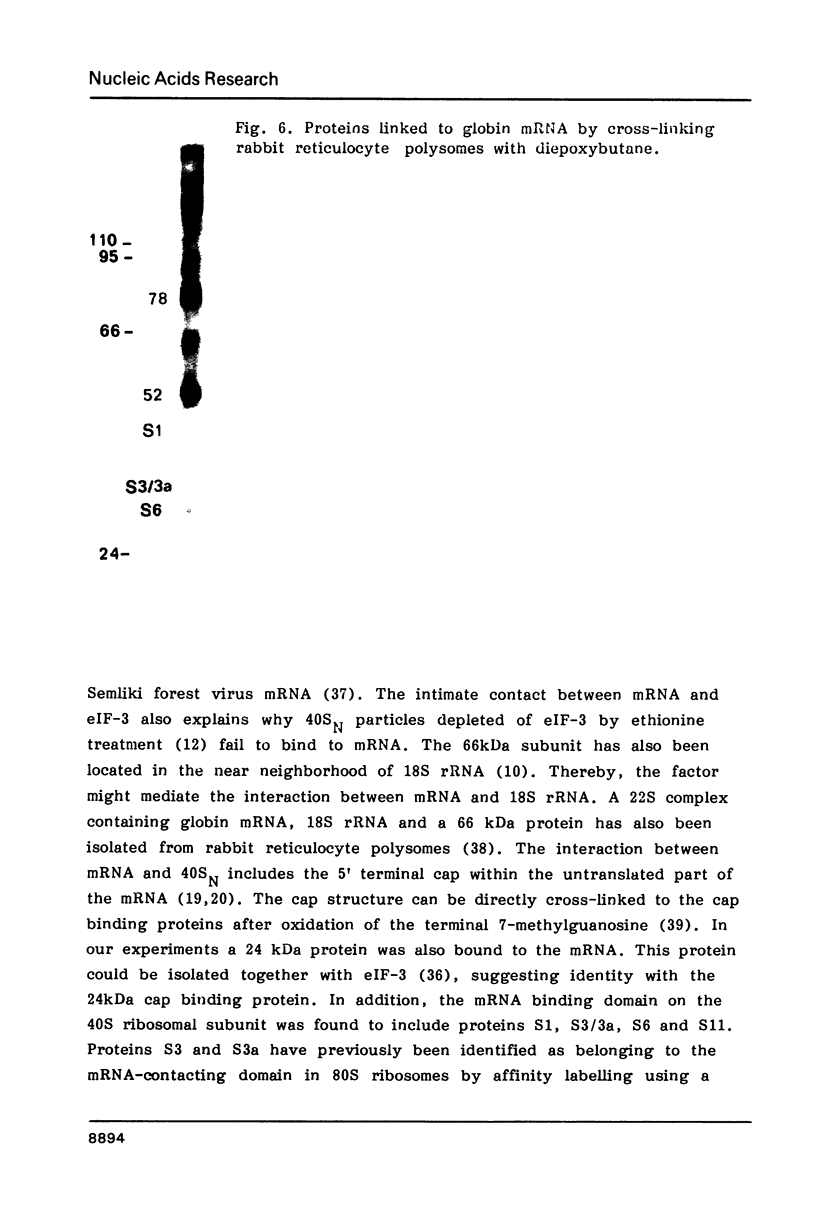
Native small ribosomal subunits from rabbit reticulocytes contain all initiation factors necessary for the formation of the mRNA-containing 48S pre-initiation complex. The complex formed in the presence of Met-tRNAf and 125I-labelled globin mRNA was cross-linked with diepoxybutane, and the covalent mRNA-protein complexes were isolated under denaturating conditions. The proteins of the covalent complex were identified as the 110, 95 and 66/64 kDa subunits of eIF-3. In addition, the 24 kDa cap binding protein and the ribosomal proteins S1, S3/3a, S6 and S11 were found covalently linked to the mRNA. Ribosomal proteins S3/3a and S6 were also involved in the ribosomal mRNA-binding domain of reticulocyte polysomes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergmann J. E., Lodish H. F. A kinetic model of protein synthesis. Application to hemoglobin synthesis and translational control. J Biol Chem. 1979 Dec 10;254(23):11927–11937. [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D., Hansen J., Ehrenfeld E. Specificity of initiation factor preparations from poliovirus-infected cells. J Virol. 1980 May;34(2):573–575. doi: 10.1128/jvi.34.2.573-575.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher P. D., Arnstein H. R. Efficient translation and polyribosome binding of 125I-labelled rabbit globin messenger ribonucleoprotein. FEBS Lett. 1983 Mar 7;153(1):119–124. doi: 10.1016/0014-5793(83)80130-6. [DOI] [PubMed] [Google Scholar]
- Bäumert H. G., Sköld S. E., Kurland C. G. RNA-protein neighbourhoods of the ribosome obtained by crosslinking. Eur J Biochem. 1978 Sep 1;89(2):353–359. doi: 10.1111/j.1432-1033.1978.tb12536.x. [DOI] [PubMed] [Google Scholar]
- Commerford S. L. Iodination of nucleic acids in vitro. Biochemistry. 1971 May 25;10(11):1993–2000. doi: 10.1021/bi00787a005. [DOI] [PubMed] [Google Scholar]
- Duncan R., McConkey E. H. Rapid alterations in initiation rate and recruitment of inactive RNA are temporally correlated with S6 phosphorylation. Eur J Biochem. 1982 Apr;123(3):539–544. doi: 10.1111/j.1432-1033.1982.tb06565.x. [DOI] [PubMed] [Google Scholar]
- Edery I., Hümbelin M., Darveau A., Lee K. A., Milburn S., Hershey J. W., Trachsel H., Sonenberg N. Involvement of eukaryotic initiation factor 4A in the cap recognition process. J Biol Chem. 1983 Sep 25;258(18):11398–11403. [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Freienstein C., Blobel G. Use of eukaryotic native small ribosomal subunits for the translation of globin messenger RNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3435–3439. doi: 10.1073/pnas.71.9.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. R. Proteins crosslinked to messenger RNA by irradiating polyribosomes with ultraviolet light. Nucleic Acids Res. 1980 Dec 11;8(23):5685–5701. doi: 10.1093/nar/8.23.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressner A. M., van de Leur E. Interaction of synthetic polynucleotides with small rat liver ribosomal subunits possessing low and highly phosphorylated protein S6. Biochim Biophys Acta. 1980 Jul 29;608(2):459–468. doi: 10.1016/0005-2787(80)90191-4. [DOI] [PubMed] [Google Scholar]
- Grifo J. A., Tahara S. M., Leis J. P., Morgan M. A., Shatkin A. J., Merrick W. C. Characterization of eukaryotic initiation factor 4A, a protein involved in ATP-dependent binding of globin mRNA. J Biol Chem. 1982 May 10;257(9):5246–5252. [PubMed] [Google Scholar]
- Gross B., Westermann P., Bielka H. Spatial arrangement of proteins within the small subunit of rat liver ribosomes studied by cross-linking. EMBO J. 1983;2(2):255–260. doi: 10.1002/j.1460-2075.1983.tb01414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Nucleotide sequences of 5'-terminal ribosome-protected initiation regions from two reovirus messages. Nature. 1977 Sep 29;269(5627):391–394. doi: 10.1038/269390a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Legon S. Characterization of the ribosome-protected regions of 125I-labelled rabbit globin messenger RNA. J Mol Biol. 1976 Sep 5;106(1):37–53. doi: 10.1016/0022-2836(76)90299-0. [DOI] [PubMed] [Google Scholar]
- Lutsch G., Bielka H., Enzmann G., Noll F. Electron microscopic investigations on the location of rat liver ribosomal proteins S3a, S5, S6, S7 and S9 by means of antibody labeling. Biomed Biochim Acta. 1983;42(6):705–723. [PubMed] [Google Scholar]
- Lutsch G., Noll F., Theise H., Enzmann G., Bielka H. Localization of proteins S1, S2, S16 and S23 on the surface of small subunits of rat liver ribosomes by immune electron microscopy. Mol Gen Genet. 1979 Oct 3;176(2):281–291. doi: 10.1007/BF00273223. [DOI] [PubMed] [Google Scholar]
- Nakashima K., Darzynkiewicz E., Shatkin A. J. Proximity of mRNA5'-region and 18S rRNA in eukaryotic initiation complexes. Nature. 1980 Jul 17;286(5770):226–230. doi: 10.1038/286226a0. [DOI] [PubMed] [Google Scholar]
- Nygård O., Hultin T. Early effects of dimethylnitrosamine on protein chain initiation and postmicrosomal polyadenylic acid-containing RNA content in mouse liver. Cancer Res. 1979 Sep;39(9):3349–3352. [PubMed] [Google Scholar]
- Nygård O., Hultin T. Separation of Met-tRNAf and Met-tRNAm by chromatography on Sepharose 4B columns. Prep Biochem. 1976;6(5):339–346. doi: 10.1080/00327487608061623. [DOI] [PubMed] [Google Scholar]
- Nygård O., Westermann P. Purification of protein synthesis initiation factor eIF-3 from rat liver microsomes by affinity chromatography on rRNA-cellulose. Biochim Biophys Acta. 1982 Jun 30;697(3):263–269. doi: 10.1016/0167-4781(82)90088-4. [DOI] [PubMed] [Google Scholar]
- Nygård O., Westermann P. Specific interaction of one subunit of eukaryotic initiation factor eIF-3 with 18S ribosomal RNA within the binary complex, eIF-3 small ribosomal subunit, as shown by cross-linking experiments. Nucleic Acids Res. 1982 Feb 25;10(4):1327–1334. doi: 10.1093/nar/10.4.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter J. D., Smith L. D. Reversible inhibition of translation by Xenopus oocyte-specific proteins. Nature. 1984 May 24;309(5966):378–380. doi: 10.1038/309378a0. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis: the importance of ribosome and initiation factor quality for the efficiency of in vitro systems. J Mol Biol. 1973 Feb 19;73(3):329–349. doi: 10.1016/0022-2836(73)90346-x. [DOI] [PubMed] [Google Scholar]
- Setyono B., Van Steeg H., Voorma H. O. Ultraviolet-crosslinking reveals specific affinity of eukaryotic initiation factors for Semliki Forest virus mRNA. Biochim Biophys Acta. 1984 Jul 18;782(3):242–246. doi: 10.1016/0167-4781(84)90058-7. [DOI] [PubMed] [Google Scholar]
- Sonenberg N. ATP/Mg++-dependent cross-linking of cap binding proteins to the 5' end of eukaryotic mRNA. Nucleic Acids Res. 1981 Apr 10;9(7):1643–1656. doi: 10.1093/nar/9.7.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Morgan M. A., Merrick W. C., Shatkin A. J. A polypeptide in eukaryotic initiation factors that crosslinks specifically to the 5'-terminal cap in mRNA. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4843–4847. doi: 10.1073/pnas.75.10.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Trachsel H., Hecht S., Shatkin A. J. Differential stimulation of capped mRNA translation in vitro by cap binding protein. Nature. 1980 May 29;285(5763):331–333. doi: 10.1038/285331a0. [DOI] [PubMed] [Google Scholar]
- Stahl J., Kobets N. D. Affinity labeling of proteins at the mRNA binding site of rat liver ribosomes by an analogue of octauridylate containing an alkylating group attached to the 3'-end. FEBS Lett. 1981 Jan 26;123(2):269–272. doi: 10.1016/0014-5793(81)80305-5. [DOI] [PubMed] [Google Scholar]
- Sundkvist I. C., Staehelin T. Structure and function of free 40 S ribosome subunits: Characterization of initiation factors. J Mol Biol. 1975 Dec 15;99(3):401–418. doi: 10.1016/s0022-2836(75)80135-5. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Ogata K. Effects of ethionine treatment of protein-synthesizing apparatus of rat liver 80 S ribosomes and 40 S ribosomal subunits. Biochim Biophys Acta. 1982 Apr 26;697(1):101–112. doi: 10.1016/0167-4781(82)90050-1. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Ogata K. Ribosomal proteins cross-linked to natural mRNA by UV irradiation of rat liver polysomes. J Biochem. 1981 Nov;90(5):1549–1552. doi: 10.1093/oxfordjournals.jbchem.a133624. [DOI] [PubMed] [Google Scholar]
- Thomas A., Goumans H., Voorma H. O., Benne R. The mechanism of action of eukaryotic initiation factor 4C in protein synthesis. Eur J Biochem. 1980;107(1):39–45. doi: 10.1111/j.1432-1033.1980.tb04621.x. [DOI] [PubMed] [Google Scholar]
- Thomas A., Spaan W., van Steeg H., Voorma H. O., Benne R. Mode of action of protein synthesis initiation factor eIF-1 from rabbit reticulocytes. FEBS Lett. 1980 Jul 11;116(1):67–71. doi: 10.1016/0014-5793(80)80530-8. [DOI] [PubMed] [Google Scholar]
- Trachsel H., Erni B., Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis. II. The assembly of the initiation complex with purified initiation factors. J Mol Biol. 1977 Nov;116(4):755–767. doi: 10.1016/0022-2836(77)90269-8. [DOI] [PubMed] [Google Scholar]
- Wagenmakers A. J., Reinders R. J., van Venrooij W. J. Cross-linking of mRNA to proteins by irradiation of intact cells with ultraviolet light. Eur J Biochem. 1980 Nov;112(2):323–330. doi: 10.1111/j.1432-1033.1980.tb07207.x. [DOI] [PubMed] [Google Scholar]
- Westermann P., Heumann W., Bommer U. A., Bielka H., Nygard O., Hultin T. Crosslinking of initiation factor eIF-2 to proteins of the small subunit of rat liver ribosomes. FEBS Lett. 1979 Jan 1;97(1):101–104. doi: 10.1016/0014-5793(79)80061-7. [DOI] [PubMed] [Google Scholar]
- Westermann P., Nygård O., Bielka H. Cross-linking of Met-tRNAf to eIF-2 beta and to the ribosomal proteins S3a and S6 within the eukaryotic inhibition complex, eIF-2 .GMPPCP.Met-tRNAf.small ribosomal subunit. Nucleic Acids Res. 1981 May 25;9(10):2387–2396. doi: 10.1093/nar/9.10.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann P., Nygård O. The spatial arrangement of the complex between eukaryotic initiation factor eIF-3 and 40 S ribosomal subunit. Cross-linking between factor and ribosomal proteins. Biochim Biophys Acta. 1983 Oct 13;741(1):103–108. doi: 10.1016/0167-4781(83)90015-5. [DOI] [PubMed] [Google Scholar]
- Wieringa B., van der Zwaag-Gerritsen J., Mulder J., Ab G., Gruber M. Translation in vivo and in vitro of mRNAs coding for vitellogenin, serum albumin and very-low-density lipoprotein II from chicken liver. A difference in translational efficiency. Eur J Biochem. 1981 Mar;114(3):635–641. doi: 10.1111/j.1432-1033.1981.tb05191.x. [DOI] [PubMed] [Google Scholar]
- Yokoe S., Tanaka M., Hibasami H., Nagai J., Nakashima K. Cross-linking of tobacco mosaic virus RNA and capped polyribonucleotides to 18S rRNA in wheat germ ribosome-mRNA complexes. J Biochem. 1983 Dec;94(6):1803–1808. doi: 10.1093/oxfordjournals.jbchem.a134532. [DOI] [PubMed] [Google Scholar]