Abstract
Background
An increased frequency of venous thromboembolism (VTE) has been shown among patients with reduced kidney function as measured by a decreased estimated glomerular filtration rate (eGFR). However, current practices with respect to VTE prevention and management in patients with a reduced eGFR in general population settings remain uncertain.
Study Design
Observational study.
Setting & Participants
Community investigation of 1,509 metropolitan Worcester (Massachusetts) residents with validated VTE during 1999, 2001, and 2003 with further follow-up for up to 3 years.
Predictor
VTE patients further classified according to their eGFR on presentation: < 30, 30-59, 60-89, or ≥ 90 ml/min/1.73m2 (reference group).
Outcomes
Recurrent VTE, major bleeding episodes, and all-cause mortality.
Measurements
Demographic and clinical characteristics, treatment practices, and study outcomes were extracted from patients’ hospital and outpatient medical records; eGFR was estimated using the Chronic Kidney Disease Epidemiology equation.
Results
VTE patients with eGFR < 30 ml/min/1.73m2 were at an increased risk for recurrent VTE (HR, 1.83; 95% CI, 1.03-3.25), major bleeding episodes (HR, 2.30; 95% CI, 1.28-4.16) and all-cause mortality (HR, 1.70; 95% CI, 1.12-2.57) over 3-year follow-up. Patients with reduced eGFR also presented with more co-morbidities and were less likely to be discharged on any form of anticoagulant therapy (72.6%, 81.0%, 82.1%, and 87.3% for eGFR < 30, 30-59, 60-89, and ≥ 90 mL/min/1.73m2, respectively; p<0.001).
Limitations
Reduced eGFR status is presumed based on creatinine values on clinical presentation. The impact of drug dosage, timing, type of anticoagulant therapy, and medication adherence on study outcomes could not be evaluated.
Conclusions
Severe reductions in eGFR are associated with an increased risk of long-term recurrent VTE, bleeding, and total mortality in patients with VTE. A greater frequency of serious co-morbidities, difficulties implementing available management strategies, and suboptimal VTE prophylaxis during hospital admissions likely contributed to our findings.
Index words: reduced kidney function, venous thromboembolism, recurrences, major bleeds, mortality
Venous thromboembolism (VTE), consisting of deep vein thrombosis and pulmonary embolism, is the third most common cardiovascular disorder in the United States with approximately 300,000 new cases diagnosed annually1-4. Patients with kidney disease have been shown to have a higher prevalence of VTE and other cardiovascular conditions in comparison to patients with normal kidney function5-9.
Despite recent advances in our understanding of the natural history of VTE1-3, 10-12, considerable uncertainty remains regarding the optimal prevention and therapeutic management of patients with decreased kidney function. Unfortunately, patients with reduced kidney function, as measured by a decreased estimated glomerular filtration rate (eGFR), are often excluded in contemporary treatment studies of VTE since they are at increased risk for bleeding5, 9, 13. As a result, guidelines on anticoagulant selection and dosing in patients with decreased estimated GFR for the prevention and treatment of VTE are not clearly established, likely leading to variation in the management of these patients in the broader community setting with an unclear impact on patient outcomes.
The objectives of this observational study carried out in residents of a large central New England metropolitan area were to describe differences in the clinical and demographic characteristics, management practices, and occurrence of all-cause mortality, VTE recurrences, and major bleeding episodes in patients with independently validated VTE further classified according to their levels of estimated GFR. Data from the population-based Worcester Venous Thromboembolism Study were used for purposes of this investigation1, 2, 14.
METHODS
Study Design, Sampling, and Data Abstraction
The Worcester Venous Thromboembolism Study is an ongoing, population-based surveillance study examining the descriptive epidemiology of VTE, including its magnitude, prophylaxis and management strategies, in-hospital and post-discharge outcomes, and long-term recurrence, bleeding, and all-cause mortality rates, among residents from a large central New England metropolitan area1, 2, 14. The catchment area for this study included all twelve hospitals serving residents of the Worcester, MA, Standard Metropolitan Statistical Area (2000 census = 478,000). Details of this study have been previously reported1, 2, 14.
A total of 7,222 medical records were obtained of greater Worcester residents diagnosed with ICD-9 codes consistent with potential VTE during the 3 study years of 1999, 2001, and 20031 (The ICD-9 codes for VTE classification provided in Item S1, available as online supplementary material). Trained physician and nurse abstractors independently validated each potential case of new onset VTE based on a modified classification schema15; each VTE event was independently adjudicated by the lead investigator (F.A.S.). Sociodemographic, clinical, and treatment variables were abstracted from hospital and outpatient medical records. Heart rate, blood pressure, and laboratory levels were obtained at the time closest to the diagnosis of VTE. Medical history variables defined as “recent” included conditions reported by a physician that were occurring or active in the 3 months prior to the diagnosis of VTE. Patients with cancer were considered to have had a malignancy that was currently being treated or palliated (except for basal cell skin cancer). The “surgery” variable included patients who had major operations where general or epidural anesthesia lasted 30 minutes or longer. The aggregated “vascular disease” variable included patients with prior coronary heart disease, those who had previously undergone a percutaneous coronary intervention or coronary artery bypass surgery, or who had been previously diagnosed with peripheral vascular or cerebrovascular disease. Similarly, cardiac procedures were defined to include electrophysiology studies, percutaneous coronary interventions, pacemaker implantations, and implantable cardiac defibrillator placements. Serious infections were limited to those involving the blood, bone and joint, central nervous, cardiovascular, gastrointestinal, and respiratory systems (pneumonia only), surgical sites, or the skin and requiring hospitalization and/or intravenous antibiotics.
Major bleeding was defined as any episode of bleeding requiring transfusion or hospitalization, or that was life threatening (e.g., resulted in myocardial infarction, stroke, or death). Potential recurrent events of VTE were classified using criteria identical to that employed for incident cases, with the exception being that a definite or probable recurrence of deep vein thrombosis or pulmonary embolism required the documentation of thrombosis in a previously uninvolved venous or pulmonary arterial segment by diagnostic imaging (compression ultrasound, ventilation perfusion imaging, or CT scan). The study’s principal investigator reviewed the medical records and diagnostic imaging reports of each case of potential VTE recurrence, and only definite or probable events were included1. Patients’ long-term vital status was determined through the review of their medical records and death certificates1.
Serum creatinine levels were obtained from each hospital’s laboratory at the time of diagnosis of VTE. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology (CKD-EPI) equation16, 17. Patients with VTE were stratified into 4 comparison groups according to baseline levels of eGFR: eGFR < 30 ml/min/1.73m2, eGFR = 30-59 ml/min/1.73m2, eGFR = 60-89 ml/min/1.73m2 and eGFR ≥ 90 ml/min/1.73m2. Patients on dialysis (n=43) were excluded from the present analysis since the natural history of VTE and its associated complications, diagnosis, and management may be significantly different from those not undergoing dialysis. In addition, patients with missing eGFR data (n=350) were excluded. The final study sample consisted of 1,509 greater Worcester residents with an independently validated episode of VTE during the study years of 1999 (n=470), 2001 (n=532), and 2003 (n=507)1.
Statistical Analysis
Differences in demographic and clinical characteristics as well as use of therapeutic modalities were compared for patients who presented with VTE across categories of eGFR using Mantel-Haenszel chi-square tests for discrete variables and ANOVA tests for continuous variables. Short-term (30-day) and long-term (3-year) rates of VTE recurrence, major bleeding (censoring subjects at the time of death), and all-cause mortality were calculated using Kaplan-Meier estimates. Short-term and long-term event rates per 100 person-years were also estimated for study outcomes.
Cox proportional hazards models were constructed to evaluate whether decreased eGFR was associated with outcomes of all-cause mortality, VTE recurrences, and major bleeding episodes while simultaneously adjusting for several demographic and clinical factors as well as duration of study follow-up; proportional hazards assumptions were satisfied for all models. Potential confounders included in our regression models were determined a priori, based on clinical relevance and findings from the published literature, for each of the individual exposure-outcome relationships. Further refinement of potential confounding factors was performed using univariate analyses identifying possible risk factors with a significance level of p < 0.25. All models were adjusted for study year and setting of VTE (hospital vs. outpatient) occurrence to account for residual confounding that may have occurred due to unmeasured covariates within each of the study cohorts and within hospital or outpatient settings. Multivariable adjusted hazards ratios (HR) and accompanying 95% CIs were computed in a standard fashion using VTE patients with eGFR ≥ 90 ml/min/1.73m2 as the reference category.
RESULTS
Demographic and Clinical Characteristics
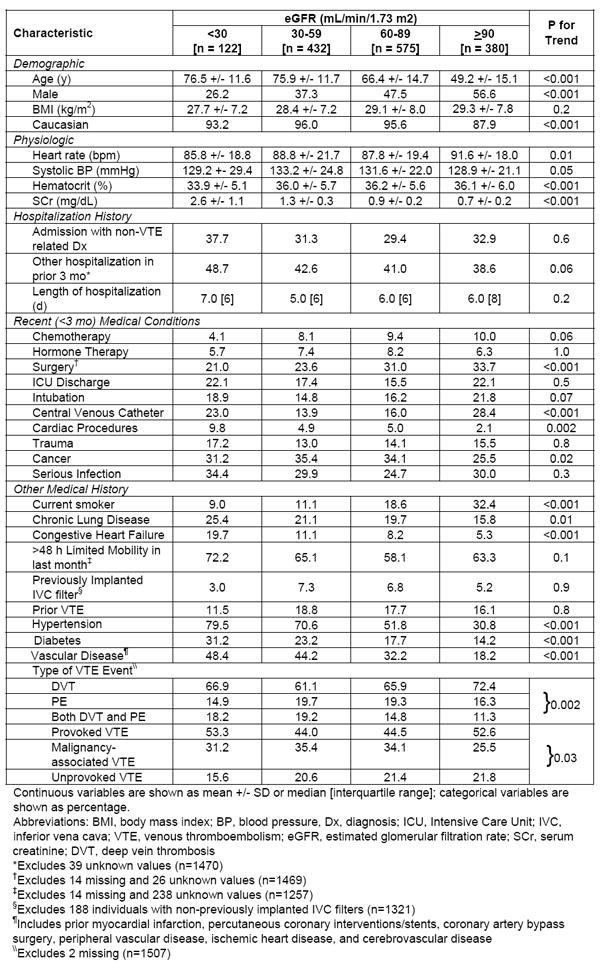
Of the 1,509 patients with confirmed VTE, 122 patients had eGFR < 30 ml/min/1.73m2, 432 had eGFR of 30-59 ml/min/1.73m2, 575 had eGFR of 60-89 ml/min/1.73m2, while the remainder (n=380) had eGFR ≥ 90 ml/min/1.73m2. The prevalence of reduced eGFR (< 60 ml/min/1.73m2) in our total study sample was essentially similar across the 3 study years; 37.7% of patients had reduced eGFR in 1999, 36.8% in 2001, and 35.7% in 2003 (p=0.8). At initial VTE presentation, deep vein thrombosis was most common among patients with eGFR ≥ 90 ml/min/1.73m2 (72.4%) while pulmonary embolism was most frequent among patients with eGFR of 30-59 ml/min/1.73m2 (19.7%) or eGFR of 60-89 ml/min/1.73m2 (19.3%); both DVT and pulmonary embolism were observed more often in patients with eGFR of 30-59 ml/min/1.73m2 (19.2%) or eGFR < 30 ml/min/1.73m2 (18.2%). Unprovoked VTE was least common among patients with eGFR < 30 ml/min/1.73m2 (Table 1).
Table 1.
Demographic and Clinical Characteristics of VTE Patients According to eGFR
|
In comparison to patients with eGFR ≥ 90 ml/min/1.73m2, VTE patients with reduced eGFR were more likely to be older, female, of Caucasian descent, and have several co-morbidities present including cancer, chronic lung disease, heart failure, hypertension, diabetes, and vascular disease; they were also more likely to have undergone a cardiac procedure (Table 1). In addition, VTE patients with reduced eGFR were more likely to have lower hematocrit levels and lower average heart rates at the time of clinical presentation. On the other hand, VTE patients with reduced kidney function were less likely to be smokers or to have undergone recent surgery compared to VTE patients with eGFR ≥ 90 ml/min/1.73m2 (Table 1). Lastly, VTE patients with either eGFR < 30 ml/min/1.73m2 (23.0%) or eGFR ≥ 90 ml/min/1.73m2 (28.4%) were more likely to have had a recent placement of a central venous catheter compared to those with eGFR = 30-59 ml/min/1.73m2 (13.9%) or eGFR of 60-89 ml/min/1.73m2 (16.0%) (Table 1).
Prior Prophylaxis of At Risk Patients
Utilization of anticoagulant VTE prophylaxis prior to a VTE event occurring during a hospitalization for another diagnosis (including heparin, low molecular weight heparin, hirudin, and warfarin) did not differ across eGFR categories (49.4%, 51.7%, 50.3%, and 51.4%, respectively, for eGFR values of < 30, 30-59, 60-89, and ≥ 90 ml/min/1.73m2; p=0.9). On average, about 43% (range, 39%-49%) of patients in each of our eGFR strata had been hospitalized in the 3 months prior to their VTE event. Use of anticoagulant prophylaxis during patient’s prior hospitalizations also did not differ by eGFR status (41.4%, 44.7%, 37.6%, and 47.2%, respectively; p=0.7).
Treatment and Management Practices
Patients with reduced eGFR and VTE were less likely to be treated with low molecular weight heparin (32.8%, 38.7%, 44.5%, and 53.2%, respectively, for eGFR <30, 30-59, 60-89, and ≥ 90 ml/min/1.73m2; p<0.001) or warfarin (60.7%, 74.5%, 75.5%, and 79.0%, respectively; p <0.001). However, patients with reduced eGFR were more likely to have an inferior vena cava filter implanted as part of their management (17.7%, 15.6%, 13.1%, and 9.1%, respectively; p = 0.003). Interestingly, almost 15% of VTE patients with eGFR < 30 ml/min/1.73m2 had received no initial anticoagulant therapy compared to only 5-6% of VTE patients in each of the remaining eGFR groups (p = 0.01). Among those who survived to hospital discharge (n=1,418), patients with reduced eGFR were significantly less likely to have been discharged on any form of anticoagulant therapy (72.6%, 81.0%, 82.1%, and 87.3%, respectively; p<0.001).
Recurrent Episodes of VTE and Decreased eGFR
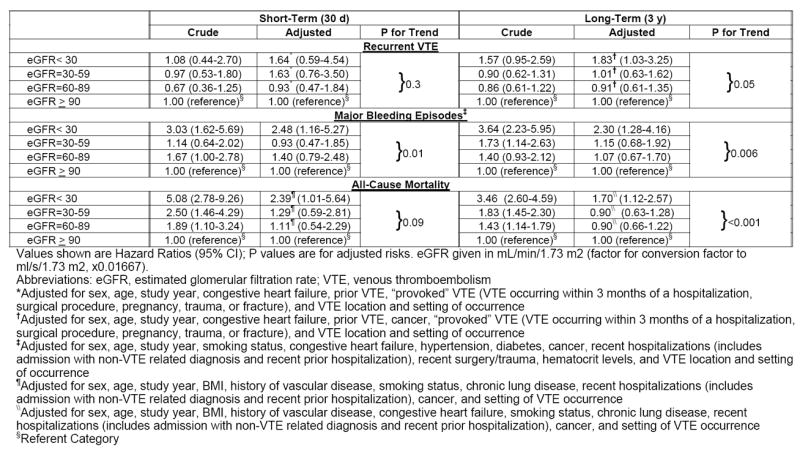
Compared with that of the higher eGFR groups, the incidence of VTE recurrences was highest among patients with eGFR < 30 ml/min/1.73m2 for both short-term and long-term follow-up (Table 2; Figure 1). After Cox regression analysis, patients with eGFR < 30 ml/min/1.73m2 were significantly more likely to have experienced a recurrent episode of VTE during long-term follow-up (HR, 1.83; 95% CI, 1.03-3.25) in comparison to those with eGFR ≥ 90 ml/min/1.73m2 (Table 3); no significant differences in the short or long-term risk of recurrent episodes of VTE were observed for patients with eGFR of 30-59 ml/min/1.73m2 or 60-89 ml/min/1.73m2 as compared to patients with eGFR ≥ 90 ml/min/1.73m2 after covariate adjustment.
Table 2.
Frequencies and Event Rates for Outcomes, by eGFR category
| Short-Term (30 d) | Long-Term (3 y) | |||
|---|---|---|---|---|
| Frequency (%) | Event Rate | Frequency (%) | Event Rate | |
| Recurrent VTE | ||||
| eGFR< 30 | 8 (6.6) | 101.9 | 23 (18.9) | 15.9 |
| eGFR=30-59 | 26 (6.0) | 84.9 | 57 (13.2) | 8.2 |
| eGFR=60-89 | 24 (4.2) | 56.9 | 74 (12.9) | 7.5 |
| eGFR ≥ 90 | 23 (6.1) | 81.0 | 60 (15.8) | 8.4 |
| Major Bleeding Episodes | ||||
| eGFR< 30 | 18 (14.8) | 246.3 | 30 (24.6) | 23.2 |
| eGFR=30-59 | 29 (6.7) | 95.9 | 64 (14.8) | 9.1 |
| eGFR=60-89 | 55 (9.6) | 136.9 | 71 (12.4) | 7.1 |
| eGFR ≥ 90 | 21 (5.5) | 74.2 | 34 (9.0) | 4.6 |
| All-Cause Mortality | ||||
| eGFR< 30 | 28 (23.0) | 340.6 | 86 (70.5) | 52.5 |
| eGFR=30-59 | 55 (12.7) | 170.4 | 206 (47.7) | 24.7 |
| eGFR=60-89 | 59 (10.3) | 134.9 | 228 (39.7) | 19.0 |
| eGFR ≥ 90 | 18 (4.7) | 60.1 | 111 (29.2) | 12.5 |
Note: event rates are shown per 100 person-years. eGFR given in mL/min/1.73 m2 (factor for conversion factor to ml/s/1.73 m2, ×0.01667).
Abbreviations: eGFR, estimated glomerular filtration rate; VTE, venous thromboembolism.
Figure 1.
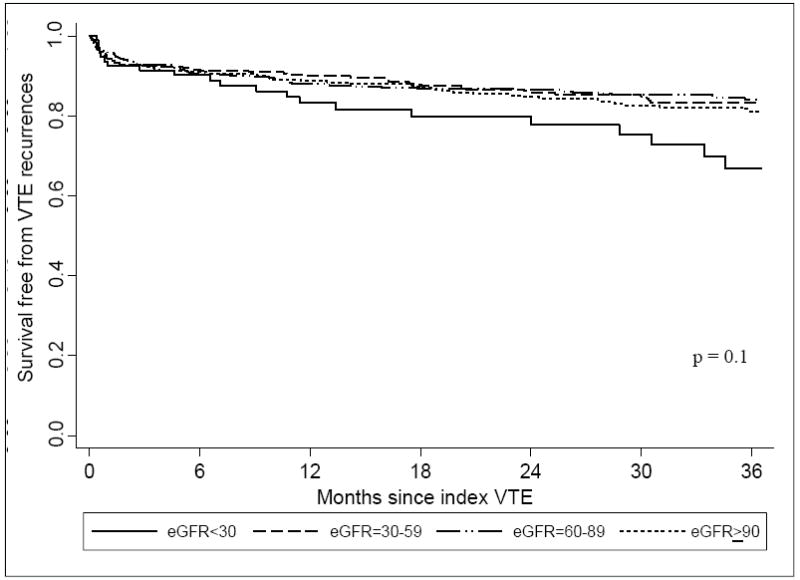
Survival free from VTE recurrence for up to 3 years following index VTE according to eGFR
Table 3.
Short- and Long-Term Risk of Outcomes, by eGFR category
|
Major Bleeding Episodes and Decreased eGFR
Compared with the higher eGFR groups, patients with eGFR < 30 ml/min/1.73m2 were significantly more likely to have developed an episode of major bleeding (Table 2; Figure 2) (p <0.001). In examining bleeding risks after controlling for several potentially confounding factors, patients with eGFR < 30 ml/min/1.73m2 were significantly more likely to have experienced a major bleeding episode as compared to patients with eGFR ≥ 90 ml/min/1.73m2 at the time of both short-term (HR, 2.48; 95% CI, 1.16-5.27) and long-term follow-up (HR, 2.30; 95% CI, 1.28-4.16) (Table 3). No difference in bleeding risk was found among VTE patients with eGFR of 30-59 or 60-89 ml/min/1.73m2 as compared to patients with eGFR ≥ 90 ml/min/1.73m2 at either 30-days or 3-years after their index VTE event following covariate adjustment.
Figure 2.
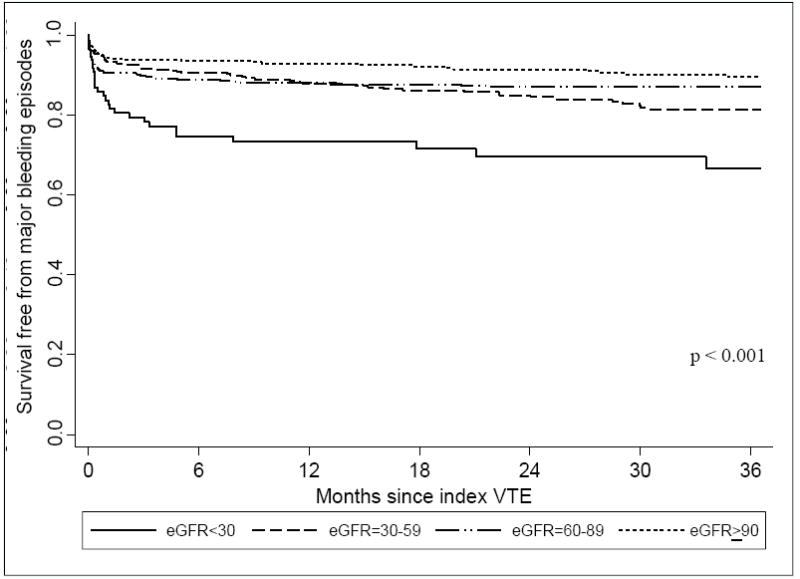
Survival free from major bleeding episodes for up to 3 years following index VTE according to eGFR
All-Cause Mortality and Decreased eGFR
Compared with the higher eGFR groups, patients with eGFR < 30 ml/min/1.73m2 were significantly more likely to have died over the course of our follow-up (Table 2; Figure 3) (p <0.001). After adjusting for differences in duration of follow-up and holding potential covariates constant, there was a significantly higher risk of dying for patients with eGFR < 30 ml/min/1.73m2 as compared to those with eGFR ≥ 90 ml/min/1.73m2 at the time of both short-term (HR, 2.39; 95% CI, 1.01-5.64) and long-term follow-up (HR, 1.70; 95% CI, 1.12-2.57) (Table 3).
Figure 3.
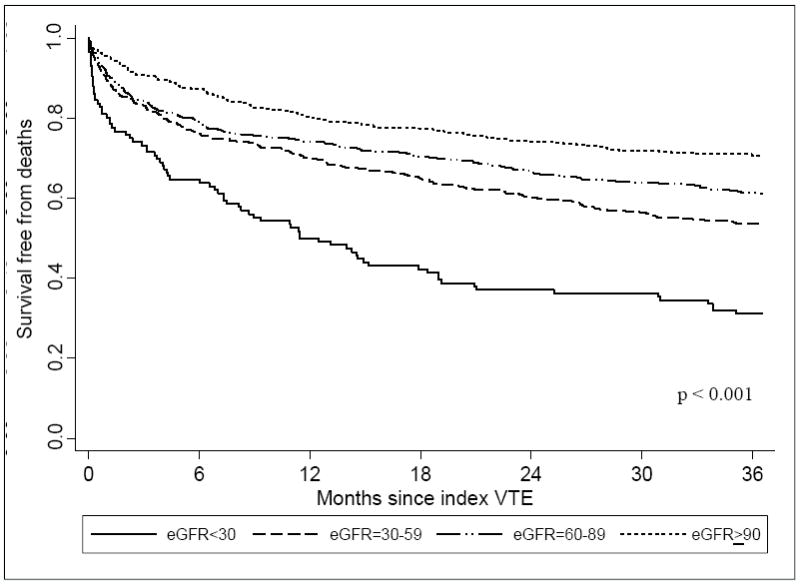
Survival free from mortality for up to 3 years following index VTE according to eGFR
DISCUSSION
The incidence and prevalence of kidney disease have nearly doubled over the past decade, presently affecting more than 20 million Americans18-20. Given the increasing magnitude of this disease, the frequency with which these patients are hospitalized, and increased bleeding risk associated with most anticoagulants, a better understanding of the clinical profile, current management, and outcomes of patients with reduced kidney function who are at risk for, and/or develop VTE, is needed.
In our population-based study, patients with decreased eGFR on clinical presentation comprised more than one-third of patients experiencing VTE in this large, central New England community. Approximately 8% of patients with VTE during our study years had severe reductions in eGFR (eGFR < 30 ml/min/1.73m2) at the time of clinical presentation. The prevalence of reduced kidney function in our study population was approximately two times greater than national estimates of kidney disease prevalence in the United States population18. Not surprisingly, patients with decreased eGFR were older and more likely to have important underlying co-morbidities present as compared to patients with eGFR ≥ 90 ml/min/1.73m2 at the time of initial VTE presentation.
Approximately 35-45% of patients with reduced eGFR experienced their VTE event during a hospitalization for another condition or had a recent (< 3 month), non-VTE related hospitalization. Accordingly, adequate prophylaxis during hospital encounters may be the most effective way to decrease the risk of VTE in these high-risk patients. Although it was encouraging that prior anticoagulant prophylaxis in these patients did not differ according to the extent of kidney function as measured by eGFR, the overall use of these anticoagulants during these at-risk periods remained woefully suboptimal (40-50%). Even though appropriate dosing of low-molecular weight heparin in patients with kidney disease for VTE prophylaxis remains somewhat controversial, there are data to suggest limited bioaccumulation of low molecular weight heparin in this population at standard doses for finite periods21, 22. Furthermore, low-dose unfractionated heparin remains an option in patients with severe kidney disease23.
Patients with reduced eGFR were significantly less likely to have been started on anticoagulant therapy for acute VTE. Approximately 1 in 6 patients with decreased eGFR had an inferior vena cava filter placed as part of their acute management. More than 20% of subjects with reduced eGFR were discharged without anticoagulation. Among patients with severe declines in kidney function not discharged on anticoagulant therapy, 32% had suffered in-hospital major bleeding episodes compared to 23% of those with eGFR ≥ 90 ml/min/1.73m2. While we cannot adequately explore the rationale for treatment decisions using these retrospective observational data, they most likely reflect a combination of perceived increased bleeding risk and early post-treatment bleeding events in patients with kidney disease.
Prior studies have demonstrated a heightened risk of developing subsequent VTE events in patients who have already experienced a first VTE episode, with the greatest risk of recurrence occurring during the first 6 to 12 months after the index event24-27. Cumulative incidence rates of recurrent events have ranged from approximately 1-5% within the first month to 15-20% within 3 years following the incident VTE episode25, 27-31. Our data fall within the range of these previously reported results; between 4-7% of our patients experienced a recurrent episode of VTE during the first 30-days of the index VTE episode and between 13-19% developed a recurrence within 3-years of the initial event. Patients with eGFR > 30 ml/min/1.73m2 were at increased risk for experiencing a recurrent episode of VTE compared to patients with eGFR ≥ 90 ml/min/1.73m2 over our extended follow-up. Although we cannot satisfactorily explore the reasons for this increased risk, which is most likely multi-factorial, an increase in thrombotic risk in patients with kidney disease can be postulated. However, it is also likely that additional co-morbidities resulting in frequent hospitalizations and interruptions of initial VTE therapy due to bleeding related issues (as well as concomitant increased use of inferior vena cava filters) contributed to these findings.
With respect to major bleeding episodes, patients with reduced kidney function often suffer from platelet dysfunction and impaired platelet-vessel wall interaction leading to an increased tendency for bleeding32-34. However, bleeding is also a common complication of VTE, primarily resulting from anticoagulant use for therapeutic management35-37. Our results show that the risk of major bleeding events is substantially increased in VTE patients with eGFR < 30 ml/min/1.73m2 as compared to patients with eGFR ≥ 90 ml/min/1.73m2. During the first 30-days after the initial VTE event, almost 15% of patients with severe reductions in eGFR experienced a major bleeding episode as compared to only 6% of patients with eGFR ≥ 90 ml/min/1.73m2. Within 3-years of the initial VTE episode, bleeding risk more than doubled for patients with severe reductions in eGFR as compared to patients with normal eGFR ranges. These findings are consistent with several studies that have evaluated the risk of bleeding in patients with chronic kidney disease (CKD), and other disease states requiring anticoagulation38-40. For example, in a nationally representative sample of patients hospitalized with myocardial infarction, higher rates of bleeding were observed in patients with severe CKD as compared to patients with mild/moderate or no CKD38. Similarly, data from the Global Registry of Acute Coronary Events (GRACE)40 as well as from the OASIS 5 trials39 found that, among patients with acute coronary syndromes, bleeding rates increased with greater severity of CKD in comparison to acute coronary syndrome patients without evidence of CKD.
Long-term mortality was very high in patients with reduced eGFR and VTE in our study, mirroring rates generally seen in patients with heart failure in the community41, 42. Between 40-50% of patients with eGFR of 30-59 or 60-89 ml/min/1.73m2, and 70% of patients with eGFR < 30 ml/min/1.73m2, died over our 3-year follow-up. In agreement with previously published results, significant differences in mortality were observed in VTE patients with decreased eGFR in comparison to patients with normal kidney function9, 13. Accelerated rates of cardiovascular disease in patients with kidney disease have been shown to increase their risk of dying as compared to patients without kidney disease42. However, whether or not complications of VTE, such as recurrent pulmonary embolism, contribute to the observed mortality rates in this high-risk population warrants further study beyond the scope of this present investigation.
The strengths of the present study include the generalizability of our population-based design that represents all patients with independently validated VTE from a well-defined, large central New England metropolitan area and our independent review and validation of patients’ medical records.
Similar to the design and conduct of any observational study, the present study has several limitations that must be kept in mind when interpreting the study results. Although a broad screening was conducted for identifying all possible VTE cases that occurred in the greater Worcester population, complete ascertainment of index VTE events, VTE recurrences, or major bleeding episodes may not have been obtained, especially for residents who sought care outside of our catchment area following initial presentation for VTE. Failure to capture these patients likely underestimated the magnitude of these endpoints in our study sample, potentially diluting our study results. Deaths due to VTE recurrences or bleeding episodes were unable to be estimated in this study because of the low autopsy rates. Because patients with moderate/severe kidney disease are more likely to receive transfusions because of their lower baseline hemoglobins, the occurrence of major bleeding episodes in these patients may be overestimated. We cannot comment on the use of non-pharmacological approaches to prophylaxis, including the use of sequential compression devices, or the influence of patients with nephrotic syndrome, proteinuria, or an elevated serum urea nitrogen on study outcomes since these data were not collected; hence, the utilization of prophylactic procedures in our population may not be generalizable to additional patient populations. In addition, we cannot differentiate between patients with acute or CKD since our methods of stratification were based on initial creatinine values. Finally, we cannot comment on the impact of drug dosage, timing, type of anticoagulant therapy during VTE-related adverse events, and issues related to medication adherence on the subsequent risks of death, VTE recurrence, or bleeding. Further study of the influence of long-term treatment strategies on selected patient outcomes remains of crucial importance in decreasing the overall risks of adverse complications associated with VTE.
The results of our population-based study demonstrate significant differences in the clinical characteristics, management approaches, and occurrence of short and long-term outcomes in VTE patients with reduced eGFR on presentation as compared to patients with normal eGFR ranges. Given the increased prevalence, clinical impact, and economic burden of kidney disease, our results may be used to inform efforts designed to improve the monitoring and treatment of VTE complications by health practitioners in these high risk patients. Future studies evaluating novel anticoagulants for the prophylaxis and treatment of deep vein thrombosis should include these high-risk patients given current limitations in available treatments.
Supplementary Material
Acknowledgments
Support: This research study is supported by a grant from the National Heart, Lung, and Blood Institute (R01-HL70283).
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
Supplementary Material Item S1: Venous thrombosis ICD-9 codes.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spencer FA, Lessard D, Emery C, Reed G, Goldberg RJ. Venous thromboembolism in the outpatient setting. Arch Intern Med. 2007 Jul 23;167(14):1471–1475. doi: 10.1001/archinte.167.14.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spencer FA, Emery C, Lessard D, et al. The Worcester Venous Thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med. 2006 Jul;21(7):722–727. doi: 10.1111/j.1525-1497.2006.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heit JA. Venous thromboembolism epidemiology: implications for prevention and management. Semin Thromb Hemost. 2002 Jun;28(Suppl 2):3–13. doi: 10.1055/s-2002-32312. [DOI] [PubMed] [Google Scholar]
- 4.Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007 Feb 6;115(5):e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 5.Cook LM, Kahn SR, Goodwin J, Kovacs MJ. Frequency of renal impairment, advanced age, obesity and cancer in venous thromboembolism patients in clinical practice. J Thromb Haemost. 2007 May;5(5):937–941. doi: 10.1111/j.1538-7836.2007.02507.x. [DOI] [PubMed] [Google Scholar]
- 6.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003 Oct 28;108(17):2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 7.McClellan WM, Langston RD, Presley R. Medicare patients with cardiovascular disease have a high prevalence of chronic kidney disease and a high rate of progression to end-stage renal disease. J Am Soc Nephrol. 2004 Jul;15(7):1912–1919. doi: 10.1097/01.asn.0000129982.10611.4c. [DOI] [PubMed] [Google Scholar]
- 8.Wattanakit K, Cushman M, Stehman-Breen C, Heckbert SR, Folsom AR. Chronic kidney disease increases risk for venous thromboembolism. J Am Soc Nephrol. 2008 Jan;19(1):135–140. doi: 10.1681/ASN.2007030308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monreal M, Falga C, Valle R, et al. Venous thromboembolism in patients with renal insufficiency: findings from the RIETE Registry. Am J Med. 2006 Dec;119(12):1073–1079. doi: 10.1016/j.amjmed.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 10.Heit JA. The epidemiology of venous thromboembolism in the community: implications for prevention and management. J Thromb Thrombolysis. 2006 Feb;21(1):23–29. doi: 10.1007/s11239-006-5572-y. [DOI] [PubMed] [Google Scholar]
- 11.Kearon C. Natural history of venous thromboembolism. Circulation. 2003 Jun 17;107(23 Suppl 1):I22–30. doi: 10.1161/01.CIR.0000078464.82671.78. [DOI] [PubMed] [Google Scholar]
- 12.White RH. The epidemiology of venous thromboembolism. Circulation. 2003 Jun 17;107(23 Suppl 1):I4–8. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]
- 13.Rondina MT, Pendleton RC, Wheeler M, Rodgers GM. The treatment of venous thromboembolism in special populations. Thromb Res. 2007;119(4):391–402. doi: 10.1016/j.thromres.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Spencer FA, Gore JM, Lessard D, Douketis JD, Emery C, Goldberg RJ. Patient outcomes after deep vein thrombosis and pulmonary embolism: the Worcester Venous Thromboembolism Study. Arch Intern Med. 2008 Feb 25;168(4):425–430. doi: 10.1001/archinternmed.2007.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ., 3rd Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998 Mar 23;158(6):585–593. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevens LA, Schmid CH, Greene T, et al. Comparative performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis. 2010 Sep;56(3):486–495. doi: 10.1053/j.ajkd.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prevalence of chronic kidney disease and associated risk factors--United States, 1999-2004. MMWR Morb Mortal Wkly Rep. 2007 Mar 2;56(8):161–165. [PubMed] [Google Scholar]
- 19.Snively CS, Gutierrez C. Chronic kidney disease: prevention and treatment of common complications. Am Fam Physician. 2004 Nov 15;70(10):1921–1928. [PubMed] [Google Scholar]
- 20.Thorp ML, Eastman L, Smith DH, Johnson ES. Managing the burden of chronic kidney disease. Dis Manag. 2006 Apr;9(2):115–121. doi: 10.1089/dis.2006.9.115. [DOI] [PubMed] [Google Scholar]
- 21.Mahe I, Aghassarian M, Drouet L, et al. Tinzaparin and enoxaparin given at prophylactic dose for eight days in medical elderly patients with impaired renal function: a comparative pharmacokinetic study. Thromb Haemost. 2007 Apr;97(4):581–586. [PubMed] [Google Scholar]
- 22.Tincani E, Mannucci C, Casolari B, et al. Safety of dalteparin for the prophylaxis of venous thromboembolism in elderly medical patients with renal insufficiency: a pilot study. Haematologica. 2006 Jul;91(7):976–979. [PubMed] [Google Scholar]
- 23.Schmid P, Fischer AG, Wuillemin WA. Low-molecular-weight heparin in patients with renal insufficiency. Swiss Med Wkly. 2009 Aug 8;139(31-32):438–452. doi: 10.4414/smw.2009.11284. [DOI] [PubMed] [Google Scholar]
- 24.Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol. 2008 Mar;28(3):370–372. doi: 10.1161/ATVBAHA.108.162545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heit JA, Mohr DN, Silverstein MD, Petterson TM, O’Fallon WM, Melton LJ., 3rd Predictors of recurrence after deep vein thrombosis and pulmonary embolism: a population-based cohort study. Arch Intern Med. 2000 Mar 27;160(6):761–768. doi: 10.1001/archinte.160.6.761. [DOI] [PubMed] [Google Scholar]
- 26.Schulman S, Lindmarker P, Holmstrom M, et al. Post-thrombotic syndrome, recurrence, and death 10 years after the first episode of venous thromboembolism treated with warfarin for 6 weeks or 6 months. J Thromb Haemost. 2006 Apr;4(4):734–742. doi: 10.1111/j.1538-7836.2006.01795.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhu T, Martinez I, Emmerich J. Venous thromboembolism: risk factors for recurrence. Arterioscler Thromb Vasc Biol. 2009 Mar;29(3):298–310. doi: 10.1161/ATVBAHA.108.182428. [DOI] [PubMed] [Google Scholar]
- 28.Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996 Jul 1;125(1):1–7. doi: 10.7326/0003-4819-125-1-199607010-00001. [DOI] [PubMed] [Google Scholar]
- 29.Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica. 2007 Feb;92(2):199–205. doi: 10.3324/haematol.10516. [DOI] [PubMed] [Google Scholar]
- 30.Hansson PO, Sorbo J, Eriksson H. Recurrent venous thromboembolism after deep vein thrombosis: incidence and risk factors. Arch Intern Med. 2000 Mar 27;160(6):769–774. doi: 10.1001/archinte.160.6.769. [DOI] [PubMed] [Google Scholar]
- 31.Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002 Nov 15;100(10):3484–3488. doi: 10.1182/blood-2002-01-0108. [DOI] [PubMed] [Google Scholar]
- 32.Livio M, Mannucci PM, Vigano G, et al. Conjugated estrogens for the management of bleeding associated with renal failure. N Engl J Med. 1986 Sep 18;315(12):731–735. doi: 10.1056/NEJM198609183151204. [DOI] [PubMed] [Google Scholar]
- 33.Molino D, De Lucia D, Gaspare De Santo N. Coagulation disorders in uremia. Semin Nephrol. 2006 Jan;26(1):46–51. doi: 10.1016/j.semnephrol.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 34.Daneschvar HL, Seddighzadeh A, Piazza G, Goldhaber SZ. Deep vein thrombosis in patients with chronic kidney disease. Thromb Haemost. 2008 Jun;99(6):1035–1039. doi: 10.1160/TH08-02-0107. [DOI] [PubMed] [Google Scholar]
- 35.Wittkowsky AK. New oral anticoagulants: a practical guide for clinicians. J Thromb Thrombolysis. Feb;29(2):182–191. doi: 10.1007/s11239-009-0409-0. [DOI] [PubMed] [Google Scholar]
- 36.Palareti G, Cosmi B. Bleeding with anticoagulation therapy - who is at risk, and how best to identify such patients. Thromb Haemost. 2009 Aug;102(2):268–278. doi: 10.1160/TH08-11-0730. [DOI] [PubMed] [Google Scholar]
- 37.Cook DJ, Douketis J, Arnold D, Crowther MA. Bleeding and venous thromboembolism in the critically ill with emphasis on patients with renal insufficiency. Curr Opin Pulm Med. 2009 Sep;15(5):455–462. doi: 10.1097/MCP.0b013e32832ea4dd. [DOI] [PubMed] [Google Scholar]
- 38.Fox CS, Muntner P, Chen AY, et al. Use of evidence-based therapies in short-term outcomes of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction in patients with chronic kidney disease: a report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network registry. Circulation. 2010 Jan 26;121(3):357–365. doi: 10.1161/CIRCULATIONAHA.109.865352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fox KA, Bassand JP, Mehta SR, et al. Influence of renal function on the efficacy and safety of fondaparinux relative to enoxaparin in non ST-segment elevation acute coronary syndromes. Ann Intern Med. 2007 Sep 4;147(5):304–310. doi: 10.7326/0003-4819-147-5-200709040-00005. [DOI] [PubMed] [Google Scholar]
- 40.Santopinto JJ, Fox KA, Goldberg RJ, et al. Creatinine clearance and adverse hospital outcomes in patients with acute coronary syndromes: findings from the global registry of acute coronary events (GRACE) Heart. 2003 Sep;89(9):1003–1008. doi: 10.1136/heart.89.9.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henkel DM, Redfield MM, Weston SA, Gerber Y, Roger VL. Death in heart failure: a community perspective. Circ Heart Fail. 2008 Jul;1(2):91–97. doi: 10.1161/CIRCHEARTFAILURE.107.743146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sosnov J, Lessard D, Goldberg RJ, Yarzebski J, Gore JM. Differential symptoms of acute myocardial infarction in patients with kidney disease: a community-wide perspective. Am J Kidney Dis. 2006 Mar;47(3):378–384. doi: 10.1053/j.ajkd.2005.11.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


