Abstract
Background
Many alcoholic patients have serum protein deficiency that contributes to their systemic problems. The unfolded protein response (UPR) is induced in response to disequilibrium in the protein folding capability of the endoplasmic reticulum (ER) and is implicated in hepatocyte lipid accumulation and apoptosis, which are associated with alcoholic liver disease. We investigated whether alcohol affects ER structure, function and UPR activation in hepatocytes in vitro and in vivo.
Methods
HepG2 cells expressing human cytochrome P450 2E1 and mouse alcohol dehydrogenase (VL-17A) were treated for up to 48 hours with 50 and 100 mM ethanol. Zebrafish larvae at 4 days post fertilization were exposed to 350 mM ethanol for 32 hours. ER morphology was visualized by fluorescence in cells and transmission electron microscopy in zebrafish. UPR target gene activation was assessed using quantitative PCR, in situ hybridization and Western blotting. Mobility of the major ER chaperone, BIP, was monitored in cells by fluorescence recovery after photobleaching (FRAP).
Results
VL-17A cells metabolized alcohol, yet only had slight activation of some UPR target genes following ethanol treatment. However, ER fragmentation, crowding and accumulation of unfolded proteins as detected by immunofluorescence and FRAP, demonstrating that alcohol induced some ER dysfunction despite the lack of UPR activation. Zebrafish treated with alcohol, however, showed modest ER dilation and several UPR targets were significantly induced.
Conclusion
Ethanol metabolism directly impairs ER structure and function in hepatocytes. Zebrafish are a novel in vivo system for studying alcoholic liver disease.
Keywords: ethanol, liver, unfolded protein response, alcoholic liver disease, zebrafish, fluorescence recovery after photobleaching
Introduction
Both chronic and acute alcohol abuse causes damage and dysfunction in the liver. Predisposition to bleeding, lower extremity edema and ascites are complications of alcoholic liver disease (ALD), which develop as a consequence of impaired serum protein secretion by the liver (Marsano et al., 2003). Given that a comparable extent of secretory dysfunction is not typically observed in patients with similar levels of hepatocellular injury due to other pathologies, it is likely that alcohol directly impairs secretory pathway function in hepatocytes. Indeed, ER dilation occurs in hepatocytes from alcoholics, even within hours of alcohol ingestion (Lane and Lieber, 1966, Rubin and Lieber, 1967, Porta et al., 1970).
Proteins destined for the plasma membrane or extracellular space are cotranslationally translocated into the endoplasmic reticulum (ER) where they are modified, folded, and exported to the Golgi apparatus. The secretory capacity of hepatocytes is tremendous: one million hepatocytes secrete ~2 mg of albumin per hour, and this is matched by similar secretion rates of other proteins (Guguen-Guillouzo et al., 1983, Le Rumeur et al., 1983, Guillouzo et al., 1984). The ability to process such a volume of secreted proteins is enabled by the unfolded protein response (UPR). This complex network of three interconnected pathways is essential for homeostasis (Rutkowski and Hegde, 2010), but aspects of this pathway can also contribute to several different pathologies, including ALD (Kaplowitz and Ji, 2006). An imbalance between the protein load and the ability of the ER to fold, process or degrade nonfoldable proteins further induces the UPR in an attempt to restore equilibrium. Failure to resolve the misfolded protein burden results in ER stress and can cause apoptosis (Tabas and Ron, 2011). Thus, the benefit of the UPR to adapt cells to a moderate accumulation of unfolded proteins is juxtaposed with cell pathology and death due to prolonged UPR activation (Tabas and Ron, 2011).
The three UPR pathways are regulated by BIP, a protein chaperone, which binds and releases mediators of each pathway: EIF2AK3 (also called PERK), ERN1 (also called IRE1A) and ATF6. These pathways collaborate to augment the protein folding capacity of the ER by upregulating levels of BIP, other chaperones, and components of ER-associated degradation (ERAD). In addition, phosphorylation of EIF2S1 (also known as eIF2α) via EIF2AK3 attenuates global mRNA translation, reducing the load of new proteins entering the ER, while selectively activating translation of ATF4 (Harding et al., 2000). In cells with ER stress, the ER is grossly dilated, dysfunctional and there is robust and prolonged UPR activation. When this persists, pro-apoptotic transcription factors including DDIT3 (also called CHOP) are induced (Tabas and Ron, 2011).
Some UPR target genes are induced in the liver in response to alcohol (Esfandiari et al., 2005, Kaplowitz and Ji, 2006, Magne et al., 2010, Shinohara et al., 2010) and functional studies have demonstrated that DDIT3 and ATF4 contribute to hepatocellular injury in response to alcohol (Ji and Kaplowitz, 2003, Ji et al., 2004, Ji et al., 2005, Magne et al., 2010). Our working model is that hepatocyte exposure to alcohol results in ER damage or functional impairment, leading to accumulation of unfolded proteins and UPR activation. Interesting new data suggests that UPR activation can also contribute to lipid accumulation in hepatocytes (steatosis) (Rutkowski et al., 2008, Yamamoto et al., 2010, Cinaroglu et al., in press), a common finding in ALD.
Zebrafish are a widely used system for studying embryogenesis (Chu and Sadler, 2009) and toxicology (Truong et al., 2011). The genetic and pharmacological tools, large sample sizes, controlled environment, uniform nutrient delivery from the yolk, small size and the expression of the full cadre of cytochrome P450 (CYP) enzymes required for xenobiotic metabolism (Goldstone et al., 2010), including Cyp2e1, make zebrafish larvae an excellent system for alcohol research. Zebrafish embryos have been used in multiple alcohol studies (Blader and Strahle, 1998, Bilotta et al., 2004, Carvan et al., 2004, Reimers et al., 2004, Arenzana et al., 2006, Dlugos and Rabin, 2007, Loucks and Ahlgren, 2009, Dlugos and Rabin, 2010), and we induced steatosis and edema in zebrafish larvae by adding ethanol to their water between 4-5.5 days post fertilization (dpf) (Passeri et al., 2009, Howarth et al., 2011).
Here, we investigate the effect of alcohol on hepatocyte ER structure, function and UPR activation using human hepatoma cells engineered to metabolize ethanol and using zebrafish larvae exposed to alcohol. We found a striking effect on the ER of both systems. However, our results from human hepatoma cells do not mimic the complexity of the response of hepatocytes in vivo. We therefore suggest zebrafish as a useful new model to study protein secretion, the UPR, and ALD.
Materials and Methods
Chemicals
All chemicals were from Sigma Aldrich (Saint Louis, MO) except ethanol (Pharmco-AAPER, Brookfield, CT) and tunicamycin (Calbiochem/EMD Chemicals, Gibbstown, NJ).
Antibodies
Rabbit polyclonal anti-fibromodulin and goat polyclonal anti-calnexin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-mouse-HRP, anti-goat-HRP, and anti-rabbit-HRP were purchased from Millipore (Billerica, MA), Jackson Immunoresearch Laboratories (Bar Harbor, ME) and Promega (Madison, WI). Rabbit polyclonal anti-CYP2E1 was a gift from J. Lasker (Hackensack University Medical Center).
Plasmids
ER-TdTomato (a gift from Michael Davidson, Florida State University) was targeted to the ER via the calreticulin signal sequence and KDEL ER retention signal (Snapp et al., 2006) and cloned into a modified N1 EGFP plasmid (Clontech, Mountain View, CA), in which the EGFP was replaced with TdTomato. Hamster BIP-sfGFP-KDEL and ER-RFP were previously described (Snapp et al., 2006, Lai et al., 2010).
Cell culture
D. Clemens (University of Nebraska Medical Center) generously provided HepG2 cells stably transfected with mouse alcohol dehydrogenase (ADH) and human CYP2E1 (VL-17A) (Donohue et al., 2006), as well as an empty vector control line (VI-7) (Clemens et al., 2002). Untransfected HepG2 cells were obtained from ATCC (Manassas, VA). Cells were grown in DMEM with 10% fetal bovine serum (FBS, Cellgro, Manassas, VA). Within 16-24 hours of transfection with Lipofectamine 2000 (Invitrogen), medium was replaced with DMEM containing 2% FBS and 0, 50, or 100 mM ethanol and changed every 24 hours. Ethanol-treated cells were cultured in an ethanol-saturated incubator as previously described (Wu et al., 2010). Cells were treated with 1 µg/mL tunicamycin for 5-6 hours, and 5 mM dithiothreitol (DTT) for 1 hour.
Fluorescence microscopy and cell counting
Cells were fixed with 4% paraformaldehyde (PFA, Electron Microscopy Sciences, Hatfield, PA). Coverslips were mounted using Vectashield containing DAPI (Vector Laboratories, Burlingame, CA) and visualized using a Leica SP5 DM confocal microscope (Leica Microsystems, Bannockburn, IL) with a 63x NA 1.4 oil objective.
ER structure was scored as normal if a regular, reticular network was present and abnormal if the network was only partially present and/or replaced by dilated or globular structures. Experiments were repeated 3 times and approximately 30 random fields were scored for each treatment.
Oil red O staining
Oil red O staining was performed as described previously (Wu et al., 2010). Lipid deposits were observed as red droplets and imaged using an Olympus BX41 microscope with a 100x NA 1.3 oil objective and a Nikon Digital Sight-Ri1 camera.
Quantitative real-time PCR
cDNA was prepared from total RNA isolated using QIAshredder and RNeasy Mini Kits (QIAGEN, Valencia, CA) and reverse transcribed using qScript cDNA SuperMix (Quanta Biosciences, Gaithersburg, MD). Quantitative, real-time PCR (qPCR) was carried out using a Light Cycler 480 (Roche, Basel, Switzerland) as previously described (Passeri et al., 2009) using PerfeCTa SYBRGreen FastMix (Quanta Biosciences). Values for target gene were normalized to reference genes GAPDH and rpp0 in human and zebrafish samples, respectively, using the equation 2-(CpTarget-CpReference).
Immunoblotting
Cells were scraped in lysis buffer (20 mM Tris, 150 mM NaCl, 1% v/v NP-40, 10% v/v glycerol, 2 mM EDTA) supplemented with 2x protease inhibitor cocktail (Roche). 60-75 livers per sample were dissected from 5.5 dpf zebrafish larvae and collected in lysis buffer. Cells and livers were lysed by sonication. Protein concentration was measured using the Bradford assay (BioRad, Hercules, CA). Lysates were mixed with an equal volume of loading buffer (10% β-mercaptoethanol/126 mM Tris/Cl (pH 6.8), 20% glycerol/4% SDS/0.02% bromophenol blue), heated for 10 minutes at 94°C and 15 μg (liver lysate) or 50 μg (cell lysate) was resolved by 10% SDS-PAGE, transferred to a PVDF membrane (Millipore) and incubated overnight with primary antibodies. For the zebrafish liver samples, total protein was stained with Amido Black (Kodak) as previously described (Aldridge et al., 2008) and quantified using ImageJ software.
Genomic DNA quantification
Dissected livers from control and ethanol-treated larvae were counted, pooled, total genomic DNA was extracted using QIAamp DNA Mini Kit (Qiagen) and quantified in triplicate using absorbance at 260 nm and averaged for each sample. The amount of DNA was divided by the number of livers per sample to obtain the amount of DNA per liver. The fold difference in genomic DNA/liver was averaged from two experiments.
Fluorescence recovery after photobleaching (FRAP)
VL-17A cells were plated in glass-bottomed Labtek slides (Nunc, Rochester, NY) and treated as above. Medium was replaced 2-6 hours prior to FRAP with phenol-red free RPMI (Invitrogen, supplemented with 0.25 mM HEPES buffer/2% FBS/ethanol). Live cells were imaged at 37°C on a Duoscan confocal microscope system (Carl Zeiss Microimaging, Thornwood, NY) with a 63x NA 1.4 oil objective, a 489 nm 100 mW diode laser with a 500–550 nm bandpass filter for GFP, and a 40 mW 561 nm diode laser with a 565 longpass filter for mRFP and TdTomato. A region of interest was photobleached at full laser power of the 489 nm line and monitoring fluorescence recovery over time. No photobleaching of the adjacent cells during the processes was observed. D measurements were calculated as described previously (Siggia et al., 2000, Snapp et al., 2003).
Zebrafish maintenance
Maintenance of adult and larval zebrafish and exposure of larvae to ethanol was performed as previously described (Passeri et al., 2009, Monson and Sadler, 2010). Briefly, 4 dpf larvae were exposed to either 0 or 2% (350 mM) ethanol for 32 hours by addition of ethanol to their water in sealed dishes to prevent evaporation. Whole livers were dissected, counted and all livers from a single clutch were pooled for protein or RNA extraction.
In situ hybridization
The probe for bip and the whole mount in situ hybridization protocol were previously described (Cinaroglu et al., in press). The probe for dnajc3 (PubMed ID: NM_199610) was generated using the primers: dnajc3F: 5’-CCTAGCCATGGGAAAGTCAA-3’, dnajc3R: 5’-GATCCTGGTCCAGCTTCAAA-3’.
Transmission electron microscopy
Larvae were anesthetized in tricaine, fixed in a 4% PFA/1% glutaraldehyde solution and processed as described (Howarth et al., 2010). Imaging was performed using a Jeol 1200EX electron microscope (Tokyo, Japan) equipped with an Advantage CCD camera (Advanced Microscopy Techniques Corporation, Danvers, MA).
Image processing, graphics and statistical analysis
Images were cropped and minimally processed using Adobe Photoshop CS4 (Adobe Systems, San Jose, CA). Graphs and statistical analyses were created and performed using Prism 5.0c (GraphPad Software Inc., La Jolla CA) and KaleidaGraph Synergy Software, Reading, PA). For qPCR data and protein content analysis, an unpaired, two-tailed t-test compared the delta Cp values between groups. For human qPCR data, either 1-way ANOVA followed by Tukey's post-hoc test or 2-way ANOVA followed by Bonferroni's post-hoc test was performed as appropriate. For cell counting, chi-square analyses with Yates’ correction were used.
Results
Ethanol causes ER fragmentation in hepatocytes
Ethanol is metabolized in hepatocytes primarily by ADH and, under saturating conditions, by CYP2E1 (Lu and Cederbaum, 2008). HepG2 cells have a secretory capacity that resembles mature hepatocytes (Knowles et al., 1980, Slany et al., 2010), but expression of ADH and CYP2E1 is very low or absent (Figure S1A) (Westerink and Schoonen, 2007b, Westerink and Schoonen, 2007a). We investigated whether HepG2 cells stably transfected with human CYP2E1 and mouse ADH (VL-17A) (Donohue et al., 2006) were a suitable model for investigating the relationship between ethanol and the ER. CYP2E1 and ADH transcripts are expressed in VL-17A cells (Figure S1A) and, as expected, that alcohol stabilized CYP2E1 (Figure S1B). In control cells, (untransfected HepG2 cells or VI-7 cells, which are HepG2 cells transfected with an empty vector (Clemens et al., 2002)) we found negligible levels of CYP2E1 mRNA (Figure S1A) and no CYP2E1 protein in VI-7 cells (Figure S1B)
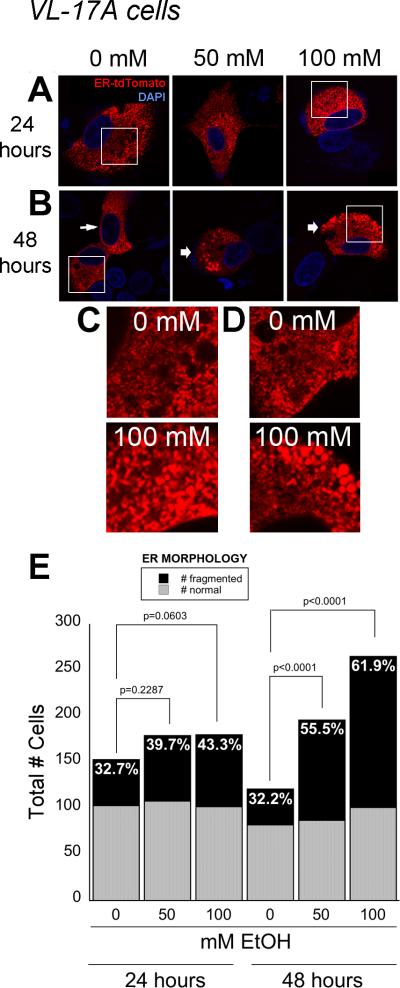
Acute and chronic alcohol exposure of hepatocytes in vivo causes ER dilation and fragmentation (Lane and Lieber, 1966, Rubin and Lieber, 1967, Baker et al., 1980). Whether this reflects ER stress has not been examined at the cellular level. We assessed ER stress based on ER structure, UPR activation and ER function. Most of the ER in untreated VL-17A cells appeared in a reticular pattern. (Figure 1A, B and 4A). However, over 55% and 60% of cells exposed to 50 and 100 mM ethanol, respectively, had fragmented ER by 48 hours of treatment (Figure 1E). This did not appear to be due to ER-tdTomato over expression, as the same finding was obtained by immunostaining with anti-calnexin (not shown) and in live cells expressing RFP or BIP-GFP targeted to the ER (see Figure 4A). Interestingly, although we found that VI-7 cells, which had a limited or absent capacity to metabolize ethanol, lacked significant changes in ER morphology in response to alcohol (Figure S2A-C). We did not see any changes in HepG2 cells exposed to alcohol (not shown). These results suggest that ethanol metabolism is necessary to produce structural changes in the ER.
Figure 1. Hepatocytes treated with alcohol in vitro have fragmented ER.
A and B: VL-17A cells were transfected with ER-TdTomato and treated with 0, 50 and 100 mM ethanol for 24 (A) or 48 (B) hours. Thin arrow represents normal ER network, while thick arrows represent fragmented and globular ER. Bar = 10 μm. C and D: Magnifications of boxed regions in A and B. E: Quantification of cells displaying fragmented and normal ER morphology after 24 and 48 hours of ethanol treatment. The percent of cells with fragmented ER is labeled on each bar. P values were calculated by Chi square analysis with Yates’ correction.
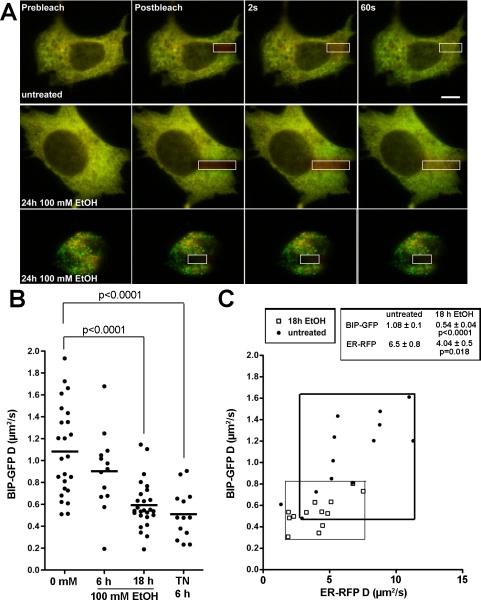
Figure 4. BIP-GFP mobility is decreased in VL-17A cells treated with alcohol.
A: VL-17A cells co-expressing BIP-GFP and ER-RFP were untreated or treated with 100 mM ethanol for 24 hours. Cells were photobleached in the region of interest indicated by the white box and fluorescence recovery was monitored over 1 minute. Recovery was observed for both channels in control cells (top two series) but not in ethanol treated cells (bottom with representative cell with fragmented ER). Scale bar = 10 um. B: FRAP showed that BIP-GFP diffusion decreased in VL-17A cells after 18 hours of 100 mM ethanol exposure. Cells treated with 1 μg/mL tunicamycin (TN) for 6 hours were used as a positive control. No significant change in BIP mobility was noted after 6 hours of ethanol exposure. C: The decrease in BIP-GFP mobility correlates with a global increase in ER luminal viscosity, as revealed by the decrease in ER-RFP mobility.
Ethanol does not cause ER stress in hepatocytes
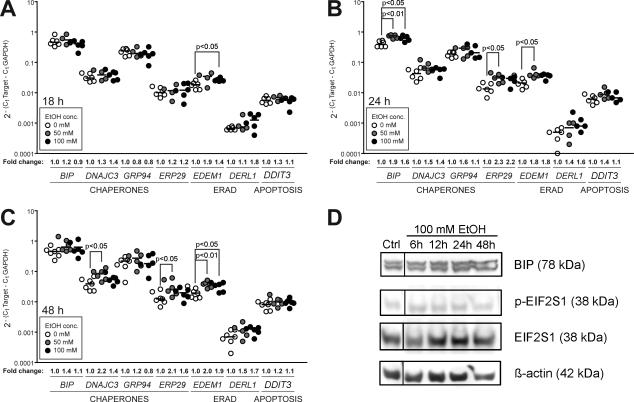
Hepatocytes are highly secretory cells and, as such, have constitutive UPR activity under normal physiological conditions. Alcohol exposure further induces the UPR in hepatocytes (Ji and Kaplowitz, 2003, Ji et al., 2004, Ji et al., 2005, Nishitani and Matsumoto, 2006, Passeri et al., 2009), but it is unclear whether UPR upregulation reflects ER stress or an adaptive response to mitigate an increased load of unfolded proteins in the ER. We differentiated between these two by examining a panel of UPR target genes and assessing the splicing and activation of XBP1, a main UPR mediator. Only some UPR target genes were slightly, but significantly, induced in VL-17A cells in response to ethanol (Figure 2A-C). However, alcohol did not affect most UPR targets, XBP1 splicing (Figure S3), expression of BIP protein or EIF2S1 phosphorylation (Figure 2D). We tested whether this could be due to an inability of these cells to mount a UPR by treating them with tunicamycin and DTT, widely used inducers of ER stress. In HepG2 cells, 6 hours of exposure to tunicamycin induces the expression of several UPR target genes (BIP, DNAJC3, DDIT3; Figure S3A) and during 2-6 hours of treatment, the amount of XBP1 splicing dramatically increases (Figure S3B). In VL-17A cells, tunicamycin also significantly induced UPR target gene expression (BIP, GRP94, EDEM1, DERL1, DDIT3; Figure S3A). However, tunicamycin did not induce XBP1 splicing to the same extent as in HepG2 cells (Figure S3B), suggesting that VL-17A cells do not fully respond to tunicamycin. Consistent with this, BIP protein was not induced by tunicamycin, and EIF2S1 phosphorylation was only observed when cells were treated with DTT (Figure S3C). This suggests that VL-17A cells may be slightly compromised in their ability to mount a robust UPR, although the reason for this is not known. Nevertheless, the degree to which the UPR is activated in VL-17A cells in response to alcohol is much less than observed when cells are treated with tunicamycin. This indicates that alcohol does not cause ER stress in hepatoma cells that can metabolize alcohol.
Figure 2. Alcohol induces some UPR target genes in VL-17A cells.
A, B, C: Expression of UPR target genes was assessed by qPCR in VL-17A cells treated with 0, 50 and 100 mM ethanol for 18 (A), 24 (B) and 48 (C) hours. Horizontal lines show the median values of at least four independent experiments. Fold changes are shown as compared to their corresponding untreated samples. Statistical significance was determined via 1-way ANOVA and Tukey's post-hoc test. D: Representative immunoblot of VL-17A cells treated with alcohol. BIP, phosphorylated EIF2S1, and total EIF2S1 levels were normalized to β-actin.
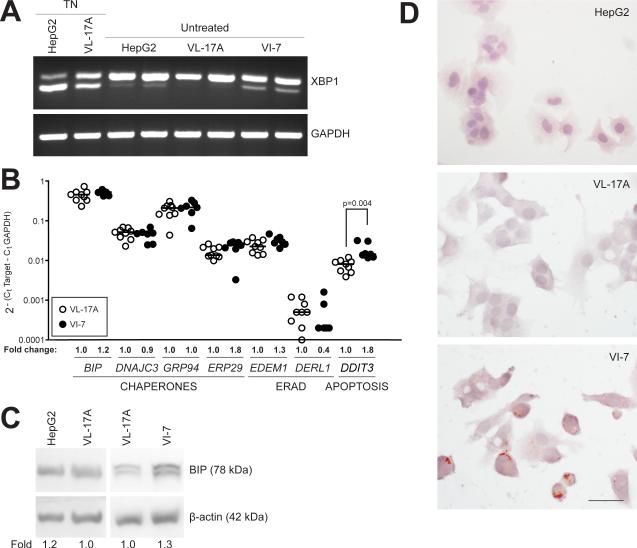
VL-7 cells have constitutive XBP1 splicing and lipid accumulation
UPR activation is associated with fatty liver disease from different etiologies (Malhi and Kaufman, 2010). However, whether UPR activation is a cause or consequence of hepatic lipid accumulation is not clear. We (Cinaroglu et al., in press) and others (Finnie, 2001, Wu et al., 2007, Rutkowski et al., 2008, Yamamoto et al., 2010) have demonstrated that tunicamycin causes ER stress and steatosis, but it is not known whether UPR activation causes fatty liver disease in other scenarios. We made a surprising discovery that under normal cell culture conditions, VI-7 cells had constitutive UPR activation as marked by XBP1 splicing (Figure 3A), increased expression of DDIT3 mRNA (Figure 3B) and BIP protein (Figure 3C), and marked lipid accumulation whereas untreated VL-17A and HepG2 cells have no lipid (Figure 3D). Additionally, VI-7 cells also grew slowly, were difficult to transfect and were susceptible to death (not shown). Although the mechanism by which the UPR is induced in these cells is unknown, these data are consistent with the hypothesis that UPR activation causes steatosis, impairs proliferation and induces apoptosis in hepatocytes.
Figure 3. VI-7 cells have constitutive XBP1 splicing, DDIT3 induction and lipid accumulation.
A: VL-17A cells treated with 1 μg/mL tunicamycin (TN) for 6 hours, and untreated HepG2, VL-17A and VI-7 cells were subject to standard PCR with XBP1 primers and the products were run in 4% agarose gel to achieve efficient separation of unspliced (U) and spliced (S) bands. VI-7 cells exhibited constitutive splicing of XBP1, which was stronger than the tunicamycin-treated VL-17A cells. B: Expression of UPR genes in untreated VL-17A and VI-7 cells. Fold changes show expression of genes compared to that of VL-17A cells. Statistical significance was determined by two-tailed, unpaired t-test. C: Representative immunoblot of BIP and β-actin on HepG2, VL-17A and VI-7 cells. Fold represents quantification of BIP normalized to actin in VI-7 cells relative to VL-17A cells in 3 experiments. * p<0.05 as calculated by one sample t-test. D: Representative images of Oil red O and hematoxylin stained HepG2, VL-17A and VI-7 cells. Bar = 10 μm.
We found that VL-17A cells did not have any detectable oil red O staining following exposure to alcohol (not shown). This indicates that the ER fragmentation and UPR target gene induction caused by alcohol treatment of these cells cannot be attributed to lipid accumulation.
BIP mobility is impaired in hepatocytes in response to alcohol
Gross accumulation of unfolded proteins in the ER increases their association with BIP, but does not generally impact the viscosity or crowdedness of the ER lumen (Dayel et al., 1999, Lai et al., 2010). We measured BIP occupancy in live cells by quantifying the mobility of hamBIP-sfGFP-KDEL (BIP-GFP) using FRAP (Figure 4A) (Lai et al., 2010). We found that 18 hours of 100 mM ethanol exposure significantly reduced BIP-GFP mobility by half (from 1.08 to 0.54 μm2/second), comparable to the change caused by tunicamycin (Figure 4B). However, tunicamycin does not alter the mobility of luminal ER reporters such as ER-RFP (Lai et al., 2010). In contrast to other UPR stressors, the decrease in BIP-GFP mobility observed after 18 hours of 100 mM ethanol exposure was likely attributed to an increase in both BIP-GFP binding to unfolded proteins and the viscosity of the ER lumen. The mobility of the ER-RFP reporter in the same cells similarly decreased (from 6.5 to 4.04 μm2/second; Figure 4C). Therefore, ethanol causes more than just misfolded protein accumulation. It globally alters the ER lumen in a way that reduces protein mobility. It is perplexing that 6 hour exposure to tunicamycin, a strong ER stress inducing agent, causes a comparable decrease BIP-GFP observed in VL-17A cells treated with alcohol for 18 hours. However, even with this similar degree of ER dysfunction, the UPR is not equivalent: tunicamycin is more effective at activating UPR target genes (compare Figure 2A with Figure S3A). Thus, despite the intriguing finding that ethanol causes changes in ER environment in VL-17A cells, our data collectively indicate that this cell system is not ideal for studying the relationship between alcohol induced ER dysfunction and UPR activation.
Hepatocytes in zebrafish with ALD have ER dilation and UPR activation
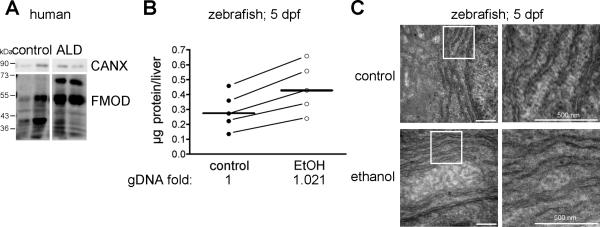
Chronic alcohol intake impairs hepatic protein secretion, as illustrated by a decrease in concentrations of secreted proteins in the serum in parallel with an increase in protein concentration in hepatocytes (Baraona and Lieber, 1982). Hepatocytes secrete a large repertoire of glycoproteins, including fibromodulin (Cubero et al., 2009). We found a 50% increase in the total amount of fibromodulin in the liver of alcoholic patients compared to healthy controls (Figure 5A). Similarly, the protein content in the liver of zebrafish exposed to alcohol was more than double to that detected in the liver of their untreated siblings (Figure 5B). While processes unrelated to secretion could explain these findings, the amount of genomic DNA did not differ between samples, consistent with other findings (Baraona et al., 1977, Baraona and Lieber, 1982, Sorrell et al., 1983) that alcohol causes secretory protein retention in hepatocytes .
Figure 5. Alcohol causes hepatocyte secretion and ER defects.
A: Liver lysates obtained from samples of healthy liver or from ALD patients were immunoblotted for fibromodulin (FMOD) and calnexin (CALX). There are multiple FMOD species that likely represent glycosylated forms of the protein. B: Livers dissected from control and ethanol treated larvae were counted and the total protein concentration was measured from five independent experiments (connected by diagonal lines). The median values are indicated by horizontal lines. The relative amount of genomic DNA per liver, as averaged from two individual experiments, is noted below the graph for each treatment group. C: Transmission electron micrographs of hepatocytes from 5.5 dpf larvae exposed to either 0 mM (-) or 350 mM (+) ethanol for 32 hours. Bar = 500 nm. The white boxes in the right panels are magnified in the left panels.
Next, we determined whether ER ultrastructure was affected by alcohol. We found that the ER lumen was expanded in the hepatocytes of ethanol treated larvae (Figure 5C), similar to what has been reported in human and rodent hepatocytes following alcohol ingestion (Lane and Lieber, 1966, Rubin and Lieber, 1967, Baraona and Lieber, 1982). This is markedly different, however, from the dilated, vesiculated structures observed in hepatocytes with ER stress (Wu et al., 2007, Yamamoto et al., 2010, Cinaroglu et al., in press).
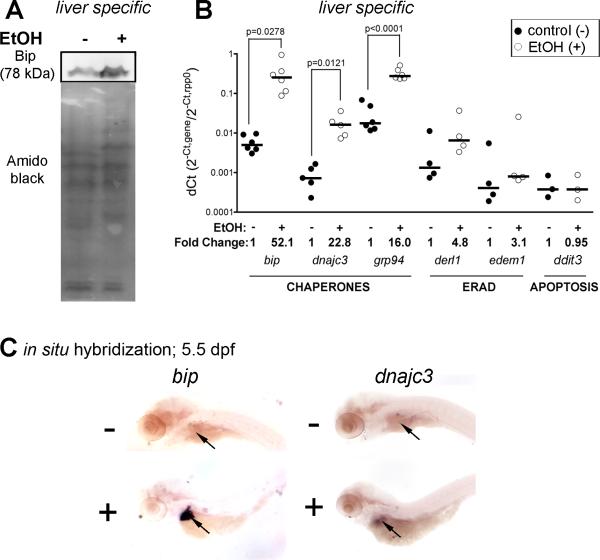
We have previously reported that alcohol causes a marked increase in hepatic bip expression in zebrafish (Passeri et al., 2009). This is reflected in an over 2-fold increase in Bip protein (Figure 6A) as normalized to total protein detected by Amido black staining. In contrast, we found no difference in the total genomic DNA in livers treated with alcohol, suggesting that the increase in total hepatic protein in alcohol treated fish is not due to an overall increase in liver cell number. While many UPR targets were not induced, protein chaperones (bip, dnajc3, and grp94) were markedly upregulated (Figure 6B). Whole mount in situ hybridization confirmed that this response was primarily observed in the liver (Figure 6C). However, xbp1 splicing does not occur in this model, suggesting that ERN1 is not activated (Passeri et al., 2009). This is similar to what we observed in VL-17A cells (Figure S3). The activation status of EIF2AK3 has not yet been determined in this system. These data demonstrate that alcohol does not cause ER stress in hepatocytes. Instead, we propose that alcohol causes an increase in misfolded and/or unfolded proteins in the ER, which induces an adaptive UPR.
Figure 6. The UPR is induced in zebrafish larvae exposed to ethanol.
A: Bip immunoblot of livers from control (-) and ethanol-treated (+; 350 mM) zebrafish. B: Quantitative, real-time PCR data from liver specific cDNA of control (black dots) and ethanol-treated (white dots) zebrafish larvae. The median is indicated by horizontal lines. Fold change is indicated below. C: Whole mount in situ hybridization for bip and dnajc3 in control and ethanol treated larvae. Arrow points to the liver.
Discussion
Serum protein secretion is significantly impaired in alcoholics (Murray-Lyon et al., 1972, Baraona and Lieber, 1982), resulting in severe extrahepatic problems (Marsano et al., 2003). Changes in hepatocyte ER morphology occur following alcohol exposure (Lane and Lieber, 1966, Rubin and Lieber, 1967, Porta et al., 1970), and therefore it is possible that ER dysfunction underlies these secretion deficiencies. However, a direct effect of alcohol on the ER has not been established. Here, we investigated the impact of alcohol on ER structure and function in vitro and in vivo. We found evidence of ER structural changes and protein folding abnormalities in both systems, however, although the hepatoma cells are human in origin, the changes observed in zebrafish hepatocytes more closely resembled to those found in rodents or humans after alcohol ingestion (Lane and Lieber, 1966, Rubin and Lieber, 1967, Porta et al., 1970, Baker et al., 1980).
There is growing appreciation of the causative role for ER stress, characterized by persistent UPR activation, gross structural ER abnormalities and dysfunction, in several diseases. In ALD, prolonged UPR activation worsens ALD (Ji and Kaplowitz, 2003, Ji et al., 2005) and improving the protein folding capacity of the ER can alleviate alcohol-induced hepatocyte injury (Neuman et al., 1995, Colell et al., 2001). Thus, it is hypothesized that UPR activation contributes to ALD. However, even though we and others (Magne et al., 2010) have shown signs of ER dysfunction and UPR activation in HepG2 cells, zebrafish hepatocytes and rodents exposed to alcohol (Ji and Kaplowitz, 2003, Ji et al., 2004, Ji et al., 2005, Nishitani and Matsumoto, 2006, Shinohara et al., 2010), this response is clearly not ER stress. Instead, these data collectively point to alcohol causing accumulation of unfolded proteins in the ER, but this has not yet progressed to ER stress, characterized by gross structural ER abnormalities, ER dysfunction and prolonged UPR activation. Previous studies demonstrated that VL-17A cells exposed to ethanol for 72 hours of ethanol causes decreased proteasome function (Osna et al., 2003). One possibility is that alcohol-induced proteasome dysfunction, could lead to the accumulation of misfolded proteins in the ER. These findings correlate with our findings of increased EDEM1 transcripts, signifying increased ERAD activity in VL-17A cells treated with ethanol (Figure 2A-C).
How does alcohol cause unfolded proteins and ER dysfunction? ADH is the primary enzyme responsible for ethanol metabolism by converting ethanol to acetaldehyde. CYP2E1 is an ER protein that metabolizes ethanol via a secondary, but important, pathway that generates reactive oxygen species in the process (Lu and Cederbaum, 2008). Oxidative stress/ROS generation by CYP2E1 plays a role in the upregulation of ATF4 and the integrated stress response following ethanol exposure in vitro (Magne et al., 2010). In addition, acetaldehyde, the product of ethanol metabolism by ADH, creates stable, covalent post-translational modifications to newly synthesized proteins and lipids as malondialdehyde adducts (Tuma and Casey, 2003). These adducts may accumulate in the ER, causing fragmentation. Finally, steatosis leads to lipid peroxidation, and this can damage ER membranes. We propose a model whereby alcohol induces the accumulation of unfolded proteins within the ER lumen, perhaps due to oxidative damage or adduct formation that result from alcohol metabolism. This then leads to UPR activation, which works to mitigate the increased load of unfolded proteins.
Cells in culture are extremely useful for studying many aspects of ALD and are the most straightforward system for live imaging studies. However, alcohol treatment of VL-17A cells did not recapitulate the secretory defects that were a well-characterized feature of ALD. Moreover, these cells appear to be partially resistant to ER stress inducing agents. It is possible that these cells already have a mild increase in the UPR, and are therefore adapted to additional ER stressors. Alternatively, they may be compromised in their ability to activate one or more UPR mediators. Given that we do not see a significantly higher baseline expression of UPR target genes in VL-17A cells compared to HepG2 cells, we favor the second hypothesis. This inability to fully activate the UPR in VL-17A cells could explain the striking difference between the large increase in UPR target gene expression observed in zebrafish, which are fully competent to induce the UPR, compared to VL-17A cells.
Nevertheless, alcohol caused significant ER fragmentation in VL-17A cells, suggesting pathological disruption of the secretory pathway. This is supported by our finding of decreased BIP mobility as a direct measure of the accumulation of unfolded proteins and decreased viscosity in the ER. In contrast, only modest induction of a few UPR target genes was detected and most markers of UPR activation were unchanged. Moreover, we were unable to detect any secretion deficiency in response to alcohol (not shown). Together, these data indicate that short-term ethanol exposure does not dramatically impair the secretory pathway of VL-17A cells. In zebrafish hepatocytes, however, alcohol disrupts ER structure and significantly induces several ER chaperones. We also observe increased protein levels in livers of treated larvae despite no change in total genomic DNA content. These findings suggest that alcohol causes protein retention in hepatocytes, and mirrors observations seen in alcoholic patients that retain fibromodulin, a secreted glycoprotein (Figure 5). However, it does not exclude the potential for increased protein synthesis in the liver.
Alcohol also causes steatosis and edema (Passeri et al., 2009, Howarth et al., 2011), suggesting disruption of hepatic lipid metabolism and serum protein deficiency as observed in alcoholics. It is possible that the differences in hepatic lipid accumulation between our in vitro cell cultures and zebrafish hepatocytes after alcohol exposure are due to variations in VLDL secretion, and this will be investigated in future studies. We propose zebrafish as a new tool in ALD research. Importantly, zebrafish embryos are transparent. This allows the use of live imaging techniques such as FRAP as a means to study the relationship between ER function and the clinically relevant phenotypes exhibited by this model: fatty liver, protein secretion defects and edema.
Supplementary Material
Acknowledgments
We are grateful to Meghan Walsh, Alex Mir, William Janssen, Chuan Gao, Peter Zhao and Defeng Wu for technical assistance, and to Dahn Clemens for VI-7 and VL-17A cells. Funding was generously provided by the American Gastroenterological Association Research Scholar Award (KCS), the NIAAA (1R011AA0188886-01 to KCS, 3R01AA017733, 3R01AA017733-01S1 to NN and p20AA017067 to KCS and NN) and the NIGMS (R01GM086530–01 to ELS). DLH was supported by the NIDDK (5T32DK007792 to S. Friedman). Confocal laser scanning microscopy performed at the MSSM-Microscopy Shared Resource Facility was supported by the NIH (5R24 CA095823-04, 1 S10 RR0 9145-01) and the NSF (DBI-9724504).
References
- Aldridge GM, Podrebarac DM, Greenough WT, Weiler IJ. The use of total protein stains as loading controls: an alternative to high-abundance single-protein controls in semi-quantitative immunoblotting. J Neurosci Methods. 2008;172:250–254. doi: 10.1016/j.jneumeth.2008.05.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenzana FJ, Carvan MJ, 3rd, Aijon J, Sanchez-Gonzalez R, Arevalo R, Porteros A. Teratogenic effects of ethanol exposure on zebrafish visual system development. Neurotoxicol Teratol. 2006;28:342–348. doi: 10.1016/j.ntt.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Baker M, Holbrook JP, Viskup RW, Penniall R. Effects of chronic ethanol feeding on rat hepatocytes. Adv Exp Med Biol. 1980;132:519–525. doi: 10.1007/978-1-4757-1419-7_53. [DOI] [PubMed] [Google Scholar]
- Baraona E, Leo MA, Borowsky SA, Lieber CS. Pathogenesis of alcohol-induced accumulation of protein in the liver. J Clin Invest. 1977;60:546–554. doi: 10.1172/JCI108806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraona E, Lieber CS. Effects of alcohol on hepatic transport of proteins. Annu Rev Med. 1982;33:281–292. doi: 10.1146/annurev.me.33.020182.001433. [DOI] [PubMed] [Google Scholar]
- Bilotta J, Barnett JA, Hancock L, Saszik S. Ethanol exposure alters zebrafish development: a novel model of fetal alcohol syndrome. Neurotoxicol Teratol. 2004;26:737–743. doi: 10.1016/j.ntt.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Blader P, Strahle U. Ethanol impairs migration of the prechordal plate in the zebrafish embryo. Dev Biol. 1998;201:185–201. doi: 10.1006/dbio.1998.8995. [DOI] [PubMed] [Google Scholar]
- Carvan MJ, 3rd, Loucks E, Weber DN, Williams FE. Ethanol effects on the developing zebrafish: neurobehavior and skeletal morphogenesis. Neurotoxicol Teratol. 2004;26:757–768. doi: 10.1016/j.ntt.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Chu J, Sadler KC. New school in liver development: lessons from zebrafish. Hepatology. 2009;50:1656–1663. doi: 10.1002/hep.23157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinaroglu A, Gao C, Imrie D, Sadler KC. Atf6 plays protective and pathologic roles in fatty liver disease due to endoplasmic reticulum stress. Hepatology. doi: 10.1002/hep.24396. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens DL, Forman A, Jerrells TR, Sorrell MF, Tuma DJ. Relationship between acetaldehyde levels and cell survival in ethanol-metabolizing hepatoma cells. Hepatology. 2002;35:1196–1204. doi: 10.1053/jhep.2002.32668. [DOI] [PubMed] [Google Scholar]
- Colell A, Coll O, Garcia-Ruiz C, Paris R, Tiribelli C, Kaplowitz N, Fernandez-Checa JC. Tauroursodeoxycholic acid protects hepatocytes from ethanol-fed rats against tumor necrosis factor-induced cell death by replenishing mitochondrial glutathione. Hepatology. 2001;34:964–971. doi: 10.1053/jhep.2001.28510. [DOI] [PubMed] [Google Scholar]
- Cubero FJ, Urtasun R, Nieto N. Alcohol and liver fibrosis. Semin Liver Dis. 2009;29:211–221. doi: 10.1055/s-0029-1214376. [DOI] [PubMed] [Google Scholar]
- Dayel MJ, Hom EF, Verkman AS. Diffusion of green fluorescent protein in the aqueous-phase lumen of endoplasmic reticulum. Biophys J. 1999;76:2843–2851. doi: 10.1016/S0006-3495(99)77438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlugos CA, Rabin RA. Ocular deficits associated with alcohol exposure during zebrafish development. J Comp Neurol. 2007;502:497–506. doi: 10.1002/cne.21320. [DOI] [PubMed] [Google Scholar]
- Dlugos CA, Rabin RA. Structural and functional effects of developmental exposure to ethanol on the zebrafish heart. Alcohol Clin Exp Res. 2010;34:1013–1021. doi: 10.1111/j.1530-0277.2010.01176.x. [DOI] [PubMed] [Google Scholar]
- Donohue TM, Osna NA, Clemens DL. Recombinant Hep G2 cells that express alcohol dehydrogenase and cytochrome P450 2E1 as a model of ethanol-elicited cytotoxicity. Int J Biochem Cell Biol. 2006;38:92–101. doi: 10.1016/j.biocel.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Esfandiari F, Villanueva JA, Wong DH, French SW, Halsted CH. Chronic ethanol feeding and folate deficiency activate hepatic endoplasmic reticulum stress pathway in micropigs. Am J Physiol Gastrointest Liver Physiol. 2005;289:G54–63. doi: 10.1152/ajpgi.00542.2004. [DOI] [PubMed] [Google Scholar]
- Finnie JW. Effect of tunicamycin on hepatocytes in vitro. J Comp Pathol. 2001;125:318–321. doi: 10.1053/jcpa.2001.0510. [DOI] [PubMed] [Google Scholar]
- Goldstone JV, McArthur AG, Kubota A, Zanette J, Parente T, Jonsson ME, Nelson DR, Stegeman JJ. Identification and developmental expression of the full complement of Cytochrome P450 genes in Zebrafish. BMC Genomics. 2010;11:643. doi: 10.1186/1471-2164-11-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guguen-Guillouzo C, Clement B, Baffet G, Beaumont C, Morel-Chany E, Glaise D, Guillouzo A. Maintenance and reversibility of active albumin secretion by adult rat hepatocytes co-cultured with another liver epithelial cell type. Exp Cell Res. 1983;143:47–54. doi: 10.1016/0014-4827(83)90107-6. [DOI] [PubMed] [Google Scholar]
- Guillouzo A, Delers F, Clement B, Bernard N, Engler R. Long term production of acute-phase proteins by adult rat hepatocytes co-cultured with another liver cell type in serum-free medium. Biochem Biophys Res Commun. 1984;120:311–317. doi: 10.1016/0006-291x(84)91255-5. [DOI] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- Howarth DL, Law SH, Law JM, Mondon JA, Kullman SW, Hinton DE. Exposure to the synthetic FXR agonist GW4064 causes alterations in gene expression and sublethal hepatotoxicity in eleutheroembryo medaka (Oryzias latipes). Toxicol Appl Pharmacol. 2010;243:111–121. doi: 10.1016/j.taap.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth DL, Passeri M, Sadler KC. Drinks Like a Fish: Using Zebrafish to Understand Alcoholic Liver Disease. Alcohol Clin Exp Res. 2011 doi: 10.1111/j.1530-0277.2010.01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji C, Deng Q, Kaplowitz N. Role of TNF-alpha in ethanol-induced hyperhomocysteinemia and murine alcoholic liver injury. Hepatology. 2004;40:442–451. doi: 10.1002/hep.20309. [DOI] [PubMed] [Google Scholar]
- Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124:1488–1499. doi: 10.1016/s0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- Ji C, Mehrian-Shai R, Chan C, Hsu YH, Kaplowitz N. Role of CHOP in hepatic apoptosis in the murine model of intragastric ethanol feeding. Alcohol Clin Exp Res. 2005;29:1496–1503. doi: 10.1097/01.alc.0000174691.03751.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplowitz N, Ji C. Unfolding new mechanisms of alcoholic liver disease in the endoplasmic reticulum. J Gastroenterol Hepatol. 2006;21:S7–S9. doi: 10.1111/j.1440-1746.2006.04581.x. [DOI] [PubMed] [Google Scholar]
- Knowles BB, Howe CC, Aden DP. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980;209:497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- Lai CW, Aronson DE, Snapp EL. BiP availability distinguishes states of homeostasis and stress in the endoplasmic reticulum of living cells. Mol Biol Cell. 2010;21:1909–1921. doi: 10.1091/mbc.E09-12-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane BP, Lieber CS. Ultrastructural alterations in human hepatocytes following ingestion of ethanol with adequate diets. Am J Pathol. 1966;49:593–603. [PMC free article] [PubMed] [Google Scholar]
- Le Rumeur E, Guguen-Guillouzo C, Beaumont C, Saunier A, Guillouzo A. Albumin secretion and protein synthesis by cultured diploid and tetraploid rat hepatocytes separated by elutriation. Exp Cell Res. 1983;147:247–254. doi: 10.1016/0014-4827(83)90293-8. [DOI] [PubMed] [Google Scholar]
- Loucks EJ, Ahlgren SC. Deciphering the role of Shh signaling in axial defects produced by ethanol exposure. Birth Defects Res A Clin Mol Teratol. 2009;85:556–567. doi: 10.1002/bdra.20564. [DOI] [PubMed] [Google Scholar]
- Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med. 2008;44:723–738. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magne L, Blanc E, Legrand B, Lucas D, Barouki R, Rouach H, Garlatti M. ATF4 and the integrated stress response are induced by ethanol and cytochrome P450 2E1 in human hepatocytes. J Hepatol. 2010 doi: 10.1016/j.jhep.2010.07.023. [DOI] [PubMed] [Google Scholar]
- Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. 2010 doi: 10.1016/j.jhep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsano LS, Mendez C, Hill D, Barve S, McClain CJ. Diagnosis and treatment of alcoholic liver disease and its complications. Alcohol Res Health. 2003;27:247–256. [PMC free article] [PubMed] [Google Scholar]
- Monson CA, Sadler KC. Inbreeding depression and outbreeding depression are evident in wild-type zebrafish lines. Zebrafish. 2010;7:189–197. doi: 10.1089/zeb.2009.0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray-Lyon IM, Clarke HG, McPherson K, Williams R. Quantitative immunoelectrophoresis of serum proteins in cryptogenic cirrhosis, alcoholic cirrhosis and active chronic hepatitis. Clin Chim Acta. 1972;39:215–220. doi: 10.1016/0009-8981(72)90318-x. [DOI] [PubMed] [Google Scholar]
- Neuman MG, Cameron RG, Shear NH, Bellentani S, Tiribelli C. Effect of tauroursodeoxycholic and ursodeoxycholic acid on ethanol-induced cell injuries in the human Hep G2 cell line. Gastroenterology. 1995;109:555–563. doi: 10.1016/0016-5085(95)90345-3. [DOI] [PubMed] [Google Scholar]
- Nishitani Y, Matsumoto H. Ethanol rapidly causes activation of JNK associated with ER stress under inhibition of ADH. FEBS Lett. 2006;580:9–14. doi: 10.1016/j.febslet.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Osna NA, Clemens DL, Donohue TM., Jr Interferon gamma enhances proteasome activity in recombinant Hep G2 cells that express cytochrome P4502E1: modulation by ethanol. Biochem Pharmacol. 2003;66:697–710. doi: 10.1016/s0006-2952(03)00252-1. [DOI] [PubMed] [Google Scholar]
- Passeri MJ, Cinaroglu A, Gao C, Sadler KC. Hepatic steatosis in response to acute alcohol exposure in zebrafish requires sterol regulatory element binding protein activation. Hepatology. 2009;49:443–452. doi: 10.1002/hep.22667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta EA, Koch OR, Hartroft WS. Recent advances in molecular pathology: a review of the effects of alcohol on the liver. Exp Mol Pathol. 1970;12:104–132. doi: 10.1016/0014-4800(70)90078-x. [DOI] [PubMed] [Google Scholar]
- Reimers MJ, Flockton AR, Tanguay RL. Ethanol- and acetaldehyde-mediated developmental toxicity in zebrafish. Neurotoxicol Teratol. 2004;26:769–781. doi: 10.1016/j.ntt.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Rubin E, Lieber CS. Early fine structural changes in the human liver induced by alcohol. Gastroenterology. 1967;52:1–13. [PubMed] [Google Scholar]
- Rutkowski DT, Hegde RS. Regulation of basal cellular physiology by the homeostatic unfolded protein response. J Cell Biol. 2010;189:783–794. doi: 10.1083/jcb.201003138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski DT, Wu J, Back SH, Callaghan MU, Ferris SP, Iqbal J, Clark R, Miao H, Hassler JR, Fornek J, Katze MG, Hussain MM, Song B, Swathirajan J, Wang J, Yau GD, Kaufman RJ. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell. 2008;15:829–840. doi: 10.1016/j.devcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M, Ji C, Kaplowitz N. Differences in betaine-homocysteine methyltransferase expression, endoplasmic reticulum stress response, and liver injury between alcohol-fed mice and rats. Hepatology. 2010;51:796–805. doi: 10.1002/hep.23391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siggia ED, Lippincott-Schwartz J, Bekiranov S. Diffusion in inhomogeneous media: theory and simulations applied to whole cell photobleach recovery. Biophysical journal. 2000;79:1761–1770. doi: 10.1016/S0006-3495(00)76428-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slany A, Haudek VJ, Zwickl H, Gundacker NC, Grusch M, Weiss TS, Seir K, Rodgarkia-Dara C, Hellerbrand C, Gerner C. Cell characterization by proteome profiling applied to primary hepatocytes and hepatocyte cell lines Hep-G2 and Hep-3B. J Proteome Res. 2010;9:6–21. doi: 10.1021/pr900057t. [DOI] [PubMed] [Google Scholar]
- Snapp EL, Altan N, Lippincott-Schwartz J. Measuring protein mobility by photobleaching GFP chimeras in living cells. 2003. Current protocols in cell biology / editorial board, Juan S. Bonifacino ... [et al Chapter 21:Unit 21 21.
- Snapp EL, Sharma A, Lippincott-Schwartz J, Hegde RS. Monitoring chaperone engagement of substrates in the endoplasmic reticulum of live cells. Proc Natl Acad Sci U S A. 2006;103:6536–6541. doi: 10.1073/pnas.0510657103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrell MF, Nauss JM, Donohue TM, Jr., Tuma DJ. Effects of chronic ethanol administration on hepatic glycoprotein secretion in the rat. Gastroenterology. 1983;84:580–586. [PubMed] [Google Scholar]
- Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong L, Harper SL, Tanguay RL. Evaluation of embryotoxicity using the zebrafish model. Methods Mol Biol. 2011;691:271–279. doi: 10.1007/978-1-60761-849-2_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuma DJ, Casey CA. Dangerous byproducts of alcohol breakdown--focus on adducts. Alcohol Res Health. 2003;27:285–290. [PMC free article] [PubMed] [Google Scholar]
- Westerink WM, Schoonen WG. Cytochrome P450 enzyme levels in HepG2 cells and cryopreserved primary human hepatocytes and their induction in HepG2 cells. Toxicol In Vitro. 2007a;21:1581–1591. doi: 10.1016/j.tiv.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Westerink WM, Schoonen WG. Phase II enzyme levels in HepG2 cells and cryopreserved primary human hepatocytes and their induction in HepG2 cells. Toxicol In Vitro. 2007b;21:1592–1602. doi: 10.1016/j.tiv.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Wu D, Wang X, Zhou R, Cederbaum A. CYP2E1 enhances ethanol-induced lipid accumulation but impairs autophagy in HepG2 E47 cells. Biochem Biophys Res Commun. 2010;402:116–122. doi: 10.1016/j.bbrc.2010.09.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Rutkowski DT, Dubois M, Swathirajan J, Saunders T, Wang J, Song B, Yau GD, Kaufman RJ. ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell. 2007;13:351–364. doi: 10.1016/j.devcel.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Takahara K, Oyadomari S, Okada T, Sato T, Harada A, Mori K. Induction of liver steatosis and lipid droplet formation in ATF6alpha-knockout mice burdened with pharmacological endoplasmic reticulum stress. Mol Biol Cell. 2010;21:2975–2986. doi: 10.1091/mbc.E09-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.