Introduction
Drug addiction is a chronic, relapsing disease and a persistent health problem in the United States with few available treatment options. While there are multiple aspects of the addiction process, a critical component is the development of sensitization, which may explain drug craving, believed to strongly contribute to relapse (Kalivas and Stewart, 1991; Robinson and Berridge, 1993). Sensitization to psychomotor stimulants such as cocaine can be observed at the behavioral, cellular and molecular levels. Recent insights into the mechanisms underlying drug sensitization have come from the study of molecular changes in the mesolimbic dopaminergic system, the major reward pathway known to be affected by cocaine and other drugs of abuse. In particular, adaptive changes in synaptic plasticity within the mesolimbic system accompany drug sensitization and may be an underlying mechanism responsible for drug craving (Vanderschuren and Kalivas, 2000; Kauer and Malenka, 2007; Thomas et al., 2008, Gong et al., 2006)
The mammalian target of rapamycin (mTOR) pathway has emerged as a regulator of neuroplasticity in the CNS (Jaworski and Sheng, 2006). The mTOR is a Ser/Thr protein kinase complex which responds to multiple extracellular stimuli such as nutrients, energy, growth factors, and mitogens that regulate cell growth, cell survival, transcription and protein synthesis (Sarbassov et al., 2005). The mTOR activation phosphorylates its downstream targets S6K1 and Akt which, in turn, regulate protein translation and cell survival. The mTOR pathway is implicated in neuronal development and synaptic plasticity, presumably via influencing axon growth, dendritic arborization, changes in neuronal morphology, as well as synaptogenesis (Schratt et al., 2004; Tavazoie et al., 2005; Jaworski and Sheng, 2006; Park et al., 2008). The mTOR pathway regulates hippocampal long-term potentiation (LTP), long-term depression (LTD) and fear memory formation (Horwood et al., 2006; Parsons et al., 2006). Apparently, the mTOR activity is regulated during synaptic transmission. For example, activation of NMDA and dopamine receptors activates mTOR (Lenz and Avruch, 2005; Gong et al., 2006). Importantly, recent studies have also revealed that mTOR regulates synaptic plasticity in the ventral tegmental area (VTA) (Mameli et al., 2007), suggesting that mTOR may be an important mediator of drug sensitization, and consequently, drug addiction.
Sensitization to cocaine and other psychomotor stimulants is often characterized behaviorally by measuring increases in locomotor activity observed following a period of withdrawal from chronic drug exposure. Generally, sensitization requires an initial induction phase followed by drug withdrawal and then a re-exposure to the drug to allow for the expression of sensitization. In this study, we examined whether cocaine exposure influences the mTOR pathway. We also assessed whether treatment with rapamycin, an inhibitor of mTOR, influences the induction and/or expression of cocaine-induced locomotor sensitization.
Materials and Methods
Animals and drugs
Female Sprague-Dawley (SD) rats weighing approximately 225-250g upon arrival were obtained from Taconic Farms (Germantown, NY). Rats were individually-housed in a room maintained on a 12-hr light/dark cycle (lights on 0700). Food and water were provided ad libitum. Cocaine hydrochloride was dissolved in saline, and rapamycin was dissolved in 4% ethanol and 5% Tween-20 in water at a concentration of 5mg/ml. Both drugs were injected intraperitoneally (i.p.) at a volume of 1 ml/kg. All experiments were performed in accordance with National Institutes of Health guidelines for the care and use of laboratory animals and were approved by our Institutional Animal Care and Use Committees.
Acute cocaine and rapamycin administration
Rats were injected with 15 mg/kg cocaine or 15 mg/kg cocaine plus 5 mg/kg rapamycin (i.p.). Rats received a single rapamycin injection 60 min before cocaine injection. One hour after cocaine injection, brain cortex, ventral tegmental area (VTA), and nucleus accumbens (NAc) were dissected on an ice-cold platform from 1 mm-thick coronal sections using a micropunch technique as described (Palkovits, 1973). Tissues were homogenized in a buffer containing 50 mM Tris·HCl pH 7.4, protease inhibitor cocktail, 2 mM sodium orthovanadate, 10 mM sodium fluoride, 100 μM PMSF. After adding the equivalent volume of 2X LDS sample buffer, brain tissue lysates were further boiled at 90°C for 5 min. Samples were centrifuged at 16,000 g for 10 min and the resulted supernatants were applied to western blot.
Western blot
Protein samples were separated in 8% Bis-Tris gel and then transferred onto a 0.45 μM nitrocellulose membrane. The membrane was blocked by 5% nonfat dry milk in TBST (25 mM Tris-HCl, pH 7.4; 150 mM NaCl; 0.05% Tween-20) for 1 hr at room temperature. The antibodies of rabbit anti-S6 and anti-phosphorylated S6 (Cell Signaling Technologies Inc. Boston, MA) were diluted at 1: 1000 in TBST with 3% BSA and incubated with membrane overnight at 4°C. The HRP-conjugated secondary antibody (GE Healthcare, Piscataway, NJ) was diluted at 1:5000 in 5% milk in TBST and incubated with membrane for 1 hr at room temperature. Signals were detected by ECL solutions (Pierce, Rockford, IL) for 3 min and scanned by FUJI image Analyzer LAS-4000. The intensity of phosphorylated S6 detected by western blot was normalized to total S6. Additionally, GAPDH was also used as a loading control.
Locomotor activity test
The experimental paradigm was performed essentially as described by Szumlinski et al. (1999). Behavioral testing was conducted in cylindrical (60-cm) photocell activity chambers, each equipped with three intersecting photobeams. All photocells and detectors were interfaced to a microprocessor that continuously recorded photobeam interruptions throughout a session using software supplied by Med Associates, Inc. (St. Albans, VT, USA). Prior to behavioral testing, each rat was habituated in the locomotor-activity testing room for 20 min daily. A 30 min baseline period was recorded before receiving an intraperitoneal (i.p.) injection of 15 mg/kg cocaine or 1 ml/kg saline, followed by a 2-hr locomotor activity test session. This procedure was conducted for 5 consecutive days. Rats were subsequently withdrawn from cocaine for 13 days and remained in the colony room during this time. On the test day (Day 19), after the 30 min baseline activity period, a challenge dose of 15 mg/kg cocaine was given to all rats immediately prior to the 2 hr activity test. For the rapamycin treatment, rats were randomly divided into 4 groups: cocaine with rapamycin (CR) or vehicle (CV), and saline control with rapamycin (SR) or vehicle (SV). All activity tests were performed during the day.
Induction of sensitization
To examine the effect of rapamycin on induction of cocaine-induced locomotor sensitization, rats either received rapamycin or vehicle 5 minutes prior to cocaine or saline via i.p. injection during the first 5 days (Figure 2C). For the rapamycin dose-response experiment, rats were treated with 0, 0.1, 1, 2.5 or 5 mg/kg rapamycin, respectively.
Figure 2.
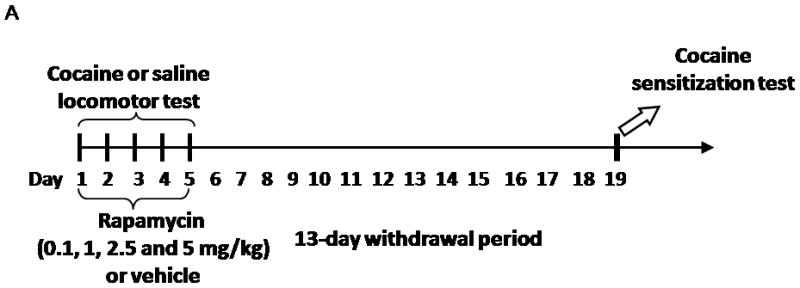
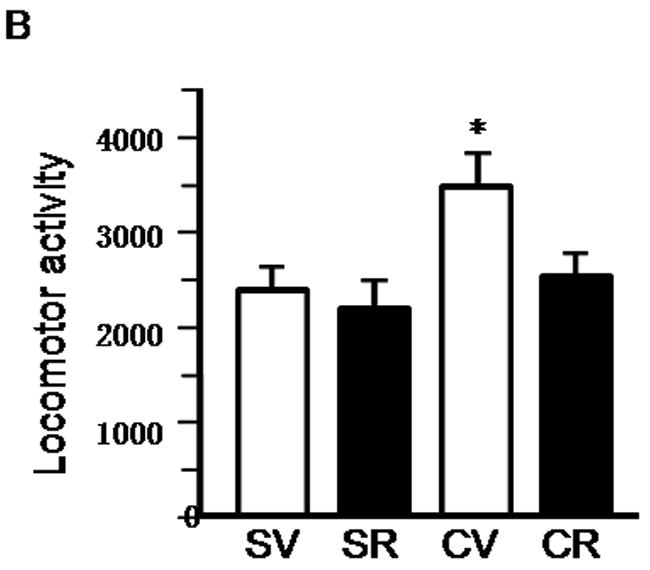
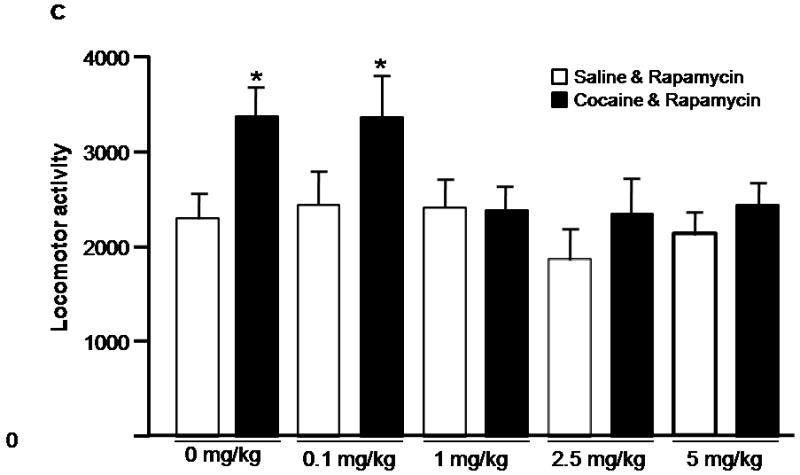
Inhibition of mTOR by rapamycin blocks induction of cocaine-induced locomotor sensitization. A, Schematic diagram showing timing of drug treatment and locomotor activity monitored. B, Rapamycin blocks cocaine induced locomotor sensitization. Rats were co-injected with rapamycin (5 mg/kg) and cocaine (15 mg/kg) or vehicle, during the first five days of treatment. Locomotor activity data (the number of beam breaks) acquired from the test day are presented as mean and SEM (n=8). The cocaine-treated group (CV) showed significantly higher locomotor activity than that of cocaine and rapamycin-treated (CR), saline/rapamycin-treated (SR), and control groups (SV). * indicates p<0.05 following one-way ANOVA. C, Dose-response of rapamycin’s effects on induction of cocaine-induced locomotor sensitization. Rapamycin at 1, 2.5 and 5 mg/kg, but not at 0.1 mg/kg, blocked induction of cocaine-induced locomotor sensitization. Data are presented as mean and SEM (0 mg/kg and 5 mg/kg, n=8; 0.1 mg/kg, n=6; 1 mg/kg, n=10; and 2.5 mg/kg, n=4). * indicates significant difference between cocaine-vehicle (CV) and saline-vehicle (SV) groups following individual t-tests (p=0.019). No significant differences were found between CR and vehicle groups (SR, SV; p > 0.05 using individual t-tests).
Expression of sensitization
To investigate the effect of rapamycin on expression of locomotor sensitization, rats were given injections of rapamycin or vehicle for 4 consecutive days beginning on day 15, just prior to the test for sensitization on Day 19.
Data analysis
Statistical analysis was performed using STATISTICA (StatSoft, Inc., Tulsa, OK, USA). Groups were compared using one- or two-way repeated measures ANOVA with Fisher LSD post-hoc tests, or student’s t-test where appropriate.
Results
Cocaine activates mTOR in vivo
To determine whether mTOR is involved in mediating cocaine’s effects, we examined the effect of cocaine on phosphorylation of S6, a downstream target of the mTOR, in rat brains. A single injection of cocaine (15 mg/kg) markedly stimulated S6 phosphorylation in the cortex within one hour (Figure 1A & 1B). The increased S6 phosphorylation observed was completely depleted by the mTOR inhibitor rapamycin (5mg/kg), suggesting that the levels of phospho-S6 reflect in vivo mTOR activity (control vs cocaine: p=0.004; cocaine vs cocaine & rapamycin: p=0.005; control vs cocaine & rapamycin: 0.592; t-test; n=6). We further examined mTOR activity in the mesolimbic dopamine pathway, specifically the ventral tegmental area (VTA) and nucleus accumbens (NAc). We found that cocaine also evoked S6 phosphorylation in these regions (control vs cocaine: p=0.026; cocaine vs cocaine & rapamycin: p=0.011; control vs cocaine & rapamycin: 0.697; t-test; n=6 in VTA) (control vs cocaine: p=0.031; cocaine vs cocaine & rapamycin: p=0.042; control vs cocaine & rapamycin: 0.881; t-test; n=6 in NAc) (Figure 1C, 1D, 1E and 1F). Again, S6 phosphorylation was nearly completely abolished by rapamycin. However, cocaine exposure did not increase S6 phosphorylation in cerebellum and hippocampus (Supplemental Figure 1).
Figure 1.
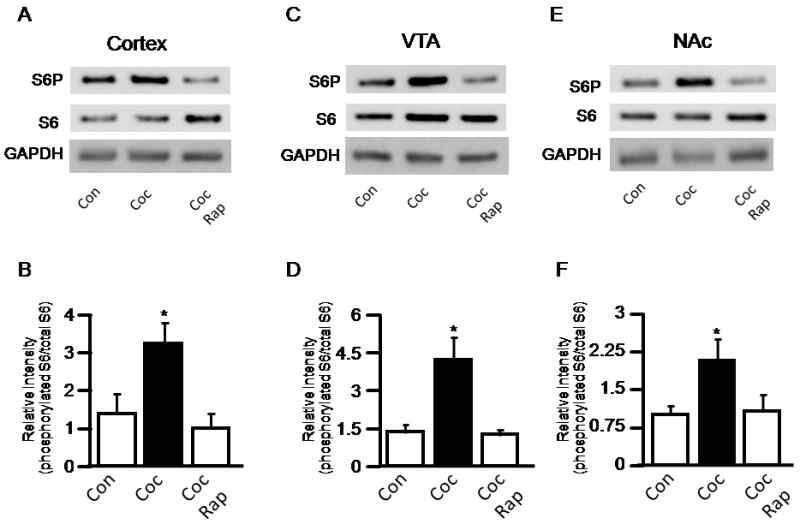
Cocaine stimulates S6 phosphorylation in rat brain. Cocaine activates mTOR in rat brain cortex (A), ventral tegmental area (VTA) (C), and Nucleus accumbens (NAc) (E). Cocaine was delivered via ip injection, and regions were dissected 1 hr after injection. S6 phosphorylation was detected by western blot. Quantification of S6 phosphorylation in cortex (B), ventral tegmental area (VTA) (D), and nucleus accumbens (NAc) (F). Data were presented as relative intensities of phosphorylated S6 to total S6 with GAPDH as an additional loading control. * indicates significant difference between control vs cocaine and cocaine (Coc) vs cocaine plus rapamycin (Rap) (p< 0.05) (t-test, n=6).
Inhibition of mTOR by rapamycin blocks the induction of cocaine-induced locomotor sensitization
The induction phase of sensitization is associated with synaptic adaptive changes (Vanderschuren and Kalivas, 2000). Having demonstrated that the mTOR pathway, known to mediate neuronal plasticity, is activated by cocaine, we next examined whether cocaine-induced locomotor sensitization in this induction period involves mTOR. To test this possibility, rats were injected with cocaine (0 or 15 mg/kg) and rapamycin (0 or 5 mg/kg) for 5 consecutive days as indicated in Figure 2A. We started with rapamycin at 5mg/kg because as shown in Figure 1 and recently studies (Zeng et al., 2009; Huang et al., 2010) this dose can effectively inhibit mTOR in rat brains. After a 13-day withdrawal period, a challenge injection of cocaine (15 mg/kg) was given to test for locomotor sensitization. Rats that were pre-exposed to cocaine (CV group) for 5 days showed significantly greater locomotor activity in the 2 hr test sesssion than vehicle-treated rats (SV group; F (3,27) = 4.05, p < 0.01; Figure 2B), reflecting cocaine-induced locomotor sensitization. However, locomotor activity in rats receiving both cocaine and rapamycin (CR group) was significantly lower than that of cocaine-sensitized rats (CV; p < 0.05) and indistinguishable from that of vehicle-treated rats (SR; Figure 2B), suggesting that mTOR inhibition by rapamycin during the induction phase completely suppresses the induction of locomotor sensitization. No differences in locomotor activity were observed in vehicle-treated rats given rapamycin (SR) compared to those vehicle rats given saline injections (SV), confirming that rapamycin itself exhibited no effects on locomotor activity (Figure 2B). We tested additional doses of rapamycin (0.1, 1 and 2.5 mg/kg). The locomotor sensitization was still significantly inhibited by the doses at 1 and 2.5 mg/kg, but not at 0.1 mg/kg (Fig. 2C).
Rapamycin blocks the expression of cocaine-induced locomotor sensitization
After initial repeated exposure to cocaine followed by a two-week withdrawal period, rats normally develop hyperlocomotor activity when re-exposed to cocaine. To determine whether a later rapamycin treatment can suppress the expression of locomotor sensitization, we treated rats with rapamycin daily on the last 4 days before the test day, when a challenge injection of cocaine was given (see Figure 3A). A one-way ANOVA by group revealed a significant difference between treatment groups (F (3, 20) = 7.24; p < 0.01). Rapamycin treatment significantly reduced the locomotor activity in rats pre-exposed to cocaine (CR group) such that locomotor activity was significantly lower than that of sensitized rats (CV group; p < 0.05; Figure 3B), but not different from that of the saline-treated rats (SV group; p > 0.05). Mean activity counts for the 30-minute habituation period prior to cocaine injection were calculated for cocaine-vehicle and cocaine-rapamycin treated animals on Day 19 and there were no differences between the two groups (cocaine-vehicle = 510.7 ± 24.3 vs. cocaine-rapamycin 396.5 ± 70.1; p =0.15; data not shown). When ten-minute activity bins were examined on Day 19 (Fig. 3C), a repeated-measures ANOVA revealed a main effect of group (F (3, 20) = 7.24, p < 0.01) and post-hoc analyses indicated higher activity counts in cocaine-vehicle treated rats than those given cocaine and rapamycin (p < 0.05), with no significant differences between the saline-rapamycin and saline-vehicle treated groups, further indicating that rapamycin alone does not inhibit locomotor activity. These data suggest that the inhibition of mTOR by rapamycin at a later stage can also block the expression of locomotor sensitization to cocaine.
Figure 3.
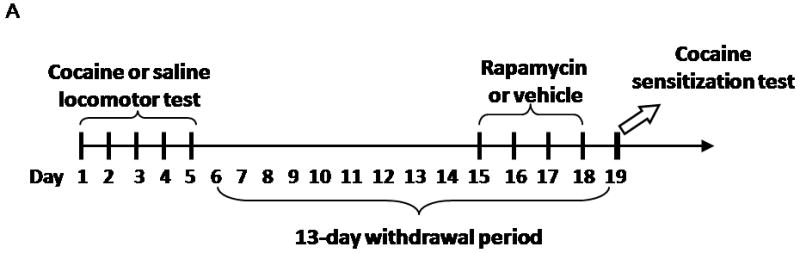
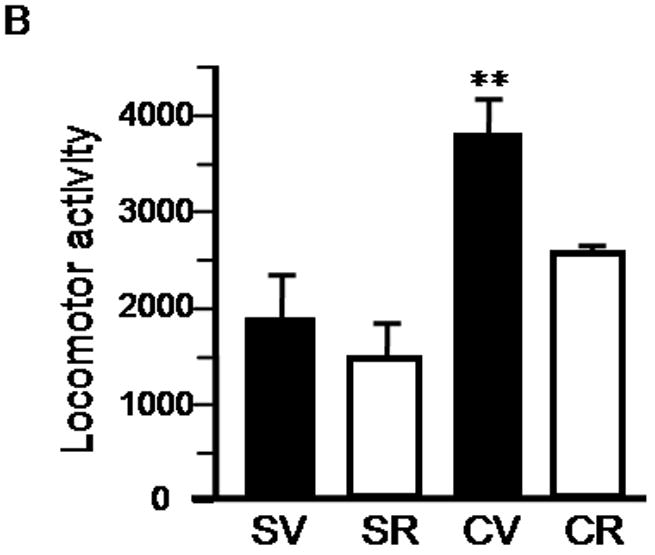
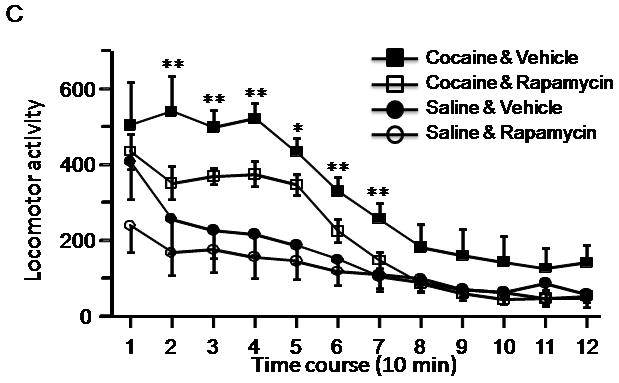
Rapamycin blocks the expression of cocaine-induced locomotor sensitization. A, Schematic diagram showing timing of drug treatment and locomotor activity tests. B, Data from the sensitization test on Day 19 are presented as mean and SEM (n=6). Rats repeatedly exposed to cocaine (CV) showed significantly higher locomotor activity (determined by beam breaks) than that of cocaine and rapamycin-treated (CR; p < 0.05), rapamycin-treated (SR; p < 0.01), and control groups (SV; p < 0.01, using post-hoc tests following one-way ANOVA). There were no differences between CR, SR and SV groups. C, A plot of locomotor activity data acquired in 10-minute intervals. * indicates significant differences (p<0.05) from saline-rapamycin group (SR) (two-way repeated measures ANOVA, followed by post-hoc test).
Discussion
Here we report that acute cocaine exposure activates the mTOR in cortical forebrain as well as in ventral tegmental area (VTA) and nucleus accumbens (NAc). Furthermore, inhibition of mTOR by systemically administered rapamycin blocks both the induction and expression of cocaine-induced locomotor sensitization, suggesting that the mTOR pathway may be critically involved in cocaine sensitization and perhaps play a role in the development of drug addiction.
Cocaine-induced locomotor sensitization involves two separate stages: the initial induction and the later expression phase; these stages are associated with different cellular and/or molecular changes and tend to be differentially mediated within the mesolimbic dopamine pathway. For instance, Mameli and colleagues (Mameli et al., 2009) showed that a single exposure to cocaine triggered synaptic plasticity only in VTA dopamine neurons whereas repeated cocaine exposure led to persistent plasticity in the nucleus accumbens (NAc). The adaptive changes in the NAc tend to be longer-lasting than those in the VTA (Wolf, 1998). The mTOR inhibitor rapamycin prevented both the induction and the expression of sensitization, suggesting that the mTOR pathway is engaged during multiple stages of the sensitization process, and/or mTOR activation in both VTA and NAc contributes to cocaine sensitization. Our findings are also consistent with recently reported observations that rapamycin reduces craving in heroin addicts (Shi et al., 2009) and methamphetamine sensitization in rodents (Narita et al., 2005).
Rapamycin apparently specifically blocks cocaine sensitization, rather than having nonspecific general suppression on locomotor activity because it does not significantly affect locomotor activity in vehicle-treated rats (i.e. no difference between SV and SR groups; Figure 2B). Rapamycin’s half life is about 60 hrs (Zimmerman and Kahan, 1997). We would expect that there is only little, if any, residual rapamycin after two weeks of abstinence. Therefore, the long lasting effect of rapamycin treatment on cocaine-induced locomotor sensitization could be through blocking cocaine-induced synaptic modification during the induction phase.
We observed that acute cocaine treatment significantly increases mTOR activity in brain cortex, VTA and NAc with little effect in hippocampus and cerebellum. The detailed mechanism by which cocaine exposure activates the mTOR remains to be elucidated. One of the most well studied mechanisms of action of cocaine in the central nervous system is the blockade of the dopamine transporters which leads to accumulation of dopamine in synaptic cleft, resulting in an enhanced and prolonged activation of dopamine receptors at postsynaptic neurons that receive dopaminergic input (Ritz et al., 1987). Of particular note, dopaminergic signaling represents one of the key components in the reward circuitry composed of VTA, NAc and the prefrontal cortex in the regulation of reward and movement. In fact, previous studies reported that dopamine treatment increases phosphorylation of S6K and its downstream target S6 in primary cultured neurons (Lenz and Avruch, 2005). Therefore, it is conceivable that acute cocaine exposure would stimulate dopaminergic transmission, which in turn leads to mTOR activation in this reward circuitry.
The precise molecular mechanism of mTOR in cocaine sensitization remains entirely unclear. The mTOR pathway is implicated in various neuronal processes including influencing axon growth and navigation, dendritic arborization, changes in soma and spine morphology, synaptogenesis, as well as expression of receptor/ channels (Schratt et al., 2004; Tavazoie et al., 2005; Jaworski and Sheng, 2006; Park et al., 2008). It is possible that inactivation of mTOR by rapamycin could attenuate the adaptive changes or neuronal plasticity elicited by cocaine exposure, therefore abolishing locomotor sensitization. Additionally, the mTOR pathway is known to be regulated by glutamatergic and dopaminergic systems which, in VTA, NAc and subregions of the cortex, are critically involved in drug sensitization, providing a potential link between cocaine exposure, aberrant activation of the mTOR, and locomotor sensitization (Lenz and Avruch, 2005, Huang et al, 2007).
Psychomotor stimulant-induced locomotor sensitization presumably shares similar cellular and molecular mechanisms with compulsive drug-seeking behavior. Moreover, increasing evidence suggests that the behavioral changes, i.e. locomotor activity and drug seeking, reflect adaptations in synaptic transmission that bear some resemblance to hippocampal learning (del Olmo et al., 2006; Chen et al., 2008). For example, it has been reported that drugs of abuse such as cocaine induce long-term potentiation (LTP) at excitatory synapses in the VTA (Chen et al., 2008). The mTOR pathway, which regulates protein translation, plays a critical role in synaptic plasticity, learning and memory and associated behavioral changes. Genetic studies have revealed that conditional knock out of the rapamycin-binding FKBP12 in the mTOR pathway leads to an enhanced basal mTOR activity along with enhancement of LTP, contextual fear memory formation and perseverative/repetitive behavior (Hoeffer et al., 2008). The mTOR pathway is also involved in mGluR-mediated long-term depression (LTD) in the VTA through control of rapid local protein synthesis (Mameli et al., 2007). In the present study, we demonstrated that cocaine exposure stimulates mTOR activity and inhibition of mTOR by rapamycin abolishes cocaine-induced locomotor sensitization, suggesting that the mTOR pathway may be involved in cellular and molecular adaptation processes underlying the transition from initial drug use to sensitization. It is noteworthy that rapamycin blocked the expression of cocaine sensitization when administered after cocaine. Since an effective pharmacotherapy for cocaine addiction would likely be used after cocaine exposure, the ability of rapamycin to attenuate both the induction and the expression of cocaine sensitization may have some clinical relevance. Future studies will be necessary in order to fully characterize the inhibitory effect of rapamycin on drug seeking, such as conditioned place preference and self administration. Nevertheless, our results suggest that the mTOR pathway could be a new therapeutic target to alleviate drug addiction. Furthermore, since most drugs of abuse share common cellular and molecular mechanisms of action, it is possible that rapamycin may also be an effective treatment for abuse of drugs other than cocaine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chen BT, Bowers MS, Martin M, Hopf FW, Guillory AM, Carelli RM, Chou JK, Bonci A. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron. 2008;59:288–297. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Olmo N, Miguens M, Higuera-Matas A, Torres I, Garcia-Lecumberri C, Solis JM, Ambrosio E. Enhancement of hippocampal long-term potentiation induced by cocaine self-administration is maintained during the extinction of this behavior. Brain Res. 2006;1116:120–126. doi: 10.1016/j.brainres.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Gong R, Park CS, Abbassi NR, Tang SJ. Roles of glutamate receptors and the mammalian target of rapamycin (mTOR) signaling pathway in activity-dependent dendritic protein synthesis in hippocampal neurons. J Biol Chem. 2006;281:18802–18815. doi: 10.1074/jbc.M512524200. [DOI] [PubMed] [Google Scholar]
- Hoeffer CA, Tang W, Wong H, Santillan A, Patterson RJ, Martinez LA, Tejada-Simon MV, Paylor R, Hamilton SL, Klann E. Removal of FKBP12 enhances mTOR-Raptor interactions, LTP, memory, and perseverative/repetitive behavior. Neuron. 2008;60:832–845. doi: 10.1016/j.neuron.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwood JM, Dufour F, Laroche S, Davis S. Signalling mechanisms mediated by the phosphoinositide 3-kinase/Akt cascade in synaptic plasticity and memory in the rat. Eur J Neurosci. 2006;23:3375–3384. doi: 10.1111/j.1460-9568.2006.04859.x. [DOI] [PubMed] [Google Scholar]
- Huang Y, Kang BN, Tian J, Liu Y, Luo HR, Hester L, Snyder SH. The cationic amino acid transporters CAT1 and CAT3 mediate NMDA receptor activation-dependent changes in elaboration of neuronal processes via the mammalian target of rapamycin mTOR pathway. J Neurosci. 2007;27(3):449–58. doi: 10.1523/JNEUROSCI.4489-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Zhang H, Yang J, Wu J, McMahon J, Cao Z, Lin Y, Gruenthal M, Huang Y. Pharmacological inhibition of the mammalian target of rapamycin pathway suppresses acquired epilepsy. Neurobio Dis. 2010 doi: 10.1016/j.nbd.2010.05.024. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski J, Sheng M. The growing role of mTOR in neuronal development and plasticity. Mol Neurobiol. 2006;34:205–219. doi: 10.1385/MN:34:3:205. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Lenz G, Avruch J. Glutamatergic regulation of the p70S6 kinase in primary mouse neurons. J Biol Chem. 2005;280:38121–38124. doi: 10.1074/jbc.C500363200. [DOI] [PubMed] [Google Scholar]
- Mameli M, Balland B, Lujan R, Luscher C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science. 2007;317:530–533. doi: 10.1126/science.1142365. [DOI] [PubMed] [Google Scholar]
- Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, Luscher C. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci. 2009;12:1036–1041. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- Narita M, Akai H, Kita T, Nagumo Y, Sunagawa N, Hara C, Hasebe K, Nagase H, Suzuki T. Involvement of mitogen-stimulated p70-S6 kinase in the development of sensitization to the methamphetamine-induced rewarding effect in rats. Neuroscience. 2005;132:553–560. doi: 10.1016/j.neuroscience.2004.12.050. [DOI] [PubMed] [Google Scholar]
- Palkovits M. Isolated removal of hypothalamic or other brain nuclei of the rat. Brain Res. 1973;59:449–450. doi: 10.1016/0006-8993(73)90290-4. [DOI] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons RG, Gafford GM, Helmstetter FJ. Translational control via the mammalian target of rapamycin pathway is critical for the formation and stability of long-term fear memory in amygdala neurons. J Neurosci. 2006;26:12977–12983. doi: 10.1523/JNEUROSCI.4209-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Schratt GM, Nigh EA, Chen WG, Hu L, Greenberg ME. BDNF regulates the translation of a select group of mRNAs by a mammalian target of rapamycin-phosphatidylinositol 3-kinase-dependent pathway during neuronal development. J Neurosci. 2004;24:7366–7377. doi: 10.1523/JNEUROSCI.1739-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Jun W, Zhao LY, Xue YX, Zhang XY, Kosten TR, Lu L. Effect of rapamycin on cue-induced drug craving in abstinent heroin addicts. Eur J Pharmacol. 2009;615:108–112. doi: 10.1016/j.ejphar.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Maisonneuve IM, Glick SD. Ibogaine enhances the expression of locomotor sensitization in rats chronically treated with cocaine. Pharmacol Biochem Behav. 1999;63:457–464. doi: 10.1016/s0091-3057(99)00038-6. [DOI] [PubMed] [Google Scholar]
- Tavazoie SF, Alvarez VA, Ridenour DA, Kwiatkowski DJ, Sabatini BL. Regulation of neuronal morphology and function by the tumor suppressors Tsc1 and Tsc2. Nat Neurosci. 2005;8:1727–1734. doi: 10.1038/nn1566. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008;154:327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Zeng LH, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci. 2009;29(21):6964–72. doi: 10.1523/JNEUROSCI.0066-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JJ, Kahan BD. Pharmacokinetics of sirolimus in stable renal transplant patients after multiple oral dose administration. J Clin Pharmacol. 1997;37(5):405–15. doi: 10.1002/j.1552-4604.1997.tb04318.x. [DOI] [PubMed] [Google Scholar]


