Abstract
Aims
Binge drinking after chronic ethanol consumption is one of the important factors contributing to the progression of steatosis to steatohepatitis. The molecular mechanisms of this effect remain poorly understood. We have therefore examined in rats the effect of single and repeat ethanol binge superimposed on chronic ethanol intake on liver injury, activation of mitogen activated protein kinases and gene expression.
Methods
Rats were chronically treated with ethanol in liquid diet for four weeks followed by single ethanol binge (5 gm/kg body weight) or three similar repeated doses of ethanol. Serum alcohol and ALT levels were determined by enzymatic methods. Steatosis was assessed by histology and hepatic triglycerides. Activation of MAPKs, RSK, and caspase-3 were evaluated by western blot. Levels of mRNA for TNFα, egr-1, and plasminogen activator inhibitor -1 (PAI-1) were measured by real time qRT-PCR.
Results
Chronic ethanol treatment resulted in mild steatosis and necrosis, whereas chronic ethanol followed by binge group exhibited marked steatosis and significant increase in necrosis. Chronic-binge group also showed significant increase (compared to chronic ethanol alone) in the phosphorylation of ERK1&ERK2, and RSK. Phosphorylation of JNK and p38 MAPK did not increase by the binge. Ethanol binge, after chronic ethanol intake, caused increase in mRNA for of egr-1 and PAI-1, but not TNFα.
Conclusions
Chronic ethanol exposure increases the susceptibility of rat liver to increased injury by one or three repeat binge. Among other alterations, the activated levels of ERK1, and more so ERK2, were remarkably amplified by binge suggesting a role of these isotypes in the binge amplification of the injury. In contrast, p38MAPK and JNK1/2 activities were not amplified. These binge induced changes were also reflected in the increases in the RNA levels for egr-1 and PAI -1. This study offers chronic followed by repeat-binge as a model for the study of progression of liver injury by ethanol and highlights the involvement of ERK1 and ERK2 isotypes in the amplification of liver injury by binge ethanol.
Keywords: Alcoholic steatohepatitis, binge ethanol, chronic ethanol, liver injury, MAP Kinase, necrosis
INTRODUCTION
Alcoholic liver disease is one of the major causes of illness and death in USA. At least 80% of heavy drinkers develop steatosis, 10–35% develop alcoholic hepatitis, and about 10% develop cirrhosis (Adachi and Brenner, 2005). The spectrum of steatohepatitis includes early or mild steatohepatitis characterized by patchy necrosis and mild inflammatory response (necroinflammation) and late or severe steatohepatitis accompanied by Mallory bodies, significant leukocyte infiltration and liver failure (Hall et al., 2001). The progression of steatosis to steatohepatitis has been shown to be dependent on second hit. In this regard endotoxin, nutritional factors, and other disease states such as hepatitis C viral infection have been considered as second hit for the progression of liver injury (Adachi and Brenner, 2005). Endotoxemia is present in patients admitted to the hospital with alcoholic liver injury and the degree of endotoxemia is related to the degree of alcoholic intoxication in chronic alcoholic liver diseases (Schafer et al., 2002). However, the changes in the levels of endotoxin were not significantly different in various stages of alcoholic liver disease. Moreover, serum endotoxin levels in alcoholic patients with cirrhosis but without hepatitis were higher compared to hepatitis alone (Schafer et al., 2002; Fukui, 2005). These findings suggest some additional modulating factors determining the severity of endotoxemia and liver injury. In this regard, alcoholic hepatitis usually occurs by the consumption of additional alcohol in the form of heavy binge in individuals already consuming alcohol for a longer period of time, thus favoring binge drinking habit as a cofactor for progression of alcoholic liver injury (Rivara et al., 1993; Crosse and Anania, 2002; Ceccanti et al., 2006; Zakhari and Li, 2007). Binge drinking is alarmingly on the rise throughout the globe (Mathurin and Deltenre, 2009). In this context, the effect of binge administration of ethanol in liver has not been examined in animals chronically pre-exposed to ethanol.
Among various signal processes, mitogen activated protein kinases (MAPKs) i.e. extracellular regulated kinases 1/2 (ERK1/2), c-Jun N-terminal kinase (JNK), and p38 MAPK play pivotal role(s) in transducing extracellular signals to various subcellular compartments by phosphorylating various substrates (Mor and Philips, 2006; Pimienta and Pascual, 2007; Boutros et al., 2008). There is overwhelming evidence that different MAP kinases are modulated by ethanol but their specific roles and downstream targets are yet to be ascertained (Aroor and Shukla, 2004; Apte et al., 2007; Mandrekar and Szabo, 2009). In primary culture of hepatocytes, ethanol activates all three MAPKs. Persistent activation of p38 MAPK leads to histone phosphorylation, and activation of JNK is related to apoptosis (Aroor and Shukla, 2004; Lee and Shukla, 2005; Venugopal et al., 2007; Lee and Shukla, 2007). It has also been proposed that acute effects of ethanol may be related to the prolongation of ERK1/2 activation whereas inhibition of heptaocyte proliferation by chronic ethanol may be due to inhibition of p38 MAPK (Nguyen and Gao, 2002, Hsu et al., 2006). ERK1/2 phosphorylation has been shown to be increased or decreased after chronic ethanol use depending on duration, endotoxin administration and partial hepatectomy (Nguyen and Gao, 2002; Kotesh et al., 2002a; Kotesh et al., 2002b). However, the behavior of the activation of all three MAPKs during chronic ethanol administration as well as ethanol binge superimposed on chronic administration of ethanol remains to be elucidated.
TNF α expression in liver has been shown to be either higher or not significantly different in early alcoholic steatohepatits stage compared to alcoholic steatosis in humans (Kotesh et al., 2002a; Deaciuc et al., 2004). Expression of PAI-1 and egr-1 is increased after chronic ethanol and lipopolysaccharide induced alcoholic liver injury in animals (Pritchard and Nagy, 2005; Beier et al., 2009). Therefore, we have determined the effects of binge ethanol administration in rats treated chronically with ethanol on liver injury, activation of MAPKs and mRNA levels of specific genes to gain insight into the significance of MAPK signaling in the progression of alcoholic liver injury.
MATERIALS AND METHODS
Materials
Antibodies to phospho-ERK1/2, ERK1/2 protein, phospho-p38 MAPK, p38 MAPK protein, phospho-JNK1/2, JNK1/2 protein and cleaved caspase 3 were purchased from Cell Signaling (Beverly, MA). Oligonucleotides were synthesized by IDT, Integrated DNA Technology Inc. Coralville, IA. Enzymes for reverse transcriptase reaction were purchased from Applied Biosystems (Foster City, CA). IQ SYBR green super mix for real time qRT-PCR reaction was purchased from Bio-Rad (Hercules, CA). Enhanced chemiluminescence (ECL) detection system was form Pierce Chemical (Rockford, IL). Protease inhibitors cocktail (p8340) and anti β-actin antibody were obtained from the Sigma-Aldrich (St. Louis, MO).
Chronic ethanol feeding and binge administration
Seven week old male Sprague–Dawley rats, each weighing between 250–300 g, were purchased from Harlan (Indianapolis, IN). They were housed under a 12-h/12-h light/dark cycle and were permitted ad libitum consumption of standard laboratory rat chow. After a 1-week equilibration period, the animals were fed Lieber–DeCarli liquid diet (Dyets, Inc., Bethlehem, PA; Lieber and Decarli, 1982). Ethanol was progressively introduced into the liquid diet starting at 1.25% (wt. /vol.) for day 1, increased to 1.67% (wt./vol.) for day 2 and to 2.5% (wt./vol.) for days 3 and 4, and, finally, maintained at 5% (wt./vol.) for 4 weeks. Each day, the previous day's intake was measured, and the pair-fed control rat was fed control liquid diet in which ethanol was replaced by dextrin/maltose to maintain the isocaloric intake in the two groups. After 4 weeks, rats were divided into four groups: Control, chronic ethanol, control- water and chronic ethanol-binge. Chronic ethanol-binge group were given either single or three binge intragastric administration of ethanol (5 gm/kg body weight). Ethanol was diluted to 32% (v/v) in sterile water and injected through the oral cavity to the stomach using an 18 gauge stainless steel blunt tipped needle. The average amount of injected alcohol was about 7.5 ml. In the control-water group ethanol was replaced by water. In addition to chronic feeding, single acute ethanol binge was administered to rats chow- fed regular solid diet (acute ethanol –binge group). In the control for acute group, ethanol was replaced with water. For three ethanol binge, ethanol was administered at 12 hr intervals. Four groups of rats for three ethanol binge were: control(pairfed) group, chronic ethanol group, control-three binge and chronic ethanol – three binge. Animals were given liquid diet without ethanol during three binge. Blood sample and liver were collected 4 hr after the last binge ethanol administration. Liver was rapidly perfused with cold phosphate buffered saline containing phosphatase inhibitors. A small portion of the liver was placed in formalin and reminder of the liver was frozen in liquid nitrogen and stored at −70°C. It should be mentioned that we have selected one and three binge after careful evaluation of the model and existing literature. The goal was to determine rapid (single binge) effects of ethanol and compare it with the repeat prolonged administration (three binge) of ethanol. Furthermore, in chronic alcohol abusers, both single and repeat binge mode of drinking is common. Another rationale for selecting one and three binge conditions was to monitor the MAPK signaling (and associated responses) in these two treatments. It was based on the fact that most of the signaling responses are rapid and transient. It also tested whether they remained sustained after repeat ethanol binge.
The animal care and protocol for their use were approved by the University of Missouri Animal Care & Use Committee.
Determination of serum ethanol and ALT
Blood alcohol was determined by alcohol dehydrogenase assay kit from Genzyme Diagnostics Framingham, MA. Serum ALT levels are determined by kinetic ALT assay in an automated analyzer.
Determination of hepatic triglyceride
For the determination of triglycerides, 100 mg of liver was homogenized in 8 volumes of hypotonic buffer containing 20 mM Tris, 2% triton X -100 and Sigma protease inhibitor cocktail (p8340). The sample was heated to 60°C followed by centrifugation at 10,000 g for 5 min. The supernatant was used for triglyceride estimation using the assay kit as detailed by the supplier (Sigma-Aldrich, St. Louis, MO).
Histopathology
After formalin fixation, specimens were sectioned and stained with hematoxylin and eosin (H&E) and used for light microscopy.
Preparation of total liver extracts
Frozen liver was homogenized with hypotonic buffer containing 10 mM HEPES, pH 7.4, 10 mM β-glycerophosphate, 0.5 mM EDTA, 0.5 mM EGTA, 10 mM sodium fluoride, 2.5 mM sodium pyrophosphate 1 mM Na-orthovanadate, 2 mM MgCl2, 1 mM DTT and Sigma p8340 protease inhibitor cocktail. The proteins in the homogenate was extracted and denatured by adding SDS to 1%. After boiling for 5 minutes, the homogenate was sonicated for 5 s and centrifuged at 12000 g for 10 min. The supernatant was used for protein assay and western blotting. Protein concentration was measured using the Bio-Rad DC protein assay kit.
Immunoblot analysis
The whole liver lysate protein (40 to 80 µg) was subjected to 10% SDS-PAGE and electrophoretically transferred onto nitrocellulose membrane (Bio-Rad) by using Bio-Rad Trans-Blot apparatus. The membrane was washed with 20 mM Tris, pH 7.5, containing 0.1% Tween 20 and 150 mM NaCl (TBST) and incubated with TBST containing 5% nonfat dry milk for 2 h at room temperature. The membrane was next incubated with antibody to phospho- or total p42/p44 ERK1/2, p38 MAPK, JNK overnight at 4°C. For western blot of cleaved caspase 3, membrane was incubated with antibody to cleaved caspase 3 (1:1000 dilution). After wahsing with TBST, the membrane was incubated with secondary antibody conjugated with horseradish peroxidase for 1 h at room temperature. Immunoblots were visualized with a chemiluminescent reagent (Pierce) and the chemiluminescence was captured with a LSA-3000 imaging system (Fujifilm life science) and quantified using Multi Gauge ™ software. The intensity of chemiluminescence was always monitored within the linear range of detection. For repeat immunoblotting, membrane was stripped using Restore Western blot stripping buffer (Pierce).
Real time qRT-PCR
Total RNA was extracted from the livers using the TRIzol reagent (Invitrogen) followed by DNase I treatment and clean up with Qiagen RNeasy midi kit (Qiagen). RNA content was measured using 260/280 UV spectrophotometry. First strand cDNA was synthesized from one microgram of total RNA using the cDNA synthesis kit (Applied Biosystems). qRT -PCR reaction mixture was prepared from SYBR green supemix from Bio-Rad using primers as shown in Table 1. PCR reactions were set up in a volume of 20 µl, containing 5 µl of diluted cDNA, 10 µl of 2× iQ SYBR Green Supermix (BioRad) and 1 µl each of forward and reverse primers and 3 µl of RNAse free water. Thermal cycling conditions for TNF α, PAI-1, egr-1 and GAPDH were 95°C for 3 min as initial denaturation and enzyme-activating step followed by 40 cycles of 95°C for 15 s denaturation, and 57°C for 1 min annealing and extension. After amplification, a melting curve analysis was performed by increasing the temperature by 0.5°C increments from 55°C to 95°C and measuring fluorescence at each temperature for a period of 10 s. Melting curve analysis was conducted to rule out primer-dimer artifacts, and contamination. All cDNA samples were analyzed in triplicate and each run contained a relative standard curve. The expression of each gene was normalized to GAPDH and calculated to relative pair fed control using comparative cycle threshold method (Peinnequinn et al., 2004).
Table 1.
Primers used for real time PCR
| Forward | Reverse | |
|---|---|---|
| GAPDH | 5’-AGACAGCCGCATCTTCTTGT-3’ | 5’-CTTGCCGTGGGTTAGAGTCAT-3’ |
| TNF α | 5’-AAATGGGCTCCCTCTCATCAG-3’ | 5’-TTCTCTGCTTGGTGGTTTGCTACGAC-3’ |
| PAI-1 | 5’-AACCCA GGC CGA CTT CA-3’ | 5’-CATGCGGGCTGAGACTAGAAT-3’ |
| Egr-1 | 5’- TCACCTATACTGGCCGCTTCT C-3’ | 5’ – AAGACGATGAAGCAGCTGGAG-3’ |
Data analysis
All results are expressed as mean ± S.E.M. and were obtained by combining data from individual experiments. Statistical analyses were made using the Student t test (two-tailed, paired and non -paired). Differences with a P value of < 0.05 were considered statistically significant.
RESULTS
Body weight and serum ethanol levels in rats treated chronically with ethanol
In single and three ethanol binge groups, administration of ethanol in liquid diet had no effect on the weight of the animal compared to pair fed animals (Table 2). The average consumption of liquid diet was about 80 ml per day. These results were similar to the liquid diet feeding paradigm reported by Lieber and DeCarli (Lieber and Decarli, 1982; Hall et al., 2001). Binge mode of ethanol administration to rats chronically treated with ethanol (chronic ethanol-single binge) resulted in two fold increase in blood ethanol levels compared to chronic ethanol alone treated rats (Table 2). Serum ethanol levels after ethanol-single binge administration to control rats (chow-fed, 12 weeks old) were similar to chronic-single binge administration (Table 2). Serum ethanol levels were very low in chronic ethanol treated rats in three binge experiments since they are on control liquid diet during 28 hr experimental (ie. water binge) period (Table 2). Serum ethanol level was increased after three doses of ethanol in control rats (control- three binge) and further increased in chronic ethanol treated rats (chronic ethanol – three binge) (Table 2).
Table 2.
Body weight and serum ethanol levels in different treatment groups: Rats were treated chronically with ethanol or without (pair-fed) for 4 weeks followed by single or three binge of ethanol as indicated.
| Treatment Group | Body weight (gm) | Serum ethanol (mM) |
|---|---|---|
| Single binge | ||
| Control (pair-fed) | 354.8 ± 10.4 | 0.75 ± 0.43 |
| Chronic ethanol | 341.3 ± 7.4 | 21.98 ± 9.13 |
| Control (chow-fed) | 355.3 ± 1.6 | 0.75 ± 0.47 |
| Control (chow-fed)-single binge | 355.3 ± 5.9 | 29.20 ± 3.16 |
| Control (pair-fed) | 375.3 ± 12.9 | 0.00 ± 0.00 |
| Chronic ethanol-single binge | 380.8 ± 10.3 | 38.05 ± 12.84 |
| Three binge | ||
| Control (pair-fed) | 308.7 ± 5.7 | 0.50 ± 0.50 |
| Chronic ethanol | 320.0 ± 3.5 | 4.50 ± 0.28 |
| Control (pair-fed)-three binge | 310.0 ± 1.2 | 89.67 ± 14.24 |
| Chronic ethanol-three binge | 312.0 ± 5.6 | 117.0 ± 15.72 |
Effect of single and three ethanol binge on liver injury in rats treated chronically with ethanol
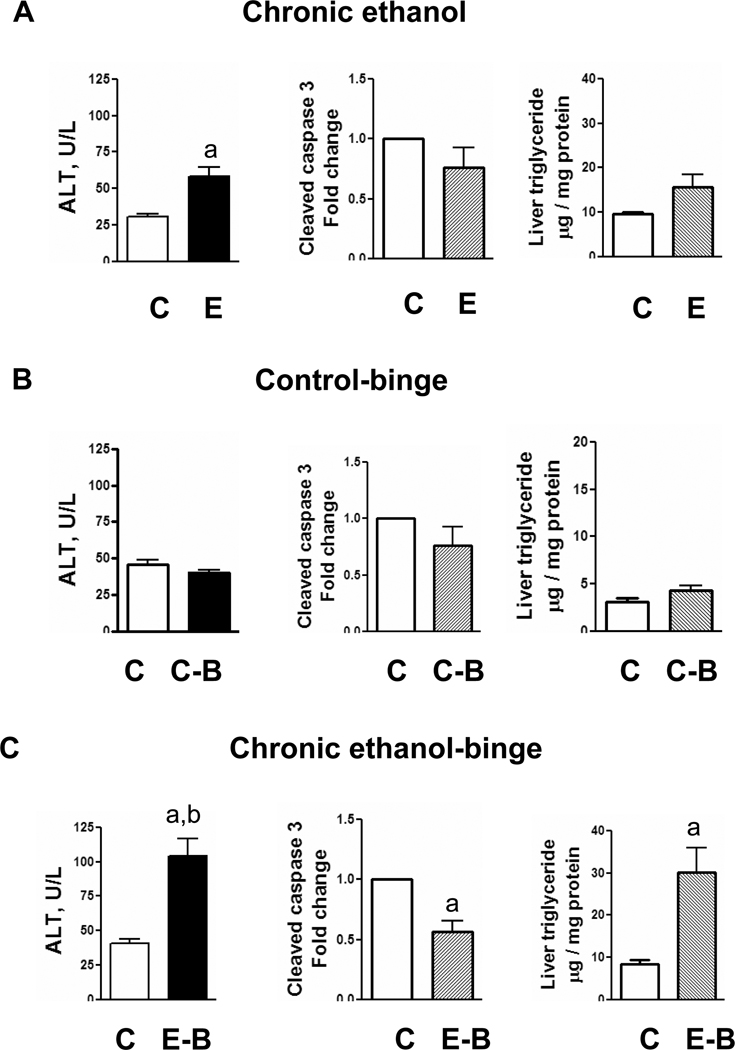
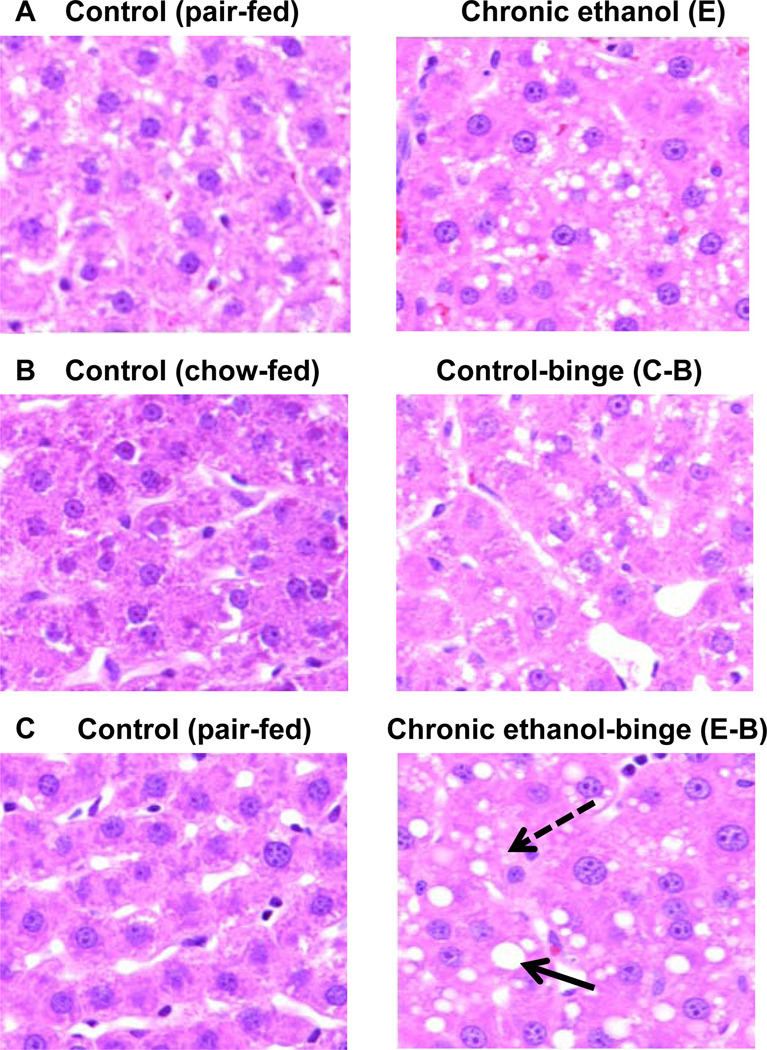
To determine the effect of ethanol binge on liver injury after chronic ethanol intake, rats were fed liquid diet (pairfed control) or liquid diet containing ethanol for four weeks. This was followed by either administration of ethanol (5 gm/kg body weight as 32% ethanol solution in water) for ethanol treated rats (chronic-binge) or equal amount of water (control). After 4 hr, blood sample was withdrawn and liver was excised. Serum ALT levels, an index of liver necrosis, were moderately increased after chronic ethanol treatment (Fig. 1A), and increased higher after ethanol-single binge (Fig. 1C). Increase in ALT over and above chronic ethanol alone treated rats also reached statistical significance. Apoptosis was evaluated by western blot analysis for cleaved caspase 3. Apoptosis was mildly suppressed in chronic ethanol group compared to control but was not statistically significant (Fig. 1B). In chronic ethanol – single binge group, apoptosis was decreased significantly (Fig. 1C). Hepatic triglyceride content was moderately increased in chronic ethanol (Fig. 1A) and it increased further in chronic ethanol – single binge group (Fig. 1C). Binge ethanol effect in chow fed rats and chronic ethanol fed rats were also compared. Although, serum ethanol levels after ethanol binge administration to control rats (chow-fed, 12 weeks old) was higher similar to chronic ethanol-single binge administration (Table 1), serum ALT levels did not increase (Fig. 1). Control-single binge administration also caused a small increase in triglycerides in liver which was statistically not significant (Fig. 1B). Cleaved caspase 3 levels were also not significantly affected in control-single binge samples (Fig. 1B). Histochemical examination of liver sections revealed mild steatosis in chronic ethanol treated rat liver (Fig. 2A) whereas steatosis was more pronounced in chronic ethanol–single binge group (Fig. 2C). Thus, microvesicular steatosis in chronic ethanol treated group progressed to macrovesicular steatosis in chronic ethanol- single binge group (Fig. 2A and Fig. 2C). Single binge administration to control (chow fed) rats was characterized by mild steatosis (Fig. 2B).
Fig. 1.
Parameters of liver injury in chronic and chronic ethanol-single binge model. Rats were fed ethanol in liquid diet chronically for 4 weeks and then given a single ethanol binge dose (5 gm/kg). Samples were collected after 4 hrs. The levels of serum ALT, hepatic triglyceride and hepatic cleaved caspase-3 were determined as described under materials and methods. Values are mean ± SE (n=4 rats). Groups represent Chronic ethanol; Control-binge; Chronic ethanol-binge; a: significant from control group (p<0.05); b: significant from chronic ethanol group (p<0.05); C: Control; E: Chronic ethanol; C-B: Control-binge; E-B: Chronicethanol- binge. Control represents pair–fed animals for chronic and chronic-ethanol binge experiments. In control-binge experiments, the control represents chow-fed animals.
Fig. 2.
Histology of liver from chronic, and chronic ethanol-single binge treatment. Rats were fed ethanol in liquid diet chronically for 4 weeks and then given single ethanol binge dose (5 gm/kg). Samples were collected after 4 hrs. Sections of liver samples were stained with hematoxylin and eosin (x 200X). Values are mean ± SE (n=4 rats). Groups represent Chronic ethanol; Control-binge; C: Control; E: Chronic ethanol; C-B: Control-binge; E-B: Chronic-ethanol binge. Control represents pair–fed animals for chronic and chronic-ethanol binge experiments. In control-binge experiments, the control represents chow-fed animals. Solid arrow represents macrovesicular steatosis and broken arrow represents microvesicular steatosis.
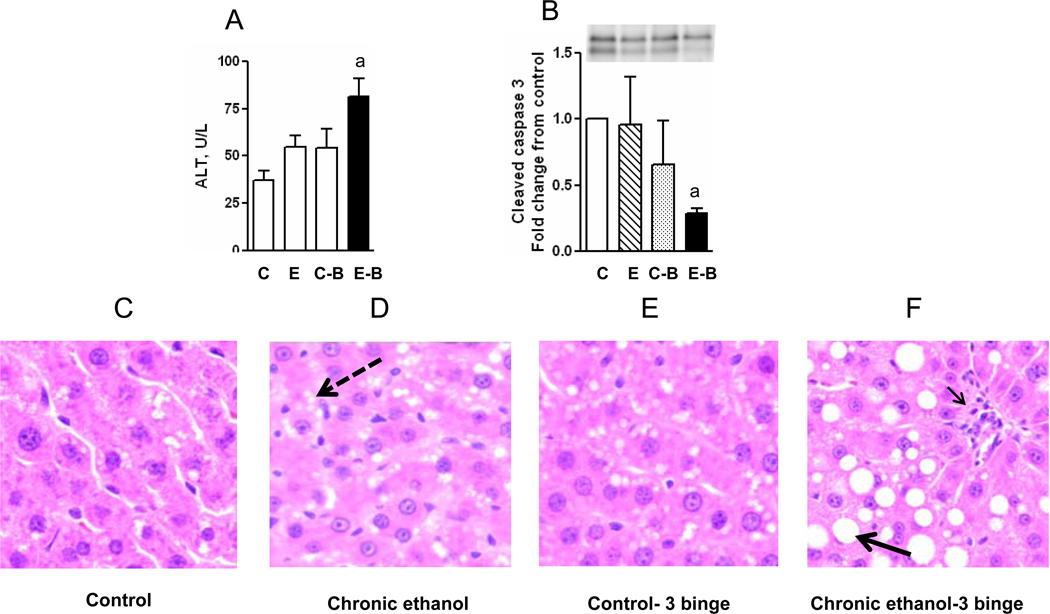
In another series of experiments, we determined the effect of repeat ethanol binge on liver injury in rats chronically treated with ethanol. In this study, we also included pair fed rats for acute ethanol binge (Fig. 3). Serum ethanol levels were very low in chronic ethanol treated rats since they are on control liquid diet during 28 hr experimental (ie. water binge) period (Table 2). Liver injury was mild in control rats treated with three doses of ethanol binge (control –three binge) in control (pair fed) rats and increase in ALT was not significant (Fig. 3A). Chronic ethanol treated rats also showed mild injury (Fig. 3A). However, the administration of three repeat ethanol binge (chronic ethanol – three binge) resulted in significant augmentation of injury as reflected by increased serum ALT levels (Fig. 3A). Similar to single ethanol binge results, apoptosis as determined by cleaved caspase 3, was lower after chronic ethanol – three binge administration (Fig. 3B). Progression of microvesicular steatosis in chronic ethanol treated group (Fig. 3D) to macrovesicular steatosis was also seen in chronic ethanol- three binge group (Fig. 3F). In contrast to single binge administration, the magnitude of leukocyte infiltration was more predominant in three ethanol binge (Fig. 3F, arrows) compared to single ethanol binge (Fig. 2C).
Fig. 3.
Parameters of liver injury and histology in chronic and chronic ethanol- three binge model. Rats were fed ethanol in liquid diet chronically for 4 weeks and then given three binge (5 gm/kg) at 12 hr intervals. Four hr after the last dose, the levels of serum ALT, and hepatic cleaved caspase-3 were determined as described under materials and methods. Sections of liver samples were stained with hematoxylin and eosin. Control represents pair –fed animals and were given water for binge control. Values are mean ± SE (n=4 rats). Western blot represents a typical experiment. A: Serum ALT; B: hepatic cleaved caspase 3; C-F: Hemotoxylin and eosin staining (x 200X); a: significant from control group (p<0.05); C: Control (pair fed); E: Chronic ethanol; C-B: Control- ethanol binge; E-B: Chronic-ethanol binge. Large solid arrow represents macrovesicular steatosis. Large broken arrow represents microvesicular steatosis. Small solid arrow represents leukocyte infiltration.
Activation of MAPKs during single ethanol binge after chronic ethanol administration
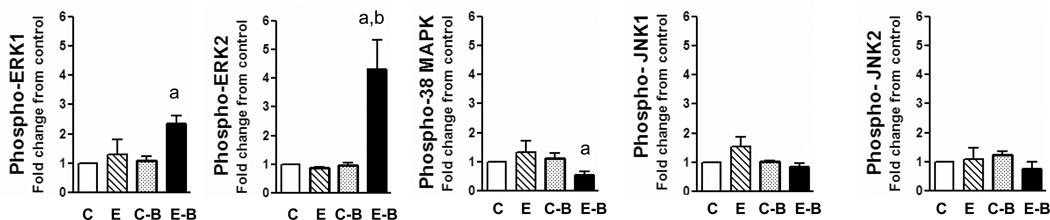
We next determined the phosphorylation of MAPKs in the liver of rats from different groups. Activation of ERK1/2 was determined in whole liver extracts by evaluating the tyrosine and threonine phosphorylation of ERK1/2 using phosphospecific antibodies. The results are shown in Fig. 4. In liver extracts from chronic ethanol treated rats, ERK1/2 phosphorylation was not significantly altered compared to normal (control) rats (Fig. 4). In contrast, single binge ethanol treatment of chronic ethanol treated rats (chronic ethanol- single binge group) resulted in significant increase in the phosphorylation of ERK1/2 (Fig. 4). The mean increases in phospho-ERK1 and phospho-ERK2 were 2.3 and 4.3 fold, respectively. The phosphorylation of ERK2 was consistently higher than ERK1. The levels of ERK1/2 protein were similar in all the three groups (data not shown). The levels of β actin, a house keeping protein, remained same in ethanol treatment group and served as a loading control (data not shown). These results suggest that increase in ERK1/2 activity is not due to changes in the protein content of ERK1 and ERK 2. The p38 MAPK is a stress activated protein kinase affected by ethanol in vivo and vitro (Aroor and Shukla, 2004; Hsu et al., 2006). The activation status of p38 MAPK in chronic ethanol alone treated rats was not different from controls (Fig. 4). However, phospho-p38 MAPK levels were significantly lower in chronic ethanol – single binge group and were statistically significant (Fig. 4). Similar to ERK1/2, the p38 MAPK protein did not change after chronic ethanol, or chronic ethanol- single binge treatments (data not shown). We next determined the activation of JNK (Fig. 4) and observed that JNK activation was not altered after chronic ethanol, or chronic ethanol-single binge treatments. Acute ethanol binge treatment of normal (control, chow-fed) rats did not increase phosphorylation of ERK1/2, p38 MAPK and JNK.
Fig. 4.
Levels of phosphorylated ERK1/2, p38 MAPK and JNK1/2 in chronic and chronic-single ethanol binge treated rats. The chronic ethanol feeding (4 weeks) and binge (single) treatment was as in Fig. 1. The whole cell extracts from liver were subjected to western blotting with respective antibodies followed by densitometry of bands (see methods). Values are mean ± SE (n=4 rats). a: significant compared to control (p<0.05); b: significant from chronic ethanol group (p<0.05); C: Control; E: Chronic ethanol; C-B: Control- binge; E-B: Chronicethanol-binge. Control represents pair-fed animals for chronic and chronic-ethanol binge experiments. In control-binge experiments, the control represents chow-fed animals.
Activation of MAPKs after chronic-three binge ethanol treatment
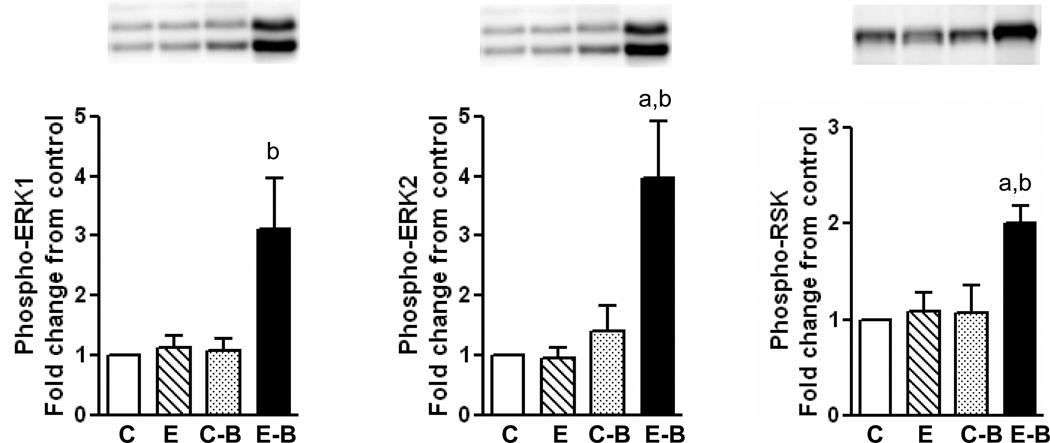
In ethanol three binge experiments, significant activation of ERK1 and ERK2 was observed in chronic ethanol – three binge group (Fig. 5). Phospho-ERK1 levels increased (3.1 fold increase) in chronic ethanol – three binge group but not in chronic ethanol or control- three binge group. The increase in the levels of phospho-ERK2 were more marked (4.0 fold increase) in chronic ethanol – three binge group, modest in control-binge group. In these experiments we also determined the activation of RSK, a downstream substrate of ERK1/2 implicated in the suppression of apoptosis (Anjum and Blenis, 2008). Phosphorylation of RSK was significant (2.0 fold increase) in chronic ethanol – three binge group compared to other groups and the pattern of activation paralleled activation of ERK1/2 (Fig. 5). In contrast to the activation of ERK1 & ERK2, the phosphorylation of p38 MAPK and JNK1/2 were not significantly different in any ethanol treated group in this series, compared to pair fed rats (data not shown).
Fig. 5.
Levels of phosphorylated ERK1/2 and RSK in chronic and chronic-three ethanol binge treated rats. The chronic ethanol feeding (4 weeks) and three binge treatment was similar to Fig. 3. The whole cell extracts from liver were subjected to western blotting with respective antibodies followed by densitometry of bands (see methods). Values are mean ± SE (n=4 rats). A representative western blot is shown for each. a: significant compared to control (p<0.05); b: significant from chronic ethanol group (p<0.05); C: Control; E: Chronic ethanol; C-B: Control-binge; E-B: Chronic ethanol-binge.
Gene expression after chronic-ethanol binge
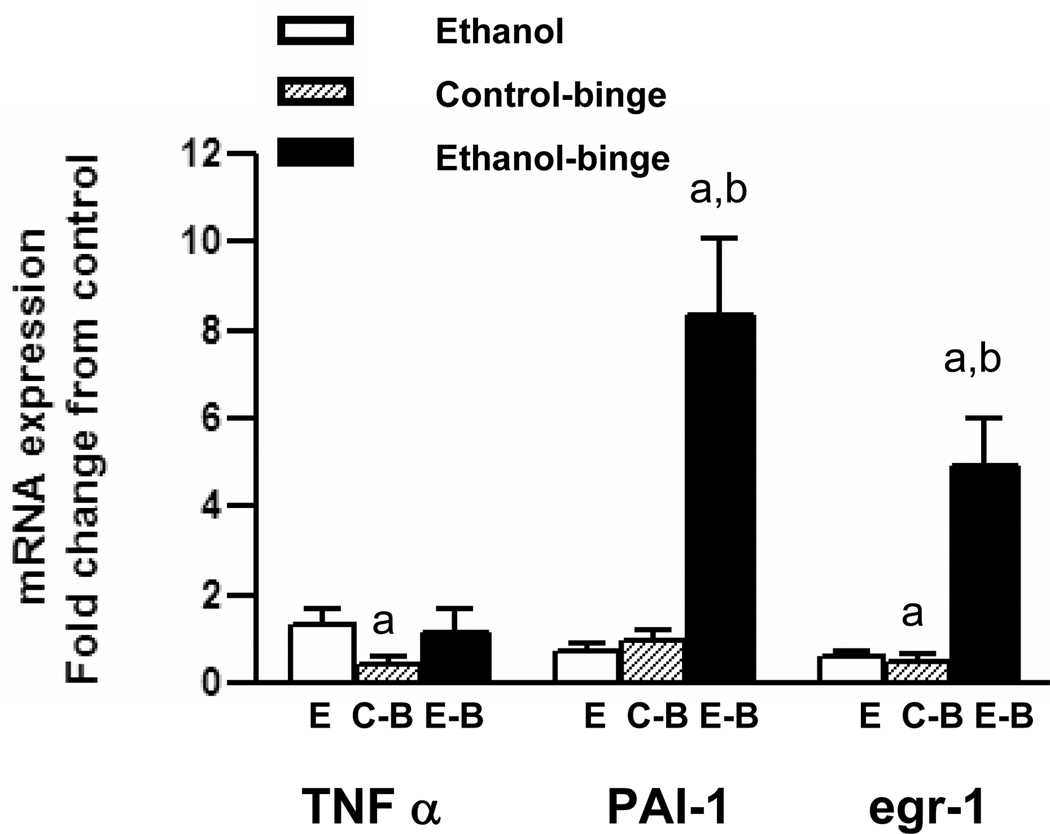
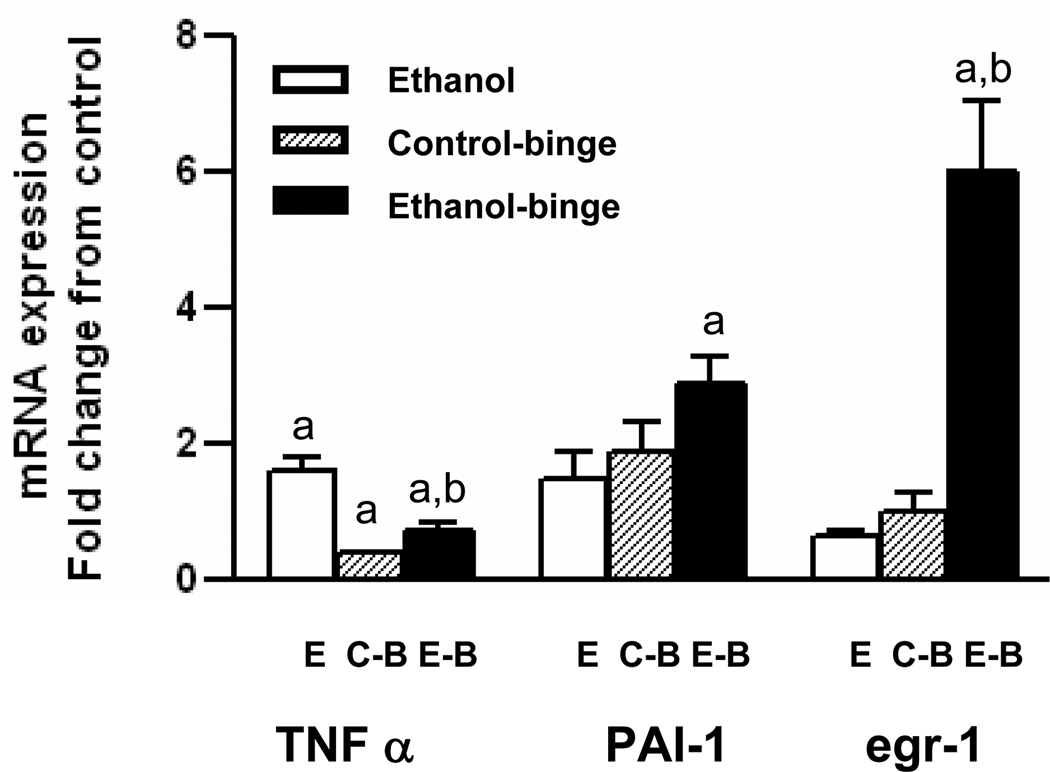
To ascertain the alterations in the altered gene expression in the binge treatments, we determined levels of mRNA for TNF-α, egr-1 and PAI-1 by qRT-PCR. TNF α mRNA level was not altered either in chronic ethanol, or chronic ethanol-single binge (Fig. 6). Although mRNA levels for PAI-1 and egr-1 decreased in chronic ethanol alone treated group, their levels were significantly increased in chronic ethanol – single binge group. In control – single binge, mRNA levels of TNF-α and egr-1 decreased and with no change for PAI-1. In chronic ethanol-three binge group, (Fig. 7), TNF α mRNA level increased in chronic ethanol group but `decreased after chronic ethanol – three binge, and control- three binge. PAI-1 mRNA level in chronic ethanol, and control - three binge increased slightly but was significantly elevated after chronic ethanol-three binge. The mRNA level for egr-1 was suppressed after chronic ethanol control – three binge where as its level was significantly increased after chronic ethanol-three binge (Fig. 7).
Fig. 6.
TNFα, PAI-1 and egr-1 mRNA levels in chronic and chronic-ethanol single binge treated rats. After 4 weeks of chronic ethanol feeding, binge was administered as in Fig. 1. Total RNA was isolated from liver and reverse transcribed to cDNA. Aliquots of the cDNA preparations were amplified by real time qRT-PCR. The fold increase in mRNA levels was determined after normalizing the differences in level of GAPDH mRNA. Values are mean ± SE (n= 4 rats). a: significant compared to control (p<0.05); b: significant from chronic ethanol group (p<0.05); E: Chronic ethanol; C-B: Control- binge; E-B: Chronic –ethanol binge. Control represents pair–fed animals for chronic and chronic-ethanol binge experiments. In control-binge experiments, the control represents chow-fed animals.
Fig. 7.
TNFα, PAI-1 and egr-1 mRNA levels in chronic and chronic-ethanol three binge treated rats. After 4 weeks of chronic ethanol feeding, three binge was administered as in Fig. 3. Total RNA was isolated from liver and reverse transcribed to cDNA. Aliquots of the cDNA preparations were amplified by real time qRT-PCR. The fold increase in mRNA levels was determined after normalizing the differences in level of GAPDH mRNA. Values are mean ± SE (n= 4 rats). a: significant compared to control (p<0.05); b: significant from chronic ethanol group (p<0.05); E: Chronic ethanol; C-B: Control- ethanol binge; E-B: Chronic-ethanol binge.
DISCUSSION
This is the first report demonstrating augmentation of liver injury after single, and repeated three binge ethanol administration in chronically ethanol treated rats. This pathophysiologically relevant animal model of alcoholic liver injury has several features worthy of comment. First, augmentation of liver injury is seen without administration of any external agent. Only ethanol is used in this model and is analogous to the situation encountered in chronic alcoholics. Second, the magnitude of injury seen in this model (i.e. 4 weeks chronic followed by binge) closely resembles the liver injury seen in rats chronically treated with ethanol for longer periods eg, 12 to 16 weeks and low dose of LPS administration after chronic ethanol intake (Bhagwandeen et al., 1987; Deacuic et al, 2004). Third, this model is clinically relevant since heavy binge drinking episode in patients chronically consuming alcohol is the most common trigger for admission of patients with stetaohepatitis (Rivara et al., 1993; Crosse and Anania, 2002). A recent study in large cohort of drinkers with consecutive biopsies, suggested the concept of multiple hits of alcoholic hepatitis occurring in the same patients as the prime determinant in the progression of alcoholic liver injury (Mathurin et al, 2007). Our animal data clearly demonstrate that ethanol-binge intake is an important factor in augmenting liver damage during chronic ethanol intake. Although a recent study showed binge induced augmentation of liver injury in mice, ethanol administration in this study was for a shorter period of time and mice are more sensitive to the effects of ethanol (Ki et al., 2010).
The significant finding in relation to MAP kinase signaling is the dramatic increase in ERK1/2 activation in both single-binge and three-binge in chronically treated rats. This finding may be of clinical significance since activation of ERK1/2 is also seen in human alcoholic liver disease (Nguyen and Gao, 2002). The role of ERK1/2 activation in hepatic steatosis and necrosis is gradually being appreciated. Ischemic–reperfusion liver injury has been shown to cause significant phosphorylation of ERK1/2 and administration of MEK1/2 inhibitor U-0126 decreased liver injury (Kaizu et al., 2008). Recently, administration of MEK inhibitor U-0126 was shown to suppress endotoxin induced liver injury after sensitization induced by acute administration of ethanol in mice (Beier et al., 2009). ERK1/2 activation has been shown to mediate arachidonic acid induced hepatocyte necrosis in oxidatively stressed CYP2E1 overexpressing rat hepatocytes (Schattenberg et al., 2004). Increased loss of arachidonic acid from cellular membrane is seen after chronic ethanol consumption (Caro and Cederbaum, 2006). A specific role of cytosolic ERK2 in the phosphorylation of dynein to mediate formation of large lipid droplets has also been reported (Ericson et al., 2006). It is of interest that in our study increase in ERK2 phosphorylation is more pronounced than ERK1 in the chronic-ethanol binge group. Therefore, a role of ERK2 in the transition from microvesicular to macrovesicular steatosis is likely. Hepatic microvesicular steatosis seen in chronic ethanol group is not accompanied by significant increase in ERK1/2 activation. We have recently shown that acetate induced acetylation of histones H3 and enhancement of HAT activity occurs by MAPK independent mechanism (Park et al., 2005). Increased expression of ACC (acetyl CoA carboxylase) as well as increased transcriptional activity of SREBP-1c at the ACC promoter after chronic ethanol administration, have been linked to histone acetylation and SREBP-1c acetylation (You et al., 2008). Thus increased accumulation of fat by chronic ethanol or acute ethanol alone may be due to ethanol metabolic effect in the absence of enhanced ERK1/2 activation, whereas increased accumulation of fat in chronic-binge group may be due to increased phosphorylation of ERK1/2.
In the present study, activation of p38 MAPK was significantly depressed after single ethanol binge administration in chronic ethanol treated rats where as it was unaffected after three binge ethanol administration. These results suggest that p38 MAPK activation may not have a significant role in the augmentation of liver injury by binge ethanol although p38 MAPK has been shown to modulate hepatic metabolic functions and liver injury (Amersi et al., 2002 Xiong et al., 2007). In the present study, JNK does not appear to be involved in exaggerated liver injury in chronic–binge model. The increase in phosphorylation of JNK by chronic ethanol alone is not significant, and levels of phosphorylated JNK are not increased after ethanol binge. This is in agreement with our previous study in hepatocytes isolated from chronic ethanol treated rats (Lee & Shukla 2002; Aroor and Shukla, 2004). Moreover, JNK activation is suppressed in chronic ethanol treatment followed by endotoxin administration in mice (Kotesh et al., 2002a). However, the role of JNK1/2 mediating hepatoprotective effects may not be excluded since JNK1/2 plays a complex dual role of hepatoprotection and hepatotoxicity depending on the condition (Ni et al., 2008; Singh et al., 2009).
Although apoptosis by ethanol has been shown to occur in liver from acute and chronic ethanol treated rats the results are conflicting. Both increased and decreased apoptosis of hepatocytes have been reported after chronic ethanol intake (Kotesh et al., 2002a; Fukumura et al., 2003; Deaciuc et al., 2004). These contradictory results may be due to feeding paradigm with different levels of ethanol consumption and duration of ethanol treatment affecting levels of ATP and oxidative stress (Kotesh et al., 2002a; Fukumura et al., 2003). In the present study, apoptosis was not seen in chronic ethanol treated group, but it decreased after single and three ethanol binge. In humans, heavy ethanol binge during chronic ethanol intake is associated with increased necrosis of liver as reflected by increased serum transaminase levels (Rivera et al, 1993). ERK1/2 activation has also been considered anti-apoptotic in ethanol treated primary hepatocytes (Lee and Shukla, 2005), thus raising the possibility of dual role for activation of ERK1/2 after chronic ethanol-binge. Moreover, RSK, one of the downstream substrate of ERK1/2 has been shown to exert antiapoptotic affect by phosphorylating BAD (Shimamura et al., 2000; Anjum and Blenis, 2008). RSK activation was higher in chronic-ethanol binge group in our study and thus supports its aniapoptotic role in binge effects. Persistent activation of RSK has been observed in hepatic fibrosis, in both experimental animals and humans (Buck and Chojkier, 2007). In the present study, changes in MAPKs represented the whole liver and not any specific cell type. Further studies are needed to determine the cell specific changes in MAPK and RSK in liver injury seen after ethanol binge during chronic ethanol intake.
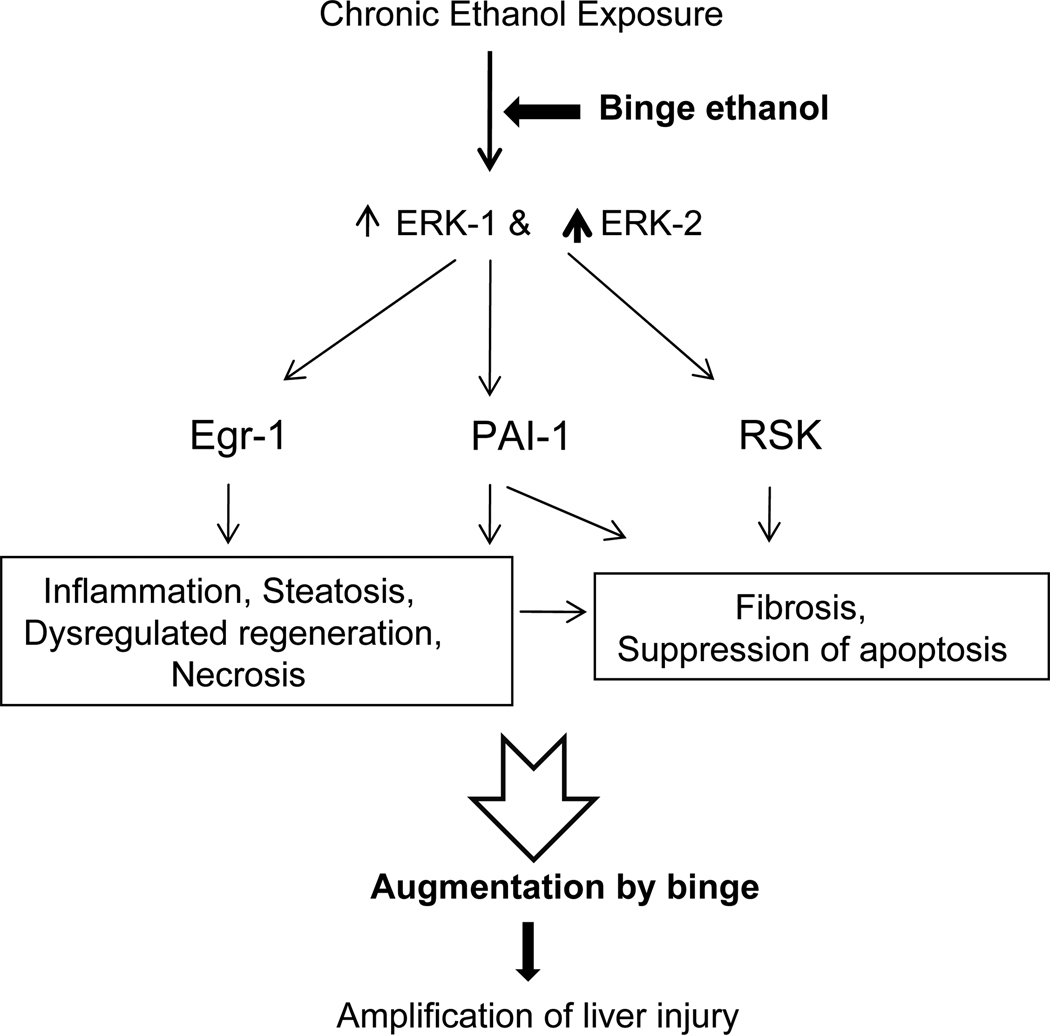
The levels of TNFα mRNA did not increase in livers of chronic ethanol binge group. In contrast, the expression of TNFα was lower compared to controls after repeat three ethanol binge in pair fed as well as chronic ethanol treated rats. Although, TNFα has been implicated in the augmentation of alcoholic liver injury after endotoxin administration to chronic ethanol treated rats or mice, mRNA level of TNFα in liver was not different between pair fed and ethanol treated rats after endotoxin administration (Kotesh et al., 2002a; Deaciuc et al., 2004). In the present study, although TNF α mRNA level was increased in chronic ethanol group in three binge study, it did not increase in chronic ethanol group in single binge study. In this regard, it may be noted that ethanol was absent (Fig. 3) in three ethanol binge study (removal of liver 28 hr after stopping of ethanol liquid diet) whereas blood ethanol levels were elevated (Fig. 1) in chronic ethanol group in single binge study. Treatment with ethanol results in decreased production of TNFα from unstimulated or endotoxin stimulated moncytes obtained from alcoholic hepatitis patients (Honchel et al., 1992), and also in alveolar macrophages obtained from chronic ethanol treated rats (Nelson et al., 1989). Although expression of TNFα was shown to be dependent on ERK1/2 and p38 MAPK activation in vitro in Kupffer cells (Thakur et al., 2007), administration of MEK inhibitor was not effective in decreasing TNFα expression in endotoxin treated animals after acute administration of ethanol in vivo (Beier et al., 2009). Moreover, activation of ERK1/2 by TNFα in rat hepatcoytes was not significant (Aroor et al, 2002) and ERK1/2 activation was not decreased in TNF knockout mice compared to wild mice after LPS treatment (Oguro et al, 2002). These findings suggest that TNFα independent pathways are also important in mediating progression of alcoholic liver injury. In this regard, acute ethanol induced AP-1 dependent expression of CD-14 in vivo (Wheeler and Thurman, 2003) and chronic ethanol induced endoplasmic reticular stress in vivo, were not suppressed in TNFα knock out mice (Ji et al., 2004). Moreover, ischemic injury after chronic ethanol intake was exaggerated but TNFα expression was not altered by ethanol treatment (Yamagishi et al., 2002). In our study, significant increases in egr-1 and PAI-1 mRNA levels were observed after chronic-ethanol binge. Activation of ERK1/2 is one of the signaling pathways involved in the induction of these genes in hepatocytes (Tsai et al., 2001; Liu et al., 2004). Expression of PAI-1 gene associated with the activation of ERK1/2 after endotoxin induced liver injury, was suppressed by administration of MEK inhibitor (Beier et al., 2009). Both egr-1 and PAI-1 have been implicated in alcoholic liver injury (Pritchard and Nagy, 2005; Beier et al., 2009). However, mRNA levels do not always result in changes in protein levels. Based on our data presented here, a scheme depicting mechanistic involvement of ERK1/2, RSK, PAI-1, and egr-1 in the binge augmentation and progression of liver injury is shown in Fig. 8.
Fig. 8.
A schematic diagram showing role of activation of ERK1, ERK2 isoforms, and RSK in the progression of liver injury by ethanol binge superimposed on chronic ethanol intake in the rat model. The scheme shows that chronic ethanol treatment sensitizes liver to binge ethanol induced enhancement of liver injury. The consequences of binge, after chronic ethanol intake, on dysregulated regeneration and fibrosis are speculative.
In summary, results presented here suggest that binge ethanol clearly augments liver injury after chronic ethanol intake in the rat model. A particular role for enhanced ERK1/2 activation appears likely. It is also suggested that accumulation of fat by chronic ethanol or acute ethanol alone may be due to mechanisms independent of ERK1/2 activation, whereas increased accumulation of fat by binge (in chronic-binge group) is likely due to increased ERK1 and more so ERK2, activation.
ACKNOWLEDGEMENTS
This work was supported in part by NIH grant AA11962 and AA16347.
List of abbreviations
- ALT
alanine amino transferase
- egr-1
early growth response -1
- ERK
exracellular regulated kinase
- MAPK
mitogen activated protein kinase
- PAI-1
plasminogen activator inhibitor -1
- RSK
90S ribosomal kinase
- TNF α
tumor necrosis factor alpha
- qRT-PCR
quantitative reverse transcriptase polymerase chain reaction
Footnotes
A part of this work was presented at Experimental Biology 2009 meeting (Aroor and Shukla, 2009) and International Society for Biomedical Research on Alcoholism 2010 meeting (Aroor et al; 2010).
REFERENCES
- Adachi M, Brenner DA. Clinical syndromes of alcoholic liver disease. Dig Dis. 2005;23:255–263. doi: 10.1159/000090173. [DOI] [PubMed] [Google Scholar]
- Amersi F, Shen Xiu-Da, Anselmo D, Anselmo D, Melinek J, Iyer S, Southard DJ, Katori M, Volk HD, Busuttil RW, Buelow R, Kupiec-Weglinski JW. Ex vivo exposure to carbon monoxide prevents hepatic ischemia /reperfusion injury through p38 MAP kinase pathway. Hepatology. 2002;35:815–823. doi: 10.1053/jhep.2002.32467. [DOI] [PubMed] [Google Scholar]
- Anjum R, Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol. 2008;9:747–758. doi: 10.1038/nrm2509. [DOI] [PubMed] [Google Scholar]
- Apte M, McCarroll J, Pirola R, Wilson J. Pancreatic MAP kinase pathways and acetaldehyde. Novartis Found Symp. 2007;285:200–211. doi: 10.1002/9780470511848.ch15. [DOI] [PubMed] [Google Scholar]
- Aroor AR, Custer GW, Weng YI, Lee YJ, Shukla SD. Phosphatidylethanol mimics ethanol modulation of p42/44 mitogen-activated protein kinase signalling in hepatocytes. Alcohol Alcohol. 2002;37:534–539. doi: 10.1093/alcalc/37.6.534. [DOI] [PubMed] [Google Scholar]
- Aroor AR, Shukla SD. MAP kinase signaling in diverse effects of ethanol. Life Sci. 2004;74:2339–2364. doi: 10.1016/j.lfs.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Aroor AR, Shukla SD. Activation of ERK1/2 MAP kinase in liver afetr binge and chronic ethanol-binge intake may augment liver injury. FASEB J. 2009;23:225A. [Google Scholar]
- Aroor AR, Jackson DE, Shukla SD. Chronic ethanol intake increases suceptibility to liver injury by repeat ethanol binge: A novel clinically relevant model for progression of alcoholic liver injury. Alcoholism Clin Exp Res. 2010;34:144A. [Google Scholar]
- Bhagwandeen BS, Apte M, Manwarring L, Dickeson J. Endotoxin induced hepatic necrosis in rats on an alcohol diet. J Pathol. 1987;152:47–53. doi: 10.1002/path.1711520107. [DOI] [PubMed] [Google Scholar]
- Beier JI, Luyendyk JP, Guo L, von Montfort C, Staunton DE, Arteel GE. Fibrin accumulation plays a critical role in the sensitization to lipopolysaccharide-induced liver injury caused by ethanol in mice. Hepatology. 2009;49:1545–1553. doi: 10.1002/hep.22847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck M, Chojkier M. A ribosomal S-6 kinase-mediated signal to C/EBP-beta is critical for the development of liver fibrosis. PLoS One. 2007;2:e1372. doi: 10.1371/journal.pone.0001372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death, and cancer. Pharmacol Rev. 2008;60:261–310. doi: 10.1124/pr.107.00106. [DOI] [PubMed] [Google Scholar]
- Caro AA, Cederbaum AI. Role of cytochrome p450 in phospholipase A2-arachidonic acid mediated toxicity. Free Radical Med Biol. 2006;40:364–375. doi: 10.1016/j.freeradbiomed.2005.10.044. [DOI] [PubMed] [Google Scholar]
- Ceccanti M, Attili A, Balducci G, Attilia F, Giacomelli S, Rotondo C, Sasso GF, Xirouchakis E, Attilia ML. Acute alcoholic hepatitis. J Clin Gastroenterol. 2006;40:833–841. doi: 10.1097/01.mcg.0000225570.04773.5d. [DOI] [PubMed] [Google Scholar]
- Crosse KI, Anania FK. Alcoholic hepatitis. Curr Treatment options in gastroenterology. 2002;5:417–423. doi: 10.1007/s11938-002-0029-7. [DOI] [PubMed] [Google Scholar]
- Deaciuc IVD, Souza NB, Burkhanov R, Nasser MS, Voskresensky IV, De Villiers WJ, McClain CJ. Alcohol but not lipopolysachharide-induced liver apoptosis involves changes in intracellular compartmentalization of apoptotic regulators. Alcoholism Clin Exp Res. 2004;28:160–172. [PubMed] [Google Scholar]
- Ericson J, Rutberg M, Magnusson B, Andersson L, Boström P. PLD1 and ERK2 regulate cytosolic lipid droplet formation. J Cell Sci. 2006;119:2246–2257. doi: 10.1242/jcs.02941. [DOI] [PubMed] [Google Scholar]
- Fukumura A, Tsutsumi M, Tsuchishima M, Takase S. Correlation between adenosine triphosphate content and apoptosis in liver treated with alcohol. Alcoholism Clin Exp Res. 2003;27:12S–15S. doi: 10.1097/01.ALC.0000078609.36825.48. [DOI] [PubMed] [Google Scholar]
- Fukui H. Relation of endotoxin, endotoxin binding proteins and macrophages to severe alcoholic injury and multiple organ failure. Alcoholism Clin Exp Res. 2005;29:172S–179S. doi: 10.1097/01.alc.0000189278.30237.e9. [DOI] [PubMed] [Google Scholar]
- Hall MP, de la Lieber CS, DeCarli LM, French SW, Lindros KO, Jarvelainen H, Bode C, Parlesak A, Bode JC. Models of alcoholic liver disease in rodents. A critical evaluation. Alcoholism Clin Exp Res. 2001;25:254S–261S. doi: 10.1097/00000374-200105051-00041. [DOI] [PubMed] [Google Scholar]
- Honchel R, Ray MB, Marsano L, Cohen D, Lee E, Shedlosky S, McClain CJ. Tumor necrosis factor in alcohol enhanced endotoxin liver injury. Alcohol Clin Exp Res. 1992;16:665–669. doi: 10.1111/j.1530-0277.1992.tb00656.x. [DOI] [PubMed] [Google Scholar]
- Hsu MKH, Qiao L, Ho VB, Zhang H, Zhang H, Teoh N, Dent P, Farrrel GC. Ethanol reduces p38 kinase activation and cyclin D1 protein expression after partial hepatectomy in rats. J Hepatol. 2006;44:375–382. doi: 10.1016/j.jhep.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Ji C, Deng Q, Kaplowitz N. Role of TNF-α in Ethanol-Induced hyperhomocysteinemia and murine alcoholic liver injury. Hepatology. 2004;40:442–451. doi: 10.1002/hep.20309. [DOI] [PubMed] [Google Scholar]
- Kaizu T, Ikeda A, Nakao A. Protection of transplant induced hepatic ischemia/reperfusion injury with carbon monoxide via MEK/ERK1/2 pathway down regulation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G236–G244. doi: 10.1152/ajpgi.00144.2007. [DOI] [PubMed] [Google Scholar]
- Ki SH, Park O, Zheng M, Morales-Ibanez O, Kolls JK, Bataller R, Gao B. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: Role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–1300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotesh A, Yang S, Lin H, Huang X, Diehl AM. Chronic ethanol exposure potentiates lipopolysaccharide liver injury despite inhibiting jun N-terminal kinase and caspase 3 activation. J Biol Chem. 2002a;44:13037–13044. doi: 10.1074/jbc.M101632200. [DOI] [PubMed] [Google Scholar]
- Kotesh A, Yang S, Lin H, Huang J, Diehl AM. Ethanol induces redox-sensitive cell-cycle inhibitors and inhibits liver regeneration after partial hepatectomy. Alcoholism Clin Exp Res. 2002b;26:1710–1718. doi: 10.1097/01.ALC.0000036923.77613.59. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Aroor AR, Shukla SD. Temporal activation of p42/44 MAPK and C-Jun N-terminal kinase by acetaldehyde in rat hepatocytes and its loss after chronic ethanol exposure. J. Pharm Exp Ther. 2002;301:908–914. doi: 10.1124/jpet.301.3.908. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Shukla SD. Pro- and anti-apoptotic roles of c-Jun N-terminal kinase (JNK) in ethanol and acetaldehyde exposed rat hepatocytes. Eur J Pharmacol. 2005;508:31–45. doi: 10.1016/j.ejphar.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Shukla SD. Histone H3 phosphorylation at serine 10 and serine 28 is mediated by p38 MAPK in rat hepatocytes exposed to ethanol and acetaldehyde. Eur J Pharmacol. 2007;573:29–38. doi: 10.1016/j.ejphar.2007.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber CS, De Carli LM. The feeding of alcohol and liquid diets: two decades of applications and 1982 update. Alcoholism Clin Exp Res. 1982;6:523–531. doi: 10.1111/j.1530-0277.1982.tb05017.x. [DOI] [PubMed] [Google Scholar]
- Liu Q, Möller U, Flügel D, Kietzmann T. Induction of plasminogen activator inhibitor I gene expression by intracellular calcium via hypoxia-inducible factor-1. Blood. 2004;104:3993–4001. doi: 10.1182/blood-2004-03-1017. [DOI] [PubMed] [Google Scholar]
- Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol. 2009;50:1258–1266. doi: 10.1016/j.jhep.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathurin P, Beuzin F, Louvet A, Carrié-Ganne N, Balian A, Trinchet JC, Dalsoglio D, Prevot S, Naveau S. Fibrosis progression occurs in a subgroup of heavy drinkers with typical histological features. Aliment Pharmacol Ther. 2007;25:1047–1054. doi: 10.1111/j.1365-2036.2007.03302.x. [DOI] [PubMed] [Google Scholar]
- Mathurin P, Deltenre P. Effect of binge drinking on the liver: an alarming public health issue? Gut. 2009;58:613–617. doi: 10.1136/gut.2007.145573. [DOI] [PubMed] [Google Scholar]
- Mor A, Philips MR. Compartmentalized Ras/MAPK signaling. Annu Rev Immunol. 2006;24:771–800. doi: 10.1146/annurev.immunol.24.021605.090723. [DOI] [PubMed] [Google Scholar]
- Nguyen VA, Gao B. Expression of interferon alfa signaling components in human alcoholic liver disease. Hepatology. 2002;35:425–432. doi: 10.1053/jhep.2002.31169. [DOI] [PubMed] [Google Scholar]
- Nelson S, Baghy GJ, Bainton BG, Summer WR The effects of acute and chronic alcoholism on tumor necrosis factor and the inflammatory response. J Inf Dis. 1989;160:422–429. doi: 10.1093/infdis/160.3.422. [DOI] [PubMed] [Google Scholar]
- Ni HM, Chen X, Ding WX, Schuchmann M, Yin X. Differential roles of JNK in ConA/GalN and ConA-induced liver injury in mice. Am J Pathol. 2008;173:962–972. doi: 10.2353/ajpath.2008.080358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguro T, Takahasi Y, Ashino T, Takaki A, Shidoja S, Horai R, Asano M, Sekikawa K, Iwakura Y, Yoshida T. Involvement of tumor necrosis factor alpha rather than interleukin 1-α/β or nitric oxides in the heme oxygenase-1 gene expression by lipopolysaccharide in the mouse liver. FEBS letters. 2002;516:63–66. doi: 10.1016/s0014-5793(02)02502-4. [DOI] [PubMed] [Google Scholar]
- Park PH, Lim RW, Shukla SD. Involvement of histone acetyltransferase (HAT) in ethanol-induced acetylation of histone H3 in hepatocytes: potential mechanism for gene expression. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1124–G1136. doi: 10.1152/ajpgi.00091.2005. [DOI] [PubMed] [Google Scholar]
- Peinnequin A, Mouret C, Birot O, Alonso A, Mathieu J, Clarençon D, Agay D, Chancerelle Y, Multon E. Rat pro-inflammatory cytokine and cytokine related mRNA quantification by real-time polymerase chain reaction using. SYBR green BMC Immunol. 2004;5:3. doi: 10.1186/1471-2172-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard MT, Nagy LE. Ethanol-induced liver injury: potential roles for egr-1. Alcohol Clin Exp Res. 2005;11 Suppl:146S–150S. doi: 10.1097/01.alc.0000189286.81943.51. [DOI] [PubMed] [Google Scholar]
- Pimienta G, Pascual J. Canonical and alternative MAPK signaling. Cell Cycle. 2007;6:2628–2632. doi: 10.4161/cc.6.21.4930. [DOI] [PubMed] [Google Scholar]
- Rivara PF, Jurkovich GJ, Gurney JG, Seguin D, Fligner CL, Ries R, Raisys VA, Copass M. The magnitude of acute and chronic alcohol abuse in trauma patients. Arch Surg. 1993;128:907–913. doi: 10.1001/archsurg.1993.01420200081015. [DOI] [PubMed] [Google Scholar]
- Schafer C, Parlesak A, Schutt C. Concentrations of lipopolysaccharide binding protein, bactericidal/ permeability-increasing protein, soluble CD-14 and plasma lipids in relation to endotoxemia in patients with alcoholic liver disease. Alcohol Alcoholism. 2002;37:81–86. doi: 10.1093/alcalc/37.1.81. [DOI] [PubMed] [Google Scholar]
- Schattenberg JM, Wang Y, Rigoli RM, Koop DR, Czaja MJ. CYP2E1 overexpression alters hepatocyte death from menadione and fatty acids by activation of ERK1/2 signaling. Hepatology. 2004;39:444–455. doi: 10.1002/hep.20067. [DOI] [PubMed] [Google Scholar]
- Shimamura A, Ballif BA, Richards SA, Blenis J. Rsk1 mediates a MEK-MAP kinase survival signal. Current Biology. 2000;10:127–135. doi: 10.1016/s0960-9822(00)00310-9. [DOI] [PubMed] [Google Scholar]
- Singh R, Wang Y, Xiang Y. Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance. Hepatology. 2009;49:87–96. doi: 10.1002/hep.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur V, McMullen MR, Pritchard MT, Nagy LE. Regulation of macrophage activation in alcoholic liver disease. J Gastroenterol Hepatol. 2007;22:S53–S56. doi: 10.1111/j.1440-1746.2006.04650.x. [DOI] [PubMed] [Google Scholar]
- Tsai JC, Liu L, Zhang J, Spokes KC, Topper JN, Aird WC. Epidermal growth factor induces Egr-1 promoter activity in hepatocytes in vitro and in vivo. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1271–G1278. doi: 10.1152/ajpgi.2001.281.5.G1271. [DOI] [PubMed] [Google Scholar]
- Venugopal SK, Chen J, Zhang Y, Clemens D, Follenzi A, Zern MA. Role of MAPK phosphatase-1 in sustained activation of JNK during ethanol-induced apoptosis in hepatocyte-like VL-17A cells. J Biol Chem. 2007;282:31900–31908. doi: 10.1074/jbc.M703729200. [DOI] [PubMed] [Google Scholar]
- Wheeler MD, Thurman RG. Up-regulation of CD14 in liver caused by acute ethanol involves oxidant-dependent AP-1 pathway. J Biol Chem. 2003;278:8435–8441. doi: 10.1074/jbc.M212076200. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Collins QF, An J, Lupo E, Jr, Liu HY, Liu D, Robidoux J, Liu Z, Cao W. p38 mitogen-activated protein kinase plays an inhibitory role in hepatic lipogenesis. J Biol Chem. 2007;282:4975–4982. doi: 10.1074/jbc.M606742200. [DOI] [PubMed] [Google Scholar]
- Yamagishi Y, Horie Y, Kato S, Kajihara S, Tamai H, Granger DN, Ishii H. Ethanol modulates gut ischemia/reperfusion-induced injury in rats. Am J Physiol Gastrointestinal Liver Physiol. 2002;282:G640–G646. doi: 10.1152/ajpgi.00171.2001. [DOI] [PubMed] [Google Scholar]
- You M, Liang X, Ajmo JM, Ness GC. Involvement of mammalian sirtuin 1 in the action of ethanol in the liver. Am J Physiol Gastrointest Liver Physiol. 2008;294:G892–G898. doi: 10.1152/ajpgi.00575.2007. [DOI] [PubMed] [Google Scholar]
- Zakhari S, Li TK. Determinants of alcohol use and abuse: impact of quantity and frequency patterns on liver disease. Hepatology. 2007;46:2032–2039. doi: 10.1002/hep.22010. [DOI] [PubMed] [Google Scholar]
- Zhuang S, Kinsey GR, Yan Y. Extracellular signal-regulated kinase activation mediates mitochondrial dysfunction and necrosis induced by hydrogen peroxide in renal proximal tubular cells. J Pharmacol Exp Ther. 2008;325:732–740. doi: 10.1124/jpet.108.136358. [DOI] [PubMed] [Google Scholar]