The language used to characterize the sensory quality of dyspnea has been studied in numerous investigations,1-28 and it is generally accepted that dyspnea comprises a variety of distinct, potentially discriminable unpleasant respiratory sensations that vary in intensity.29 However, only a few clinical studies of dyspnea sensory quality have involved patients with acute or exacerbated chronic dyspnea,13,22,23 only one of which focused on heart failure (HF),22 despite the fact that dyspnea is a major feature of emergency department visits30-33 and hospital admissions33-36 for HF.
Most studies of dyspnea sensory quality have used exploratory data analysis methods, such as open-ended interviews,2,16 cluster analysis,4,5,8,11,25 multidimensional scaling,3,17 or exploratory factor analysis22,23,28,37 to categorize groupings of related sensations. A major limitation of these methods is that they are essentially inductive (ie, descriptive, not hypothesis-driven); hence, there is no guarantee that results will generalize beyond the sample being described. Not surprisingly, results of these studies have varied widely in terms of the number of higher order groupings of descriptors identified and in the number and identity of descriptors said to characterize the various clusters or factors. The clinical usefulness of such findings has been hampered by a lack of replication or validation in independent samples.
With respect to HF, a further limitation of most studies of dyspnea sensory quality has been either an exclusive12-14,23,37 or predominant2,5,8,11,25 focus on patients with chronic respiratory disease. Few studies of dyspnea sensory quality have focused on patients with HF.22,26 Although the need for valid clinical measures of dyspnea in acutely decompensated HF is recognized,35,36,38 so far, only unidimensional measures of dyspnea severity have been studied in that context.31,32,39,40 To date, no clinical studies have tested a specific, hypothesized measurement model of dyspnea sensory quality during acutely exacerbated HF, and no studies have attempted to validate a measurement model derived from patients of one diagnosis in patients of a different diagnosis using confirmatory factor analysis.
The purpose of this study was to validate a 3-factor measurement model of dyspnea sensory quality in adults hospitalized for HF using confirmatory factor analysis (CFA), a hypothesis-driven method in which a prespecified model is tested against data from an independent sample; evidence of good model fit implies that the model is reproducible.41,42 The model was originally derived from an exploratory factor analysis of dyspnea descriptor ratings (Table 1) during emergency department visits for chronic obstructive pulmonary disease (COPD).23 The specified model consisted of 3 sensory quality factors (latent variables, UPPERCASE) characterized by 7 sensory quality descriptors (indicators / items, lowercase italics) with adequate reliability (internal consistency): SMOTHERING-AIR HUNGER (smothering, suffocating, hunger for air; Cronbach’s α = .87); WORK-EFFORT (work, effort; α = .87), TIGHTNESS (tight, constricted; α = .74).23 In addition, exploratory analyses, based on having found 2 additional weaker (less internally consistent) factors pertaining to amount and depth of ventilation in the original COPD study,23 were conducted with additional descriptors to determine whether other sensory quality factors were supported.
TABLE 1. Variable Names and Corresponding Questionnaire Itemsa.
| Variable name | Questionnaire item |
|---|---|
| constrict | My chest feels/felt constricted |
| smother | I feel/felt I am/was smothering |
| notout | My breath does not/did not go out all the way |
| tight | My chest feels/felt tight |
| shallow | My breathing is/was shallow |
| couldnot | I feel/felt that I cannot/could not breathe |
| work | My breathing requires/required work |
| notin | My breath does not/did not go in all the way |
| oob | I feel/felt out of breath |
| hunger | I feel/felt a hunger for air |
| heavy | My breathing is/was heavy |
| effort | My breathing requires/required effort |
| More b | I feel/felt I am/was breathing more |
| rapid | I feel/felt my breathing is/was rapid |
| suffocate | I feel/felt that I am/was suffocating |
| notenough | I cannot/could not get enough air in |
All descriptors scaled from 0-10 (0 = no [absent]; 1 = just barely noticeable; 10 = as intense or severe as that sensation could possibly be). Bold type = descriptors included in 3-factor / 7-indicator measurement model.
Deleted from factor analyses because of questionable item performance (cf. Ref. 23).
Methods
Design and Setting
The study used a prospective, longitudinal correlational design. Participants were adults admitted for treatment of new-onset or decompensated HF to an urban academic medical center or a Department of Veterans Affairs medical center (VAMC) in the southwestern United States. The ethical review bodies of both institutions approved the study, and signed informed consent was obtained from all participants.
Eligibility
Participants were at least 18 years of age, able to speak and understand English, and of sufficient cognitive capacity to give informed consent and participate in questionnaire administration. HF was either the admitting diagnosis or a major focus of inpatient medical management. Reasons for exclusion were admissions for pulmonary embolism, active malignant or metastatic cancer, trauma, pregnancy, or a B-type natriuretic peptide (BNP) level less than 100 pg/ml or N-terminal proBNP (NT proBNP) level less than 300 pg/ml. Patients admitted for ST-elevation myocardial infarction (STEMI) were excluded, as were patients with any other acute coronary syndrome who received percutaneous coronary intervention. In addition, any patients admitted or transferred to the cardiothoracic surgery service (eg, for coronary artery bypass or valve surgery) were excluded.
Sample
A total of 465 adult inpatients with a known history or admitting diagnosis of any type of HF, cardiomyopathy, or fluid overload were assessed for eligibility, of whom 260 (56%) were excluded (Figure 1). The most frequent exclusion was that HF was not a major focus of the hospitalization, based on clinical judgment and discussion between study personnel and members of the cardiology service. The most common excluded diagnoses were dysrhythmias for which the focus of inpatient treatment was primarily rate or rhythm control (Figure 1). Other exclusions were for STEMI (or non-STEMI or unstable angina with percutaneous coronary intervention), syncope, cerebrovascular disease, hypertensive urgency, and noncardiac fluid overload (eg, chronic kidney disease, iatrogenic).
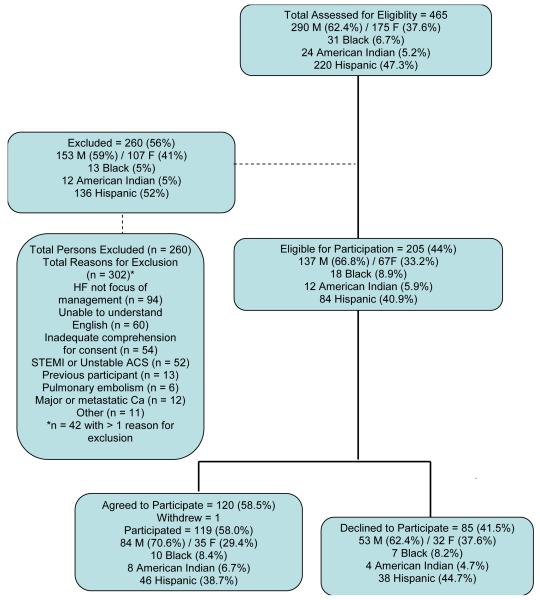
Figure 1.
Assessed for eligibility, exclusions, eligible to participate, and agreed versus declined to participate. M = male; F = female; HF = heart failure; STEMI = ST-elevation myocardial infarction; ACS = acute coronary syndrome; Ca = cancer.
Of the 205 patients deemed eligible for participation (44% of all screened), 120 agreed to participate, one of whom subsequently withdrew, leaving a final sample of 119 (58% of eligible, 25.6% of all screened; Figure 1). Participants were enrolled as soon as possible after admission, consistent with their clinical condition and willingness to participate. The day of enrollment was considered Study Day 1.
Demographic and clinical characteristics of participants are shown in Table 2. There were no significant differences by sex in the relative numbers excluded versus eligible, or participating versus declining to participate. Proportions of Blacks and American Indians were similar across screened, excluded, eligible, participant, and declining groups (Figure 1). Proportions of Hispanics were similar between participants and those declining to participate (P = .471), but because only English-speaking patients were enrolled, a larger proportion of Hispanics was excluded (136/260; 52.3%) than deemed eligible (84/205, 40.9%), χ2 (df = 1) = 5.46, P = .019 (Figure 1). Mean (SD) age of participants was 58.9 (13.7) years and was approximately 6 years younger than those who declined or were excluded (P ≤ .003).
Table 2. Demographic and clinical characteristics (N = 119).
| Sex | n | % |
|---|---|---|
| Male | 84 | 70.6 |
| Female | 35 | 29.4 |
| Ethnicity | ||
| Hispanic | 46 | 38.7 |
| Not Hispanic | 73 | 61.3 |
| Race | ||
| Black | 10 | 8.4 |
| American Indian | 8 | 6.7 |
| White (not Hispanic) | 55 | 46.2 |
| Other admitting diagnoses | ||
| Hypertension | 20 | 16.8 |
| Dysrhythmias | 19 | 16.0 |
| SOB / respiratory distress | 11 | 9.2 |
| COPD | 9 | 7.6 |
| Unstable angina | 7 | 5.9 |
| Pneumonia | 6 | 5.0 |
| NSTEMI | 5 | 4.2 |
| Renal failure | 5 | 4.2 |
| Median | (25th, 75th %ile) | |
|---|---|---|
| LV ejection fraction (%) | 34.5 | (20, 45) |
| BNP (pg/ml) | 1143 | (741, 1,756) |
| NT-proBNP (pg/ml) | 3815 | (2,020, 12,000) |
SOB = shortness of breath; COPD = chronic obstructive pulmonary disease; NSTEMI = non ST-elevation myocardial infarction; LV = left ventricular; BNP B-type natriuretic peptide;, N NT-proBNP = N-terminal prohormone BNP
Measures
The dyspnea questionnaire included 16 sensory quality descriptor items (Table 1). Content validity of these descriptors was initially developed through interviews with healthy subjects exposed to unpleasant respiratory stimuli and patients with cardiopulmonary disease.4,5 In subsequent studies, content validity has been supported by frequency of endorsement from patients of various diagnoses5,11,13,22,23,25,26 and assessment of open-ended characterizations of dyspnea prior to checklist administration.22,23 In this study, participants were asked to rate each descriptor (sensation) as absent (no) or present (yes) and then to rate the intensity for any yes response (0 = no [absent]; 1 = just barely noticeable; 10 = as intense or severe as possible).22,23
Participants completed the dyspnea questionnaire in a today version on Study Day 1 (N = 119) and up to 2 consecutive days thereafter (Study Days 2 and 3, n = 114 and 98, respectively). After enrollment of 22 participants, a protocol amendment was approved to add a recall version of the questionnaire pertaining to the day of admission (Study Day 0; n = 97) because it was evident that participants had already experienced very substantial dyspnea relief by the time of enrollment. The Day 0 questionnaire was administered on Study Day 1, immediately following the Day 1 version.
Statistical Analysis
Descriptive analyses included frequencies and percentages for nominal categorical data and estimates of central tendency and dispersion appropriate to the level of measurement and distribution of continuous variables. We used χ2 tests to assess differences in proportions and Wilcoxon signed rank tests for paired comparisons of continuous variables. The criterion for statistical significance was P < .05.
Psychometric analysis included inter-item correlations and Cronbach’s α for internal consistency of scaled questionnaire scores. CFA was conducted with Mplus, version 4.1 (Muthén & Muthén, Los Angeles, CA),43 using a maximum likelihood χ2 estimator with adjusted means and variances (MLMV). The MLMV estimator is robust to non-normally distributed data and, for small samples, provides more appropriate standard error estimates, global and local tests of model fit, and parameter estimates than conventional maximum likelihood estimation.44 In CFA, the overall model χ2 test (in this case, the MLMV χ2 test) is a goodness of fit test for which the null hypothesis is that the model fits the data (ie, P > .05 indicates good fit to the data). Additional indices of model fit included: relative χ2 (ie, the model χ2 divided by its degrees of freedom [df]), the comparative fit index (CFI) and non-normed fit index (NNFI), and the standardized root mean square residual (SRMR) and root mean square error of approximation (RMSEA). Criteria for good model fit include a relative χ2 < 3.0,45 CFI and NNFI > 0.95,45,46 SRMR < 0.08,46,47 and RMSEA as small as possible: values < .05 indicate good fit, ≤ .08 close fit, and > .10 poor fit.42,45-48
We tested the 3-factor measurement model and several alternative models of up to 5-factors. Model testing used the Study Day 1 and Day 0 questionnaires only, because a majority of participants gave intensity ratings of zero to most descriptors by Study Day 2. Because the original COPD study23 used a different estimation method (exploratory principal axis factor analysis with varimax rotation), those results were not directly comparable to the results of the present study. Therefore, we also reanalyzed, and report here for the first time, a secondary analysis of the original COPD data using the MLMV estimator.
Results
The median and modal hospital day on Study Day 1 was hospital day 1 (25th to 75th %ile: hospital day 1 to 2; range: hospital day 1 to 5). Cumulatively, 24% of the sample (n = 29) were enrolled on the day of admission, 72% (n = 86) by hospital day 1, 87% by hospital day 2 (n = 104), and 96% (n = 114) by hospital day 3. Only 5 participants (4%) were enrolled after hospital day 3 (4 on hospital day 4 and 1 on hospital day 5). All descriptors were endorsed by at least 60% of participants for Day 0 (Figure 2a) For each of the descriptors, the Day 1 scores were significantly lower than Day 0 scores (Wilcoxon signed rank test, |z| > 6.5, P < .0001 for all comparisons; Figure 2b).
Figure 2.
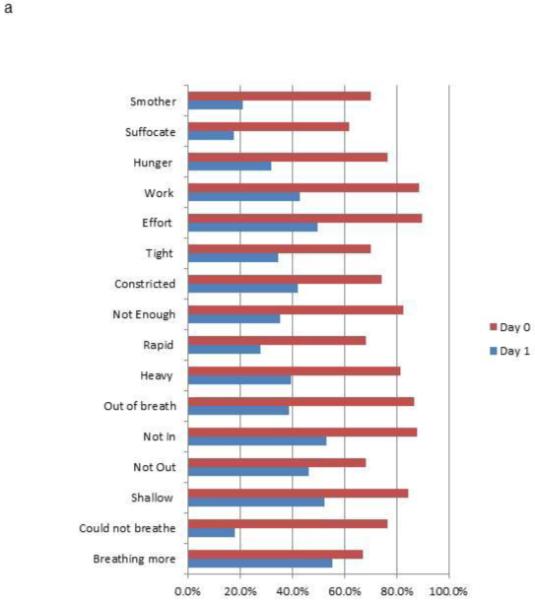
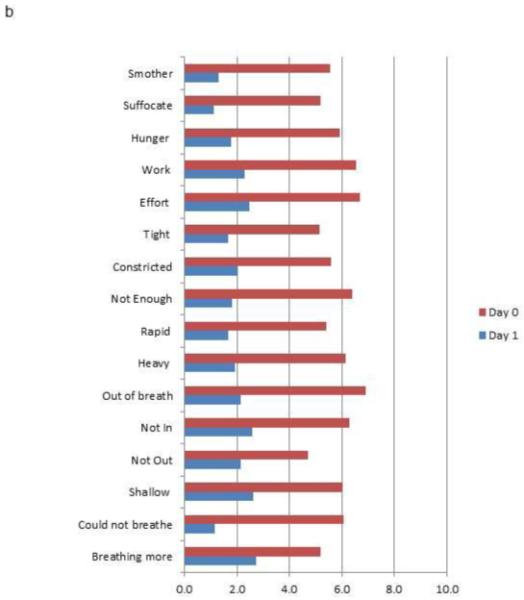
Dyspnea descriptor percent endorsement and mean ratings for Study Day 1 and Study Day 0. (2A) Percentage of participants endorsing dyspnea descriptors (rating > 0); (2B) Mean intensity ratings for dyspnea descriptors (All Day 0 to Day 1 comparisons, |z| > 6.5, P < .0001, paired Wilcoxon test)
CFA of the 3-Factor / 7-Indicator Model
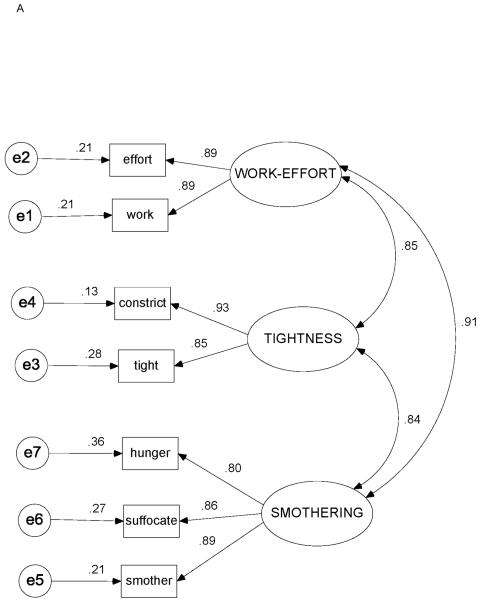
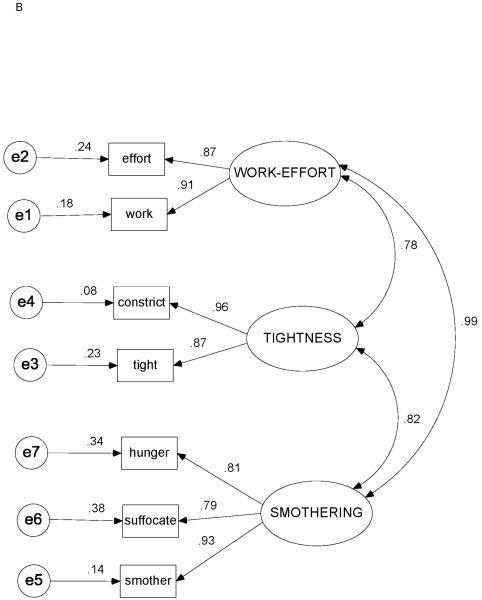
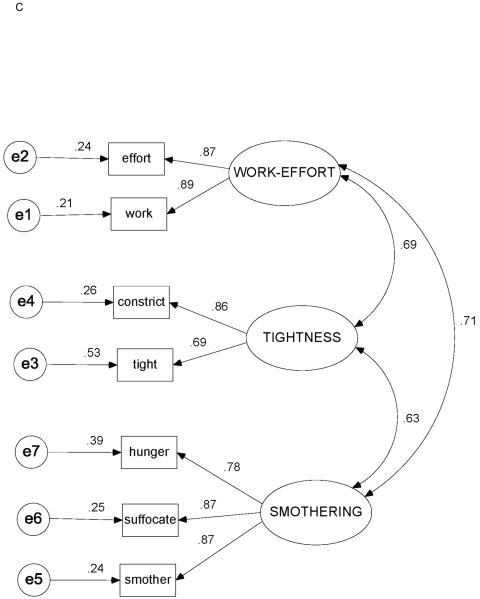
Correlation matrices with item means and standard deviations for Study Day 1 and Study Day 0 are shown in Tables 3 and 4. Figures 3A-3C depict the factor loadings (standardized partial regression coefficients), interfactor correlations, and standardized residual (error) estimates for Study Day 1, Study Day 0, and the original COPD study data,23 respectively.
TABLE 3. Correlation Matrix, Means, and SDs for Study Day 1a (N = 119).
| constrict | smother | tight | work | hunger | effort | suffocate | |
|---|---|---|---|---|---|---|---|
| smother | .682 | ||||||
| tight | .789 | .604 | |||||
| work | .722 | .723 | .612 | ||||
| hunger | .662 | .697 | .717 | .630 | |||
| effort | .700 | .693 | .650 | .787 | .696 | ||
| suffocate | .645 | .789 | .579 | .693 | .646 | .701 | |
|
| |||||||
| Mean | 2.03 | 1.29 | 1.68 | 2.29 | 1.77 | 2.49 | 1.11 |
| SD | 2.88 | 2.91 | 2.73 | 3.16 | 3.14 | 3.24 | 2.77 |
Study Day 1 = day of enrollment in study.
P < .001 for all correlation coefficients
TABLE 4. Correlation Matrix, Means, and SDs for Study Day 0a (N = 97).
| constrict | smother | tight | work | hunger | effort | suffocate | |
|---|---|---|---|---|---|---|---|
| smother | .726 | ||||||
| tight | .841 | .626 | |||||
| work | .670 | .839 | .639 | ||||
| hunger | .671 | .720 | .711 | .726 | |||
| effort | .665 | .819 | .595 | .792 | .691 | ||
| suffocate | .603 | .743 | .521 | .701 | .707 | .642 | |
|
| |||||||
| Mean | 5.59 | 5.57 | 5.15 | 6.53 | 5.91 | 6.70 | 5.17 |
| SD | 3.86 | 4.04 | 3.87 | 3.26 | 3.87 | 3.18 | 4.29 |
Study Day 0 = day admitted to hospital (recall rating).
P < .001 for all correlation coefficients
Figure 3.
Standardized estimates for 3-factor / 7-indicator model. (3A) Heart failure, Study Day 1 (N = 119). (3B) Heart failure, Study Day 0 (n = 97). (3C) Chronic obstructive pulmonary disease (data from ref. 23; N = 100). Ovals = factors (latent variables); rectangles = indicators (measured variables, descriptors); circles = unique measurement error; capital letters = factor names; SMOTHERING = SMOTHERING-AIR HUNGER factor; lowercase letters = variable (descriptor) names (see Table 1 for complete items); double-headed curved arrows = correlations between factors; single-headed arrows from factors to indicators = factor loadings (standardized partial regression coefficients, ie, β-weights); single-headed arrows from errors to indicators = standardized residuals.
The 3-factor / 7-indicator model fit the data for both Study Day 0 and Study Day 1, as well as for the reanalyzed COPD data (P > .10 for all). All fit indices were in satisfactory ranges (Table 5). Relative χ2 values for each data set were all ≤ 1.7, and all SRMR and RMSEA values were ≤ .08.
TABLE 5. Three-Factor / 7-Indicator Measurement Model: CFA χ2 Statistics and Fit Indices.
| Diagnosis | Study Day |
n | Model χ2 |
dfb | p | Relative χ2 |
CFI | NNFI | SRMR | RMSEA |
|---|---|---|---|---|---|---|---|---|---|---|
| HF | 1 | 119 | 7.91 | 7 | 0.34 | 1.13 | 0.99 | 0.99 | 0.03 | 0.03 |
| HF | 0 | 97 | 9.92 | 6 | 0.13 | 1.65 | 0.98 | 0.97 | 0.03 | 0.08 |
| COPDa | ED | 100 | 10.00 | 6 | 0.12 | 1.67 | 0.96 | 0.94 | 0.05 | 0.08 |
CFA, confirmatory factor analysis; HF, heart failure; COPD, chronic obstructive pulmonary disease; ED, emergency department; Study Day 1, dyspnea ratings on day of HF study enrollment; Study Day 0, recall dyspnea rating for day of admission obtained on Study Day 1; df, degrees of freedom for model χ2; relative χ2, model χ2/df; CFI, comparative fit index; NNFI, non-normed fit index; RMSEA, root mean square error of approximation.
Data from ref 23.
The df for the maximum likelihood mean- and variance-adjusted (MLMV) χ2 are estimated and rounded to the nearest integer; hence, the df for the same model can vary.
Cronbach’s alpha coefficients for the 7 items on Study Day 1 and Day 0 were > .93 and for each factor were all > .87 for both Study Day 1 and Day 0. For both Study Day 1 and Day 0, all estimated factor loadings (Table 6), covariances (Table 7), and variances (Table 8) were statistically significant (P ≤ 0.003). For comparison, the corresponding MLMV parameter estimates from the reanalyzed COPD data are included in Tables 6, 7, and 8.
TABLE 6. Regression Weights and Standard Errors for 3-Factor / 7-Indicator Model in Patients with Heart Failure and Chronic Obstructive Pulmonary Disease.
| HF: Study Day 1 (N = 119) |
HF: Study Day 0 (n = 97) |
COPD (n = 100)a |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item → FACTOR | B | SE | CRb | β | B | SE | CRb | β | B | SE | CRb | β |
| work → EFFORT | c | d | d | 0.89 | c | d | d | 0.91 | c | d | d | 0.89 |
| effort → EFFORT | 1.03 | 0.06 | 18.14 | 0.89 | 0.94 | 0.09 | 10.67 | 0.87 | 0.90 | 0.09 | 10.26 | 0.87 |
| tight → TIGHTNESS | c | d | d | 0.85 | c | d | d | 0.88 | c | d | d | 0.69 |
| constrict → TIGHTNESS | 1.16 | 0.14 | 8.03 | 0.93 | 1.10 | 0.09 | 12.40 | 0.96 | 1.31 | 0.20 | 6.58 | 0.86 |
| smother → SMOTHER | c | d | d | 0.89 | c | d | d | 0.93 | c | d | d | 0.87 |
| suffocate → SMOTHER | 0.92 | 0.07 | 12.61 | 0.86 | 0.90 | 0.07 | 12.92 | 0.79 | 1.15 | 0.10 | 11.69 | 0.87 |
| hunger → SMOTHER | 0.97 | 0.10 | 9.56 | 0.80 | 0.84 | 0.07 | 12.90 | 0.81 | 0.90 | 0.10 | 9.26 | 0.78 |
HF, heart failure; COPD, chronic obstructive pulmonary disease; Study Day 1, day enrolled in study (today rating); Study Day 0, day of admission (recall rating, obtained immediately after Study Day 1 rating); B, unstandardized regression weights; SE, standard error; CR, critical ratio (B/SE); β, standardized regression (beta-) weights.
Data from ref 23.
P < 0.0001 for all estimates.
Scaling constant (one constant required per factor; value set to 1.0).
Not estimated.
TABLE 7. Inter-Factor Covariances, Standard Error Estimates, Critical Ratios, and Correlations for 3-Factor / 7-Indicator Model in Patients with Heart Failure and Chronic Obstructive Pulmonary Disease.
| HF: Study Day 1 (N = 119) |
HF: Study Day 0 (n = 97) |
COPD (n = 100)a |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor covariances | COV | SE | CRb | r | COV | SE | CRb | r | COV | SE | CRc | r |
| EFFORT ↔ SMOTHERING | 6.56 | 1.44 | 4.56 | 0.91 | 10.86 | 1.11 | 9.76 | 0.99 | 5.13 | 1.26 | 4.07 | 0.71 |
| EFFORT↔TIGHTNESS | 5.49 | 1.08 | 5.09 | 0.85 | 7.75 | 1.22 | 6.35 | 0.78 | 4.28 | 0.97 | 4.42 | 0.69 |
| SMOTHERING ↔ TIGHTNESS | 4.99 | 1.20 | 4.15 | 0.84 | 10.29 | 1.30 | 7.90 | 0.82 | 4.57 | 1.16 | 3.92 | 0.63 |
HF, heart failure; COPD, chronic obstructive pulmonary disease; Study Day 1, day enrolled in study (today rating); Study Day 0, day of admission (recall rating, obtained immediately after Study Day 1 rating); COV, unstandardized covariance estimate between factors; SE, standard error, CR, critical ratio (COV/SE, t-statistic); P, P value for t-statistic with df, n-1; r, interfactor correlation (standardized covariance estimate).
Data from ref 23.
P< .0001 for all estimates.
P < .0002 for all estimates.
TABLE 8. Factor and Residual (Error) Variances, Standard Errors, Critical Ratios, and Standardized Estimates for 3-Factor / 7-Indicator Model in Patients with Heart Failure and Chronic Obstructive Pulmonary Disease.
| HF: Study Day 1 (N = 119) |
HF: Study Day 0 (n = 97) |
COPD (n = 100)a |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factors | VAR | SE | CR | P | Stdb | VAR | SE | CR | P | Stdb | VAR | SE | CR | P | Stdb |
| EFFORT | 7.77 | 1.37 | 5.68 | c | 8.63 | 1.36 | 6.35 | c | 6.19 | 1.59 | 3.90 | c | |||
| TIGHTNESS | 5.32 | 1.27 | 4.19 | c | 11.39 | 1.68 | 6.76 | c | 6.19 | 1.66 | 3.74 | c | |||
| SMOTHERING | 6.65 | 1.77 | 3.77 | c | 13.87 | 1.32 | 10.54 | c | 8.47 | 1.72 | 4.93 | c | |||
| Residuals | VAR | SE | CR | P | SR | VAR | SE | CR | P | SR | VAR | SE | CR | P | SR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| work | 2.11 | 0.54 | 3.93 | c | 0.21 | 1.86 | 0.64 | 2.92 | c | 0.18 | 1.64 | 0.83 | 1.99 | .05 | 0.21 |
| effort | 2.19 | 0.66 | 3.32 | c | 0.21 | 2.37 | 0.66 | 3.62 | c | 0.24 | 1.59 | 0.46 | 3.46 | c | 0.24 |
| tight | 2.09 | 0.45 | 4.67 | c | 0.28 | 3.47 | 1.15 | 3.03 | c | 0.23 | 6.86 | 1.64 | 4.17 | c | 0.53 |
| constrict | 1.09 | 0.49 | 2.22 | c | 0.13 | 1.12 | 0.52 | 2.14 | c | 0.08 | 3.78 | 1.53 | 2.48 | .02 | 0.26 |
| smother | 1.77 | 0.57 | 3.09 | c | 0.21 | 2.29 | 0.66 | 3.50 | c | 0.14 | 2.76 | 1.08 | 2.54 | .01 | 0.25 |
| suffocate | 2.03 | 0.62 | 3.27 | c | 0.27 | 6.93 | 1.48 | 4.67 | c | 0.38 | 3.66 | 1.14 | 3.22 | c | 0.25 |
| hunger | 3.55 | 0.87 | 4.11 | c | 0.36 | 5.02 | 0.78 | 6.42 | c | 0.34 | 4.32 | 1.24 | 3.50 | c | 0.39 |
HF, heart failure; COPD, chronic obstructive pulmonary disease; Study Day 1, day enrolled in study (today rating); Study Day 0, day of admission (recall rating, obtained immediately after Study Day 1 rating); VAR, variance estimate; SE, standard error, CR, critical ratio (VAR/SE); P, P value for CR (t-statistic) with df, n-1; Std, standardized variance; SR, standardized residual.
Data from ref 23.
Value standardized to 1.0 (ie, not estimated).
P ≤.004
The WORK-EFFORT factor showed the greatest similarity across study days and diagnoses in terms of factor loadings (Figures 3A-3C; Table 6), as well as variance and standard error estimates (Table 8). For the other 2 factors, variance estimates, though not standard errors, were substantially larger for Study Day 0 compared with Study Day 1 and the COPD data (Table 8), and factor loadings were somewhat more variable across study days (Table 6; Figures 3A-3B) and diagnoses (Table 6; Figure 3C).
The most notable difference in the model across diagnoses was the substantially stronger interfactor correlations for HF (r > .78) compared with COPD (r < .71; Table 7). For Study Day 0 and the COPD study data, factor variances from largest to smallest were SMOTHERING-AIR HUNGER > TIGHTNESS > WORK-EFFORT. For Study Day 1, factor variances from largest to smallest were WORK-EFFORT > SMOTHERING-AIR HUNGER > TIGHTNESS (Table 8).
Exploratory Analyses of Alternative Factor Models
We conducted exploratory analyses to determine whether a model with 4 or 5 factors fit the HF data. Initial specification of a 5-factor model with 14 descriptors (Table 2) was: AMOUNT (heavy, rapid, not out all the way); DEPTH (not enough air in, shallow); TIGHTNESS (tight, constricted); and augmented SMOTHERING-AIR HUNGER (smothering, suffocating, hunger for air, could not breathe) and WORK-EFFORT (work, effort, out of breath) factors.23 This model fit the original COPD data, as would be expected, but did not fit the HF data for Study Day 0 or Study Day 1.
We combined the putative AMOUNT and DEPTH of ventilation factors, above, into a single factor pertaining to awareness of ventilation. We examined several alternate specifications for this 4-factor model. We found a 4-factor / 15-indicator model (VENTILATION: heavy, rapid, not out all the way, not in all the way, shallow; SMOTHERING-AIR HUNGER: smothering, suffocating, hunger for air, could not breathe, not enough air in; and the same WORK-EFFORT and TIGHTNESS factors as in the preceding paragraph) demonstrated adequate fit to the Study Day 0 data, but fit poorly to the Study Day 1 data.
Because the WORK-EFFORT and SMOTHERING-AIR HUNGER factors were highly correlated in the HF study data (especially for Study Day 0), we also conducted an unplanned exploratory analysis of a 2-factor / 7-descriptor model consisting of the 2-descriptor TIGHTNESS factor (tight, constricted) while combining the WORK-EFFORT and SMOTHERING-AIR HUNGER factors into a single factor (smothering, suffocating, hunger for air, work, effort). The fit of this 2-factor model was comparable to the 3-factor model for the HF Study Day 0 and Study Day 1 data, but the fit to the COPD study data was markedly worse. A single-factor model with all 7 descriptors fit the data very poorly across the HF Study Day 1 and Study Day 0 and the COPD data.
Discussion
Overall, the same 3-factor / 7-indicator measurement model fit the Study Day 1 and Day 0 data and the COPD data very satisfactorily and substantially better than any alternative model. All 3 factors had strong internal consistency reliability regardless of diagnosis or study day. To our knowledge, this is the first time that a specific measurement model of dyspnea sensory quality has been validated in an independent sample using CFA methods (which supports the internal validity of the 3-factor measurement model), and it also is the first time a model derived in patients of one diagnosis has been validated in patients of another diagnosis (which supports its external validity). External validity of the measurement model is further supported by replication of the WORK-EFFORT, SMOTHERING-AIR HUNGER, and TIGHTNESS factors in an independent sample41,42,45 among patients in a different geographic region than the original study. In addition, robustness of fit of the 3-factor model over successive days characterized by very substantial change in ratings supports its construct validity.
We considered whether coexisting HF and COPD might be a possible explanation for the model fitting data from patients of either diagnosis. However, we believe this is unlikely because less than 10% of our HF sample had a diagnosis of COPD, and, in all cases, we confirmed that HF was the diagnosis driving the admission. In the earlier study, only 15% of participants had a history of HF in addition to COPD, but unless they were being treated with corticosteroids or beta-agonists (neither of which is indicated in HF), patients with HF were excluded.
As with any correlational method, CFA does not establish a causal basis for observed associations. Distinct sensory-perceptual mechanisms underlying effort perception, tightness, and air hunger are supported by substantial experimental and neuroimaging evidence.1,6,7,9,10,12,14,18-21,24,27,49-59 However, it would not be valid to infer that there are only three sensory quality factors or that only these three matter to patients with acute or exacerbated cardiopulmonary disease. The findings of this study also do not rule out the possibility that there may be other sensory quality factors that may be more diagnosis-specific. Rather, the 3-factor / 7-descriptor model represents sensory quality factors that can be reliably measured with relatively few items, at least for patients with exacerbated HF or COPD. Further research would be required to determine how well this measurement model fits other diagnoses.
A number of earlier studies focused on the possibility that certain combinations of sensory descriptor clusters might better characterize a particular diagnosis versus others and therefore might be useful in differential diagnosis.2,5,8,11 However, at least one study concluded that such clusters of descriptors may not be sufficiently specific for that purpose.25 Regardless of diagnosis, the number of afferent pathways and subcortical to cortical brain regions involved in processing and awareness of respiratory sensations is finite.60-65 Which stimuli and which pathways predominate at any given time may differ between diagnoses or even within diagnoses as clinical condition changes, but there is no compelling evidence that afferent or higher central nervous system pathways differ by diagnosis in patients with cardiopulmonary disease.66-71
Although few, if any, participants in the present study or the earlier COPD study spontaneously reported “air hunger” in open-ended statements prior to seeing the descriptor questionnaire, many patients in both samples endorsed the statement “I feel [or felt] a hunger for air” on the questionnaire, and ratings for that item correlated more strongly and consistently with ratings for smothering and suffocating than with the other descriptors. Numerous physiological investigations have focused on the sensation of “air hunger” in relation to increases in CO2-mediated reflex inspiratory drive,6,7,18,49-52,54,59 or, more generally, when inspiratory drive exceeds a constrained ventilatory capacity.1,15,21,53,56,57 Few participants in either the HF or the COPD study had arterial blood gas measurements, and, of those who did, only a small number had elevated Pco2. Therefore, it seems likely that the confluence of ratings of air hunger, smothering, and suffocating in both samples was due to factors other than increased Pco2.
Limitations of the present study include convenience sampling, a preponderance of male participants, and a relatively small sample size for CFA.45 In addition, there was a lack of direct correspondence between study day and hospital day, and, in consequence, a variable recall interval for the day 0 ratings.
Convenience sampling is difficult to avoid in a clinical study with prospective data collection in an acute care setting. Recruitment was necessarily contingent on clinical condition and confirmation that HF was, indeed, a major focus of inpatient care. In addition, participation necessarily was limited to those who were willing to participate in an observational study that did not offer any prospect of direct clinical benefit. Although our sample was disproportionately male, there were no statistically significant differences by sex between those excluded versus eligible, nor, among the eligible, between those agreeing versus those declining to participate. Excluding non-English speakers was a limitation, but, even so, nearly 40% of the sample was Hispanic.
The possible impact of the relatively small sample and non-normally distributed data on statistical analyses was mitigated by using a robust estimator appropriate for small samples in which multivariate normality cannot be assumed. We preferred this approach to statistical transformations of variables because transformation is generally ineffective when scores are constrained to a narrow range,72,73 and the model was robust to substantial differences in item scores and factor variances as well as the reversal in the direction of skewness from Study Day 0 to Study Day 1).
Despite the lack of one-to-one correspondence between study day and hospital day, the recall interval, though not standardized, was relatively short. In the original COPD study, test-retest reliability of recall descriptor ratings during an ED visit was strong.23 In the present study, the changes in descriptor means and in the skewness of ratings between Day 0 and Day 1 reflect expected characteristics of score distributions in relation to aggressive, inpatient treatment over a relatively short interval.
Lastly, it is important to note that dyspnea encompasses multiple sensory qualities that vary not only in intensity,29 but in their unpleasantness,74 and in emotional responses (eg, overall distress and judgments about the seriousness and significance of what is felt).27,37,74-76 Thus, validation of a measurement model of dyspnea sensory quality in conjunction with ratings of descriptor intensity does not, by itself, capture the emotional dimension of the symptom. Research to address that limitation is ongoing.
Even with these limitations, this study demonstrates several strengths beyond using CFA. Confirmation of a diagnosis of HF among participants was essentially 100%. Any exclusions based on judgments that HF was not a major focus of inpatient management were made prospectively, based on clinical information available during rounds and through discussion with members of the cardiology services of the 2 facilities. There were no post hoc exclusions. Moreover, we did not exclude on the basis of presumed mechanism of HF (systolic vs. diastolic dysfunction) or functional consequence (eg, reduced vs. preserved ejection fraction), because HF is a heterogeneous condition, and ejection fraction and the underlying pathophysiology of HF may not always be ascertainable at the time of admission.
Our results have several notable clinical and research implications. First, nearly all currently available dyspnea measures have been developed and tested primarily in ambulatory settings.29,77,78 Therefore, their construct validity is uncertain with respect to patients in an acute care setting. Secondly, many such measures are diagnosis-specific, but in the context of an emergency department visit or hospital admission, the underlying diagnosis is not always immediately known, and chronic pulmonary and cardiac conditions frequently coexist. Our findings suggest that diagnosis-specific measures may not be necessary to reliably assess changes in dyspnea in response to treatment of exacerbated chronic cardiopulmonary disease in acute care settings.
Conclusion
This study demonstrates that intensity ratings for 3 sensory quality factors can be measured consistently and reproducibly with only a few descriptors both across diagnoses and across time points within a diagnosis. Although a single-item rating of intensity or distress has demonstrated responsiveness to clinical change in acute HF syndromes,31,39,40 using a single-item assumes that differences in sensory quality are irrelevant to feeling better or worse. As far as we are aware, such an assumption has never been rigorously tested, and risks underestimating clinically meaningful change or missing it altogether. For example, in asthma sensations of tightness differ mechanistically from sensations of work,20,24 and tightness responds more rapidly than sensations of work or effort to bronchodilator treatment.13 The extent to which different sensory qualities of dyspnea in diseases other than asthma may also have different time-courses of response to acute treatment has not been studied. Whether home-monitoring of sensory qualities of dyspnea might predict deterioration or exacerbation of previously stable HF or COPD has also never been studied. Both of those possibilities are worthwhile areas for future investigation. Lastly, given the heterogeneity of both HF and COPD, it would be reasonable to hypothesize in a future study that this 3-factor measurement model may also fit other diagnoses.
Acknowledgments
Funded by a grant from the National Institute of Nursing Research (1 R15 NR008883). The opinions expressed in this article are the authors’ alone.
The authors thank all the patients who participated or were willing to consider participating in the study, as well as the members of the inpatient cardiology services and the nursing staff of the participating hospitals. The authors are grateful for the assistance of: Jessica Benton, RN, MSN, ACNP-C; Rebecca Mayo, PhD, RN, FNP-C; Eleanor Miranda, RN, MSN, FNP-C; Susie Stokely, RN, MSN, ACNP-C; Julia Silva, RN, BSN, and Vivian Yarmola, RN, MSN. The authors also thank Anne Mattarella of the UNM College of Nursing for editorial assistance with the preparation of this article. The principal investigator wishes to thank Richard Schwartzstein, Robert Banzett, and Paula Meek for critical feedback that strengthened the original research proposal and for ongoing discussions that have contributed to this work. Lastly, the authors wish to thank the anonymous reviewers of an earlier version of this article for their thoughtful and constructive critiques.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement:
The authors declare they have no competing interests with any commercial entity.
References
- 1.Wright GW, Branscomb B. The origin of the sensations of dyspnea? Trans Am Clin Cimatol Assoc. 1955;66:116–125. [PMC free article] [PubMed] [Google Scholar]
- 2.Janson-Bjerklie S, Carrieri VK, Hudes M. The sensations of pulmonary dyspnea. Nurs Res. 1986;35(3):154–159. [PubMed] [Google Scholar]
- 3.Harver A, Baird JC, McGovern JF, Daubenspeck JA. Grouping and multidimensional organization of respiratory sensations. Percept Psychophys. 1988;44(3):285–292. doi: 10.3758/bf03206297. [DOI] [PubMed] [Google Scholar]
- 4.Simon PM, Schwartzstein RM, Weiss JW, et al. Distinguishable sensations of breathlessness induced in normal volunteers. Am Rev Respir Dis. 1989;140(4):1021–1027. doi: 10.1164/ajrccm/140.4.1021. [DOI] [PubMed] [Google Scholar]
- 5.Simon PM, Schwartzstein RM, Weiss JW, Fencl V, Teghtsoonian M, Weinberger SE. Distinguishable types of dyspnea in patients with shortness of breath. Am Rev Respir Dis. 1990;142(5):1009–1014. doi: 10.1164/ajrccm/142.5.1009. [DOI] [PubMed] [Google Scholar]
- 6.Banzett RB, Lansing RW, Reid MB, Adams L, Brown R. ‘Air hunger’ arising from increased Pco2 in mechanically ventilated quadriplegics. Respir Physiol. 1989;76(1):53–67. doi: 10.1016/0034-5687(89)90017-0. [DOI] [PubMed] [Google Scholar]
- 7.Banzett RB, Lansing RW, Brown R, et al. ‘Air hunger’ from increased Pco2 persists after complete neuromuscular block in humans. Respir Physiol. 1990;81(1):1–17. doi: 10.1016/0034-5687(90)90065-7. [DOI] [PubMed] [Google Scholar]
- 8.Elliott MW, Adams L, Cockcroft A, MacRae KD, Murphy K, Guz A. The language of breathlessness. Use of verbal descriptors by patients with cardiopulmonary disease. Am Rev Respir Dis. 1991;144(4):826–832. doi: 10.1164/ajrccm/144.4.826. [DOI] [PubMed] [Google Scholar]
- 9.Shea SA, Andres LP, Shannon DC, Guz A, Banzett RB. Respiratory sensations in subjects who lack a ventilatory response to CO2. Respir Physiol. 1993;93(2):203–219. doi: 10.1016/0034-5687(93)90006-v. [DOI] [PubMed] [Google Scholar]
- 10.Raj H, Singh VK, Anand A, Paintal AS. Sensory origin of lobeline-induced sensations: a correlative study in man and cat. J Physiol (Lond) 1995;482(Pt 1):235–246. doi: 10.1113/jphysiol.1995.sp020513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahler DA, Harver A, Lentine T, Scott JA, Beck K, Schwartzstein RM. Descriptors of breathlessness in cardiorespiratory diseases. Am J Respir Crit Care Med. 1996;154(5):1357–1363. doi: 10.1164/ajrccm.154.5.8912748. [DOI] [PubMed] [Google Scholar]
- 12.O’Donnell DE, Bertley JC, Chau LK, Webb KA. Qualitative aspects of exertional breathlessness in chronic airflow limitation: pathophysiologic mechanisms. Am J Respir Crit Care Med. 1997;155(1):109–115. doi: 10.1164/ajrccm.155.1.9001298. [DOI] [PubMed] [Google Scholar]
- 13.Moy ML, Lantin ML, Harver A, Schwartzstein RM. Language of dyspnea in assessment of patients with acute asthma treated with nebulized albuterol. Am J Respir Crit Care Med. 1998;158(3):749–753. doi: 10.1164/ajrccm.158.3.9707088. [DOI] [PubMed] [Google Scholar]
- 14.O’Donnell DE, Chau LK, Webb KA. Qualitative aspects of exertional dyspnea in patients with interstitial lung disease. J Appl Physiol. 1998;84(6):2000–2009. doi: 10.1152/jappl.1998.84.6.2000. [DOI] [PubMed] [Google Scholar]
- 15.Harty HR, Corfield DR, Schwartzstein RM, Adams L. External thoracic restriction, respiratory sensation, and ventilation during exercise in men. J Appl Physiol. 1999;86(4):1142–1150. doi: 10.1152/jappl.1999.86.4.1142. [DOI] [PubMed] [Google Scholar]
- 16.Hardie GE, Janson S, Gold WM, Carrieri-Kohlman V, Boushey HA. Ethnic differences: word descriptors used by African-American and white asthma patients during induced bronchoconstriction. Chest. 2000;117(4):935–943. doi: 10.1378/chest.117.4.935. [DOI] [PubMed] [Google Scholar]
- 17.Harver A, Mahler DA, Schwartzstein RM, Baird JC. Descriptors of breathlessness in healthy individuals: distinct and separable constructs. Chest. 2000;118(3):679–690. doi: 10.1378/chest.118.3.679. [DOI] [PubMed] [Google Scholar]
- 18.Lansing RW, Im BS, Thwing JI, Legedza AT, Banzett RB. The perception of respiratory work and effort can be independent of the perception of air hunger. Am J Respir Crit Care Med. 2000;162(5):1690–1696. doi: 10.1164/ajrccm.162.5.9907096. [DOI] [PubMed] [Google Scholar]
- 19.Moosavi SH, Topulos GP, Hafer A, et al. Acute partial paralysis alters perceptions of air hunger, work and effort at constant PCO2 and V □E. Respir Physiol. 2000;122(1):45–60. doi: 10.1016/s0034-5687(00)00135-3. [DOI] [PubMed] [Google Scholar]
- 20.Moy ML, Weiss JW, Sparrow D, Israel E, Schwartzstein RM. Quality of dyspnea in bronchoconstriction differs from external resistive loads. Am J Respir Crit Care Med. 2000;162(2 Pt 1):451–455. doi: 10.1164/ajrccm.162.2.9907138. [DOI] [PubMed] [Google Scholar]
- 21.O’Donnell DE, Hong HH, Webb KA. Respiratory sensation during chest wall restriction and dead space loading in exercising men. J Appl Physiol. 2000;88(5):1859–1869. doi: 10.1152/jappl.2000.88.5.1859. [DOI] [PubMed] [Google Scholar]
- 22.Parshall MB, Welsh JD, Brockopp DY, Heiser RM, Schooler MP, Cassidy KB. Reliability and validity of dyspnea sensory quality descriptors in heart failure patients treated in an emergency department. Heart Lung. 2001;30(1):57–65. doi: 10.1067/mhl.2001.112499. [DOI] [PubMed] [Google Scholar]
- 23.Parshall MB. Psychometric characteristics of dyspnea descriptor ratings in emergency department patients with exacerbated chronic obstructive pulmonary disease. Res Nurs Health. 2002;25(5):331–344. doi: 10.1002/nur.10051. [DOI] [PubMed] [Google Scholar]
- 24.Binks AP, Moosavi SH, Banzett RB, Schwartzstein RM. “Tightness” sensation of asthma does not arise from the work of breathing. Am J Respir Crit Care Med. 2002;165(1):78–82. doi: 10.1164/ajrccm.165.1.2105061. [DOI] [PubMed] [Google Scholar]
- 25.Wilcock A, Crosby V, Hughes A, Fielding K, Corcoran R, Tattersfield AE. Descriptors of breathlessness in patients with cancer and other cardiorespiratory diseases. J Pain Symptom Manage. 2002;23(3):182–189. doi: 10.1016/s0885-3924(01)00417-1. [DOI] [PubMed] [Google Scholar]
- 26.de Souza Caroci A, Lareau SC. Descriptors of dyspnea by patients with chronic obstructive pulmonary disease versus congestive heart failure. Heart Lung. 2004;33(2):102–110. doi: 10.1016/j.hrtlng.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Banzett RB, Pedersen SH, Schwartzstein RM, Lansing RW. The affective dimension of laboratory dyspnea: air hunger Is more unpleasant than work/effort. Am J Respir Crit Care Med. 2008;177(12):1384–1390. doi: 10.1164/rccm.200711-1675OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han J, Zhu Y, Li S, et al. The language of medically unexplained dyspnea. Chest. 2008;133(4):961–968. doi: 10.1378/chest.07-2179. [DOI] [PubMed] [Google Scholar]
- 29.American Thoracic Society Dyspnea. Mechanisms, assessment, and management: a consensus statement. Am J Respir Crit Care Med. 1999;159(1):321–340. doi: 10.1164/ajrccm.159.1.ats898. [DOI] [PubMed] [Google Scholar]
- 30.Parshall MB. Adult emergency visits for chronic cardiorespiratory disease: does dyspnea matter? Nurs Res. 1999;48(2):62–70. doi: 10.1097/00006199-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Ander DS, Aisiku IP, Ratcliff JJ, Todd KH, Gotsch K. Measuring the dyspnea of decompensated heart failure with a visual analog scale: how much improvement Is meaningful? Congest Heart Fail. 2004;10(4):188–191. doi: 10.1111/j.1527-5299.2004.03475.x. [DOI] [PubMed] [Google Scholar]
- 32.Smithline HA, Caglar S, Blank FSJ. Physician vs patient assessment of dyspnea during acute decompensated heart failure. Congest Heart Fail. 2010;16(2):60–64. doi: 10.1111/j.1751-7133.2009.00127.x. [DOI] [PubMed] [Google Scholar]
- 33.Weintraub NL, Collins SP, Pang PS, et al. Acute heart failure syndromes: emergency department presentation, treatment, and disposition: current approaches and future aims: a scientific statement from the American Heart Association. Circulation. 2010;122(19):1975–1996. doi: 10.1161/CIR.0b013e3181f9a223. [DOI] [PubMed] [Google Scholar]
- 34.Adams KF, Jr, Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149(2):209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Gheorghiade M, Zannad F, Sopko G, et al. Acute heart failure syndromes: current state and framework for future research. Circulation. 2005 December 20;112(25):3958–3968. doi: 10.1161/CIRCULATIONAHA.105.590091. 2005. [DOI] [PubMed] [Google Scholar]
- 36.West RL, Hernandez AF, O’Connor CM, Starling RC, Califf RM. A review of dyspnea in acute heart failure syndromes. Am Heart J. 2010;160(2):209–214. doi: 10.1016/j.ahj.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 37.Yorke J, Moosavi SH, Shuldham C, Jones PW. Quantification of dyspnoea using descriptors: development and initial testing of the Dyspnoea-12. Thorax. 2010;65(1):21–26. doi: 10.1136/thx.2009.118521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pang PS, Cleland JGF, Teerlink JR, et al. A proposal to standardize dyspnoea measurement in clinical trials of acute heart failure syndromes: the need for a uniform approach. Eur Heart J. 2008;29(6):816–824. doi: 10.1093/eurheartj/ehn048. [DOI] [PubMed] [Google Scholar]
- 39.Mebazaa A, Pang PS, Tavares M, et al. The impact of early standard therapy on dyspnoea in patients with acute heart failure: the URGENT-dyspnoea study. Eur Heart J. 2010;31(7):832–841. doi: 10.1093/eurheartj/ehp458. [DOI] [PubMed] [Google Scholar]
- 40.Teerlink JR. Dyspnea as an end point in clinical trials of therapies for acute decompensated heart failure. Am Heart J. 2003;145(2, Part 2):S26–S33. doi: 10.1067/mhj.2003.151. [DOI] [PubMed] [Google Scholar]
- 41.Bollen KA. Structural equation modeling with latent variables. John Wiley & Sons; New York: 1989. [Google Scholar]
- 42.Thompson B. Exploratory and confirmatory factor analysis: understanding concepts and applications. American Psychological Association; Washington, DC: 2004. [Google Scholar]
- 43.MPlus. Muthén & Muthén; Los Angeles: 1998-2007. [computer program]. Version 4.1. [Google Scholar]
- 44.Muthén LK, Muthén BO. MPlus user’s guide. 5th ed. Muthén & Muthén; Los Angeles, CA: 1998-2007. [Google Scholar]
- 45.Kline R. Principles and practice of structural equation modeling. 3rd ed. Guilford Press; New York: 2010. [Google Scholar]
- 46.Hu L-t, Bentler PM. Fit indices in covariance structure modeling: sensitivity to underparameterized model misspecification. Psychol Methods. 1998;3(4):424–453. [Google Scholar]
- 47.Kenny DA. [Accessed December 28, 2010];Measuring model fit. 2010 http://davidakenny.net/cm/fit.htm.
- 48.Hu L-t, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equation Model. 1999;6(1):1–55. [Google Scholar]
- 49.Banzett R, Lansing R, Evans K, Shea S. Stimulus-response characteristics of CO2-induced air hunger in normal subjects. Respir Physiol. 1996;103(1):19–31. doi: 10.1016/0034-5687(95)00050-x. [DOI] [PubMed] [Google Scholar]
- 50.Banzett RB. Dynamic response characteristics of CO2-induced air hunger. Respir Physiol. 1996;105(1-2):47–55. doi: 10.1016/0034-5687(96)00042-4. [DOI] [PubMed] [Google Scholar]
- 51.Corfield DR, Fink GR, Ramsay SC, et al. Activation of limbic structures during CO2-stimulated breathing in awake man. Adv Exp Med Biol. 1995;393:331–334. doi: 10.1007/978-1-4615-1933-1_62. [DOI] [PubMed] [Google Scholar]
- 52.Corfield DR, Fink GR, Ramsay SC, et al. Evidence for limbic system activation during CO2-stimulated breathing in man. J Physiol (Lond) 1995;488(Pt 1):77–84. doi: 10.1113/jphysiol.1995.sp020947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Demediuk BH, Manning H, Lilly J, et al. Dissociation between dyspnea and respiratory effort. Am Rev Respir Dis. 1992;146(5 Pt 1):1222–1225. doi: 10.1164/ajrccm/146.5_Pt_1.1222. [DOI] [PubMed] [Google Scholar]
- 54.Evans KC, Banzett RB, Adams L, McKay L, Frackowiak RS, Corfield DR. BOLD fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger. J Neurophysiol. 2002;88(3):1500–1511. doi: 10.1152/jn.2002.88.3.1500. [DOI] [PubMed] [Google Scholar]
- 55.Fink GR, Corfield DR, Murphy K, et al. Human cerebral activity with increasing inspiratory force: a study using positron emission tomography. J Appl Physiol. 1996;81(3):1295–1305. doi: 10.1152/jappl.1996.81.3.1295. [DOI] [PubMed] [Google Scholar]
- 56.Gandevia SC, Killian K, McKenzie DK, et al. Respiratory sensations, cardiovascular control, kinaesthesia and transcranial stimulation during paralysis in humans. J Physiol (Lond) 1993;470:85–107. doi: 10.1113/jphysiol.1993.sp019849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manning HL, Shea SA, Schwartzstein RM, Lansing RW, Brown R, Banzett RB. Reduced tidal volume increases ‘air hunger’ at fixed Pco2 in ventilated quadriplegics. Respir Physiol. 1992;90(1):19–30. doi: 10.1016/0034-5687(92)90131-f. [DOI] [PubMed] [Google Scholar]
- 58.Murphy K, Mier A, Adams L, Guz A. Putative cerebral cortical involvement in the ventilatory response to inhaled CO2 in conscious man. J Physiol (Lond) 1990;420:1–18. doi: 10.1113/jphysiol.1990.sp017898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwartzstein RM, Simon PM, Weiss JW, Fencl V, Weinberger SE. Breathlessness induced by dissociation between ventilation and chemical drive. Am Rev Respir Dis. 1989;139(5):1231–1237. doi: 10.1164/ajrccm/139.5.1231. [DOI] [PubMed] [Google Scholar]
- 60.Guz A. Respiratory sensations in man. Br Med Bull. 1977;33(2):175–177. doi: 10.1093/oxfordjournals.bmb.a071419. [DOI] [PubMed] [Google Scholar]
- 61.Paintal AS. Thoracic receptors connected with sensation. Br Med Bull. 1977;33(2):169–174. doi: 10.1093/oxfordjournals.bmb.a071418. [DOI] [PubMed] [Google Scholar]
- 62.Banzett R, Lansing R. Respiratory sensations arising from chemoreceptors and pulmonary receptors: air hunger and lung volume. In: Adams L, Guz A, editors. Respiratory Sensation. Vol. 90. Marcel Dekker; New York: 1996. pp. 155–180. [Google Scholar]
- 63.Guz A. Brain, breathing and breathlessness. Respir Physiol. 1997;109(3):197–204. doi: 10.1016/s0034-5687(97)00050-9. [DOI] [PubMed] [Google Scholar]
- 64.Evans KC. Cortico-limbic circuitry and the airways: insights from functional neuroimaging of respiratory afferents and efferents. Biol Psychol. 2010;84(1):13–25. doi: 10.1016/j.biopsycho.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scano G, Innocenti-Bruni G, Stendardi L. Do obstructive and restrictive lung diseases share common underlying mechanisms of breathlessness? Respir Med. 2010;104(7):925–933. doi: 10.1016/j.rmed.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 66.Buchanan GF, Richerson GB. Role of chemoreceptors in mediating dyspnea. Respir Physiol Neurobiol. 2009;167(1):9–19. doi: 10.1016/j.resp.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee L-Y. Respiratory sensations evoked by activation of bronchopulmonary C-fibers. Respir Physiol Neurobiol. 2009;167(1):26–35. doi: 10.1016/j.resp.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Donnell DE, Ora J, Webb KA, Laveneziana P, Jensen D. Mechanisms of activity-related dyspnea in pulmonary diseases. Respir Physiol Neurobiol. 2009;167(1):116–132. doi: 10.1016/j.resp.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 69.Ravi K, Kappagoda T. Rapidly adapting receptors in acute heart failure and their impact on dyspnea. Respir Physiol Neurobiol. 2009;167(1):107–115. doi: 10.1016/j.resp.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 70.Undem BJ, Nassenstein C. Airway nerves and dyspnea associated with inflammatory airway disease. Respir Physiol Neurobiol. 2009;167(1):36–44. doi: 10.1016/j.resp.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 71.Widdicombe J. Lung afferent activity: Implications for respiratory sensation. Respir Physiol Neurobiol. 2009;167(1):2–8. doi: 10.1016/j.resp.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 72.Emerson JD, Stoto MA. Transforming data. In: Hoaglin DC, Mosteller F, Tukey JW, editors. Understanding robust and exploratory data analysis. John Wiley & Sons; New York: 1983 / 2000. [Google Scholar]
- 73.Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression / correlation analysis for the behavioral sciences. 3rd ed. Lawrence Erlbaum; Mahwah, NJ: 2003. [Google Scholar]
- 74.Lansing RW, Gracely RH, Banzett RB. The multiple dimensions of dyspnea: Review and hypotheses. Respir Physiol Neurobiol. 2009;167(1):53–60. doi: 10.1016/j.resp.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Williams M, Cafarella P, Olds T, Petkov J, Frith P. Affective descriptors of the sensation of breathlessness are more highly associated with severity of impairment than physical descriptors in people With COPD. Chest. 2010;138(2):315–322. doi: 10.1378/chest.09-2498. [DOI] [PubMed] [Google Scholar]
- 76.von Leupoldt A, Sommer T, Kegat S, et al. Dyspnea and pain share emotion-related brain network. NeuroImage. 2009;48(1):200–206. doi: 10.1016/j.neuroimage.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 77.Bausewein C, Farquhar M, Booth S, Gysels M, Higginson IJ. Measurement of breathlessness in advanced disease: a systematic review. Respir Med. 2007;101(3):399–410. doi: 10.1016/j.rmed.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 78.Dorman S, Byrne A, Edwards A. Which measurement scales should we use to measure breathlessness in palliative care? a systematic review. Palliat Med. 2007;21(3):177–191. doi: 10.1177/0269216307076398. [DOI] [PubMed] [Google Scholar]