Abstract
Background
Prenatal alcohol exposure is related to a wide range of neurocognitive effects. Eyeblink conditioning (EBC), which involves temporal pairing of a conditioned with an unconditioned stimulus, has been shown to be a potential biomarker of fetal alcohol exposure. A growing body of evidence suggests that white matter may be a specific target of alcohol teratogenesis, and the neural circuitry underlying EBC is known to involve the cerebellar peduncles. Diffusion tensor imaging (DTI) is a magnetic resonance imaging (MRI) technique which has proven useful for assessing central nervous system white matter integrity. This study used DTI to examine the degree to which the fetal alcohol-related deficit in EBC may be mediated by structural impairment in the cerebellar peduncles.
Methods
13 children with fetal alcohol spectrum disorder (FASD) and 12 matched controls were scanned using DTI and structural MRI sequences. The DTI data were processed using a voxelwise technique, and the structural data were used for volumetric analyses. Prenatal alcohol exposure group and EBC performance were examined in relation to brain volumes and outputs from the DTI analysis.
Results
FA and perpendicular diffusivity group differences between alcohol-exposed and nonexposed children were identified in the left middle cerebellar peduncle. Alcohol exposure correlated with lower fractional anisotropy (FA) and greater perpendicular diffusivity in this region, and these correlations remained significant even after controlling for total brain and cerebellar volume. Conversely, trace conditioning performance was related to higher FA and lower perpendicular diffusivity in the left middle peduncle. The effect of prenatal alcohol exposure on trace conditioning was partially mediated by lower FA in this region.
Conclusions
This study extends recent findings that have used DTI to reveal microstructural deficits in white matter in children with FASD. This is the first DTI study to demonstrate mediation of a fetal alcohol-related effect on neuropsychological function by deficits in white matter integrity.
Keywords: fetal alcohol spectrum disorder, fetal alcohol syndrome, diffusion tensor imaging, DTI, white matter, eyeblink conditioning
Introduction
Descriptive studies spanning three decades have identified a broad range of irreversible cognitive and behavioral deficits in children with fetal alcohol spectrum disorder (FASD). Fetal alcohol syndrome (FAS), which is characterized by a distinctive craniofacial dysmorphology (short palpebral fissures, thin upper lip, flat or smooth philtrum), small head circumference, and pre- and/or postnatal growth retardation, is the most severe of the fetal alcohol disorders (Hoyme et al., 2005). Partial FAS (PFAS) is diagnosed when there is a history of heavy maternal drinking during pregnancy, the presence of two of the three key alcohol-related facial anomalies, and at least one of the following—small head circumference, growth retardation, or cognitive and/or behavioral dysfunction. Children with alcohol-related neurodevelopmental disorder exhibit significant cognitive impairment but lack the distinctive facial anomalies. We have identified eyeblink conditioning (EBC), a Pavlovian paradigm that involves contingent temporal pairing of a conditioned stimulus (a tone) with an unconditioned stimulus (an air puff), as a remarkably consistent potential biomarker of fetal alcohol exposure (Jacobson et al., 2008). The neural circuitry that mediates EBC has been documented in considerable detail in animal models (Christian & Thompson, 2003; Lavond & Steinmetz, 1989). In the 5-year follow-up assessment of our Cape Town Longitudinal Cohort, not a single child with full FAS met criterion for conditioning in a delay EBC task, as contrasted with 75.0% of the controls. This finding corroborates a report of poorer delay EBC in a small sample of school-aged, alcohol-exposed children (Coffin et al., 2005). We have recently replicated and extended this finding in a new study of delay and trace EBC in the cross-sectional sample of school-age Cape Town children who provided the data reported in this paper (Jacobson et al., 2011).
A growing body of evidence suggests that white matter is a specific target of alcohol teratogenesis. Archibald et al. (2001) found a disproportionate reduction in cerebral white matter in their FAS group, suggesting an effect on myelination that has also been observed in ethanol-exposed animals (Bichenkov & Ellingson, 2001; Zoeller et al., 1994). White matter lesions have also been observed in preterm infants with heavy prenatal alcohol exposure (Holzman et al., 1995) and in fetal alcohol exposed sheep (Watari et al., 2006). Studies with fetal alcohol exposed rodents have reported decreases in axon size, increased packing density, and thinner myelin sheaths (Miller & Al-Rabiai, 1994), as well as abnormalities in the oligodendrocytes that produce the myelin sheath (Chiappelli et al., 1991; Guerri et al., 2001). Diffusion tensor imaging (DTI) is a magnetic resonance imaging (MRI) technique that is used to assess white matter structural integrity (Basser et al., 1994). Fractional anisotropy (FA), calculated as the normalized variance of the diffusion tensor eigenvalues, represents the degree to which water diffuses preferentially along the length of the axonal axis rather than perpendicular to it. Mean diffusivity (MD) is a direction-independent measure of local diffusivity. Healthy, highly organized white matter is indicated by high FA and relatively low MD values. The directional diffusivities derived from DTI measurements are commonly separated into eigenvalues parallel (λ1) and perpendicular (λ2 and λ3) to the white matter tract. These components are described as parallel or axial diffusivity λ∥ = λ1 and perpendicular or radial diffusivity λ⊥ = (λ1+λ2)/2. Research with animal models has demonstrated that the myelin structure impedes perpendicular diffusivity, while some forms of injury to the axon itself impede parallel diffusivity by reducing the continuity of the axonal cylinders and myelin sheaths. Increased perpendicular and parallel diffusivity are thus indicators of myelin damage and axonal health, respectively (Beaulieu, 2002; Song et al., 2002, 2003, 2005). However, this distinction is not always clear, as demonstrated by Werring at al. (2000), who showed that axonal degeneration can lead to increases in water diffusion in both the parallel and perpendicular directions, and by Beaulieu (2002), who demonstrated anisotropy in non-myelinated nerves.
To date, eight DTI studies have been performed on individuals with FASD. The most consistent alcohol-related FA and MD differences have been seen in corpus callosum (Ma et al., 2005; Wozniak et al., 2006; Fryer et al., 2009; Li et al., 2009; Wozniak et al., 2009), but white matter deficits have also been linked to fetal alcohol exposure in the lateral splenium, posterior cingulate, deep white matter in the right lateral temporal lobe, medial portions of the frontal and occipital lobes, and inferior parietal white matter (Sowell et al., 2008; Fryer et al., 2009). Lebel et al. (2008) identified several FA and MD changes using fiber tracking, and Lebel et al. (2010) identified several regions where FA was correlated with arithmetic achievement test scores.
In this study we investigate FASD-associated differences in white matter microstructure in the cerebellar peduncles. These are large bundles of myelinated nerve fibers that connect the cerebellum to the brainstem and constitute the principal white matter element of the EBC circuit. Two EBC tasks are examined: a delay task, in which the air puff is presented during the final 100 ms of a 750 ms tone, and a trace task, in which a 500 ms interval occurs between the offset of a 750 ms tone and the onset of an air puff (Jacobson et al., 2011). Information about the tone (conditioned stimulus; CS) is conveyed from the pontine nucleus in the brainstem to the cerebellum via the middle cerebellar peduncle; information about the air puff (unconditioned stimulus; US) is conveyed from the inferior olive via the inferior peduncle. Both inputs reach Purkinje cells in the cerebellar cortex and send collateral input to the cerebellar deep nuclei. Neural plasticity in these cerebellar regions produced by appropriately timed CS and US inputs underlies EBC. The essential efferent pathway for the conditioned response is via the superior cerebellar peduncle to the red nucleus, where the conditioned response is projected to motor neurons that generate the conditioned eyeblinks. Although the brainstem-cerebellar circuitry described here is sufficient for delay conditioning, trace conditioning also engages the hippocampus (Cheng et al., 2008; Woodruff-Pak & Disterhoft, 2008; Tran & Thomas, 2007).
In this study we use DTI to examine the degree to which the fetal alcohol-related deficit in EBC may be mediated by structural impairment in the cerebellar peduncles. The DTI data were analyzed using a voxelwise approach. FA, MD, and parallel and perpendicular diffusivity were measured to better characterize white matter structural deficits. We also examined the relation of the FA and diffusivity values to the children's performance on the delay and trace conditioning tasks. Multiple regression analysis was used to test the hypothesis that structural deficits in the cerebellar peduncles play a role in mediating the effect of prenatal alcohol exposure on delay and trace EBC.
1 Methods
1.1 Participants
DTI data were obtained from 25 right-handed children, age 9.7 – 13.7 years, from the Cape Coloured (mixed ancestry) community in Cape Town, South Africa, 13 of whom were heavily exposed to alcohol and 12 of whom were controls. Fifteen were older siblings of the children from our Cape Town Longitudinal Cohort (Jacobson et al., 2008); and 10 were identified by screening all of the children in this age range from an elementary school in a rural section of Cape Town, where there is a very high incidence of alcohol abuse among Cape Coloured farm workers (Jacobson et al., 2011; Dodge et al., 2009). Alcohol abuse and dependence are unusually prevalent in women of child-bearing age in the Cape Coloured community, which has one of the highest incidences of FAS in the world (May et al., 2000; Jacobson et al., 2008).
1.2 Procedure
Demographic background and maternal alcohol consumption
When the children were recruited to participate in this cross-sectional study, the mothers were interviewed regarding their demographic background and alcohol consumption during pregnancy, using a timeline follow-back approach (Sokol et al., 1985; Jacobson et al., 2002) to determine incidence and amount of drinking on a day-by-day basis. Volume was recorded for each type of beverage consumed and converted to ounces (oz) of absolute alcohol (AA), using multipliers developed by Bowman et al. (1975). Any child whose mother reported consuming at least 14 standard drinks per week (1.0 oz AA/day) on average or engaged in binge drinking (4 or more drinks/occasion) during pregnancy was considered heavily exposed. Mothers were interviewed about their children's school and health history. Children in this community are rarely prescribed psychoactive medications, such as methylphenidate (Ritalin). None of the mothers reported their children to be taking any such medications.
Dysmorphology assessment and fetal alcohol disorder diagnosis
The children were examined for growth and FAS anomalies by three expert dysmorphologists (H.E. Hoyme, L.K. Robinson, N. Khaole), using a standard protocol (Hoyme et al., 2005) (see Jacobson et al., 2008). Following Hoyme et al.'s (2005) Revised Institute of Medicine (IOM) criteria, FAS was diagnosed when the child exhibited at least two of three facial anomalies (short palpebral fissures, thin upper lip, flat philtrum), microcephaly, and weight or height below the 10th percentile; PFAS when the mother drank heavily during pregnancy (as defined above) and the child had at least two of the three features and microcephaly, weight or height below the 10th percentile, or low IQ (< 70).
Neuropsychological and EBC assessments
Each child was assessed for IQ and EBC at the University of Cape Town Child Development Research Laboratory. IQ was estimated from seven subtests from the Wechsler Intelligence Scales for Children, third edition (WISC-III)—Similarities, Arithmetic, Digit Span, Symbol Search, Coding, Block Design, and Picture Completion—and one subtest from the WISC-IV, Matrix Reasoning. Sattler (1992) provides a method for calculating an estimated IQ score based on any number of subtests. Validity coefficients for estimated IQ based on this method when at least five subtests are included consistently exceed r = 0.90. Each child was administered the WISC subtests in the language used in his/her school; for 18 (72.0%) the language was Afrikaans, for the others, English. In the 5-year follow-up for our Cape Town Longitudinal Study (Jacobson et al., 2008), we had administered the Junior South African Intelligence Scale (JSAIS), which has been normed for South African children. Sixty-two of those children were subsequently administered the WISC IQ test at 9 years (Jacobson et al., 2011). IQ scores based on the JSAIS were strongly correlated with the 9-year WISC IQ scores, r = 0.77, p < 0.001, confirming the validity of the WISC for use with this population. Although the IQ scores are low, the mean for the controls in this economically disadvantaged sample (Table 1) is comparable to IQ scores reported for U.S. inner-city children, whose scores are consistently lower than suburban, nonminority children. For example, in our Detroit inner city, African American cohort the mean IQ was 81.9 at 7.5 and 14 years (Burden et al., 2010). The EBC assessments were administered using a commercially available human EBC system (San Diego Instruments, Model #2325-0145-W; see Jacobson et al., 2008, 2011). The child sat facing a monitor, on which s/he watched a video (Milo and Otis). The child wore a lightweight headgear, which supports a flexible plastic tube that delivers an air puff to the right eye, at a distance of approximately 2.5 cm. Eyelid closure was measured using a photodiode device placed at the corner of the right eye. Two small 7 Ohm speakers were directed to the child's ears for delivery of the auditory conditioned stimulus (CS), a 1 kHz, 80 dB tone. The EBC system generated the tone CS and an air puff unconditioned stimulus (US), processed the eyeblink signal, and integrated the data from the peripheral devices on a desk top computer. Each session consisted of five 10-trial blocks. Two 50-trial sessions were administered on the same day about 2 hr apart. Trace conditioning was administered 1.3-1.8 years after the delay task (M = 1.6, SD = 0.1). The procedure was the same as in the delay task except that a 500 ms stimulus-free interval occurred between the offset of the 750 ms tone and the onset of the air puff. Eyeblinks within 350 ms prior to the air puff onset are considered conditioned responses (CRs) (see Herbert et al., 2003). The principal dependent measure for both the delay and trace tasks was whether the child met the criterion of 40% CRs within the two sessions.
Table 1.
Sample characteristics by alcohol exposure group (N = 25)
| Control (12) | Alcohol exposed (13) | t or χ2 | |
|---|---|---|---|
| Maternal characteristics | |||
| Age at delivery | 25.2 (3.4) | 26.5 (5.4) | -0.72 |
| Years of education | 8.4 (2.2) | 7.0 (2.7) | 1.44 |
| Married (%) | 38.5 | 50.0 | -0.56 |
| Parity | 2.6 (1.4) | 2.3 (1.1) | 0.54 |
| Alcohol during pregnancy | |||
| oz AA/day | 0.004 (0.01) | 2.7 (2.3) | -4.18*** |
| oz AA/occasion | 0.17 (0.4) | 6.5 (3.3) | -6.84*** |
| Frequency (days/week) | 0.03 (0.09) | 2.8 (1.4) | -7.24*** |
| Binges (frequency) | 0.08 (0.08) | 10.5 (5.8) | -6.46*** |
| Alcohol dependent (%)a | 11.1 | 76.9 | -9.21** |
| Cigarettes/day during pregnancy | 3.4 (3.4) | 8.6 (7.9) | -2.00† |
| Child characteristics | |||
| Child gender (% male) | 41.7 | 23.1 | -0.32 |
| Age at EBC visit | |||
| Delay conditioningb | 11.1 (1.2) | 11.1 (1.2) | 0.00 |
| Trace conditioningb | 12.7 (1.2) | 12.7 (1.3) | -0.04 |
| Years between delay and trace conditioningb | 1.5 (0.2) | 1.6 (0.1) | -0.36 |
| Age at DTI scan | 11.8 (1.2) | 11.8 (1.2) | 0.16 |
| Grade in school | 4.3 (1.3) | 3.7 (1.4) | 1.19 |
| Weight (kg)c | 34.2 (7.8) | 28.7 (7.2) | 1.82† |
| Height (cm)c | 136.5 (7.5) | 131.3 (9.6) | 1.49 |
| Head circumference (cm)c | 53.1 (1.5) | 51.1 (2.7) | 2.30* |
| WISC IQ | 76.6 (13.1) | 62.8 (9.3) | 3.05** |
| Volumetric measures (mm3)d | |||
| Total brain volume | 1.28 × 106 (1.52 × 105) | 1.14 × 106 (1.33 × 105) | 2.40* |
| Cerebellar volume | 1.49 × 105 (1.48 × 105) | 1.30 × 105 (1.94 × 105) | 2.60* |
| Cerebellar white matter | 3.21 × 104 (5.87 × 103) | 2.61 × 104 (4.73 × 103) | 2.74** |
| Cerebellar gray matter | 1.17 × 105 (1.05 × 104) | 1.04 × 105 (1.60 × 104) | 2.25 |
Values are means (SD).
Based on the NAWS; missing for three mothers.
N = 17 for whom both delay and trace data were available.
At dysmorphology assessment.
Missing for one child.
p < 0.10.
p < 0.05.
p < 0.01.
p < 0.001.
MRI data acquisition
Each child was scanned on a 3T Allegra MR scanner (Siemens, Erlangen Germany). A magnetization-prepared rapid gradient echo (MPRAGE) structural image was acquired in a sagittal orientation with the following parameters: TR = 2300 ms, TE = 3.93 ms, TI = 1100 ms, 160 slices, flip angle = 12 degrees, voxel size = 1.3×1.0×1.0 mm3, scan time = 6:03. Diffusion weighted imaging was performed in 30 directions with b-value of 1000 sec/mm2, and one volume was acquired with b=0 sec/mm2. Thirty-six slices were acquired in an oblique axial orientation which included largely infra-tentorial structures. Other imaging parameters included TR = 5000 ms, TE = 88 ms, acquisition matrix = 120×120, resolution = 2×2 mm2, slice thickness = 2.2 mm. Five identical acquisitions were performed, with each acquisition taking 2 min, 40 s.
1.3 Data Analysis
Preprocessing of DTI data
Eddy current correction was performed separately in each of the five DTI acquisitions using affine transformations in FSL (Oxford Center for MRI of the Brain, Oxford, UK). Images were then imported into custom MATLAB (MathWorks, Natick, MA) tools for further processing. Co-registration across acquisitions was performed across the five acquisitions using affine transformations with the unweighted image of the first acquisition as a reference. For each of the five acquisitions, diffusion tensors were calculated and outliers were rejected by first calculating Z-scores based on 25 and 75 percentile limits, and then discarding data points more than 3 standard deviations beyond the mean. The five acquisitions were then averaged and the mean diffusivity (MD) was calculated. Diffusion tensors and corresponding eigenvalues were determined, and from these, fractional anisotropy (FA), parallel diffusivity (λ∥) and perpendicular diffusivity (λ⊥) images were derived. Perpendicular diffusivity is defined here as the average of the second and third eigenvalues. A binary mask was obtained from the unweighted image (b0), using a brain extraction algorithm with a fractional threshold of 0.5, and applied to the FA, MD, λ∥ and λ⊥ images.
Voxelwise comparison of DTI measures
The structural MPRAGE images were interpolated to have isometric 1×1×1 mm3 voxels, and tools from Diedrichsen (2006) were used to crop the images to include only the cerebellum and brainstem. For each subject the masked b0 image was registered to the cropped structural image using SPM5 (Wellcome Department of Imaging and Neuroscience, London, UK). A two-step registration process was used, comprising an affine transformation followed by a high-dimensional warping algorithm. For the intra-subject registration, the FA, MD, , and images were warped using the same transforms.
The FA image from a model brain was selected from among the control subjects, and all FA images were co-registered to it using the same two-step registration process. A mean FA template image was then calculated from all subjects, and a further two-step registration was applied to align all of the FA images to the template FA image. The MD, , and images were co-aligned using all transforms applied to the FA images. No smoothing was performed.
All images were masked by manually defining the inferior, middle, and superior cerebellar peduncle volumes on the template FA image, and further applying an FA threshold > 0.25. One-way analysis of variance (ANOVA) was then performed at a voxel level to identify where the FA, MD, λ∥, and λ⊥ data differed between the two groups. Type I error was controlled by setting a cluster volume threshold at a voxel level significance of p < 0.01. This was performed using a Monte Carlo simulation (Forman et al., 1995) with an alpha rate of 0.01, a 1 mm connectivity radius, and assuming uncorrelated voxels. A cluster size threshold of 84 mm3 was identified for the mask defining the cerebellar peduncles (17990 mm3). The co-registration efficacy was visually scrutinized on all subjects for every cluster.
Volumetric analysis
Total brain volume, cerebellar volume, and cerebellar white matter and gray matter volume were measured from the MPRAGE images using FreeSurfer (Fischl et al., 2002).
Relation of DTI clusters to alcohol exposure group, FAS diagnosis, and eyeblink conditioning
Pearson correlation analysis was used to examine the magnitude of the relation between the prenatal alcohol exposure group (exposed vs. controls) and the FA and diffusivity measures for the DTI clusters where the exposed and control children differed. Nine control variables were assessed for consideration as potential confounders of the relation of exposure to the DTI measures: maternal age at delivery, parity, years of education, marital status, smoking during pregnancy, child gender, age at assessment, language used for neuropsychological assessment, and postnatal lead exposure based on a venous blood lead sample obtained from the child. Multiple regression analysis was used to determine whether the effects of exposure group on each DTI outcome remained significant after adjustment for all potential confounders that were related even weakly (at p < 0.10) to that outcome. The relation of severity of fetal alcohol diagnosis (FAS/PFAS/controls) to the DTI measures on which the exposed and control groups differed was examined using ANOVA.
Pearson correlation analysis was used to examine the relation of the FA and diffusivity measures to delay and trace conditioning, assessed as dichotomous variables indicating whether the child reached criterion for conditioning. A box plot was run to compare the FA and diffusivity distributions for the children who met criterion for conditioning with those who did not. Chi-square was used to confirm that the adverse effects of prenatal alcohol exposure on delay and trace conditioning found in our previous studies (Jacobson et al., 2008, 2011) were also seen in the sample of children for whom DTI data are available.
The hypothesis that FA in the cerebellar peduncles mediates the effect of prenatal alcohol exposure on EBC was tested using hierarchical multiple regression analysis. Alcohol exposure group was entered in the first step of the regression; the mediator (FA in the left middle peduncle) was entered in the second step. Mediation was inferred if the addition of FA substantially reduced the magnitude of the regression coefficient for exposure group. The Difference in Coefficients Test (Clogg et al., 1992) was used to assess whether the reduction in the magnitude of the regression coefficient was statistically significant. The Clogg Test was selected because, in a Monte Carlo study comparing 14 methods to test the statistical significance of mediation hypotheses, MacKinnon et al. (2002) found that it was one of two with the greatest power. This approach was also used to test whether the relation of prenatal alcohol exposure to EBC was mediated by four other structural brain measures—total brain volume, cerebellar volume, and cerebellar white and gray matter volume.
2 Results
Sample characteristics
There were no significant between-group differences in terms of maternal age, education, marital status, or parity or child gender, age, or grade in school (Table 1). As expected, women in the alcohol consuming group drank more alcohol per occasion and more frequently than control mothers. Ten of the 12 controls (83.3%) abstained during pregnancy; one drank 2 drinks/occasion once/month, and the other drank 2 drinks on a single occasion during pregnancy. More than three-fourths of the alcohol users met criterion for alcohol dependence/abuse on the NAWS, as compared to only one control, who no longer used alcohol. Heavy alcohol users also tended to smoke more cigarettes/day than controls. Five of the children met Revised IOM (Hoyme et al., 2005) criteria for full FAS, 8 for PFAS. As expected, children in the alcohol-exposed group had lower IQ scores, tended to weigh less, and had significantly smaller head circumference than controls. The exposed children also had smaller brain and cerebellar volumes and smaller cerebellar white and gray matter volumes compared with control children.
Voxelwise analysis
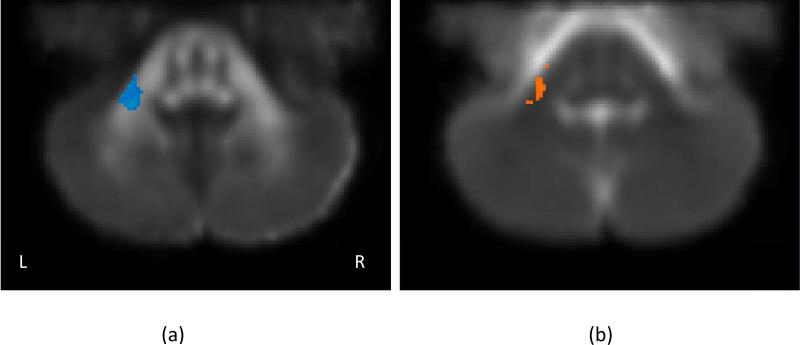
Data were excluded for three alcohol-exposed children due to poor co-registration in the superior cerebellar peduncles and one control child for whom no MPRAGE image was available. The voxelwise analysis revealed no significant group differences on any of the DTI measures in the inferior or superior peduncles. Significant group differences were identified in the left middle cerebellar peduncle for two DTI measures: FA and λ⊥ (Figure 1). Table 2 shows the group means and total cluster volumes for the Regions of Interest (ROI's) defined by the analyses of the exposure group differences. FA is decreased and λ⊥ is increased in alcohol-exposed children compared to controls.
Figure 1.
ROIs consisting of at least 84 contiguous voxels at which the exposed and control groups differed at p < 0.01 (corrected). (a) FA – fractional anisotropy, and (b) λ⊥ – perpendicular diffusivity. No differences were noted for mean or parallel diffusivity. Blue indicates lower values for the alcohol-exposed children; orange indicates lower values for the controls. The grayscale image corresponds to the mean template.
Table 2.
Regions with significant differences between the alcohol-exposed and normal control groups (n = 21, exposed = 10, controls = 11, cluster volume > 84 mm3, p < 0.01, corrected)
| Location | Controls | Alcohol exposed | MNI coords. (at min p) x, y, z | Cluster vol. (mm3) | Min p | |
|---|---|---|---|---|---|---|
| FA | LMP | 0.40±0.03 | 0.36±0.03 | -24, -46, -44 | 126 | 0.001 |
| λ ⊥ | LMP | 0.53±0.03 | 0.62±0.06 | -23, -45, -38 | 114 | 0.00001 |
Values are mean ± standard deviation; FA units are dimensionless and diffusivity units are represented as × 10-3 mm2/sec.
FA = fractional anisotropy; λ⊥ = perpendicular diffusivity; LMP = left middle cerebellar peduncle.
Relation of alcohol exposure group and diagnosis to FA and perpendicular diffusivity
The analyses in Table 3 show strong correlations of alcohol exposure group with lower FA and greater perpendicular diffusivity in the ROIs in the left middle cerebellar peduncle identified in Figure 1. Among the nine control variables assessed as potential confounders, only one tended to be related to these outcomes; all p's were greater than 0.15 except for lead exposure, where p = 0.09 for both outcomes. Regression analyses indicated that exposure group continued to be significantly related to both FA and perpendicular diffusivity, after controlling for lead exposure, β = -0.50, p < 0.05, and β = 0.62, p = 0.005, respectively.
Table 3.
Relation of alcohol exposure group to fractional anisotropy and perpendicular diffusivity (N = 21)
| Exposure groupa | Child |
Volumeb |
Alcohol exposure controlling for |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | Gender | IQ | Brain | Cerebellar | Cerebellar WM | Brain volume | Cerebellar volume | Cerebellar WM | ||
| FA LMP | -0.62** | 0.23 | -0.03 | 0.33 | 0.62** | 0.65** | 0.75*** | -0.48* | -0.46* | -0.48* |
| λ⊥ LMP | 0.71*** | -0.15 | 0.04 | -0.36 | -0.48* | -0.50* | -0.58** | 0.64** | 0.63** | 0.64** |
Values are Pearson rs except for values controlling for brain cerebellar and cerebellar white matter volumes, which are partial rs.
FA = fractional anisotropy; λ⊥ = perpendicular diffusivity; LMP = left middle cerebellar peduncle.
Exposed vs. controls.
Missing for one child.
p < 0.05.
p < 0.01.
p < 0.0001.
Brain volume, cerebellar volume, and cerebellar white matter volume were all moderately related to higher FA and less perpendicular diffusivity (Table 3). When the relations of prenatal alcohol exposure to FA and perpendicular diffusivity were examined controlling for the brain, cerebellar, and cerebellar white matter volume measures, all of the associations between prenatal alcohol and the DTI measures remained significant, indicating that this deficit in white matter integrity is not attributable to the alcohol-related reductions in brain or cerebellar volume. Child's age, gender, and IQ were unrelated to FA and perpendicular diffusivity.
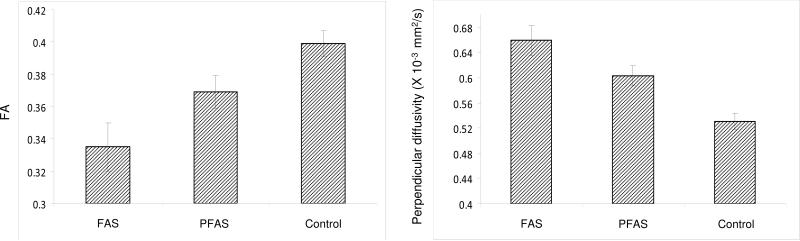
The FA and perpendicular diffusivity measures identified in Figure 1 were then examined in relation to diagnostic group (FAS, PFAS, control). As shown in Figure 2, the relations of severity of FASD diagnosis to lower FA and greater perpendicular diffusivity were dose dependent, F (2,18) = 8.50, p < 0.003 and F (2,18) = 13.53, p < 0.0001, respectively. Given that the hypothesis was unidirectional, one-tailed post-hoc comparisons were computed. For FA, results of the post-hoc comparisons were FAS < PFAS, p = 0.035; FAS < controls, p = 0.001; and PFAS < controls, p = 0.012. For perpendicular diffusivity, FAS > PFAS, p = 0.035; FAS > controls, p = 0.0001; and PFAS > controls, p = 0.001.
Figure 2.
Relation of FASD diagnostic group to FA and perpendicular diffusivity in the left middle cerebellar peduncle. Error bars indicate standard error (SE).
Relation of FA and perpendicular diffusivity measures to EBC
We examined the relation of FA and perpendicular diffusivity to EBC in the 17 children (8 exposed and 9 controls) for whom both imaging and EBC data were available. Consistent with our findings for the larger Cape Town sample from which these children were drawn (Jacobson et al., 2011), only 3 of the 8 (37.5%) fetal alcohol-exposed children reached criterion for delay conditioning, as compared to 8 of 9 (88.9%) control children, X2 (1) = 4.90, p < 0.05. Trace conditioning was more difficult for all of the children at this age. None of the 8 exposed children met criterion for trace conditioning, compared with 5 of 9 (55.6%) controls, X2 (1) = 6.30, p < 0.01. Delay conditioning was moderately associated with higher FA (r = 0.43, p < 0.10) and less perpendicular diffusivity (r = -0.31) in the left middle peduncle, although these correlations fell short of statistical significance in this small sample. Trace conditioning was strongly related to higher FA (r = 0.71, p < 0.001) and moderately related to less diffusivity (r = -0.58, p < 0.01) in that region. The box plots in Figure 3 show that the FA values were consistently higher among the children who met criterion for trace conditioning than those who did not and that there was remarkably little overlap between these groups. The correlations of the FA and diffusivity measures to percentage of trials with a conditioned response (% CRs) were particularly strong during the first trace conditioning session when learning/acquisition first occurs (r's = 0.79 and -0.71, both p's < 0.001, for FA and perpendicular diffusivity, respectively).
Figure 3.
Comparison of FA and perpendicular diffusivity in the left middle cerebellar peduncle in children who met criterion for trace conditioning with those who did not.
Because FA was measured in an ROI in which the alcohol and exposed groups differed, we also examined the correlation between FA and EBC performance (% conditioned responses (CRs) during the first trace conditioning session) for the exposed and control groups separately. For the control group only, that correlation is 0.75; for the exposed group, it is 0.57. Although the latter correlation falls short of significance, the association between FA and trace conditioning is evident in both groups. To address the concern that the correlation between FA and % CRs might be spurious, i.e., attributable to the fact that the FA ROI was selected to maximize the exposure group differences, we ran a regression relating FA to % CRs controlling for alcohol exposure group. At Step 1 of that analysis, the correlation of FA with % CRs was 0.79, p < 0.001 (as reported in the previous paragraph). When alcohol group was added at the second step, the standardized regression coefficient for FA dropped to 0.71, p < 0.01. (By contrast the coefficient for alcohol group dropped from -0.57 to -0.13.) Using the Clogg test, the decrease from 0.79 to 0.71 was not significant, t = 0.63, indicating that alcohol group did not mediate (account for) the relation of FA to trace conditioning.
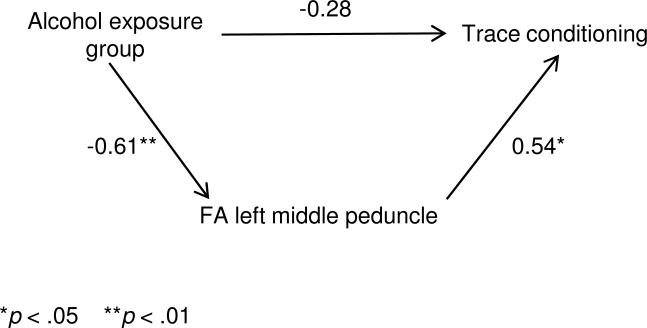
Mediation of the effect of prenatal alcohol exposure on trace conditioning by FA in the left middle peduncle was assessed using multiple regression analysis. As shown in Table 4, the standardized regression coefficient for prenatal alcohol decreased from -0.61 to -0.28 when FA in the left middle peduncle was entered into the regression, a decrease that was statistically significant. This mediation model is illustrated in Figure 4. The regression coefficient for alcohol also decreased substantially when perpendicular diffusivity in the left middle peduncle was added to the regression of trace conditioning on prenatal alcohol exposure, but this decrease was not significant. By contrast, the coefficient for prenatal alcohol was virtually unchanged when total brain volume was added to the regression, indicating that the effect of alcohol on trace conditioning was not mediated by the reduction in brain volume associated with fetal alcohol exposure. Nor was this effect mediated by the alcohol effect on cerebellar volume or cerebellar gray matter volume. The effect of alcohol exposure on trace conditioning was, however, significantly mediated by cerebellar white matter volume.
Table 4.
Mediation of the effect of prenatal alcohol exposure on trace conditioning by DTI and volumetric measures
| Alcohol during pregnancy |
|||
|---|---|---|---|
| Mediator | β 1 | β 2 | ta |
| FA LMP | -0.61** | -0.28 | -2.38* |
| λ⊥ LMP | -0.61** | -0.39 | -1.05 |
| Volume | |||
| Total brain | -0.60* | -0.56* | -0.64 |
| Cerebellum | -0.60* | -0.49† | -1.13 |
| Cerebellar WM | -0.60* | -0.43* | -2.22* |
| Cerebellar GM | -0.60* | -0.55* | -0.54 |
β1 = standardized regression coefficient for alcohol during pregnancy before controlling for mediator. β2 = standardized coefficient for alcohol during pregnancy after controlling for mediator.
FA = fractional anisotropy. LMP = left middle peduncle. λ⊥ = perpendicular diffusivity. WM = white matter. GM = gray matter.
Significance based on the difference in coefficients method (Clogg et al., 1992). For the DTI measures, df = 15, for the volumetric measures, df = 14.
p < 0.10.
p < 0.05.
p < 0.01.
Figure 4.
Mediation of the effect of fetal alcohol exposure on trace conditioning by FA in the left middle cerebellar peduncle. Values are standardized regression coefficients.
3 Discussion
This study used DTI to examine effects of fetal alcohol exposure on the structural integrity of the cerebellar peduncles, which mediate neural transmission between the brainstem and the cerebellum. The voxelwise analysis showed no significant exposure group differences in the inferior and superior cerebellar peduncles, but significant focal changes were seen in the left middle cerebellar peduncle. The principal function of the cerebellar peduncles is to transmit information between brainstem and cerebellum; in the case of eyeblink conditioning, the middle peduncle, which is the largest, conveys information about the auditory tone from the pontine nucleus to cerebellar cortex. Lebel et al. (2010) have recently reported a positive correlation between FA and age-standardized math scores specifically in the left middle cerebellar peduncle in children with FASD. Lower FA in the left middle peduncle has also been reported in studies with patients with schizophrenia (Kyriakopoulos et al., 2008) and adolescents with attention-deficit/hyperactivity disorder (ADHD; Ashtari et al., 2005). In the latter study, decreased FA values in this region were associated with increased severity of inattentive scores on the Conners (1997) ADHD scale.
It is noteworthy that the group differences were seen on perpendicular but not parallel diffusivity. Specific effects on perpendicular diffusivity were also seen in the only two other DTI studies to measure patterns of directional diffusivity in relation to FASD. Li et al. (2009) found increased perpendicular diffusivity (with no difference in parallel diffusivity) in the isthmus of the corpus callosum of young adults with fetal alcohol-related dysmorphology. In their sample of children with FASD, Lebel et al. (2010) found an association between lower math achievement test scores and higher perpendicular diffusivity in left anterior cerebellar white matter tracts connected to the middle peduncle but no association with parallel diffusivity. Experimental studies with laboratory animals have shown that the structural feature of white matter most directly involved in restricting perpendicular diffusivity is axonal packing density but that myelination plays an important secondary role (Beaulieu, 2002). Laboratory animal and human studies have shown that maternal alcohol ingestion during pregnancy reduces iron uptake, which may interfere with normal myelination due to altered expression of myelin basic protein (Connor, 1994; Connor & Menzies, 1996) and that fetal alcohol exposure leads to impairment in the oligodendrocytes that produce the myelin sheath (Ozer et al., 2000; Phillips and Krueger, 1992). These findings suggest that poor myelination may play an important role in the increased perpendicular diffusivity in the left middle peduncle seen in the children with FASD.
The principal limitations of the voxel-based analysis are the small sample size and, although care was taken to ensure satisfactory co-registration, the potential existence of regional misregistration errors, which are a limitation of any voxel-based approach. The cerebellar region is also prone to susceptibility artifacts and pulsatility artifacts. The susceptibility artifacts may amplify misregistration errors, and the pulsatility artifacts degrade the data quality in the inferior aspects of the cerebellum as the brainstem/CSF recoils when high velocity blood enters the brain just after systole. Although these artifacts did not noticeably affect the data quality in this study, future work should include steps to quantify and, where possible, correct for these.
A primary aim of this study was to test the hypothesis that microstructural defects in the cerebellar peduncles might be responsible, in part, for the poorer eyeblink conditioning seen in fetal alcohol-exposed children. As hypothesized, lower FA and greater diffusivity in these ROIs were moderately associated with poorer performance on the delay task, but the correlations were not significant in the small sample examined in this study. The strong correlations between these DTI measures and trace conditioning performance were impressive. Since the peduncles presumably play the same role in both the delay and trace conditioning tasks, the stronger effect seen on trace compared with delay may reflect the greater difficulty of the trace task, in which there is a 500 ms interval between the offset of the 750 ms tone and the onset of the air puff. The notably stronger correlations with percent conditioned responses during the first trace conditioning session suggest that the observed microstructural defects may have the greatest impact during the acquisition of trace conditioning, that is, when the child is initially learning to make the association between the tone and the air puff, and are somewhat less important during the second session, which presumably entails consolidation and retention of the learned association.
The data illustrated in Figure 4 provide support for the hypothesis that the fetal alcohol-related deficit in trace conditioning can be attributed, in part, to less optimal transmission of information from the brainstem via the left middle cerebellar peduncle. These data are consistent with the emerging evidence from eight recent DTI studies of alcohol-related deficits in the structural integrity of white matter tracts in multiple regions throughout the brain. This is, to our knowledge, the first study to use a statistical model to examine the role of a white matter deficit in mediating the effect of prenatal alcohol exposure on a specific neurobehavioral endpoint. It is significant that, although prenatal alcohol exposure was also associated with significant reductions in total brain size and cerebellar gray matter volume in these children, neither of those deficits mediated the alcohol effect on trace conditioning performance. By contrast, the addition of cerebellar white matter volume to the regression analysis significantly reduced the association of prenatal alcohol with trace conditioning, providing additional evidence that alcohol effects on white matter may mediate the fetal alcohol effect on EBC. Given that the peduncles appear to be a homogeneous white matter structure, it seems unlikely that fetal alcohol exposure affects only the particular ROI in the left middle peduncle identified in this study. The small sample size limitation has been noted previously. We suspect that, with the greater power available in a larger sample, microstructural deficits in broader regions of the cerebellar peduncles may become evident.
4 Conclusions
This study extends recent findings that have used DTI to reveal microstructural deficits in white matter in corpus callosum and throughout the brain in children with FASD to show reduced FA and increased diffusivity in the cerebellar peduncles, a white matter structure that has been shown in animal models to be critically important in EBC. The strongest group differences were seen on the perpendicular diffusivity measure, which suggests poorer axon packing density and/or myelination. The inference of a myelination deficit is supported by the animal model studies linking prenatal alcohol exposure to impairment in the oligodendrocytes and expression of myelin basic protein involved in producing the myelin sheath. Although the left middle peduncle was the only white matter cerebellar structure in which these microstructural deficits were evident in this study, similar deficits may become evident in the other peduncles in a larger sample study. The relation of fetal alcohol diagnosis to FA and perpendicular diffusivity in this region is strongly dose dependent, with the children with full FAS showing the strongest deficits. Consistent with what is known about the neurophysiology of eyeblink conditioning, lower FA and greater perpendicular diffusivity in the left middle peduncle were associated with poorer child performance in the eyeblink conditioning paradigm. Data from a mediation model suggest that lower FA in this region may play an important role in the eyeblink conditioning deficit commonly seen in fetal alcohol-exposed children.
Acknowledgments
This research was funded by a Fogarty International Research Collaboration Award from the National Institutes of Health (R03 TW007030), a Focus Area grant (FA2005040800024) from the National Research Foundation of South Africa, the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa, the Medical Research Council of South Africa, a Children's Bridge grant from the Office of the President of Wayne State University (WSU), and the Joseph Young, Sr., Fund from the State of Michigan. The dysmorphology assessments were conducted in conjunction with the National Institute on Alcohol Abuse and Alcoholism (NIAAA) Collaborative Initiative on Fetal Alcohol Spectrum Disorder (U01-AA014790 to S.W. Jacobson and U24AA014815 to K.L. Jones). We thank the three dysmorphologists H.E. Hoyme, L.K. Robinson, and N. Khaole, who performed the dysmorphology examinations of the children. We also thank our UCT research staff, M. Pienaar, M. September, M. Cronje, J. Chamberlain, and J. Minnies for their work on the maternal alcohol and neurobehavioral data collection and R. Sun for her work at WSU on the maternal alcohol data. We thank the mothers and children who have participated in our Cape Town research program. Lastly, we thank Siemens Medical Solutions South Africa and all the staff at the Cape Universities Brain Imaging Centre for their contributions to the acquisition and analysis of the DTI data.
Data analysis was funded, in part, by R01 AA06781 from the National Institute on Alcohol Abuse and Alcoholism.
References
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol. 2001;43:148–154. [PubMed] [Google Scholar]
- Ashtari M, Kumra S, Bhaskar SL, Clarke T, Thanden E, Cervellione KL, Rhinewine J, Kane JM, Adesman A, Milanaik R, Maytal J, Diamond A, Szeszko P, Ardekani BA. Attention-deficit/hyperactivity disorder: a preliminary diffusion tensor imaging study. Biol Psychiatry. 2005;57:448–455. doi: 10.1016/j.biopsych.2004.11.047. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR Diffusion tensor spectroscopy and imaging. Biophysical J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system – a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Bichenkov E, Ellingson JS. Ethanol exerts different effects on myelin basic protein and 2′,3′-cyclic nucleotide 3′-phosphodiesterase expression in differentiating CG-4 oligodendrocytes. Brain Res Dev Brain Res. 2001;128:9–16. doi: 10.1016/s0165-3806(01)00142-0. [DOI] [PubMed] [Google Scholar]
- Bowman RS, Stein LI, Newton JR. Measurement and interpretation of drinking behavior. Q J Stud Alcohol. 1975;36:1154–1172. doi: 10.15288/jsa.1975.36.1154. [DOI] [PubMed] [Google Scholar]
- Burden MJ, Jacobson JL, Westerlund AJ, Lundahl LH, Klorman R, Nelson CA, Avison MJ, Jacobson SW. An event-related potential study of response inhibition in ADHD with and without prenatal alcohol exposure. Alcohol Clin Exp Res. 2010;34:617–627. doi: 10.1111/j.1530-0277.2009.01130.x. [DOI] [PubMed] [Google Scholar]
- Carter RC, Jacobson SW, Molteno CD, Chiodo LM, Viljoen D, Jacobson JL. Effects of prenatal alcohol exposure on infant visual acuity. J Pediatr. 2005;147:473–479. doi: 10.1016/j.jpeds.2005.04.063. [DOI] [PubMed] [Google Scholar]
- Cheng DT, Disterhoft JF, Power JM, Ellis DA, Desmond JE. Neural substrates underlying human delay and trace eyeblink conditioning. Proc Nat Acad Sciences. 2008;105:8108–8113. doi: 10.1073/pnas.0800374105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappelli F, Taylor AN, Espinosa de los Monteros A, de Vellis J. Fetal alcohol delays the development expression of myelin basic protein and transferring in rat primary oligodendrocyte cultures. Int J Dev Neurosci. 1991;9:67–75. doi: 10.1016/0736-5748(91)90074-v. [DOI] [PubMed] [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learn Mem. 2003;10:427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Clogg CC, Petkova E, Shihadeh ES. Statistical methods for analyzing collapsibility in regression models. J Educ Stat. 1992;17:51–74. [Google Scholar]
- Coffin JM, Baroody S, Schneider K, O'Neill J. Impaired cerebellar learning in children with prenatal alcohol exposure: A comparative study of eyeblink conditioning in children with ADHD and dyslexia. Cortex. 2005;41:389–398. doi: 10.1016/s0010-9452(08)70275-2. [DOI] [PubMed] [Google Scholar]
- Conners CK. Conners’ Rating Scales-Revised User's Manual. Multi-Health Systems; North Tonawanda, New York: 1997. [Google Scholar]
- Connor JR. Iron acquisition and expression of iron regulatory proteins in the developing brain: Manipulation by ethanol exposure, iron deprivation and cellular dysfunction. Dev Neurosci. 1994;16:233–247. doi: 10.1159/000112115. [DOI] [PubMed] [Google Scholar]
- Connor JR, Menzies SL. Relationship of iron to oligodendrocytes and myelination. Glia. 1996;17:83–93. doi: 10.1002/(SICI)1098-1136(199606)17:2<83::AID-GLIA1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. Neuroimage. 2006;33:127–138. doi: 10.1016/j.neuroimage.2006.05.056. [DOI] [PubMed] [Google Scholar]
- Dodge NC, Jacobson JL, Molteno CD, Meintjes EM, Bangalore S, Diwadkar V, Hoyme EH, Robinson LK, Khaole N, Avison MJ, Jacobson SW. Prenatal alcohol exposure and interhemispheric transfer of tactile information: Detroit and Cape Town findings. Alcohol Clin Exp Res. 2009;33:1628–1637. doi: 10.1111/j.1530-0277.2009.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magn Res Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fryer SL, Schweinsburg BC, Bjorkquist OA, Frank LR, Mattson SN, Spadoni AD, Riley EP. Characterization of White Matter Microstructure in Fetal Alcohol Spectrum Disorders. Alcohol Clin Exp Res. 2009;33:514–521. doi: 10.1111/j.1530-0277.2008.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerri C, Pascual M, Renau-Piqueras J. Glia and fetal alcohol syndrome. Neurotoxicol. 2001;22:593–599. doi: 10.1016/s0161-813x(01)00037-7. [DOI] [PubMed] [Google Scholar]
- Herbert JS, Eckerman CO, Stanton ME. The ontogeny of human learning in delay, long-delay, and trace eyeblink conditioning. Behav Neurosci. 2003;117:1196–1210. doi: 10.1037/0735-7044.117.6.1196. [DOI] [PubMed] [Google Scholar]
- Holzman C, Paneth N, Little R, Pinto-Martin J. Perinatal brain injury in premature infants born to mothers using alcohol in pregnancy. Pediatr. 1995;95:66–73. [PubMed] [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: Clarification of the 1996 Institute of Medicine criteria. Pediatr. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatr. 2002;109:815–825. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme HE, Robinson LK, Khaole N, Jacobson JL. Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2008;32:365–372. doi: 10.1111/j.1530-0277.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Dodge NC, Fuller DS, Molteno CD, Meintjes EM, Hoyme HE, Robinson LK, Khaole N, Jacobson JL. Impaired short delay and trace eyeblink conditioning in school-age children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2011;35:250–264. doi: 10.1111/j.1530-0277.2010.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakopoulos M, Vyas NS, Barker GJ, Chitnis XA, Frangou S. A diffusion tensor imaging study of white matter in early-onset schizophrenia. Biol Psychiatr. 2008;63:519–523. doi: 10.1016/j.biopsych.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Lavond DG, Steinmetz JE. Acquisition of classical conditioning without cerebellar cortex. Behav Brain Res. 1989;33:113–164. doi: 10.1016/s0166-4328(89)80047-6. [DOI] [PubMed] [Google Scholar]
- Lebel C, Rasmussen C, Wyper K, Walker L, Andrew G, Yager J, Beaulieu C. Brain diffusion abnormalities in children with fetal alcohol spectrum disorder. Alcohol Clin Exp Res. 2008;22:1–9. doi: 10.1111/j.1530-0277.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- Lebel C, Rasmussen C, Wyper K, Andrew G, Beaulieu C. Brain microstructure is related to math ability in children with fetal alcohol spectrum disorder. Alcohol Clin Exp Res. 2010;34:354–363. doi: 10.1111/j.1530-0277.2009.01097.x. [DOI] [PubMed] [Google Scholar]
- Li L, Coles CD, Lynch ME, Hu X. Voxelwise and skeleton-based region of interest analysis of fetal alcohol syndrome and fetal alcohol spectrum disorders in young adults. Human Brain Mapping. 2009;30:3265–3274. doi: 10.1002/hbm.20747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Coles CD, Lynch ME, LaConte SM, Zurkiya O, Wang D, Hu X. Evaluation of corpus callosum anisotropy in young adults with fetal alcohol syndrome according to diffusion tensor imaging. Alcohol Clin Exp Res. 2005;29:1214–1222. doi: 10.1097/01.alc.0000171934.22755.6d. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Brooke L, Gossage JP, Croxford J, Adnams C, Jones KL, Robinson L, Viljoen D. Epidemiology of fetal alcohol syndrome in a South African community in the Western Cape Province. Am J Public Health. 2000;90:1905–1912. doi: 10.2105/ajph.90.12.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Al-Rabiai S. Effects of prenatal exposure to ethanol on the number of axons in the pyramidal tract of the rat. Alcohol Clin Exp Res. 1994;18:346–354. doi: 10.1111/j.1530-0277.1994.tb00024.x. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Nagae-Poetscher LM, Van Zijl PCM. MRI Atlas of human white matter. 1st ed. Elsevier; Amsterdam: 2005. [Google Scholar]
- Ozer E, Sarioglu S, Gure A. Effects of prenatal ethanol exposure on neuronal migration, neuronogenesis and brain myelination in the mouse brain. Clin Neuropathol. 2000;19:21–25. [PubMed] [Google Scholar]
- Phillips DE, Krueger SK. Effects of combined pre- and postnatal ethanol exposure (third trimester equivalency) on glial cell development in rat optic nerve. Int J Dev Neurosci. 1992;10:197–206. doi: 10.1016/0736-5748(92)90059-9. [DOI] [PubMed] [Google Scholar]
- Sattler JM. Assessment of children. 3rd ed. Jerome M. Sattler, Inc.; San Diego: 1992. [Google Scholar]
- Sokol RJ, Martier S, Ernhart C. Identification of alcohol abuse in the prenatal clinic. In: Chang NC, Chao HM, editors. Early identification of alcohol abuse. Alcohol, Drug Abuse, and Mental Health Administration Research Monograph No. 17; Rockville, MD: 1985. pp. 85–128. [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuromage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Johnson A, Kan E, Lu LH, Van Horn JD, Toga AW, O'Connor MJ, Bookheimer SY. Mapping white matter integrity and neurobehavioral correlates in children with fetal alcohol spectrum disorders. J Neurosci. 2008;28:1313–1319. doi: 10.1523/JNEUROSCI.5067-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TD, Thomas JD. Perinatal choline supplementation mitigates trace eyeblink conditioning deficits associated with 3rd trimester alcohol exposure in rodents. Soc Neurosci Abstracts. 2007;37:746.23. [Google Scholar]
- Watari H, Born DE, Gleason CA. Effects of first trimester binge alcohol exposure on developing white matter in fetal sheep. Pediatr Res. 2006;59:560–564. doi: 10.1203/01.pdr.0000203102.01364.de. [DOI] [PubMed] [Google Scholar]
- Werring DJ, Toosy AT, Clark CA, Parker GJM, Barker GJ, Miller DH, Thompson AJ. Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. J Neurol Neurosurg Psychiatr. 2000;69:269–272. doi: 10.1136/jnnp.69.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Disterhoft JF. Where is the trace in trace conditioning? Trends Neurosci. 2008;31:105–112. doi: 10.1016/j.tins.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Wozniak JR, Mueller BA, Chang P, Muetzel RL, Caros L, Lim KO. Diffusion tensor imaging in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2006;30:1799–1806. doi: 10.1111/j.1530-0277.2006.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak JR, Muetzel RL, Mueller BA, McGee CL, Freerks MA, Ward EE, Nelson ML, Chang P, Lim KO. Microstructural corpus callosum anomalies in children with prenatal alcohol exposure: An extension of previous diffusion tensor imaging findings. Alcohol Clin Exp Res. 2009;30:1825–1835. doi: 10.1111/j.1530-0277.2009.01021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller RT, Butnariu OV, Fletcher DL, Riley EP. Limited postnatal ethanol exposure permanently alters the expression of mRNAS encoding myelin basic protein and myelin-associated glycoprotein in cerebellum. Alcohol Clin Exp Res. 1994;18:909–916. doi: 10.1111/j.1530-0277.1994.tb00059.x. [DOI] [PubMed] [Google Scholar]