Abstract
The social motivation hypothesis posits that aberrant neural response to human faces in autism is attributable to atypical social development and consequently reduced exposure to faces. The specificity of deficits in neural specialization remains unclear, and alternative theories suggest generalized processing difficulties. The current study contrasted neural specialization for social information versus non-social information in 36 individuals with autism and 18 typically developing individuals matched for age, race, sex, handedness, and cognitive ability. Event-related potentials elicited by faces, inverted faces, houses, letters, and pseudoletters were recorded. Groups were compared on an electrophysiological marker of neural specialization (N170), as well as behavioral performance on standardized measures of face recognition and word reading/decoding. Consistent with prior results, individuals with autism displayed slowed face processing and decreased sensitivity to face inversion; however, they showed comparable brain responses to letters, which were associated with behavioral performance in both groups. Results suggest that individuals with autism display atypical neural specialization for social information but intact specialization for non-social information. They concord with the notion of specific dysfunction in social brain systems rather than non-specific information processing difficulties in autism.
Keywords: Perceptual expertise, N170, event-related potential (ERP/EEG), face perception, autism spectrum disorder
Preserved neural specialization for non-social information in autism
The ability to efficiently perceive the human face is a crucial and early-emerging social ability. Specialized processing for faces emerges in the first days of life (Bushnell, Sai & Mullin, 1989; Goren, Sarty & Wu, 1975; Johnson, Dziurawiec, Ellis & Morton, 1991; Meltzoff & Moore, 1977) and is honed by developmental experience (Nelson, 2001). Faces come to be encoded using configural processing mechanisms (Farah, Tanaka & Drain, 1995), reflected in disproportionate impairments in recognizing both upside down faces (the inversion effect (Yin, 1970) and facial features out of context (Tanaka & Farah, 1993). Functional neuroimaging studies show that faces elicit selective, right-lateralized hemodynamic activity in a portion of occipitotemporal cortex, the fusiform gyrus (Haxby et al., 1994; Kanwisher, McDermott & Chun, 1997; Puce, Allison, Gore & McCarthy, 1995), and intra-cranial electrophysiological recordings reveal face-related negative electrical activity originating from this portion of cortex (Allison, McCarthy, Novbre, Puce & Belger, 1994). Likewise, event-related potentials (ERPs) recorded from corresponding scalp regions show a negative-going electrical deflection approximately 170 milliseconds after viewing a face (N170; Bentin, Allison, Puce, Perez & McCarthy, 1996). The N170 putatively reflects structural encoding, an early stage of face processing preceding higher-order processes like recognition (Bentin, Deouell & Soroker, 1999), and is sensitive to perturbations in face configuration, including inversion (Rossion et al., 2000). Neural generators of the N170 have been localized to occipitotemporal sites including the fusiform gyrus (Itier & Taylor, 2002; Rossion, Joyce, Cottrell & Tarr, 2003; Shibata et al., 2002), as well as the superior temporal sulcus (Itier & Taylor, 2004), lingual gyrus (Shibata et al., 2002), and posterior inferotemporal gyrus (Schweinberger, Pickering, Jentzsch, Burton & Kaufmann, 2002; Shibata et al., 2002).
These processing strategies and brain regions are also observed in the perception of visual stimuli with which viewers have extensive experience (Diamond & Carey, 1986; Gauthier, 2000). Experts at perceiving and discriminating among exemplars within a visually homogenous class (e.g., Greebles, birds, or cars (Gauthier, Skudkarski, Gore & Anderson, 2000; Gauthier, Williams, Tarr & Tanaka, 1998) develop face-like patterns of brain activity, in terms of both hemodynamic (Tarr & Gauthier, 2000) and electrophysiological response, as indexed by the N170 (Rossion, Gauthier, Goffaux, Tarr & Crommelinck, 2002). According to this model, these brain regions subserve a processing style rather than specific content, and face-related brain activity reflects, in large part, human beings’ extensive experience processing human faces during development (Gauthier & Nelson, 2001).
Analogous specialization through developmental experience occurs in brain mechanisms subserving letter and word processing. Perception of printed letters (James, James, Jobard, Wong & Gauthier, 2005) and words (McCandliss, Cohen & Dehaene, 2003) selectively activates left fusiform gyrus and elicits a left-lateralized N170 in literate children as young as eight years of age (Bentin, Mouchetant-Rostaing, Giard, Echallier & Pernier, 1999; Maurer et al., 2006). A maturational course independent of higher-order phonological or semantic processes (Grossi, Coch, Coffey-Corina, Holcomb & Neville, 2001; Holcomb, Coffey & Neville, 1992) and an early time course suggest that this “letter N170” marks pre-linguistic processes related to visual perception of form (Bentin, Mouchetant-Rostaing et al., 1999) and, like the N170 elicited by faces, automatic perceptual categorization within a domain of expertise (Maurer, Brem, Bucher & Brandeis, 2005). Neural specialization for letters is revealed by enhanced N170 amplitude to familiar alphabets but not foreign alphabets or nonsensical letter approximations (pseudoletters; Wong, Gauthier, Woroch, DeBuse & Curran, 2005). Converging evidence from neuroimaging studies and ERP source localization suggest left-lateralized sources in the fusiform gyrus and the inferior occipitotemporal cortex (Cohen et al., 2000; Maurer et al., 2005; Rossion et al., 2003). Though letter-related brain activity is typically contralateral to face processing areas (Rossion et al., 2003), there is some degree of functional overlap; under special circumstances, such as precocious reading ability, right fusiform gyrus is recruited for letter and word recognition (Turkeltaub et al., 2004).
Because face perception is a well-studied social behavior, it has been employed as an avenue to understand social development in autism spectrum disorder (ASD). In ASD, decreased attention to human faces is evident by 6 to 12 months (Maestro et al., 2002; Osterling & Dawson, 1994), and abnormalities in face perception and recognition have been observed throughout the lifespan (Hobson, 1986; Hobson, Ouston & Lee, 1988; Klin et al., 1999; Langdell, 1978; Schultz, 2005; Wolf et al., 2008). Individuals with ASD often exhibit abnormal viewing patterns to faces (Jones, Carr & Klin, 2008; Klin, Jones, Schultz, Volkmar & Cohen, 2002) and hypoactivation in face-related brain areas (Schultz, 2005; Schultz et al., 2000). Studies of electrophysiological markers of face perception suggest delayed N170 to human faces and decreased sensitivity to face inversion in individuals with ASD, as well as first degree relatives (Dawson et al., 2002; Dawson, Webb, Wijsman et al., 2005; McCleery, Akshoomoff, Dobkins & Carver, 2009; McPartland, Dawson, Webb, Panagiotides & Carver, 2004; O’Connor, Hamm & Kirk, 2005, 2007; Webb, Dawson, Bernier & Panagiotides, 2006) though some studies suggest at least partially preserved face perception in some subgroups of individuals with ASD (Webb et al., 2010; Webb et al., 2009).
One theoretical explanation for these observed differences in face perception in ASD focuses on the role of developmental exposure to faces. The social motivation hypothesis (Dawson, Webb & McPartland, 2005; Schultz, 2005) posits that, due to abnormalities in social drive very early in childhood, children with ASD do not attend to faces during sensitive developmental periods. Consequently, people with ASD fail to develop typical proficiency in face processing and associated patterns of behavioral and brain specialization (Behrmann, Thomas & Humphreys, 2006). Because the social motivation hypothesis implicates social drive as the dysfunction from which face perception difficulties originate (rather than specific dysfunction of brain regions subserving face perception), it presumes that individuals with ASD, given appropriate exposure to and interest in a stimulus class, should develop both behavioral and brain specialization (Sasson, 2006). This notion is supported by a single-case study revealing behavioral and neural indices of specialization in a child with ASD during perception of cartoon characters associated with a circumscribed interest (Grelotti et al., 2005). Though others have attempted to investigate brain response associated with experience in this population (Boeschoten, Kenemans, van Engeland & Kemner, 2007), research has been stymied by difficulty finding shared areas of expertise in ASD; whereas groups of study participants experienced in perceiving faces are common, groups of individuals with ASD who share a common non-face area of expertise are rare.
The current work circumvented this difficulty by examining brain activity reflecting neural specialization for letters of the alphabet. As described above, development of specialization for letters and words is well studied and elicits brain activity similar to faces in terms of temporal characteristics and scalp topography (despite lateralization differences). This is a novel and uniquely appropriate comparison because, despite developmental disinterest towards faces and characteristic weakness in language, facility with reading has been a noted strength in ASD since Kanner’s original account (Kanner, 1943). High-functioning individuals on the autism spectrum display age-appropriate skills in single word-reading and word-decoding ability (Huemer & Mann, 2009; Nation, Clarke, Wright & Williams, 2006; Newman et al., 2006), and a subgroup possesses precocious interest and proficiency in reading, or hyperlexia (Burd, Kerbeshian & Fisher, 1985; Grigorenko et al., 2002; Klin, 2004). In this study, electrophysiological and behavioral methods were applied to compare neural specialization for faces and letters in individuals with ASD. Experiments contrasted neural response to faces versus houses, faces versus inverted faces, and letters versus pseudoletters and compared these parameters to behavioral measures assessing proficiency in face recognition and letter and word perception. Consistent with previous work, it was hypothesized that individuals with ASD would exhibit impaired face recognition and delayed brain response to faces in the right hemisphere, as well as decreased sensitivity to face inversion. In keeping with the notion that these atypicalities reflect developmental sequelae of social deficits, it was predicted that similar anomalies would not be observed for non-social stimuli; individuals with ASD would show typical skills in terms of letter and word perception and comparably enhanced response to letter stimuli with respect to unfamiliar pseudoletters.
As prior work has revealed relationships among neural correlates of face perception and behavioral measures of face recognition (McPartland et al., 2004), exploratory analyses examined relationships among neural and behavioral measures of face and letter perception.
Methods
Participants
Two groups participated in the study: individuals with ASD and medically and neuropsychiatrically healthy individuals with typical development. Exclusionary criteria for participants with ASD included seizures, neurological disease, history of serious head injury, sensory or motor impairment that would impede completion of the study protocol, active psychiatric disorder (other than ASD; screened with the Child Symptom Inventory: Fourth Edition (Gadow & Sprafkin, 1994), or medication known to affect brain electrophysiology. Additional exclusionary criteria for typical participants included the above plus learning/language disability or family history of ASD. From an existing pool of subjects involved in ongoing research at the Yale Child Study Center, participants were selected based on having a Full Scale IQ in the average range or higher (Standard Score of 80 or above; Differential Ability Scales: Second Edition (Elliott, 2007); Wechsler Intelligence Scale for Children–Fourth Edition (Wechsler, 2003); Wechsler Adult Intelligence Scale: Third Edition (Wechsler, 1997)). All individuals with ASD had a pre-existing diagnosis that was confirmed with gold standard diagnostic assessments for research: combination of parent interview (Autism Diagnostic Interview-Revised (Lord, Rutter & Le Couteur, 1994; ADI-R), semi-structured social behavior and communication assessment (Autism Diagnostic Observation Schedule; Lord et al., 2000), and clinical diagnosis based on DSM-IV-TR (American Psychiatric Association, 2000) criteria by an expert clinician. The ADI was not administered to one subject because a parent was unavailable for interviewing, and two individuals were included in the sample who failed to meet ADI-R onset criteria; for both of these high-functioning, verbal individuals, problems were not detected until enrolled in school with peers. In addition to the aforementioned exclusionary criteria, typical participants were recruited to match the ASD sample in terms of sex ethnicity, handedness (Edinburgh Handedness Inventory (Oldfield, 1971), chronological age, and Full Scale IQ (Wechsler Abbreviated Scale of Intelligence; Psychological Corporation, 1999). Groups did not significantly differ on any of these variables. Behavioral assessments could not be administered to one typical participant due to time limitations. All procedures were approved by the Human Investigation Committee at Yale School of Medicine and were carried out in accordance with the Declaration of Helsinki (1975/1983). Of an initial sample of 57 individuals with ASD and 25 typically developing participants, adequate artifact-free data was obtained from 36 and 18 participants, respectively, in the letter/pseudoletter experiment, and 32 and 17, respectively, in the face/house experiment. Table 1 displays demographic data for the complete sample; variation in sample between experiments did not introduce differences on demographic characteristics.
Table 1.
Participant Characteristics
| Typical (N=18) | ASD (N=36) | |
|---|---|---|
| Number male (Percent) | 15 (83.3) | 32 (88.9) |
| Number White (Percent) | 15 (83.3) | 34 (94.4) |
| Number right handed (Percent) | 16 (88.9) | 31 (86.1) |
| Mean age (SD) | 12.6 (2.4) | 11.2 (3.4) |
| Mean Full Scale IQ (SD) | 112.9 (13.4) | 105.2 (17.3) |
EEG procedures
Stimuli
Stimuli were administered in pseudorandom sequence in two counterbalanced blocks. The first block consisted of gray-scale digitized images of neutral faces, houses, inverted faces, and inverted houses (not included in current analyses), all displayed from a direct frontal perspective. The second block included letters and a confabulated alphabet of pseudoletters (Wong et al., 2005). Example stimuli are displayed in the legends of Figures 1 and 2. Subjects were presented with 23 stimuli from each category four times, for a total of 92 stimuli per category. Stimuli were standardized in terms of size (approximately five degrees of visual angle), background color (gray), and average luminance. To maximally engage attention to individual stimuli, participants were asked to press a button whenever a stimulus repeated (9 times for each stimulus category). Because this behavioral task was confounded with face recognition, attention to task was monitored in real time through closed-circuit video, enabling pausing of data collection and redirection of attention to stimulus presentation if needed.
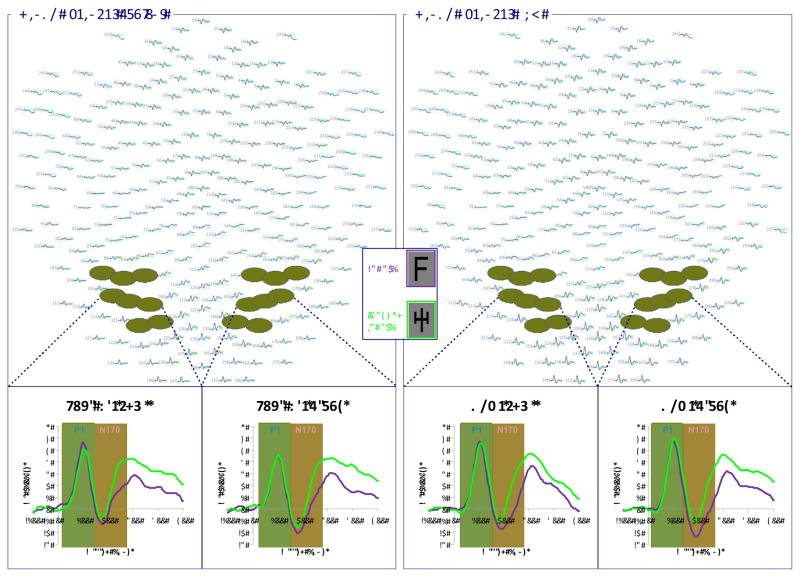
Figure 1.
Grand averaged waveforms across entire scalp for faces, houses, and inverted faces for typical participants and those with ASD. Electrodes of interest in right and left hemisphere are highlighted. Subpanels display the averaged waveform across the eight specified electrodes in each hemisphere for both groups.
Figure 2.
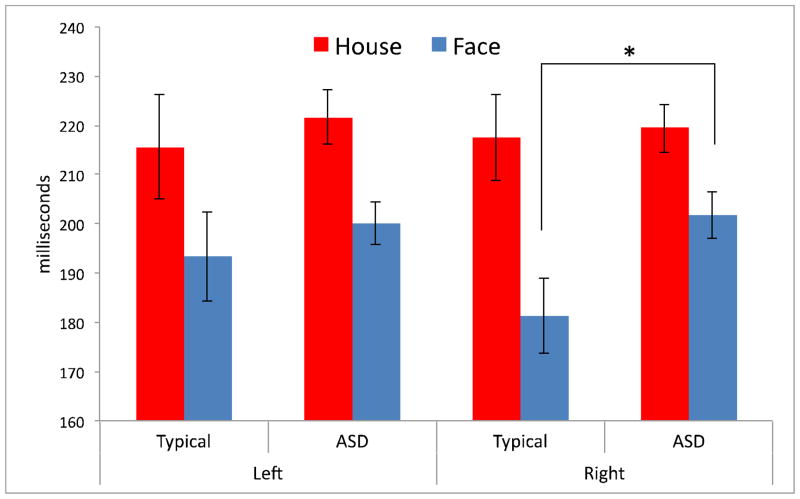
Mean latency of the N170 component (in milliseconds) elicited by faces and houses for both groups in both hemispheres. Error bars represent +/− 1 S.E. Significance at the p ≤.05 level is indicated by *.
Data collection
Stimuli were presented on a Pentium-IV computer controlling a 51 cm color monitor (75-Hz, 1024×768 resolution) running E-Prime 2.0 software (Schneider, Eschman & Zuccolotto, 2002). Displays were viewed at a distance of 90 cm in a sound attenuated room with low ambient illumination. EEG was recorded using NetStation 4.3. A 256 lead Geodesic sensor net (Electrical Geodesics Incorporated; (Tucker, 1993) was dampened with potassium-chloride electrolyte solution, placed on the participant’s head, and fitted according to the manufacturer’s specifications. Impedances were kept below 40 kilo-ohms. ERP was recorded continuously throughout each stimulus presentation trial, consisting of a fixation cross (randomly varying from 250–750 ms), stimulus (500 ms), and blank screen (500 ms). The EEG signal was amplified (×1000) and filtered (0.1 Hz high-pass filter and 100 Hz elliptical low-pass filter) via a preamplifier system (Electrical Geodesics Incorporated). The conditioned signal was multiplexed and digitized at 250 Hz using an analog-to-digital converter (National Instruments PCI-1200) and a dedicated Macintosh computer. The vertex electrode was used as a reference, and data were re-referenced to an average reference after data collection.
Data editing and reduction
Data were averaged for each subject by stimulus type across trials. Averaged data were digitally filtered with a 30 Hz low-pass filter and transformed to correct for baseline shifts. The window for segmentation of the ERP was set from 100 ms before and 500 ms after stimulus onset. NetStation artifact detection settings were set to 200 μv for bad channels, 150 μv for eye blinks, and 150 μv for eye movements. Channels with artifacts on more than 50 percent of trials were marked as bad channels and replaced through spline interpolation. Segments that contained eye blinks, eye movement, and those with more than 20 bad channels were also excluded.
Participants with less than 46 good trials for any stimulus category were excluded from analysis. Electrodes of interest were selected based on maximal observed amplitude of the N170 to faces and letters in grand averaged data and to conform to those used in previous research. Data were averaged across eight electrodes over the left (95, 96, 97, 106, 107, 108, 116, 117) and right lateral posterior scalp (151, 152, 153, 160, 161, 162, 170, 171). The time windows for P1 and N170 analysis, extending from 108 ms to 327 ms and 56 ms to 206 ms post-stimulus onset, respectively, were chosen by visual inspection of grand averaged data and then customized for each subject to confirm that the component of interest was captured at each electrode. Peak amplitude and latency to peak were averaged across each electrode group within the specified time window and were extracted for each participant for each stimulus category.
Data analysis
P1 and N170 amplitudes and latencies to peak were separately analyzed using univariate repeated measures analyses of variance (ANOVA) with two within-subject factors, condition (face/house; face/inverted face, letter/pseudoletter) and hemisphere (left/right). The between subjects factor was Group (ASD/Typical). A planned comparison using one-way ANOVA was employed to test the specific hypothesis that N170 to faces would be delayed in the right hemisphere in the ASD group relative to the typical group.
Behavioral procedures
Face perception
Face recognition was measured with the Benton Facial Recognition Test (Benton, Sivan, Hamsher, Varney & Spreen, 1994). Participants viewed a grayscale image of a face and specified one or three matches from an array of six faces, varying in shadowing and orientation.
Letter perception
The Letter-Word Identification and Word Attack subtests of the Woodcock-Johnson Tests of Achievement – Third Edition (Woodcock, McGrew & Mather, 2001) required the participant to read words aloud, with the former using genuine English words and the latter using novel words. Both subtests yielded a standard score (Mean = 100, SD = 15) derived from an age-based standardization sample.
Data analysis
Between-group differences in behavioral measures were analyzed with independent samples t-tests. Interrelationships among behavioral measures and ERP parameters (N170 latency, amplitude) were computed using Pearson Product-Moment Correlations.
Results
Electrophysiological measures
Faces versus houses: P1 Amplitude
Figure 1 displays waveforms depicting ERPs to faces and houses, and Table 2 displays mean amplitudes and standard deviations for both groups across both hemispheres and all conditions. Faces elicited smaller P1 amplitudes across hemisphere and group (main effect of Condition; F(1,47) = 18.01, p ≤.01). No other significant effects were observed (all Fs < 2.44; all ps > .05).
Table 2.
P1 and N170 amplitude
| Hemisphere | Condition | P1 | N170 | ||
|---|---|---|---|---|---|
| M (microVolts) | SD | M (microVolts) | SD | ||
| Typical group | |||||
| Left | Faces | 7.77 | 4.4 | 0.39 | 1.7 |
| Houses | 8.60 | 5.7 | 2.49 | 3.5 | |
| Inverted faces | 7.70 | 4.8 | 0.24 | 2.8 | |
| Letters | 6.28 | 3.3 | −2.27 | 3.0 | |
| Pseudoletters | 5.89 | 3.4 | −1.51 | 3.1 | |
| Right | Faces | 6.79 | 5.0 | −0.16 | 2.0 |
| Houses | 7.94 | 5.8 | 0.89 | 2.6 | |
| Inverted faces | 7.50 | 5.5 | −0.58 | 2.8 | |
| Letters | 5.03 | 2.5 | −3.03 | 4.2 | |
| Pseudoletters | 5.10 | 3.0 | −2.53 | 3.9 | |
| ASD group | |||||
| Left | Faces | 8.94 | 5.6 | −0.32 | 3.4 |
| Houses | 10.49 | 6.2 | 3.16 | 4.0 | |
| Inverted faces | 9.64 | 5.4 | 0.59 | 3.6 | |
| Letters | 6.26 | 3.8 | −3.09 | 3.6 | |
| Pseudoletters | 6.31 | 3.6 | −2.22 | 3.3 | |
| Right | Faces | 10.10 | 6.5 | 0.76 | 3.6 |
| Houses | 11.11 | 6.7 | 3.44 | 4.2 | |
| Inverted faces | 11.17 | 6.7 | 1.52 | 3.5 | |
| Letters | 6.40 | 3.3 | −3.57 | 3.1 | |
| Pseudoletters | 6.59 | 3.7 | −2.29 | 2.9 | |
Faces versus houses: P1 Latency
Table 3 displays mean latencies and standard deviations for both groups across both hemispheres and all conditions. Faces elicited shorter P1 latencies across hemisphere and group (main effect of Condition; F(1,47) = 63.92, p ≤.01). No other significant effects were observed (all Fs < 3.10; all ps > .05).
Table 3.
P1 and N170 latency
| Hemisphere | Condition | P1 | N170 | ||
|---|---|---|---|---|---|
| M (milliseconds) | SD | M (milliseconds) | SD | ||
| Typical group | |||||
| Left | Faces | 115.27 | 10.9 | 193.24 | 37.3 |
| Houses | 134.71 | 20.6 | 215.59 | 43.4 | |
| Inverted faces | 119.44 | 9.7 | 194.82 | 35.3 | |
| Letters | 106.67 | 22.0 | 177.25 | 26.9 | |
| Pseudoletters | 111.58 | 25.6 | 179.56 | 26.8 | |
| Right | Faces | 118.44 | 12.2 | 181.38 | 31.6 |
| Houses | 133.97 | 15.0 | 217.50 | 36.4 | |
| Inverted faces | 121.12 | 9.6 | 188.85 | 32.7 | |
| Letters | 104.72 | 25.8 | 181.19 | 25.9 | |
| Pseudoletters | 106.89 | 24.7 | 176.03 | 28.5 | |
| ASD group | |||||
| Left | Faces | 118.73 | 10.3 | 200.13 | 24.3 |
| Houses | 134.05 | 11.3 | 221.67 | 31.2 | |
| Inverted faces | 125.97 | 10.5 | 205.42 | 27.7 | |
| Letters | 102.68 | 13.4 | 189.21 | 26.7 | |
| Pseudoletters | 106.29 | 15.1 | 178.83 | 19.3 | |
| Right | Faces | 117.59 | 10.8 | 201.78 | 27.2 |
| Houses | 130.25 | 14.4 | 219.38 | 27.8 | |
| Inverted faces | 122.78 | 15.4 | 204.66 | 31.3 | |
| Letters | 101.53 | 14.1 | 188.42 | 28.0 | |
| Pseudoletters | 103.97 | 14.9 | 183.51 | 23.6 | |
Faces versus houses: N170 Amplitude
Faces elicited N170s with larger amplitudes (main effect of Condition; F(1,47) = 49.77, p ≤.01) across hemispheres for both groups. N170 amplitude to houses was reduced in the left hemisphere across groups (Hemisphere by Condition interaction; F(1,47) = 6.80, p ≤.01), and, relative to typical individuals, bilaterally in the ASD group (Condition by Group interaction; F(1,47) = 5.17, p ≤.05). Right-lateralization was evident only in typically developing individuals (Hemisphere by Group interaction; F(1,47) = 7.22, p ≤.01). No other significant effects were observed (all Fs < 0.90; all ps > .05).
Faces versus houses: N170 Latency
Faces elicited N170s with shorter latencies (main effect of Condition; F(1,47) = 30.10, p ≤.01) across hemispheres for both groups. A planned comparison confirmed the predicted differences in latency between groups in the right hemisphere, N170 latency to faces was significantly faster (a difference of approximately 20.4 milliseconds) in typically developing individuals than those with ASD (F(1, 47) = 5.57; p ≤.05). Figure 2 displays N170 amplitudes for faces and houses, highlighting this difference. No other significant effects were observed (all Fs < 3.09; all ps > .05).
Face versus inverted faces: P1 Amplitude
Waveforms depicting ERPs to faces and inverted faces are displayed in Figure 1. Inverted faces elicited larger P1 amplitudes across hemisphere and group (main effect of Condition; F(1,47) = 12.41, p≤ .01). P1 amplitude to inverted faces was larger relative to upright faces in the right hemisphere across groups (Hemisphere by Condition interaction; F(1,47) = 5.88, p ≤.05). No other significant effects were observed (all Fs < 3.25; all ps > .05).
Face versus inverted faces: P1 Latency
Faces elicited shorter P1 latencies across hemisphere and group (main effect of Condition; F(1,47) = 19.80, p ≤.01). No other significant effects were observed (all Fs < 3.31; all ps > .05).
Face versus inverted faces: N170 Amplitude
Across faces and inverted faces, typically developing individuals displayed enhanced amplitude in the right hemisphere, while those with ASD exhibited equivalent amplitude in both hemispheres (Hemisphere by Group interaction; F(1,47) = 7.70, p ≤.01). Across hemisphere, typically developing individuals displayed an inversion effect in the expected direction, with larger amplitude to inverted relative to upright faces, whereas individuals with ASD displayed attenuated N170 amplitudes to inverted faces relative to upright faces (Condition by Group interaction; F(1,47) = 5.84, p ≤.05). No other significant effects were observed (all Fs < 1.40; all ps > .05).
Face versus inverted faces: N170 Latency
Inverted faces elicited N170s with longer latencies than upright faces (main effect of Condition; F(1,47) = 4.66, p ≤.05) across hemispheres for both groups. No other significant effects were observed (all Fs < 2.69; all ps > .05).
Letters versus pseudoletters: P1 Amplitude
Figure 3 displays waveforms depicting ERPs to letters and pseudoletters. No significant effects were observed (all Fs < 3.08; all ps > .05).
Figure 3.
Grand averaged waveforms across entire scalp for letters and pseudoletters for typical participants and those with ASD. Electrodes of interest in right and left hemisphere are highlighted. Subpanels display the averaged waveform across the eight specified electrodes in each hemisphere for both groups.
Letters versus pseudoletters: P1 Latency
Across hemisphere and group, letters elicited shorter P1 latency than pseudoletters (main effect of Condition; F(1, 52) = 16.28, p ≤.01). Across group and condition, P1 latency was shorter in the right hemisphere (main effect of Hemisphere; F(1, 52) = 4.15, p ≤.05). No other significant effects were observed (all Fs < 1.07; all ps > .05).
Letters versus pseudoletters: N170 Amplitude
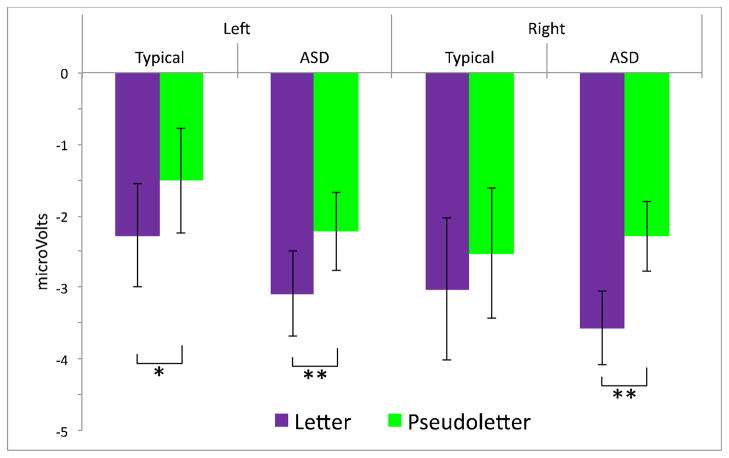
For both groups, letters elicited N170s with larger amplitudes than pseudoletters across hemispheres (main effect of Condition; F(1, 52) = 14.67, p≤ .01). As displayed in Figure 4, paired samples t-tests revealed that this effect was carried by significantly enhanced amplitude to letters versus pseudoletters in the typical group in the left hemisphere (t(1,17) = 2.12, p ≤.05) and in the ASD group in both left (t(1,35) = 2.90, p ≤.01) and right hemispheres (t(1,35) = 3.34, p ≤.01). No other significant effects were observed (all Fs < 2.68; all ps > .05).
Figure 4.
Amplitude of the N 170 component (in microVolts) elicited by letters and pseudoletters for both groups in both hemispheres. Error bars represent +/− 1 S.E. Significance at the p ≤.05 level is indicated by *, and significance at the p ≤.01 level is indicated by **.
Letters versus pseudoletters: N170 Latency
No significant effects were observed(all Fs < 3.58; all ps > .05).
Behavioral measures
Face perception
Table 2 displays mean score and standard deviation on behavioral measures for both groups. Individuals with ASD obtained significantly lower face recognition scores than typically developing individuals (t(1,51)=3.29, p ≤.01). For both groups, N170 latency to faces in the right hemisphere was correlated with face recognition skill; individuals with faster N170s displayed better face recognition performance (ASD: r = −.39, p ≤.05; Typical: r = −.53, p ≤.05). Among individuals with ASD, N170 amplitude to inverted faces was correlated with face recognition performance; those with better face recognition abilities were more likely to display an enhanced N170 associated with inversion (r = −.47, p ≤.01).
Letter perception
Groups performed comparably and in the average range on word reading and decoding tasks. Among typically developing individuals, longer N170 latency to letters in the right hemisphere was correlated with word reading score; those with longer latencies tended to perform better on the measure of single word reading (r = .64, p ≤.01).
General Discussion
The current study contrasted neural specialization for social and non-social information in individuals with ASD and a cohort of typically developing individuals of comparable age, ethnicity, sex, handedness, and cognitive ability. A critical social stimulus with which most adults possess great experience, the human face, was contrasted with a comparably complex visual stimulus without interpersonal relevance, houses. Consistent with predictions and with prior research (McPartland et al., 2004), individuals with ASD displayed a selective processing delay for human faces in the right hemisphere relative to typical counterparts. Individuals with ASD also showed reduced hemispheric specialization compared to typical counterparts, who showed a marked right lateralization effect for faces. The inversion effect, a marker of neural specialization and processing experience for faces, was evident in typically developing individuals but not those with ASD. On a behavioral measure of face recognition, individuals with ASD, despite comparable intellectual ability, performed significantly worse than typically developing counterparts. Face recognition performance was associated with processing efficiency for faces; in both groups, individuals with better face recognition abilities displayed faster N170 response. Among individuals with ASD, increased inversion effects, as reflected by a stronger response to inverted faces, were associated with better face recognition performance. This pattern of anomalies, i.e., decreased efficiency of processing, insensitivity to inversion, and impaired face recognition, is hypothesized to reflect underdeveloped specialization for faces, a downstream effect of decreased attention to faces during childhood secondary to reduced social drive from infancy (Dawson, Webb & McPartland, 2005). Indeed, the observed correlation between neural response to face inversion and recognition performance suggests that, in this case, development of expertise and processing proficiency are related.
Though differences related to condition and lateralization were observed in an earlier component, the P1, no between-group differences were detected. Given the P1’s role in basic visual attention and low-level sensory perception (Key, Dove, & Maguire, 2005), this pattern of results indicates that children with ASD did not differ from typically developing peers in terms of fundamental sensory perception. Indeed, despite observed differences in the basic sensory response to different classes of stimuli, groups responded similarly. These findings suggest normative sensory perception for visual information in ASD, with differences emerging at subsequent processing stages related to social perception.
Current findings concord with prior work describing deviant social development in ASD. However, scant evidence to-date has informed the specificity of the observed neural processing anomalies to social information. By measuring responses to non-social expert stimuli, the current study demonstrated the selectivity of perceptual deficits in ASD. Following up on work showing N170-related expertise effects for letters, ERP response to letters of the Roman alphabet were compared to a confabulated alphabet of pseudoletters (Wong et al., 2005). In contrast to the discrepancies observed during perception of social stimuli, individuals with ASD displayed neural responses comparable to typical counterparts; both groups showed enhanced N170 to familiar letters. Similar results were obtained on behavioral measures, with individuals with ASD obtaining word reading and decoding scores that were comparable to the typical participants in this study and within the average range. These results suggest intact behavioral and brain specialization for non-social information in individuals with ASD. These results are consistent with prior behavioral work demonstrating preserved word-reading and word-decoding ability in cognitively able individuals with ASD (Huemer & Mann, 2009; Nation et al., 2006; Newman et al., 2006) despite complications with reading comprehension and more sophisticated aspects of language (O’Connor & Klein, 2004). They are consistent with the notion that the social element of communication rather than language, more generally, may be most directly impacted in ASD (Paul, 2003).
The current findings have significant implications for understanding the neuropathology of autism spectrum disorders. Two prevailing classes of theories attribute autistic impairments to dysfunctional brain structures supporting social information processing (Dawson, Webb & McPartland, 2005) or altered connectivity among distributed brain regions (Minshew & Williams, 2007). The former emphasizes the import of the content that is processed, and the latter accentuates the nature of processing itself. Social brain theories posit that social information is qualitatively unique and that specific brain systems have evolved to support this type of information processing. Connectivity theories, in contrast, have traditionally argued that social information is relevant only insofar as it relies on complex or cortically distributed processing mechanisms. The current work demonstrates, for the first time in a substantial sample of children with ASD, preserved specialization for a cognitive process subserved by distributed cortical regions. Development of specialized letter processing is a process that develops over time and requires elaborate communication of anterior and posterior cortical regions (Krigolson, Pierce, Holroyd & Tanaka, 2009). The demonstration of preserved neural specialization for this type of “expert” processing in ASD is not consistent with models of non-specific, brain-wide dysfunction. Taking into account consideration considerable evidence for atypical patterns of connectivity in ASD (Minshew & Williams, 2007), current findings emphasize the potential value of studying connectivity within specific brain systems in developmental context. The observed latency delays may reflect reduced connectivity, and their specificity to social information compared to non-social information may indicate system-specificity in terms of atypical connectivity. By studying connectivity within specific neural circuits, scientists may also extricate atypical connectivity as potential cause or consequence of autistic dysfunction; it is likely that origins of dysfunction in functionally specific brain systems would, through developmental maturation, lead to broader connectivity problems. Such research may also clarify to what degree problems with connectivity uniquely differentiate autism from the diversity of developmental and psychiatric disorders also manifesting atypical connectivity, e.g. obsessive-compulsive disorder, (Garibotto et al., 2010), schizophrenia (Friston, 2002), ADHD (Murias, Swanson & Srinivasan, 2007), and intellectual impairment (Zhou et al., 2008).
This work yields clinically relevant implications for the detection and treatment of ASD. Results are supportive of the broad class of interventions designed to direct the attention of children with ASD to relevant social information. When children are appropriately engaged and attuned to information, in this case, letters, typical patterns of neural specialization develop; given the right input, the brain of a person with autism can function like that a of a typical peer, without ostensible reliance on compensatory mechanisms or alternative processing strategies. Findings add to a body of evidence that electrophysiological brain activity to faces represents a viable bio-behavioral risk marker for ASD, as temporal anomalies in neural correlates of face perception have been observed in children with ASD (Dawson, Webb, Wijsman et al., 2005) and infants at-risk for ASD (McCleery et al., 2009).
Though the current work replicates initial findings of temporal anomalies to faces (McPartland et al., 2004), these findings have not fully replicated in all samples (Kemner, Schuller & van Engeland, 2006; Senju, Tojo, Yaguchi & Hasegawa, 2005; Webb et al., 2009). Some of this variability may reflect methodological inconsistencies in terms of electrode selection (e.g., Webb et al., 2009) or employment of gold-standard diagnostic procedures (e.g., Grice, Halit, Farroni, Baron-Cohen, Bolton & Johnson, 2005); however, varied results may accurately reflect the phenotypic heterogeneity evident in ASD. Despite the unifying characteristic of social impairment, ASD presents in a remarkable diversity of manifestations, likely representing multiple etiologic pathways and developmental experiences (Jones & Klin, 2009). Considering the manner in which face processing (especially in older children and adults) has been actively shaped by experience, it is intuitive that anomalies might emerge in different ways or might not emerge universally (Jemel, Mottron & Dawson, 2006). In this regard, like any of the symptoms characterizing autism, anomalous face perception is neither necessary nor specific. It is one potential manifestation of atypical social development that, by virtue of a deep understanding of behavioral and brain bases in typical social development, is a viable avenue for investigating social disability. Variability in electrophysiological studies of face perception may also relate to differences in visual attention (Webb et al., 2009), a trend observed in hemodynamic studies (Dalton, Nacewicz, Alexander & Davidson, 2007; Dalton et al., 2005). Our employment of a pre-stimulus fixation crosshair reduces the likelihood that between-group differences are attributable to differences in visual attention. Furthermore, given comparable N170 latencies to visual fixations to eyes and mouths in typical development (McPartland, Cheung, Perszyk & Mayes, 2010) and delayed processing in ASD irrespective of point of gaze on the face (McPartland, Perszyk, Crowley, Naples & Mayes, 2011), it is unlikely that variation in visual attention alone could account for observed latency differences; resolution of this matter will ultimately require co-registration of eye-tracking and EEG.
There are several aspects of the current work that are being revisited and improved upon in ongoing research. Limiting the sample to high-functioning individuals was a necessary first step towards addressing the research questions posed in this study, but it limits generalizability to the broader range of individuals with ASD. Given that even many nonverbal children with ASD are capable of reading, these types of experiments offer a window into domains of strength and preserved neural functions of children on the autism spectrum, important goals for tailoring interventions and proscribing specific treatments. The sample in the current study focused on pre-adolescence, a time of rapid maturation of brain systems subserving face perception. Additional research in younger and older children and adults will elucidate the protracted maturational course of specialization for face perception in ASD and of letter expertise in both typical and atypical development. Of note, many participants in the current study displayed the bifid waveform morphology characteristic of pre-adult face responses (Taylor, Batty & Itier, 2004); however, this was not evident for letter N170s. Exploiting the dense spatial sampling afforded by the 256 electrode sensor net, analyses in progress are using individual-specific three-dimensional head models (computed with sensor registration images acquired with the Geodesic Photogrammetry System) to localize potentially distinct neural sources for these facets of the developing N170 (Perszyk et al., 2010).
Our current results reveal a different relationship between performance on the behavioral face processing task and brain responses in individuals with ASD than observed in a prior study (McPartland et al., 2004). Previously, results indicated slowed processing to be associated with improved face recognition. In the current study, however, the group of people with ASD, like the typically developing counterparts in the current and prior study, displayed an association between faster processing and better face recognition. We hypothesize that this reflects age-related differences in the application of compensatory strategies over time. In this younger sample, more normative brain responses correlated with more normative face recognition ability in ASD. In the older sample studied previously, the opposite trend was observed in the hemisphere contralateral to that typically associated with face perception; we interpret this as reflective of effective compensatory processing strategies. In the approximately 10 years between age 11 (current study) and age 21 (prior study), increased reliance on compensatory strategies may “overtake” weakened default processing mechanisms, ultimately resulting in better performance associated with these compensatory strategies. Though we see the pattern of results as supportive of this interpretation, it is also possible that the differences observed between studies simply reflect task effects, as the prior work relied on a visual recall task and the current study utilized a visual discrimination task with reduced memory demands. Face recognition performance in ASD is demonstrated to vary with task characteristics (McPartland, Webb, Keehn & Dawson, 2011).
Understanding developmental factors is particularly important in the current context in that neural specialization for letters is clearly a distinct phenomenon from face expertise, occurring over a relatively compressed period of time rather than from birth. Moreover, it is likely that qualitatively different types of experience are associated with the accrual of proficiency in letter versus face processing. It will thus be essential to examine development of specialized processing mechanisms for a greater variety of stimuli. Though it has been proposed that, like faces, letters are encoded using a holistic processing strategy (Martelli, Majaj & Pelli, 2005), unlike faces, letters are processed at a basic rather than subordinate level of identification (James et al., 2005). The N170 has been posited to denote specialization at this basic level of identification, while later components, such as the N250, index expertise at the subordinate level of identification (Scott, Tanaka, Sheinberg & Curran, 2006). Similar mechanisms underlying neural specialization for both faces and letters exist at early processing stages as indexed by the N170, but study of a broader range of electrophysiological components and expert stimuli will paint a clearer picture of the development and potential limitations of neural specialization in ASD.
Table 4.
Performance on behavioral measures
| Measure | Typical (N = 17) | ASD (N = 36) | ||
|---|---|---|---|---|
| M | SD | M | SD | |
| Benton Face Recognition Raw Score | 41.41 | 3.6 | 37.11 | 4.8 |
| Letter-Word Identification Standard Score | 108.41 | 9.9 | 105.67 | 15.0 |
| Word Attack Standard Score | 101.41 | 9.7 | 103.86 | 11.6 |
Acknowledgments
This research was supported by NIMH R03 MH079908, NIMH K23 MH086785, NICHD PO1HD003008, a NARSAD Atherton Young Investigator Award, and CTSA Grant Number UL1 RR024139 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH roadmap for Medical Research (USA). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
References
- Allison T, McCarthy G, Novbre A, Puce A, Belger A. Human extrastriate visual cortext and the perception of faces, words, numbers, and colors. Cerebral Cortex. 1994;5:544–554. doi: 10.1093/cercor/4.5.544. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Behrmann M, Thomas C, Humphreys K. Seeing it differently: visual processing in autism. Trends in Cognitive Sciences. 2006;10(6):258–264. doi: 10.1016/j.tics.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. Journal of Cognitive Neuroscience. 1996;8(6):551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentin S, Deouell LY, Soroker N. Selective visual streaming in face recognition: evidence from developmental prosopagnosia. Neuroreport. 1999;10(4):823–827. doi: 10.1097/00001756-199903170-00029. [DOI] [PubMed] [Google Scholar]
- Bentin S, Mouchetant-Rostaing Y, Giard MH, Echallier JF, Pernier J. ERP manifestations of processing printed words at different psycholinguistic levels: time course and scalp distribution. Journal of Cognitive Neuroscience. 1999;11(3):235–260. doi: 10.1162/089892999563373. [DOI] [PubMed] [Google Scholar]
- Benton A, Sivan A, Hamsher K, Varney N, Spreen O. Contributions to Neuropsychological Assessment. New York: Oxford University Press; 1994. [Google Scholar]
- Boeschoten MA, Kenemans JL, van Engeland H, Kemner C. Face processing in Pervasive Developmental Disorder (PDD): the roles of expertise and spatial frequency. Journal of Neural Transmission. 2007;114(12):1619–1629. doi: 10.1007/s00702-007-0780-y. [DOI] [PubMed] [Google Scholar]
- Burd L, Kerbeshian J, Fisher W. Inquiry into the incidence of hyperlexia in a statewide population of children with pervasive developmental disorder. Psychological Reports. 1985;57(1):236–238. doi: 10.2466/pr0.1985.57.1.236. [DOI] [PubMed] [Google Scholar]
- Bushnell I, Sai F, Mullin J. Neonatal recognition of the mother’s face. British Journal of Developmental Pscyhology. 1989;7:3–15. [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene-Lambertz G, Henaff MA, Michel F. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123(2):291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Alexander AL, Davidson RJ. Gaze-fixation, brain activation, and amygdala volume in unaffected siblings of individuals with autism. Biological Psychiatry. 2007;61(4):512–520. doi: 10.1016/j.biopsych.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, Alexander AL, Davidson RJ. Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience. 2005;8(4):519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Carver L, Meltzoff AN, Panagiotides H, McPartland J, Webb SJ. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Development. 2002;73(3):700–717. doi: 10.1111/1467-8624.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, McPartland J. Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Developmental Neuropsychology. 2005;27(3):403–424. doi: 10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, Wijsman E, Schellenberg G, Estes A, Munson J, Faja S. Neurocognitive and electrophysiological evidence of altered face processing in parents of children with autism: implications for a model of abnormal development of social brain circuitry in autism. Development and Psychopathology. 2005;17(3):679–697. doi: 10.1017/S0954579405050327. [DOI] [PubMed] [Google Scholar]
- Diamond R, Carey S. Why faces are and are not special: An effect of expertise. Journal of Experimental Psychology. 1986;115(2):107–117. doi: 10.1037//0096-3445.115.2.107. [DOI] [PubMed] [Google Scholar]
- Elliott C. The Differential Ability Scales. 2. San Antonio, TX: Harcourt Assessment; 2007. [Google Scholar]
- Farah MJ, Tanaka JW, Drain HM. What causes the face inversion effect? Journal of Experimental Psychology: Human Perception and Performance. 1995;21(3):628–634. doi: 10.1037//0096-1523.21.3.628. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Dysfunctional connectivity in schizophrenia. World Psychiatry. 2002;1(2):66–71. [PMC free article] [PubMed] [Google Scholar]
- Gadow K, Sprafkin J. Child Symptom Inventories manual. Stony Brook, NY: Checkmate Plus; 1994. [Google Scholar]
- Garibotto V, Scifo P, Gorini A, Alonso CR, Brambati S, Bellodi L, Perani D. Disorganization of anatomical connectivity in obsessive compulsive disorder: A multi-parameter diffusion tensor imaging study in a subpopulation of patients. Neurobiology of Disease. 2010;37(2):468–476. doi: 10.1016/j.nbd.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Nelson C. The development of face expertise. Current Opinion in Neurobiology. 2001;11:219–224. doi: 10.1016/s0959-4388(00)00200-2. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Skudkarski P, Gore J, Anderson A. Expertise for cars and birds recruits brain areas involved in face recognition. Nature Neuroscience. 2000;3(2):191–197. doi: 10.1038/72140. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Williams P, Tarr MJ, Tanaka J. Training ‘greeble’ experts: a framework for studying expert object recognition processes. Vision Research. 1998;38(15–16):2401–2428. doi: 10.1016/s0042-6989(97)00442-2. [DOI] [PubMed] [Google Scholar]
- Gauthier I. What constrains the organization of the ventral temporal cortex? Trends in Cognitive Sciences. 2000;4(1):1–2. doi: 10.1016/s1364-6613(99)01416-3. [DOI] [PubMed] [Google Scholar]
- Goren CC, Sarty M, Wu PY. Visual following and pattern discrimination of face-like stimuli by newborn infants. Pediatrics. 1975;56(4):544–549. [PubMed] [Google Scholar]
- Grelotti DJ, Klin AJ, Gauthier I, Skudlarski P, Cohen DJ, Gore JC, Volkmar FR, Schultz RT. fMRI activation of the fusiform gyrus and amygdala to cartoon characters but not to faces in a boy with autism. Neuropsychologia. 2005;43(3):373–385. doi: 10.1016/j.neuropsychologia.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Grice SJ, Halit H, Farroni T, Baron-Cohen S, Bolton P, Johnson M. Neural correlates of eye-gaze detection in young children with autism. Cortex. 2005;4(3):342–353. doi: 10.1016/s0010-9452(08)70271-5. [DOI] [PubMed] [Google Scholar]
- Grigorenko EL, Klin A, Pauls DL, Senft R, Hooper C, Volkmar F. A descriptive study of hyperlexia in a clinically referred sample of children with developmental delays. Journal of Autism and Developmental Disorders. 2002;32(1):3–12. doi: 10.1023/a:1017995805511. [DOI] [PubMed] [Google Scholar]
- Grossi G, Coch D, Coffey-Corina S, Holcomb PJ, Neville HJ. Phonological processing in visual rhyming: a developmental erp study. Journal of Cognitive Neuroscience. 2001;13(5):610–625. doi: 10.1162/089892901750363190. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Grady CL, Horwitz B, Ungerleider JM, Maisog M, Pietrini P. The functional organization of human extrastriate cortext: A pet-rCBFstudy of selective attention to faces and locations. Journal of Neuroscience. 1994;14:6336–6353. doi: 10.1523/JNEUROSCI.14-11-06336.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson R. The autistic child’s appraisal of expressions of emotion. Journal of Child Psychology and Psychiatry. 1986;27(3):321–342. doi: 10.1111/j.1469-7610.1986.tb01836.x. [DOI] [PubMed] [Google Scholar]
- Hobson R, Ouston J, Lee A. What’s in a face? The case of autism. British Journal of Psychology. 1988;79:441–453. doi: 10.1111/j.2044-8295.1988.tb02745.x. [DOI] [PubMed] [Google Scholar]
- Holcomb P, Coffey S, Neville H. Visual and auditory sentence processing: a developmental analysis using event-related brain potentials. Developmental Neuropsychology. 1992;8(2–3):203–241. [Google Scholar]
- Huemer SV, Mann V. A Comprehensive Profile of Decoding and Comprehension in Autism Spectrum Disorders. Journal of Autism and Developmental Disorders. 2009;40(4):485–493. doi: 10.1007/s10803-009-0892-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itier RJ, Taylor MJ. Inversion and contrast polarity reversal affect both encoding and recognition processes of unfamiliar faces: a repetition study using ERPs. Neuroimage. 2002;15(2):353–372. doi: 10.1006/nimg.2001.0982. [DOI] [PubMed] [Google Scholar]
- Itier RJ, Taylor MJ. Source analysis of the N170 to faces and objects. Neuroreport. 2004;15(8):1261–1265. doi: 10.1097/01.wnr.0000127827.73576.d8. [DOI] [PubMed] [Google Scholar]
- James KH, James TW, Jobard G, Wong AC, Gauthier I. Letter processing in the visual system: different activation patterns for single letters and strings. Cognitive, Affective, and Behavioral Neuroscience. 2005;5(4):452–466. doi: 10.3758/cabn.5.4.452. [DOI] [PubMed] [Google Scholar]
- Jemel B, Mottron L, Dawson M. Impaired face processing in autism: fact or artifact? Journal of Autism and Developmental Disorders. 2006;36(1):91–106. doi: 10.1007/s10803-005-0050-5. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Dziurawiec S, Ellis H, Morton J. Newborns’ preferential tracking of face-like stimuli and its subsequent decline. Cognition. 1991;40(1–2):1–19. doi: 10.1016/0010-0277(91)90045-6. [DOI] [PubMed] [Google Scholar]
- Jones W, Carr K, Klin A. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Archives of General Psychiatry. 2008;65(8):946–954. doi: 10.1001/archpsyc.65.8.946. [DOI] [PubMed] [Google Scholar]
- Jones W, Klin A. Heterogeneity and homogeneity across the autism spectrum: the role of development. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(5):471–473. doi: 10.1097/CHI.0b013e31819f6c0d. [DOI] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemner C, Schuller AM, van Engeland H. Electrocortical reflections of face and gaze processing in children with pervasive developmental disorder. Journal of Child Psychology and Psychiatry. 2006;47(10):1063–1072. doi: 10.1111/j.1469-7610.2006.01678.x. [DOI] [PubMed] [Google Scholar]
- Key AP, Dove GO, Maguire MJ. Linking brainwaves to the brain: an ERP primer. Developmental Neuropsychology. 2005;27(2):183–215. doi: 10.1207/s15326942dn2702_1. [DOI] [PubMed] [Google Scholar]
- Klin A. Understanding circumscribed interests in autism spectrum disorders. Paper presented at the The 25th Annual TEACCH Conference; Chapel Hill, NC. 2004. [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Archives of General Psychiatry. 2002;59(9):809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Klin A, Sparrow S, De Bildt A, Cicchetti D, Cohen D, Volkmar F. A normed study of face recognition in autism and related disorders. Journal of Autism and Developmental Disorders. 1999;29(6):499–508. doi: 10.1023/a:1022299920240. [DOI] [PubMed] [Google Scholar]
- Krigolson OE, Pierce LJ, Holroyd CB, Tanaka JW. Learning to become an expert: reinforcement learning and the acquisition of perceptual expertise. Journal of Cognitive Neuroscience. 2009;21(9):1834–1841. doi: 10.1162/jocn.2009.21128. [DOI] [PubMed] [Google Scholar]
- Langdell T. Recognition of faces: an approach to the study of autism. Journal of Child Psychology and Psychiatry. 1978;19(3):255–268. doi: 10.1111/j.1469-7610.1978.tb00468.x. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The Autism Diagnostic Observation Schedule--Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Maestro S, Muratori F, Cavallaro MC, Pei F, Stern D, Golse B, Palacio-Espasa F. Attentional skills during the first 6 months of age in autism spectrum disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(10):1239–1245. doi: 10.1097/00004583-200210000-00014. [DOI] [PubMed] [Google Scholar]
- Martelli M, Majaj NJ, Pelli DG. Are faces processed like words? A diagnostic test for recognition by parts. Journal of Vision. 2005;5(1):58–70. doi: 10.1167/5.1.6. [DOI] [PubMed] [Google Scholar]
- Maurer U, Brem S, Bucher K, Brandeis D. Emerging neurophysiological specialization for letter strings. Journal of Cognitive Neuroscience. 2005;17(10):1532–1552. doi: 10.1162/089892905774597218. [DOI] [PubMed] [Google Scholar]
- Maurer U, Brem S, Kranz F, Bucher K, Benz R, Halder P, Steinhausen H, Brandeis D. Coarse neural tuning for print peaks when children learn to read. Neuroimage. 2006;33(2):749–758. doi: 10.1016/j.neuroimage.2006.06.025. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends in Cognitive Sciences. 2003;7(7):293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- McCleery JP, Akshoomoff N, Dobkins KR, Carver LJ. Atypical face versus object processing and hemispheric asymmetries in 10-month-old infants at risk for autism. Biological Psychiatry. 2009;66(10):950–957. doi: 10.1016/j.biopsych.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland JC, Cheung CH, Perszyk D, Mayes LC. Face-related ERPs are modulated by point of gaze. Neuropsychologia. 2010;48(12):3657–3660. doi: 10.1016/j.neuropsychologia.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland JC, Dawson G, Webb SJ, Panagiotides H, Carver LJ. Event-related brain potentials reveal anomalies in temporal processing of faces in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2004;45(7):1235–1245. doi: 10.1111/j.1469-7610.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- McPartland JC, Perszyk D, Crowley M, Naples AJ, Mayes L. Visual attention modulates neural response to faces in autism. Paper accepted for presentation at the American Psychological Association Annual Convention.2011. Aug, [Google Scholar]
- McPartland JC, Webb SJ, Keehn B, Dawson G. Patterns of visual attention to faces and objects in autism spectrum disorder. J Autism Dev Disord. 2011;41(2):148–157. doi: 10.1007/s10803-010-1033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN, Moore MK. Imitation of facial and manual gestures by human neonates. Science. 1977;198(4312):74–78. doi: 10.1126/science.897687. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Williams DL. The new neurobiology of autism: cortex, connectivity, and neuronal organization. Archives of Neurology. 2007;64(7):945–950. doi: 10.1001/archneur.64.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murias M, Swanson JM, Srinivasan R. Functional connectivity of frontal cortex in healthy and ADHD children reflected in EEG coherence. Cerebral Cortex. 2007;17(8):1788–1799. doi: 10.1093/cercor/bhl089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nation K, Clarke P, Wright B, Williams C. Patterns of reading ability in children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2006;36(7):911–919. doi: 10.1007/s10803-006-0130-1. [DOI] [PubMed] [Google Scholar]
- Nelson C. The development and neural bases of face recognition. Infant and Child Development. 2001;10:3–18. [Google Scholar]
- Newman TM, Macomber D, Naples AJ, Babitz T, Volkmar F, Grigorenko EL. Hyperlexia in Children with Autism Spectrum Disorders. Journal of Autism and Developmental Disorders. 2006;37(4):760–774. doi: 10.1007/s10803-006-0206-y. [DOI] [PubMed] [Google Scholar]
- O’Connor K, Hamm JP, Kirk IJ. The neurophysiological correlates of face processing in adults and children with Asperger’s syndrome. Brain and Cognition. 2005;59(1):82–95. doi: 10.1016/j.bandc.2005.05.004. [DOI] [PubMed] [Google Scholar]
- O’Connor K, Hamm JP, Kirk IJ. Neurophysiological responses to face, facial regions and objects in adults with Asperger’s syndrome: An ERP investigation. International Journal of Psychophysiology. 2007;63(3):283–293. doi: 10.1016/j.ijpsycho.2006.12.001. [DOI] [PubMed] [Google Scholar]
- O’Connor IM, Klein PD. Exploration of strategies for facilitating the reading comprehension of high-functioning students with autism spectrum disorders. J Autism Dev Disord. 2004;34(2):115–127. doi: 10.1023/b:jadd.0000022603.44077.6b. [DOI] [PubMed] [Google Scholar]
- Oldfield R. The assessment and analysis of handedness; The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Osterling JA, Dawson G. Early recognition of children with autism: A study of first birthday home videotapes. Journal of Autism and Developmental Disorders. 1994;24(3):247–257. doi: 10.1007/BF02172225. [DOI] [PubMed] [Google Scholar]
- Paul R. Promoting social communication in high functioning individuals with autistic spectrum disorders. Child & Adolescent Psychiatric Clinics of North America. 2003;12(1):87–106. doi: 10.1016/s1056-4993(02)00047-0. [DOI] [PubMed] [Google Scholar]
- Perszyk D, Molfese P, Kilroy E, Mayes L, Klin A, McPartland J. Developmental brain bases of face perception in autism as revealed by ERPs. Paper presented at the International Meeting for Autism Research.2010. [Google Scholar]
- Psychological Corporation. Wechsler Abbreviated Scale of Intelligence (WASI) manual. San Antonio, TX: Author; 1999. [Google Scholar]
- Puce A, Allison T, Gore J, McCarthy G. Face-sensitive regions in human extrastriate cortex studied by functional MRI. Journal of Neurophysiology. 1995;74(3):1192–1199. doi: 10.1152/jn.1995.74.3.1192. [DOI] [PubMed] [Google Scholar]
- Rossion B, Gauthier I, Goffaux V, Tarr MJ, Crommelinck M. Expertise training with novel objects leads to left-lateralized facelike electrophysiological responses. Psychological Science. 2002;13(3):250–257. doi: 10.1111/1467-9280.00446. [DOI] [PubMed] [Google Scholar]
- Rossion B, Gauthier I, Tarr MJ, Despland P, Bruyer R, Linotte S, Crommelinck M. The N170 occipito-temporal component is delayed and enhanced to inverted faces but not to inverted objects: an electrophysiological account of face-specific processes in the human brain. Neuroreport. 2000;11(1):69–74. doi: 10.1097/00001756-200001170-00014. [DOI] [PubMed] [Google Scholar]
- Rossion B, Joyce CA, Cottrell GW, Tarr MJ. Early lateralization and orientation tuning for face, word, and object processing in the visual cortex. Neuroimage. 2003;20(3):1609–1624. doi: 10.1016/j.neuroimage.2003.07.010. [DOI] [PubMed] [Google Scholar]
- Sasson NJ. The Development of Face Processing in Autism. Journal of Autism and Developmental Disorders. 2006;36(3):381–394. doi: 10.1007/s10803-006-0076-3. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-prime user’s guide. Pittsburg: Psychology Software Tools Inc; 2002. [Google Scholar]
- Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience. 2005;23(2–3):125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, Skludlarski P, Lacadie C, Cohen DJ, Gore JC. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Archives of General Psychiatry. 2000;57(4):331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Schweinberger SR, Pickering EC, Jentzsch I, Burton AM, Kaufmann JM. Event-related brain potential evidence for a response of inferior temporal cortex to familiar face repetitions. Cognitive Brain Research. 2002;14(3):398–409. doi: 10.1016/s0926-6410(02)00142-8. [DOI] [PubMed] [Google Scholar]
- Scott LS, Tanaka JW, Sheinberg DL, Curran T. A reevaluation of the electrophysiological correlates of expert object processing. Journal of Cognitive Neuroscience. 2006;18(9):1453–1465. doi: 10.1162/jocn.2006.18.9.1453. [DOI] [PubMed] [Google Scholar]
- Senju A, Tojo Y, Yaguchi K, Hasegawa T. Deviant gaze processing in children with autism: an ERP study. Neuropsychologia. 2005;43(9):1297–1306. doi: 10.1016/j.neuropsychologia.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Shibata T, Nishijo H, Tamura R, Miyamoto K, Eifuku S, Endo S, Ono T. Generators of visual evoked potentials for faces and eyes in the human brain as determined by dipole localization. Brain Topography. 2002;15(1):51–63. doi: 10.1023/a:1019944607316. [DOI] [PubMed] [Google Scholar]
- Tanaka JW, Farah MJ. Parts and wholes in face recognition. The Quarterly Journal of Experimental Psychology Section A. 1993;46(2):225–245. doi: 10.1080/14640749308401045. [DOI] [PubMed] [Google Scholar]
- Tarr MJ, Gauthier I. FFA: a flexible fusiform area for subordinate-level visual processing automatized by expertise. Nature Neuroscience. 2000;3(8):764–769. doi: 10.1038/77666. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Batty M, Itier RJ. The faces of development: a review of early face processing over childhood. Journal of Cognitive Neuroscience. 2004;16(8):1426–1442. doi: 10.1162/0898929042304732. [DOI] [PubMed] [Google Scholar]
- Tucker DM. Spatial sampling of head electrical fields: the geodesic sensor net. Electroencephalography and Clinical Neurophysiology. 1993;87(3):154–163. doi: 10.1016/0013-4694(93)90121-b. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Flowers DL, Verbalis A, Miranda M, Gareau L, Eden GF. The neural basis of hyperlexic reading: an FMRI case study. Neuron. 2004;41(1):11–25. doi: 10.1016/s0896-6273(03)00803-1. [DOI] [PubMed] [Google Scholar]
- Webb SJ, Dawson G, Bernier R, Panagiotides H. ERP evidence of atypical face processing in young children with autism. Journal of Autism and Developmental Disorders. 2006;36(7):881–890. doi: 10.1007/s10803-006-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, Jones EJ, Merkle K, Murias M, Greenson J, Richards T, Aylward E, Dawson G. Response to familiar faces, newly familiar faces, and novel faces as assessed by ERPs is intact in adults with autism spectrum disorders. International Journal of Psychophysiology. 2010;77(2):106–117. doi: 10.1016/j.ijpsycho.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, Merkle K, Murias M, Richards T, Aylward E, Dawson G. ERP responses differentiate inverted but not upright face processing in adults with ASD. Social Cognitive and Affective Neuroscience. 2009 doi: 10.1093/scan/nsp002. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale. 3. San Antonio, Tx: The Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. The Wechsler Intelligence Scale for Children. 4. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- Wolf JM, Tanaka JW, Klaiman C, Cockburn J, Herlihy L, Brown C, South M, McPartland JC, Kaiser MD, Phillips R, Schultz RT. Specific impairment of face processing abilities in children with autism spectrum disorder using the Let’s Face It! Skills Battery. Autism Research. 2008;1(6):329–340. doi: 10.1002/aur.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AC, Gauthier I, Woroch B, DeBuse C, Curran T. An early electrophysiological response associated with expertise in letter perception. Cognitive, Affective, and Behavioral Neuroscience. 2005;5(3):306–318. doi: 10.3758/cabn.5.3.306. [DOI] [PubMed] [Google Scholar]
- Woodcock R, McGrew K, Mather N. Woodcock-Johnson III Tests of Achievement. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- Yin R. Face recognition by brain-inujred patients: A dissociable ability. Neuropsychologia. 1970;8:395–402. doi: 10.1016/0028-3932(70)90036-9. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Dougherty JH, Jr, Hubner KF, Bai B, Cannon RL, Hutson RK. Abnormal connectivity in the posterior cingulate and hippocampus in early Alzheimer’s disease and mild cognitive impairment. Alzheimer’s and Dementia. 2008;4(4):265–270. doi: 10.1016/j.jalz.2008.04.006. [DOI] [PubMed] [Google Scholar]