Abstract
Metabotropic glutamate receptors (mGluRs) have been implicated in the regulation of anxiety, stress responses, and the neurobehavioral effects of psychostimulants. The present study was designed to examine whether antagonizing mGluR5 or activating mGluR2/3 prevents stress-induced reinstatement of cocaine seeking. Male Wistar rats were trained to self-administer cocaine and then subjected to daily extinction training for 2 weeks. Subsequent exposure to 15 min of intermittent footshock elicited robust reinstatement of responding at the previously active lever. Both the selective mGluR5 antagonist MTEP (0-3 mg/kg, IP) and the selective mGluR2/3 agonist LY379268 (0-3 mg/kg, SC) prevented cocaine seeking induced by footshock stress following the same dose-response function. The data show that although mGluR2/3 and mGluR5 are differentially located on synaptic compartments, both LY379268 and MTEP produced the same behavioral effects in reducing stress-induced reinstatement. These results are important because they demonstrate that a reduction in glutamate-mediated neural excitability (albeit via different mechanisms of action) reverses footshock-induced reinstatement and suggest that pharmacological manipulations of mGluR2/3 and mGluR5 can prevent the effects of stress, a major precipitating factor for relapse. These findings further confirm that mGluR2/3 or mGluR5 are promising targets for relapse prevention.
Keywords: addiction, LY379268, MTEP, self-administration, cocaine-seeking behavior, stress
Introduction
The development of selective ligands for metabotropic glutamate receptors (mGluRs) has provided novel opportunities for investigating the role of these receptors and, more specifically, glutamate transmission in drug addiction and anxiety. Particularly interesting in this respect are Group I (mGluR1 and mGluR5) and Group II (mGluR2/3) mGluRs because of their abundance in brain regions that mediate drug reward and incentive motivation (Kenny and Markou, 2004; Ohishi et al., 1993a, b) or regulate stress and anxiety (Chojnacka-Wojcik, Klodzinska and Pilc, 2001; Kenny and Markou, 2004; Spooren et al., 2000, 2002; Swanson et al., 2005). mGluR5 located predominantly on postsynaptic neurons where they positively modulate glutamate-induced neural excitability (Schoepp et al., 2001). In contrast, mGluR2/3 are located at the presynaptic and perisynaptic level and provide negative feedback to decrease glutamate release or synaptic glutamate availability (Ferraguti and Shigemoto, 2006; Pinheiro and Mulle, 2008; Schoepp et al., 2001).
Growing evidence implicates mGluR5 and mGluR2/3 in the modulation of several neurobehavioral effects of cocaine, including the drug's psychomotor stimulant and rewarding actions (Baptista, Martin-Fardon and Weiss, 2004; Chiamulera et al., 2001; Martin-Fardon et al., 2009). With regard to the role of mGluR5 and mGluR2/3 in cocaine addiction, pharmacological manipulation of these receptors attenuates drug-seeking behavior induced by drug-associated environmental stimuli (Baptista et al., 2004; Martin-Fardon et al., 2009) that, similar to stress, are major precipitating factors for the resumption of drug use (O'Brien et al., 1998; See, 2002; Sinha et al., 2000; Weiss, 2005). What has remained unknown, however, is whether mGluR2/3 activation or mGluR5 blockade attenuates the well-established effects of stress on cocaine seeking.
The present study sought to extend the understanding of the role of mGluR5 and mGluR2/3 in behavior linked to cocaine addiction by using two specific pharmacological tools: 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]piperidine (MTEP; a selective mGluR5 antagonist) and (−)-2-oxa-4-aminobicylco[3.1.0]hexane-4,6-dicarboxylic acid (LY379268; a selective mGluR2/3 agonist). These mGluR ligands exert potent anxiolytic-like effects in animal models of anxiety (Spooren et al., 2003; Swanson et al., 2005), thus implicating mGluR5 and mGluR2/3 in the modulation of behavioral responses to anxiety and stress, which are both important risk factors for relapse to drug use. MTEP and LY379268 reduce glutamate-mediated neural excitability (albeit via different mechanisms of action) and were tested for their effects on stress-induced cocaine seeking.
Materials and Methods
Animals
Sixty-four male Wistar rats (Charles River, Wilmington, MA; 200-250 g upon arrival) were housed 2-3 per cage in a temperature- and humidity-controlled vivarium on a reverse 12 h/12 h light/dark cycle with ad libitum access to food and water. All procedures were conducted in strict adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Drugs
Cocaine hydrochloride (National Institute on Drug Abuse, Bethesda, MD) was dissolved in sterile physiological saline. Cocaine was intravenously (IV) infused in a volume of 0.1 ml over 4 s. The mGluR2/3 agonist LY379268 (generously provided by Lilly Research Laboratories, Indianapolis, IN) was dissolved in sterile water and administered subcutaneously (SC), in a volume of 1 ml/kg. The mGluR5 antagonist MTEP (generously provided by Merck, Rahway, NJ) was dissolved in 10% (v/v) Tween 80 and administered intraperitoneally (IP) in a volume of 1 ml/kg. These different routes of administration for LY379268 and MTEP were selected based on the extensive literature that demonstrated that these conditions are the most favorable for obtaining optimal pharmacological effects of these mGluR ligands (for LY379268, see Cartmell, Monn and Schoepp, 1999; Cartmell et al., 2000b; Baptista et al., 2004; Zhao et al., 2006; Imre, 2007; Aujla, Martin-Fardon and Weiss, 2008; Sidhpura, Weiss and Martin-Fardon, 2010; for MTEP, see Anderson et al., 2003; Busse et al., 2004; Martin-Fardon et al., 2009; Sidhpura et al., 2010).
Apparatus
Animals were trained and tested in standard 29 × 24 × 19.5 cm operant conditioning chambers (BRS/LVE Inc., Laurel, MD), located inside ventilated sound-attenuating cubicles (BRS/LVE Inc.). All chambers were equipped with two retractable levers (6 cm above the grid floor), a white cue light above each lever, and a house light located at the top of the chamber's front panel. Intravenous infusions were administered by a syringe pump (Razel Scientific Instruments, Stamford, CT) located outside the sound-attenuating boxes. Testing equipment and data collection were controlled by a computer.
Self-Administration Training
Before implantation of intravenous catheters, rats were food-deprived for 24 h and maintained on a restricted diet (20 g standard laboratory chow/day) to establish lever-pressing maintained by 45 mg food pellets (PJ Noyes Company, Lancaster, NH) on a fixed-ratio 1 (FR1) timeout 20 s (TO 20) schedule of reinforcement. The TO period was signaled by illumination of a white cue light above the right lever for 20 s. Operant training for food reinforcers was continued until a criterion of at least 50 pellets per 30 min session over 3 consecutive days was reached. The animals then were returned to ad libitum food availability. Two or 3 days after the last food training session, the rats were surgically implanted with chronic jugular catheters (Caine, Lintz and Koob, 1993; Martin-Fardon et al., 2000) and given 7 days of recovery before commencing self-administration training. Self-administration of cocaine (0.25 mg/0.1 ml, IV, delivered over 4 s) began on an FR1 schedule of reinforcement in daily 120 min sessions, 5 days per week. Responses at the right, active lever were reinforced and followed by a 20 s TO period signaled by illumination of a cue light above the active lever. During this time, the lever remained inactive. Responses at the left, inactive lever had no scheduled consequences.
Extinction
Following 2 weeks of cocaine self-administration training, the rats were placed on extinction conditions in daily 2 h sessions. Extinction sessions lasted 14 days and were identical to the self-administration sessions, including the 20 s signaled TO period following each lever press, but cocaine was not available. Beginning with the 8th extinction session, the rats were placed in the boxes without extension of the levers for a 15 min waiting period before the session to habituate them to the footshock exposure procedure. Moreover, on the last day of extinction, to habituate the animals to the IP or SC injections, half of the rats were administered the LY379268 vehicle SC 30 min before the 15 min waiting period, and the other half were administered the MTEP vehicle IP 60 min before the 15 min waiting period.
Stress-Induced Reinstatement
Reinstatement tests began 1 day after the final extinction session. Thirty minutes after LY378268 (0.3, 1, and 3 mg/kg, SC) or 1 h after MTEP (0.3, 1 and 3 mg/kg, IP) administration, rats were exposed to intermittent electric footshock in the self-administration chamber for 15 min. Footshock (0.5 mA; duration, 0.5 s; mean intershock interval, 40 s; range, 10-70 s) was administered via the grid floor of the chamber. Using a between-subjects design, each animal received a single dose of either LY379268 or MTEP before testing. After termination of the footshock, the levers were extended into the chambers, and responses were recorded for 2 h. Reinstatement sessions were conducted under conditions identical to those in effect during extinction, including the 20 s signaled TO period following each lever press.
Statistical Analysis
The effects of LY379268 and MTEP on stress-induced reinstatement were analyzed by two-way between-subjects analysis of variance (ANOVA) followed by individual one-way ANOVA. The cumulative number of responses for stress-induced reinstatement were analyzed by mixed-factorial ANOVA, followed by Simple Effects analysis. Significant main effects or interactions were confirmed by Newman-Keuls post hoc tests. Differences in self-administration performance between the LY379268 and MTEP groups were analyzed by unpaired t-tests. Differences in the number of responses between the last extinction session and reinstatement session conducted under vehicle or drug (LY379268 or MTEP) conditions were analyzed by paired t-tests.
Results
During the experimental procedure, six rats were lost because of catheter patency failure (n = 4) or health complications (n = 2), reducing the number of animals to n = 58. Following 2 weeks of cocaine self-administration, all 58 rats acquired cocaine-reinforced responding and maintained stable cocaine self-administration. The rats then were divided into two groups (n = 29 animals/group) to test the effects of LY379268 and MTEP. These two groups of animals were matched according to their cocaine self-administration performance (i.e., the two groups had similar self-administration baselines; LY379268 group: 29.0 ± 2.4 responses; MTEP group: 31.0 ± 2.2 responses; unpaired t-test, t56 = −0.74; NS; Fig. 1A and 2A).
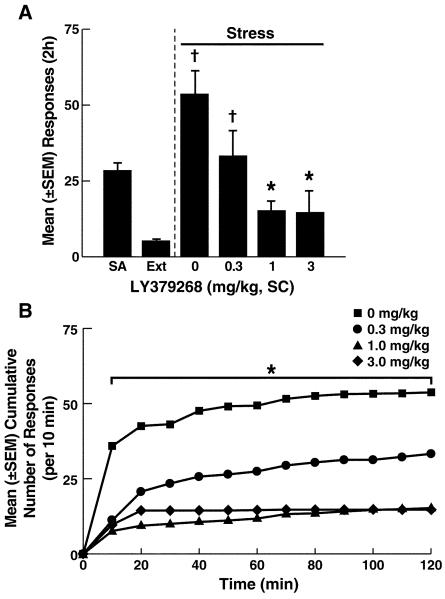
Figure 1.
Effects of LY379268 on reinstatement induced by footshock stress. A: Active lever responses during cocaine self-administration (SA) and extinction (EXT) and the effect of LY379268 on stress-induced reinstatement. Following EXT, exposure to footshock stress produced robust reinstatement of cocaine seeking (0 mg/kg), and LY379268 reversed stress-induced reinstatement at 1 and 3 mg/kg. †p < 0.05, compared with extinction (paired t-test; see Results for details); *p < 0.05, compared with 0 mg/kg. Number of animals/group: 0 mg/kg, n = 7; 0.3 mg/kg, n = 7; 1 mg/kg, n = 8; 3 mg/kg, n = 7 B: Cumulative number of responses (per 10 min intervals) throughout the 2 h reinstatement period (error bars omitted for clarity). *p < 0.05, vehicle vs. 1 and 3 mg/kg (Simple Effects analysis; see Results for details).
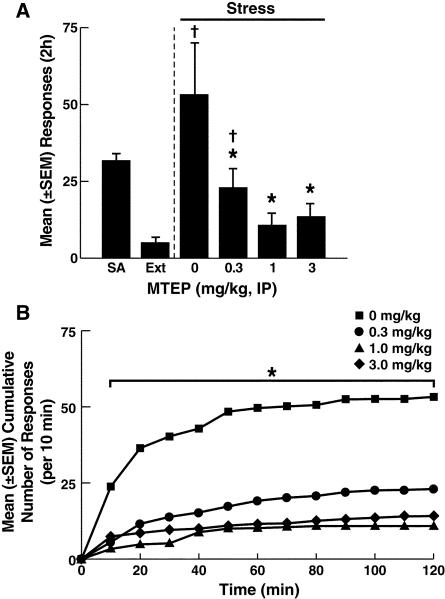
Figure 2.
Effects of MTEP on reinstatement induced by footshock stress. A: Active lever responses during cocaine self-administration (SA) and extinction (EXT) and the effect of MTEP on stress-induced reinstatement. As in Fig. 1, footshock stress produced robust reinstatement of cocaine seeking under MTEP vehicle conditions (0 mg/kg). MTEP prevented stress-induced reinstatement at 0.3, 1, and 3 mg/kg. †p < 0.05, compared with extinction (paired t-test; see Results for details), *p < 0.05, compared with 0 mg/kg Number of animals/group: 0 mg/kg, n = 7; 0.3 mg/kg, n = 7; 1 mg/kg, n = 7; 3 mg/kg, n = 8. B: Cumulative number of responses (per 10 min intervals) throughout the 2 h reinstatement period (error bars omitted for clarity). *p < 0.05, vehicle vs. 0.3, 1, and 3 mg/kg (Simple Effects analysis; see Results for details).
Fourteen days following the initiation of the extinction period, responding decreased to a mean (± SEM) of 7.3 ± 2.0 responses (Fig. 1 and 2). Exposure to footshock stress elicited significant recovery of responding in vehicle-treated (0 mg/kg) rats compared with extinction in both the LY379268 and MTEP groups (LY379268 group: paired t-test, t6 = −2.7, p < 0.05; MTEP group: paired t-test, t6 = −3.16, p < 0.05; compare Fig. 1A and 2A). Both LY379268 and MTEP similarly attenuated footshock stress-induced reinstatement of cocaine seeking, reflected by a lack of significant differences between the two groups (F1,50 = 0.35, p > 0.05) and a strong overall effect of dose (F3,50 = 7.5, p < 0.001; Fig. 1A and 2A).
Individual one-way ANOVA confirmed a significant effect of LY379268 dose (F3,25 = 3.3, p < 0.05), and the reversal of stress-induced reinstatement by LY379268 was confirmed at doses of 1 and 3 mg/kg (Newman-Keuls, p < 0.05; Fig. 1A). Moreover, LY379268 reversed footshoock-induced reinstatement at extinction levels at 1 mg/kg (paired t-test, t7 = −2.2, NS) and 3 mg/kg (paired t-test, t6 = −1.2, NS) but not at 0.3 mg/kg (paired t-test, t6 = −2.8, p < 0.05; Fig. 1A). Further examination of the temporal profile (reflected by the cumulative number of responses during the 2 h reinstatement tests) demonstrated that under vehicle conditions, reinstatement occurred during the first 50-60 min of the tests (Fig. 1B). LY379268 reduced responding associated with footshock stress per unit of time (Fig. 1B). These effects were dose-dependent, reflected by the progressive decrease in the cumulative number of responses with increasing LY379268 doses (Fig. 1B). This dose-dependent decrease of reinstatement was reflected by a main effect of LY379268 dose (F3,25 = 2.8, p < 0.05) and a dose × time (10 min intervals) interaction (F32,275 = 3.9, p < 0.001). Moreover, this analysis indicated that LY379268 decreased responding at all time-points compared with the vehicle-treated rats, with the exception of the 0.3 mg/kg dose (Simple Effects, p < 0.05; Fig 1B). Responses at the inactive lever remained minimal (≤ 5) throughout the experiment and unaltered by LY379268 (data not shown).
As confirmed by a separate analysis, MTEP reduced stress-induced reinstatement (one-way ANOVA, significant effect of dose: F3,25 = 4.5, p < 0.05; Fig. 2A), with a significant reversal of stress-induced reinstatement at all doses tested (Newman-Keuls, p < 0.05; Fig. 2A). Additionally, similar to observations in the LY379268 group, MTEP reversed footshoock-induced reinstatement at extinction levels at 1 mg/kg (paired t-test, t6 = −0.6, NS) and 3 mg/kg (paired t-test, t7 = −1.2, NS) but not at 0.3 mg/kg (paired t-test, t6 = −2.5, p < 0.05; Fig. 2A). Examination of MTEP's effects on cumulative responses confirmed that this mGluR5 antagonist modified the cumulative response profile at all doses (Fig. 2B). Similar to the observations in the LY379268 group, under MTEP vehicle conditions, reinstatement occurred during the first 50-60 min of the tests (Fig. 2B). Moreover, with increasing MTEP doses, responding decreased, reflected by a main effect of MTEP dose (F3,25 = 4.2, p < 0.05) and a dose × time interaction (F33,275 = 3.1, p < 0.001). Simple Effects analyses confirmed that MTEP decreased responding at all time-points across all doses (p < 0.05; Fig 2B). Responses at the inactive lever remained negligible (≤ 4) throughout training and testing and were not modified by MTEP (data not shown).
Discussion
The results showed that two agents known to “dampen” excitatory glutamatergic transmission by activating pre- and perisynaptic mGluR2/3 autoregulatory receptors (LY379268) or antagonizing excitatory postsynaptic mGluR5 receptors (MTEP) attenuate stress-induced reinstatement of cocaine seeking. These findings support a role for mGluR2/3 and mGluR5 receptors in anxiety-related behavior and extend evidence that showed that targeting Group I and Group II mGluRs may represent treatment targets to prevent stress-induced drug seeking during abstinence.
The modification of stress-induced reinstatement of cocaine seeking by LY379268 and MTEP cannot be attributed to nonspecific motor impairment. At the dose range used here, LY379268 and MTEP do not modify either operant responding maintained by a non-drug reinforcer (Baptista et al., 2004; Martin-Fardon et al., 2009; Zhao et al., 2006) or motor coordination and activity (Belozertseva et al., 2007; Cartmell et al., 1999, 2000a; Varty et al., 2005).
The present results clearly demonstrate that blockade of mGluR5 or activation of mGluR2/3 prevents stress-induced cocaine seeking in a similar dose-dependent manner. Although the effects of MTEP were detected at a dose as low as 0.3 mg/kg (compare Fig. 1A and 2A), no difference was detected between the effects of LY379268 and MTEP. These findings show that these two compounds, although acting at different synaptic sites, produce the same anti-stress-like behavioral effects. Because the net effect of both agents is to dampen glutamate-mediated neural excitability, and both of these agents have been described earlier to have anxiolytic-like characteristics (e.g., Spooren et al., 2003; Swanson et al., 2005; Kenny and Markou, 2004), the present results extend evidence that shows that targeting mGlu2/3 and mGlu5 receptors may prevent drug seeking in response to major triggering factors, including stress and drug-related stimuli. Consistent with these observations are earlier findings that showed that targeting these two receptor systems prevents reinstatement of a wide range of drugs of abuse, such as nicotine (Liechti et al., 2007; Palmatier et al., 2008), cocaine (Baptista et al., 2004; Martin-Fardon et al., 2009), heroin (Bossert, Busch and Gray, 2005; Bossert et al., 2006), and alcohol (Backstrom et al., 2004; Backstrom and Hyytia, 2005; Sidhpura et al., 2010; Zhao et al., 2006; for recent reviews, see Moussawi and Kalivas, 2010 [Group II mGluR]; Olive, 2009 [Group I mGluR]). Furthermore, mGluR2/3 and mGluR5 have been implicated in both drug seeking and reward function. For example, genetic or pharmacological inhibition of mGluR5 function reduces cocaine (Chiamulera et al., 2001; Hao, Martin-Fardon and Weiss, 2010; Martin-Fardon et al., 2009; Paterson and Markou, 2005), heroin (van der Kam, de Vry and Tzschentke, 2007), nicotine (Paterson and Markou, 2005), and alcohol (Cowen, Djouma and Lawrence, 2005; Olive et al., 2005; Sidhpura et al., 2010) self-administration. Similarly, in the case of Group II mGluRs, mGluR2/3 agonists attenuate the reinforcing effects of cocaine (Baptista et al., 2004; Hao et al., 2010), nicotine (Liechti et al., 2007), and alcohol (Backstrom and Hyytia, 2005; Sidhpura et al., 2010; for reviews, see Moussawi and Kalivas, 2010; Olive, 2009). Together with these earlier findings, the present data strongly suggest that targeting Group I and Group II mGluRs may be beneficial for treating several aspects of drug addiction.
The present findings have implications beyond the data that warrant further discussion. In conjunction with earlier results, the data indentify mGluR2/3 and mGluR5 as promising pharmacological targets for attenuating cocaine “craving” associated with cocaine-related environmental stimuli (Baptista et al., 2004; Martin-Fardon et al., 2009) and in the case of mGluR2/3, elevated anxiety linked to a history of cocaine dependence that persists long after withdrawal (Aujla et al., 2008). In fact, previous studies that used a conditioned reinstatement model conclusively established that both MTEP and LY379268 dose-dependently reduce reinstatement induced by cocaine-related contextual stimuli (Baptista et al., 2004; Martin-Fardon et al., 2009). However, these studies also revealed that these two compounds do not produce identical behavioral profiles. LY379268 was more effective at reversing reinstatement induced by stimuli conditioned to cocaine compared with stimuli paired with a highly palatable natural reinforcer, sweetened condensed milk (SCM) (Baptista et al., 2004). Additionally, LY379268 did not affect SCM self-administration but decreased cocaine self-administration at the highest dose tested (3 mg/kg) (Baptista et al., 2004; Hao et al., 2010), suggesting that LY379268 can be specific in the prevention of cocaine seeking induced by drug-related stimuli and stress as opposed to behavior motivated by natural reward.
MTEP dose-dependently attenuated the response-reinstating effects of both the cocaine- and SCM-related stimuli in a similar dose-dependent manner (Martin-Fardon et al., 2009). Moreover, when the effects of MTEP were tested on primary reinforcement, cocaine self-administration was found to be decreased but not behavior maintained by SCM. These data suggest that, in contrast to mGluR2/3 stimulation, blockade of mGluR5 attenuates appetitive behavior motivated by reward-related stimuli regardless of their association with a specific primary reinforcer (i.e., drug vs. conventional reinforcer) but is specific to drug reinforcers (Martin-Fardon et al., 2009). These earlier studies demonstrate that both LY379268 and MTEP are selective for drug self-administration compared with a conventional reinforcer, and MTEP has more pronounced effects than LY379268 (Baptista et al., 2004; Hao et al., 2010; Martin-Fardon et al., 2009).
The present results extend previous findings that identified mGluRs as promising targets for the prevention of relapse induced by drug-related stimuli or stress. Notably, however, drug addiction is often associated with increased drug consumption that can modify the pharmacological profile of promising therapeutic agents, possibly resulting in drug-induced neuroadaptation (for review, see Moussawi et al., 2009; Kalivas and O'Brien, 2008), a phenomenon clearly demonstrated in previous studies. Following extended-access cocaine self-administration (6 h/day), LY379268 became more efficient at preventing anxiety-like behavior and decreasing cocaine self-administration (Aujla et al., 2008; Hao et al., 2010), whereas the effects of MTEP were blunted (Hao et al., 2010). A similar behavioral pharmacological profile was observed in animals that had a history of alcohol dependence, in which LY379268 and MTEP dose-dependently reduced both alcohol self-administration and reinstatement of alcohol seeking induced by footshock stress, but LY379268 was more effective than MTEP in inhibiting both behaviors in postdependent compared with nondependent animals (Sidhpura et al., 2010). This is an important issue that requires further research to identify the most effective pharmacological tools for relapse prevention in postdependent individuals.
Conclusion
In conclusion, the results implicate mGluR2/3- and mGluR5-regulated glutamatergic transmission not only in the incentive-motivational effects of stimuli conditioned to drugs of abuse as previously established (Backstrom et al., 2004; Backstrom and Hyytia, 2005; Baptista et al., 2004; Bossert et al., 2005, 2006; Liechti et al., 2007; Martin-Fardon et al., 2009; Zhao et al., 2006), but also in the control of drug-seeking behavior induced by stress. Moreover, together with earlier findings, the data presented here suggest differential roles for mGluR2/3 and mGluR5 in drug vs. conventional reward. LY379268 appears to be more selective in preventing cocaine-seeking behavior, and mGluR5 antagonists may have substantial potential as treatment medications for cocaine addiction and relapse prevention. However, the behavioral profile of the “anti-addictive” actions of these compounds remains to be more systematically established, including verification of the absence of potential anhedonic effects and changes in pharmacological profile following dependence.
Acknowledgements
This is publication number 20098 from The Scripps Research Institute. This research was supported by NIH/NIDA grant DA07348. The authors thank Drs. James A. Monn and David McKinzie of Eli Lilly Research Laboratories (Indianapolis, IN) for providing LY379268 and Merck (Rahway, NJ) for providing MTEP. The authors also thank N. Stuempfig and J.R. Lewis for excellent technical assistance and Dr. N. Sidhpura and M. Arends for help with manuscript preparation.
Abbreviations
- LY379268
(−)-2-oxa-4-aminobicylco[3.1.0]hexane-4,6-dicarboxylic acid
- MTEP
3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]piperidine mGluR, metabotropic glutamate receptor
Footnotes
Authors Contribution
R.M.F. and F.W. participated in study concept and design. R.M.F. performed the experiments, undertook the statistical analysis, interpreted the findings, and drafted the manuscript. Both authors critically reviewed the content and approved the final version for publication.
References
- Anderson JJ, Bradbury MJ, Giracello DR, Chapman DF, Holtz G, Roppe J, King C, Cosford ND, Varney MA. In vivo receptor occupancy of mGlu5 receptor antagonists using the novel radioligand [3H]3-methoxy-5-(pyridin-2-ylethynyl)pyridine) Eur J Pharmacol. 2003;473:35–40. doi: 10.1016/s0014-2999(03)01935-6. [DOI] [PubMed] [Google Scholar]
- Aujla H, Martin-Fardon R, Weiss F. Rats with extended access to cocaine exhibit increased stress reactivity and sensitivity to the anxiolytic-like effects of the mGluR 2/3 agonist LY379268 during abstinence. Neuropsychopharmacology. 2008;33:1818–1826. doi: 10.1038/sj.npp.1301588. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Bachteler D, Koch S, Hyytia P, Spanagel R. mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology. 2004;29:921–928. doi: 10.1038/sj.npp.1300381. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Suppression of alcohol self-administration and cue-induced reinstatement of alcohol seeking by the mGlu2/3 receptor agonist LY379268 and the mGlu8 receptor agonist (S)-3,4-DCPG. Eur J Pharmacol. 2005;528:110–118. doi: 10.1016/j.ejphar.2005.10.051. [DOI] [PubMed] [Google Scholar]
- Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24:4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belozertseva IV, Kos T, Popik P, Danysz W, Bespalov AY. Antidepressant-like effects of mGluR1 and mGluR5 antagonists in the rat forced swim and the mouse tail suspension tests. Eur Neuropsychopharmacol. 2007;17:172–179. doi: 10.1016/j.euroneuro.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Busch RF, Gray SM. The novel mGluR2/3 agonist LY379268 attenuates cue-induced reinstatement of heroin seeking. Neuroreport. 2005;16:1013–1016. doi: 10.1097/00001756-200506210-00026. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Gray SM, Lu L, Shaham Y. Activation of Group II Metabotropic Glutamate Receptors in the Nucleus Accumbens Shell Attenuates Context-Induced Relapse to Heroin Seeking. Neuropsychopharmacology. 2006;3:2197–2209. doi: 10.1038/sj.npp.1300977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse CS, Brodkin J, Tattersall D, Anderson JJ, Warren N, Tehrani L, Bristow LJ, Varney MA, Cosford ND. The behavioral profile of the potent and selective mGlu5 receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine (MTEP) in rodent models of anxiety. Neuropsychopharmacology. 2004;29:1971–1979. doi: 10.1038/sj.npp.1300540. [DOI] [PubMed] [Google Scholar]
- Caine SB, Lintz R, Koob GF. Intravenous drug self-administration techniques in animals. In: Sahgal A, editor. Behavioral Neuroscience: A Practical Approach, Vol2. Oxford University Press; 1993. pp. 117–143. [Google Scholar]
- Cartmell J, Monn JA, Schoepp DD. The metabotropic glutamate 2/3 receptor agonists LY354740 and LY379268 selectively attenuate phencyclidine versus d-amphetamine motor behaviors in rats. J Pharmacol Exp Ther. 1999;291:161–170. [PubMed] [Google Scholar]
- Cartmell J, Monn JA,, Schoepp DD. Attenuation of specific PCP-evoked behaviors by the potent mGlu2/3 receptor agonist, LY379268 and comparison with the atypical antipsychotic, clozapine. Psychopharmacology (Berl) 2000a;148:423–429. doi: 10.1007/s002130050072. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Perry KW, Salhoff CR, Monn JA, Schoepp DD. The potent, selective mGlu2/3 receptor agonist LY379268 increases extracellular levels of dopamine, 3,4-dihydroxyphenylacetic acid, homovanillic acid, and 5-hydroxyindole-3-acetic acid in the medial prefrontal cortex of the freely moving rat. J Neurochem. 2000b;75:1147–1154. doi: 10.1046/j.1471-4159.2000.0751147.x. [DOI] [PubMed] [Google Scholar]
- Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- Chojnacka-Wojcik E, Klodzinska A, Pilc A. Glutamate receptor ligands as anxiolytics. Curr Opin Investig Drugs. 2001;2:1112–1119. [PubMed] [Google Scholar]
- Cowen MS, Djouma E, Lawrence AJ. The metabotropic glutamate 5 receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine reduces ethanol self-administration in multiple strains of alcohol-preferring rats and regulates olfactory glutamatergic systems. J Pharmacol Exp Ther. 2005;315:590–600. doi: 10.1124/jpet.105.090449. [DOI] [PubMed] [Google Scholar]
- Ferraguti F, Shigemoto R. Metabotropic glutamate receptors. Cell Tissue Res. 2006;326:483–504. doi: 10.1007/s00441-006-0266-5. [DOI] [PubMed] [Google Scholar]
- Hao Y, Martin-Fardon R, Weiss F. Behavioral and functional evidence of metabotropic glutamate receptor 2/3 and metabotropic glutamate receptor 5 dysregulation in cocaine-escalated rats: factor in the transition to dependence. Biol Psychiatry. 2010;68:240–248. doi: 10.1016/j.biopsych.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imre G. The preclinical properties of a novel group II metabotropic glutamate receptor agonist LY379268. CNS Drug Rev. 2007;13:444–464. doi: 10.1111/j.1527-3458.2007.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, O'Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. The ups and downs of addiction: role of metabotropic glutamate receptors. Trends Pharmacol Sci. 2004;25:265–272. doi: 10.1016/j.tips.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Lhuillier L, Kaupmann K, Markou A. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci. 2007;27:9077–9085. doi: 10.1523/JNEUROSCI.1766-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Baptista MA, Dayas CV, Weiss F. Dissociation of the effects of MTEP [3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]piperidine] on conditioned reinstatement and reinforcement: comparison between cocaine and a conventional reinforcer. J Pharmacol Exp Ther. 2009;329:1084–1090. doi: 10.1124/jpet.109.151357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Ciccocioppo R, Massi M, Weiss F. Nociceptin prevents stress-induced ethanol- but not cocaine-seeking behavior in rats. Neuroreport. 2000;11:1939–1943. doi: 10.1097/00001756-200006260-00026. [DOI] [PubMed] [Google Scholar]
- Moussawi K, Kalivas PW. Group II metabotropic glutamate receptors (mGlu2/3) in drug addiction. Eur J Pharmacol. 2010;639:115–122. doi: 10.1016/j.ejphar.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12:182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: Can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the messenger RNA for a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat. Neuroscience. 1993a;53:1009–1018. doi: 10.1016/0306-4522(93)90485-x. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: an in situ hybridization study. J Comp Neurol. 1993b;335:252–266. doi: 10.1002/cne.903350209. [DOI] [PubMed] [Google Scholar]
- Olive MF. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr Drug Abuse Rev. 2009;2:83–989. doi: 10.2174/1874473710902010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, McGeehan AJ, Kinder JR, McMahon T, Hodge CW, Janak PH, Messing RO. The mGluR5 antagonist 6-methyl-2-(phenylethynyl)pyridine decreases ethanol consumption via a protein kinase C epsilon-dependent mechanism. Mol Pharmacol. 2005;67:349–355. doi: 10.1124/mol.104.003319. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Liu X, Donny EC, Caggiula AR, Sved AF. Metabotropic glutamate 5 receptor (mGluR5) antagonists decrease nicotine seeking, but do not affect the reinforcement enhancing effects of nicotine. Neuropsychopharmacology. 2008;33:2139–2147. doi: 10.1038/sj.npp.1301623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Markou A. The metabotropic glutamate receptor 5 antagonist MPEP decreased break points for nicotine, cocaine and food in rats. Psychopharmacology (Berl) 2005;179:255–261. doi: 10.1007/s00213-004-2070-9. [DOI] [PubMed] [Google Scholar]
- Pinheiro PS, Mulle C. Presynaptic glutamate receptors: physiological functions and mechanisms of action. Nat Rev Neurosci. 2008;9:423–436. doi: 10.1038/nrn2379. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Cartmell J, Johnson BG, Salhoff CR, Wright RA, Tizzano JP, Monn JA. Metabotropic glutamate receptors: a novel approach to treat psychiatric disorders. In: Turski L, Schoepp DD, Cavalheiro EA, editors. Excitatory Amino Acids: Ten Years Later. IOS Press; Washington DC: 2001. pp. 207–216. [Google Scholar]
- See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 2002;71:517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- Sidhpura N, Weiss F, Martin-Fardon R. Effects of the mGlu2/3 agonist LY379268 and the mGlu5 antagonist MTEP on ethanol seeking and reinforcement are differentially altered in rats with a history of ethanol dependence. Biol Psychiatry. 2010;67:804–811. doi: 10.1016/j.biopsych.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O'Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl) 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Spooren W, Ballard T, Gasparini F, Amalric M, Mutel V, Schreiber R. Insight into the function of Group I and Group II metabotropic glutamate (mGlu) receptors: behavioural characterization and implications for the treatment of CNS disorders. Behav Pharmacol. 2003;14:257–277. doi: 10.1097/01.fbp.0000081783.35927.8f. [DOI] [PubMed] [Google Scholar]
- Spooren WP, Schoeffter P, Gasparini F, Kuhn R, Gentsch C. Pharmacological and endocrinological characterisation of stress-induced hyperthermia in singly housed mice using classical and candidate anxiolytics (LY314582, MPEP and NKP608) Eur J Pharmacol. 2002;435:161–170. doi: 10.1016/s0014-2999(01)01562-x. [DOI] [PubMed] [Google Scholar]
- Spooren WP, Vassout A, Neijt HC, Kuhn R, Gasparini F, Roux S, Porsolt RD, Gentsch C. Anxiolytic-like effects of the prototypical metabotropic glutamate receptor 5 antagonist 2-methyl-6-(phenylethynyl)pyridine in rodents. J Pharmacol Exp Ther. 2000;295:1267–1275. [PubMed] [Google Scholar]
- Swanson CJ, Bures M, Johnson MP, Linden AM, Monn JA, Schoepp DD. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat Rev Drug Discov. 2005;4:131–144. doi: 10.1038/nrd1630. [DOI] [PubMed] [Google Scholar]
- van der Kam EL, de Vry J, Tzschentke TM. Effect of 2-methyl-6-(phenylethynyl) pyridine on intravenous self-administration of ketamine and heroin in the rat. Behav Pharmacol. 2007;18:717–724. doi: 10.1097/FBP.0b013e3282f18d58. [DOI] [PubMed] [Google Scholar]
- Varty GB, Grilli M, Forlani A, Fredduzzi S, Grzelak ME, Guthrie DH, Hodgson RA, Lu SX, Nicolussi E, Pond AJ, Parker EM, Hunter JC, Higgins GA, Reggiani A, Bertorelli R. The antinociceptive and anxiolytic-like effects of the metabotropic glutamate receptor 5 (mGluR5) antagonists, MPEP and MTEP, and the mGluR1 antagonist, LY456236, in rodents: a comparison of efficacy and side-effect profiles. Psychopharmacology (Berl) 2005;179:207–217. doi: 10.1007/s00213-005-2143-4. [DOI] [PubMed] [Google Scholar]
- Weiss F. Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol. 2005;5:9–19. doi: 10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Dayas CV, Aujla H, Baptista MA, Martin-Fardon R, Weiss F. Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. J Neurosci. 2006;26:9967–9974. doi: 10.1523/JNEUROSCI.2384-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]