Abstract
Hybrid selection translation experiments have been carried out with genomic and cDNA relatives of two repetitive sequence families. On the basis of the in vitro translation products detected, it was found that transcripts complementary to these repeats are linked to several different mature mRNAs in stage 40 embryos of Xenopus laevis. One repeat hybridizes to mRNAs that direct the synthesis of 17 proteins. The second is present on mRNAs coding for 3 proteins. By estimating the abundance of these proteins among the translation products of total embryonic mRNA, it is inferred that all of the repeat bearing mRNAs are rare, less than one in 20,000 mRNA molecules.
Full text
PDF





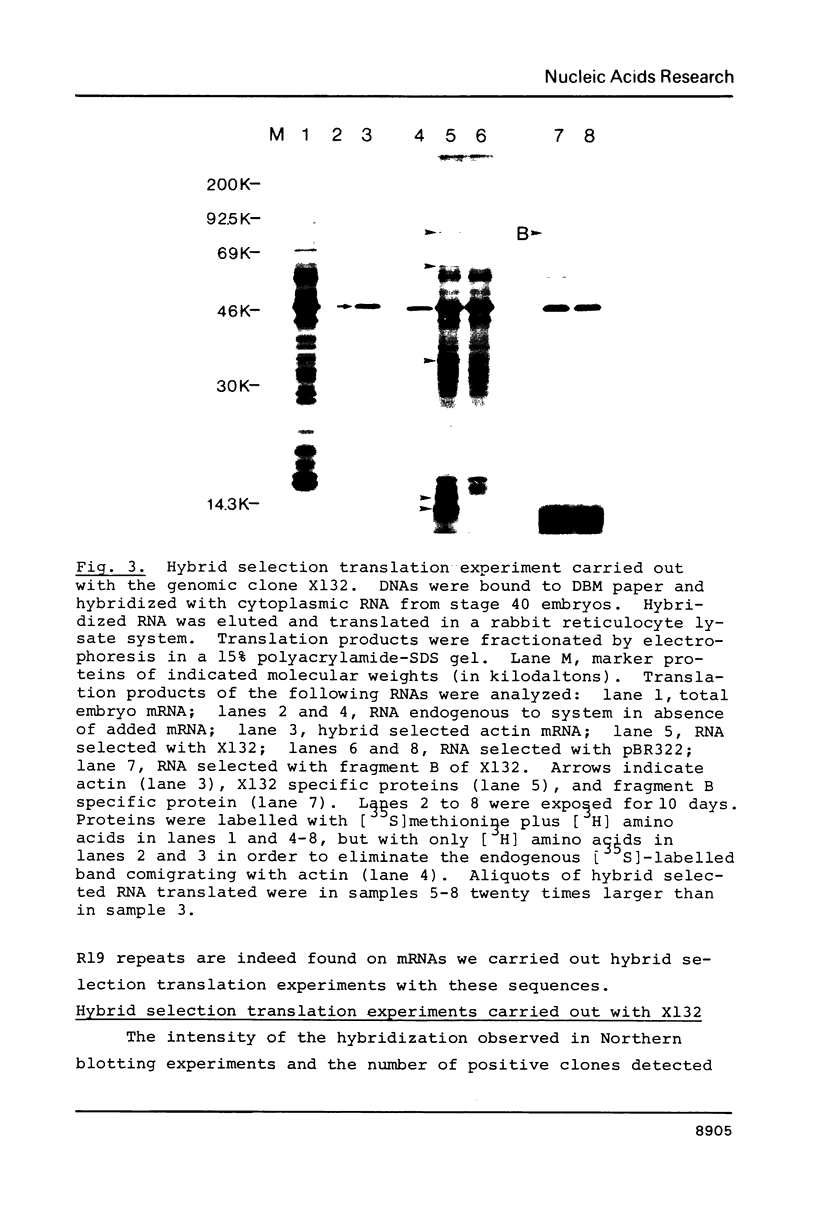











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Parker B. A., Reiser J., Renart J., Stark G. R., Wahl G. M. Detection of specific RNAs or specific fragments of DNA by fractionation in gels and transfer to diazobenzyloxymethyl paper. Methods Enzymol. 1979;68:220–242. doi: 10.1016/0076-6879(79)68017-5. [DOI] [PubMed] [Google Scholar]
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. M., Richter J. D., Chamberlin M. E., Price D. H., Britten R. J., Smith L. D., Davidson E. H. Sequence organization of the poly(A) RNA synthesized and accumulated in lampbrush chromosome stage Xenopus laevis oocytes. J Mol Biol. 1982 Mar 5;155(3):281–309. doi: 10.1016/0022-2836(82)90006-7. [DOI] [PubMed] [Google Scholar]
- Anderson D. M., Scheller R. H., Posakony J. W., McAllister L. B., Trabert S. G., Beall C., Britten R. J., Davidson E. H. Repetitive sequences of the sea urchin genome. Distribution of members of specific repetitive families. J Mol Biol. 1981 Jan 5;145(1):5–28. doi: 10.1016/0022-2836(81)90332-6. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN D. D., LITTNA E. RNA SYNTHESIS DURING THE DEVELOPMENT OF XENOPUS LAEVIS, THE SOUTH AFRICAN CLAWED TOAD. J Mol Biol. 1964 May;8:669–687. doi: 10.1016/s0022-2836(64)80116-9. [DOI] [PubMed] [Google Scholar]
- Barnett T., Pachl C., Gergen J. P., Wensink P. C. The isolation and characterization of Drosophila yolk protein genes. Cell. 1980 Oct;21(3):729–738. doi: 10.1016/0092-8674(80)90436-5. [DOI] [PubMed] [Google Scholar]
- Bozzoni I., Tognoni A., Pierandrei-Amaldi P., Beccari E., Buongiorno-Nardelli M., Amaldi F. Isolation and structural analysis of ribosomal protein genes in Xenopus laevis. Homology between sequences present in the gene and in several different messenger RNAs. J Mol Biol. 1982 Nov 5;161(3):353–371. doi: 10.1016/0022-2836(82)90244-3. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Davidson E. H. Gene regulation for higher cells: a theory. Science. 1969 Jul 25;165(3891):349–357. doi: 10.1126/science.165.3891.349. [DOI] [PubMed] [Google Scholar]
- Carpenter C. D., Bruskin A. M., Hardin P. E., Keast M. J., Anstrom J., Tyner A. L., Brandhorst B. P., Klein W. H. Novel proteins belonging to the troponin C superfamily are encoded by a set of mRNAs in sea urchin embryos. Cell. 1984 Mar;36(3):663–671. doi: 10.1016/0092-8674(84)90346-5. [DOI] [PubMed] [Google Scholar]
- Carpenter C. D., Bruskin A. M., Spain L. M., Eldon E. D., Klein W. H. The 3' untranslated regions of two related mRNAs contain an element highly repeated in the sea urchin genome. Nucleic Acids Res. 1982 Dec 11;10(23):7829–7842. doi: 10.1093/nar/10.23.7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S., Zuker C., Lodish H. F. A repetitive and apparently transposable DNA sequence in Dictyostelium discoideum associated with developmentally regulated RNAs. Nucleic Acids Res. 1983 Jul 25;11(14):4835–4852. doi: 10.1093/nar/11.14.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini F. D., Britten R. J., Davidson E. H. Message sequences and short repetitive sequences are interspersed in sea urchin egg poly(A)+ RNAs. Nature. 1980 Sep 11;287(5778):111–117. doi: 10.1038/287111a0. [DOI] [PubMed] [Google Scholar]
- Costantini F. D., Scheller R. H., Britten R. J., Davidson E. H. Repetitive sequence transcripts in the mature sea urchin oocyte. Cell. 1978 Sep;15(1):173–187. doi: 10.1016/0092-8674(78)90093-4. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Britten R. J. Regulation of gene expression: possible role of repetitive sequences. Science. 1979 Jun 8;204(4397):1052–1059. doi: 10.1126/science.451548. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Hough-Evans B. R., Britten R. J. Molecular biology of the sea urchin embryo. Science. 1982 Jul 2;217(4554):17–26. doi: 10.1126/science.6178156. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Posakony J. W. Repetitive sequence transcripts in development. Nature. 1982 Jun 24;297(5868):633–635. doi: 10.1038/297633a0. [DOI] [PubMed] [Google Scholar]
- Dina D., Meza I., Crippa M. Relative positions of the "repetitive", "unique" and poly(A) fragments of mRNA. Nature. 1974 Apr 5;248(448):486–490. doi: 10.1038/248486a0. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Fedoroff N., Wellauer P. K., Wall R. Intermolecular duplexes in heterogeneous nuclear RNA from HeLa cells. Cell. 1977 Apr;10(4):597–610. doi: 10.1016/0092-8674(77)90092-7. [DOI] [PubMed] [Google Scholar]
- Goldberg M. L., Lifton R. P., Stark G. R., Williams J. G. Isolation of specific RNA's using DNA covalently linked to diazobenzyloxymethyl cellulose or paper. Methods Enzymol. 1979;68:206–220. doi: 10.1016/0076-6879(79)68016-3. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Bonner J. Interspersion of repetitive and single-copy sequences in nuclear ribonucleic acid of high molecular weight. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1108–1112. doi: 10.1073/pnas.71.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W. R., Toomey T. P., Leinwand L., Duncan C. H., Biro P. A., Choudary P. V., Weissman S. M., Rubin C. M., Houck C. M., Deininger P. L. Ubiquitous, interspersed repeated sequences in mammalian genomes. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1398–1402. doi: 10.1073/pnas.77.3.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay B. K., Dawid I. B. The 1723 element: a long, homogeneous, highly repeated DNA unit interspersed in the genome of Xenopus laevis. J Mol Biol. 1983 Nov 5;170(3):583–596. doi: 10.1016/s0022-2836(83)80122-3. [DOI] [PubMed] [Google Scholar]
- Kimmel A. R., Firtel R. A. A family of short, interspersed repeat sequences at the 5' end of a set of Dictyostelium single-copy mRNAs. Cell. 1979 Apr;16(4):787–796. doi: 10.1016/0092-8674(79)90094-1. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations close to the AUG initiator codon affect the efficiency of translation of rat preproinsulin in vivo. Nature. 1984 Mar 15;308(5956):241–246. doi: 10.1038/308241a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lam B. S., Carroll D. Tandemly repeated DNA sequences from Xenopus laevis. II. Dispersed clusters of a 388 base-pair repeating unit. J Mol Biol. 1983 Apr 25;165(4):587–597. doi: 10.1016/s0022-2836(83)80268-x. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D., Brickell P. M., Latchman D. S., Willison K., Rigby P. W. Transcripts regulated during normal embryonic development and oncogenic transformation share a repetitive element. Cell. 1983 Dec;35(3 Pt 2):865–871. doi: 10.1016/0092-8674(83)90119-8. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D. Prevention of NH2-terminal acetylation of proteins synthesized in cell-free systems. J Biol Chem. 1977 Dec 25;252(24):8781–8783. [PubMed] [Google Scholar]
- Paterson B. M., Roberts B. E., Kuff E. L. Structural gene identification and mapping by DNA-mRNA hybrid-arrested cell-free translation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4370–4374. doi: 10.1073/pnas.74.10.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S., Rosbash M. Analysis of Xenopus laevis ovary and somatic cell polyadenylated RNA by molecular hybridization. Dev Biol. 1978 Mar;63(1):197–212. doi: 10.1016/0012-1606(78)90125-2. [DOI] [PubMed] [Google Scholar]
- Perry R. P., La Torre J., Kelley D. E., Greenberg J. R. On the lability of poly(A) sequences during extraction of messenger RNA from polyribosomes. Biochim Biophys Acta. 1972 Mar 14;262(2):220–226. doi: 10.1016/0005-2787(72)90236-5. [DOI] [PubMed] [Google Scholar]
- Posakony J. W., Flytzanis C. N., Britten R. J., Davidson E. H. Interspersed sequence organization and developmental representation of cloned poly(A) RNAs from sea urchin eggs. J Mol Biol. 1983 Jun 25;167(2):361–389. doi: 10.1016/s0022-2836(83)80340-4. [DOI] [PubMed] [Google Scholar]
- Posakony J. W., Scheller R. H., Anderson D. M., Britten R. J., Davidson E. H. Repetitive sequences of the sea urchin genome. III. Nucleotide sequences of cloned repeat elements. J Mol Biol. 1981 Jun 15;149(1):41–67. doi: 10.1016/0022-2836(81)90259-x. [DOI] [PubMed] [Google Scholar]
- Scheller R. H., Anderson D. M., Posakony J. W., McAllister L. B., Britten R. J., Davidson E. H. Repetitive sequences of the sea urchin genome. II. Subfamily structure and evolutionary conservation. J Mol Biol. 1981 Jun 15;149(1):15–39. doi: 10.1016/0022-2836(81)90258-8. [DOI] [PubMed] [Google Scholar]
- Scheller R. H., Costantini F. D., Kozlowski M. R., Britten R. J., Davidson E. H. Specific representation of cloned repetitive DNA sequences in sea urchin RNAs. Cell. 1978 Sep;15(1):189–203. doi: 10.1016/0092-8674(78)90094-6. [DOI] [PubMed] [Google Scholar]
- Smith M. J., Hough B. R., Chamberlin M. E., Davidson E. H. Repetitive and non-repetitive sequence in sea urchin heterogeneous nuclear RNA. J Mol Biol. 1974 May 5;85(1):103–126. doi: 10.1016/0022-2836(74)90132-6. [DOI] [PubMed] [Google Scholar]
- Spohr G., Reith W., Crippa M. Structural analysis of a cDNA clone from Xenopus laevis containing a repetitive sequence element. Dev Biol. 1982 Nov;94(1):71–78. doi: 10.1016/0012-1606(82)90069-0. [DOI] [PubMed] [Google Scholar]
- Spohr G., Reith W., Sures I. Organization and sequence analysis of a cluster of repetitive DNA elements from Xenopus laevis. J Mol Biol. 1981 Oct 5;151(4):573–592. doi: 10.1016/0022-2836(81)90424-1. [DOI] [PubMed] [Google Scholar]
- Spohr G., Reymond C., Reith W., Sures I., Crippa M. Structural analysis of repetitive sequence elements transcribed in early development of Xenopus laevis. Mol Biol Rep. 1983 May;9(1-2):33–38. doi: 10.1007/BF00777471. [DOI] [PubMed] [Google Scholar]
- Spradling A., Penman S., Campo M. S., Bishop J. O. Repetitious and unique sequences in the heterogeneous nuclear and cytoplasmic messenger RNA of mammalian and insect cells. Cell. 1974 Sep;3(1):23–30. doi: 10.1016/0092-8674(74)90033-6. [DOI] [PubMed] [Google Scholar]
- Tchurikov N. A., Naumova A. K., Zelentsova E. S., Georgiev G. P. A cloned unique gene of Drosophila melanogaster contains a repetitive 3' exon whose sequence is present at the 3' ends of many different mRNAs. Cell. 1982 Feb;28(2):365–373. doi: 10.1016/0092-8674(82)90354-3. [DOI] [PubMed] [Google Scholar]
- Woodland H. R. Changes in the polysome content of developing Xenopus laevis embryos. Dev Biol. 1974 Sep;40(1):90–101. doi: 10.1016/0012-1606(74)90111-0. [DOI] [PubMed] [Google Scholar]
- Zuker C., Lodish H. F. Repetitive DNA sequences cotranscribed with developmentally regulated Dictyostelium discoideum mRNAs. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5386–5390. doi: 10.1073/pnas.78.9.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]