Abstract
Rationale
Pulmonary arterial hypertension (PAH) is an incurable disease associated with viral infections and connective tissue diseases. The relationship between inflammation and disease pathogenesis in these disorders remains poorly understood.
Objective
To determine whether immune dysregulation due to absent T cell populations directly contributes to the development of PAH.
Methods and Results
Vascular endothelial growth factor receptor 2 (VEGFR2) blockade induced significant pulmonary endothelial apoptosis in T-cell deficient rats but not in immune-reconstituted (IR) rats. T cell-lymphopenia in association with VEGFR2 blockade resulted in periarteriolar inflammation with macrophages, and B cells even prior to vascular remodeling and elevated pulmonary pressures. IR prevented early inflammation and attenuated PAH development. IR with either CD8 T cells alone or with CD4-depleted spleen cells was ineffective in preventing PAH whereas CD4-depleting immunocompetent euthymic animals increased PAH susceptibility. IR with either CD4+CD25hi or CD4+CD25- T cell subsets prior to vascular injury attenuated the development of PAH. Immune reconstitution limited perivascular inflammation and endothelial apoptosis in rat lungs in association with increased FoxP3+-, IL-10- and TGF-β– expressing CD4 cells, and upregulation of pulmonary bone morphogenetic protein receptor type 2 (BMPR2)-expressing cells, a receptor that activates endothelial cell survival pathways.
Conclusions
PAH may arise when regulatory T cell (Treg) activity fails to control endothelial injury. These studies suggest that regulatory T cells normally function to limit vascular injury and may protect against the development of PAH.
Keywords: pulmonary arterial hypertension, inflammation, regulatory T cell, bone morphogenetic protein receptor type 2
INTRODUCTION
PAH is a frequently lethal disease of relatively mysterious origins. For over 50 years it has been recognized that autoimmune phenomena are associated with certain forms of PAH, but it has never been previously demonstrated that autoimmunity, itself, may be a root cause for PAH 1. Previous work by our group and others demonstrated that T-cell immunity is involved in PAH pathogenesis, but no study has yet sought to explain how immune dysregulation directly causes PAH. Human and rat lung PAH pathology demonstrate occlusive arterioles and immune cell aggregates composed of macrophages, mast cells, B and T lymphocytes and anti-endothelial cell antibodies (AECAs) adherent to injured vasculature 1-4. Conditions commonly associated with PAH, such as systemic sclerosis and HIV, are associated with abnormalities in CD4 T cell function and exhibit a similarly inflamed pulmonary vascular pathology 1, 5. To date, it has not been clear whether inflammation surrounding the pulmonary vasculature is primarily the cause or the consequence of vascular remodeling. Given that athymic animals, which are congenitally T-cell deficient, are also particularly susceptible to the development of experimental PAH 6-8, we questioned whether inappropriately exuberant inflammation due to the lack of normal immune regulation could be a key predisposing factor for the development of this disease in these animals.
Injury resolution is an established function for a subset of T cells with strong regulatory activity, commonly referred to as Tregs. These cells typically express the co-receptor CD4+, the transcription factor Forkhead box protein 3 (FoxP3), and highly express the IL-2 receptor-α chain (i.e.CD25hi) 9. In addition to these classical Treg populations, rats also exhibit regulatory activity in CD4+CD25- cells10,11, 12. Tregs participate in the regulation of immune responses following a variety of inflammatory injuries such as burns and infection 13-15. In the absence of normal Treg activity, inflammatory injury is less readily resolved, and serious disease may develop 16.
Our group and others have demonstrated that the athymic rat develops severe PAH following vascular injury induced by monocrotaline (MCT) or VEGFR2 blockade (SU5416) 6-8. Compared to other PAH models, which require chronic hypoxia or a surgical pneumonectomy, T cell immunodeficiency renders these athymic animals particularly sensitive to the development of severe PAH under normoxic conditions. The athymic rat is the result of an autosomal recessive mutation on the rnu locus (rnu/rnu) of chromosome 10 leading to T-cell deficiency, thymic aplasia and hairlessness 17. Following MCT or SU5416 administration, athymic rats of different genetic backgrounds develop significant RV remodeling, perivascular inflammation and occlusive arteriolar lesions that are similar to lesion observed in severe clinical PAH 6-8. Prior studies utilizing athymic rats were limited by the availability of inbred animals which precluded an evaluation of how immune dysregulation could account for the PAH diathesis 7. In this study, we administered SU5416 to genetically-inbred athymic animals and performed immune reconstitution (IR) experiments with MHC-identical cells to assess, for the first time, the role of immune regulation in the development of PAH.
METHODS
A detailed Methods can be found as an online supplement available at http://circres.ahajournals.org.
Animal Model
The experimental protocol was approved by the Veterans Affairs Palo Alto Animal Care and Use Committee. Inbred WAG (RT1u) ATHYMIC nude rats (rnu/rnu) and euthymic (rnu/+) rats were utilized for these studies (Biomedical Research Models, Inc, Worcester, MA). Euthymic rats served as MHC-identical controls and as cell donors for adoptive transfer experiments. Six to eight week old animals were injected s.c. with a single dose of either VEGFR2 antagonist SU5416 (20 mg/kg) dissolved in DMSO or DMSO (vehicle) alone. This dose was previously determined to be effective for inducing PAH in athymic rats 7. SU5416 was synthesized utilizing a previously described modified protocol 18. All animals were maintained in normoxic conditions.
Immune Reconstitution
20×106 unfractionated spleen cells obtained from euthymic male rats were injected into athymic rats i.p. 7d prior to SU5416 administration except for the time-course experiment in which they were injected at d-7, d-5, d-3, d0, d3, d5, d7 and d10, with d0 being the day of SU5416 administration. For CD4+ and CD8+ T cell reconstitutions 20 × 106 cells were administered i.v. to athymic rats, and for CD4+CD25hi and CD4+CD25- cell reconstitutions, approximately 3 × 106 cells were administered i.v. to athymic rats 7d prior to SU5416 administration.
Cell isolation and purification
Isolation and purification of CD4+ and CD8+ T cells
Spleens from inbred euthymic rats were processed and filtered through a 100 μm cell strainer to obtain a single cell suspension, washed with DMEM, and then counted. CD4+ T cells were purified by labeling B cells, NK cells, CD8+ T cells, CD68+ cells, and mast cells with the following purified antibodies (all of which were mouse IgG1 isotype): anti-CD45RA (OX-33), anti-CD161a (10/78), anti-CD8a (OX8), anti-mast (AR32AA4) (all BDPharmingen), and anti-CD68 (ED-1) (AbD Serotec). The labeled splenocytes were then incubated with rat anti-mouse IgG1 microbeads (Miltenyi Biotec) according to the manufacturer's description. Live cells were recovered using the magnetic activated cell sorting (MACS) system with magnetic LS columns (Miltenyi Biotech). The collected negative fraction and enriched CD4+ T cells were further purified with a cell sorter (FACSAria) (BD Bioscience). The average purity was >98%. The same procedure was used to isolate CD8+ T cells except that instead of labeling the splenocytes with anti-CD8a prior to incubation with microbeads, they were labeled with purified anti-CD4 (OX-38) (BD Pharmingen) (average purity>98%). To obtain the CD4+ T cell depleted splenocytes, the splenocyte suspension was incubated with anti-CD4 (OX-38 and OX-35) antibodies (BD Pharmingen) and then the negative fraction was isolated with microbeads as described above.
Isolation and purification of Tregs (CD4+ CD25hi) and CD4+ CD25- cells
Spleens were removed from inbred euthymic rats and prepared as a single cell suspension as described above. CD4+ T cells were purified as described above. To obtain CD4+CD25hi and CD4+CD25- subsets, pure CD4+ T cells were labeled with mouse anti-CD25-PE antibody (OX39) (BD Pharmingen) followed by incubation with anti-PE microbeads. LS columns (Miltenyi Biotec) were used with the MACS system to fractionate the two populations. Both cell fractions were then sorted with a fluorescent activated cell sorting machine, FACSAria (BD Biosciences). The average purity obtained was >97.5%. Expression of FoxP3 in both of these populations was assessed by using the FoxP3 staining set buffers (eBioscience) for intracellular staining with anti-FoxP3 antibody (150D) (Biolegend) according to the manufacturer's protocol. These cells were co-stained with antibodies to CD4 (OX-38), CD8a (OX8), CD25 (OX39), IL-10 (A5-4), CD45RA (OX-33) (all BD Pharmingen), and TCR (R73) (eBioscience). Flow cytometry was performed on a FACSCalibur (BD Biosciences) and the data was analyzed with CellQuest software (BD Biosciences).
Hemodynamic Measurements
The rats were anesthetized with ketamine hydrochloride (70 mg/kg) and xylazine (10 mg/kg) injected i.p. before right and left heart catheterization. Right ventricular systolic pressure (RVSP) measurements were obtained by insertion of a Micro Tip pressure transducer catheter (model SPR-671, 1.4F) (Millar Instruments) through the jugular vein into the RV. Signals were recorded continuously with a TC-510 pressure control unit (Millar Instruments) coupled to a Bridge Amp (AD Instruments). Data was collected with the Powerlab4/30 data acquisition system (AD Instruments) and analyzed with Chart Pro software (AD Instruments). LV combined pressure-volume signals were recorded by insertion of a pressure- volume conductance (PV) catheter (model SPR-838, 2F) (Millar Instruments) through the carotid artery into the LV. Subsequently, a median sternotomy was performed, the pericardium was opened and the PV catheter was introduced into the RV for measurements of RV pressure loops and pulmonary artery pressures. The PV catheter was connected to a MPVS Ultra single segment pressure volume system (Millar Instruments) and signals were recorded digitally. All data were acquired and analyzed with Chart Pro software (AD Instruments). The RV was dissected from the left ventricle (LV) and the septum (S) and each was weighed. The ratio of RV/(LV+S) was calculated to determine the degree of RVH.
Statistical Methods
GraphPad Prism® was used for statistical analysis. Differences between multiple groups were compared using one way ANOVA with Bonferonni multiple comparisons test for post hoc analyses. For datasets with non-parametric data, multiple groups were compared using the Kruskal-Wallis test and Dunn's multiple comparisons test was used for the post test analysis. For the cytokine, histologic and western blot analyses, unpaired T-tests assuming unequal variances were utilized with the comparisons being negative athymic control vs. athymic SU and athymic SU vs. athymic IR-SU groups. All data are represented as means ± SEM, and P-value < 0.05 is considered significant.
RESULTS
IR of athymic rats with MHC-identical splenocytes attenuates the development of PAH when injected prior to SU5416 administration
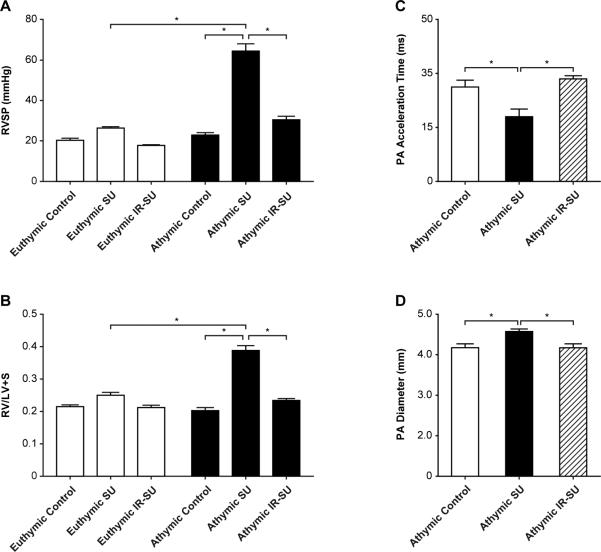
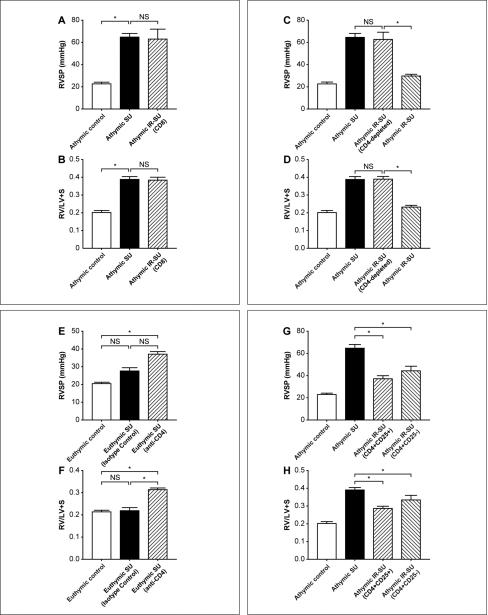
We first investigated whether inbred athymic rats exhibited more profound PAH than euthymic rats on a WAG strain (RT1u) genetic background. The heterozygous rnu/+ euthymic rats used in this study are T cell-replete and are MHC-identical to the athymic rats; a characteristic that makes them suitable as T cell donors for the athymic rat. Right ventricle systolic pressures (RSVPs) were used to estimate PAH. SU5416-treated athymic rats developed severe PAH and right ventricular hypertrophy (RVH) compared to immune-replete SU-5416-treated euthymic rats, and vehicle (DMSO)-treated athymic controls. To test whether IR with splenocytes from euthymic donors was protective against the development of PAH, athymic rats underwent IR with 20 × 106 spleen cells 7d prior to SU5416 administration; IR was later confirmed by flow cytometry of peripheral blood 7d after cell injection. IR prevented the development of PAH (Figures 1A, B). PAH was associated with histologically-evident pulmonary vascular remodeling which was prevented by IR (Online Figure I). IR led to a larger percentage of vessels being open compared to athymic SU5416 animals (Online Figure II). Additionally, pressure-volume studies were performed, the RVSP and dp/dt max data from these studies supported the hypothesis that athymic SU5416 animals develop significant PAH and that the PAH is minimal in IR-athymic SU5416 animals (Online Figure III; Online Tables I and II).
Figure 1.
Immune reconstitution of athymic rats prevents PAH development. (A) RVSP measurements in athymic and euthymic rats treated with SU5416 at d21 and vehicle-treated rats (athymic control, euthymic control). (B) RVH measurements as assessed by RV/(LV+S) ratio in athymic and euthymic rats treated with SU5416 at d21 and vehicle-treated control rats (n=10-16/group). (C-D) Sequential echocardiography of athymic controls, athymic SU, and athymic IR-SU at d21 (n=4/group). Data are shown as means with error bars representing SEM. * p<0.05.
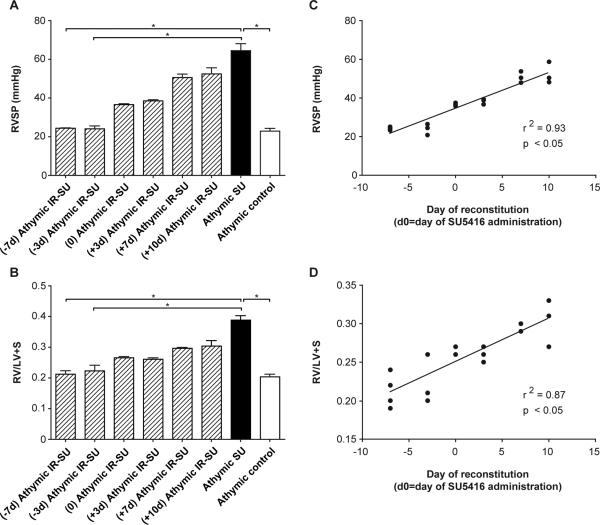
To confirm PAH by echocardiography, animals from the three athymic treatment groups were compared. SU5416-treated athymic rats had significantly faster pulmonary artery acceleration times (PAAT) (Fig. 1C), and an expanded pulmonary artery diameter (PADia) (Fig. 1D); parameters all indicative of worsening PAH. No significant echocardiographic left ventricle differences were noted between the three groups (data not shown). To address whether IR protection from PAH required that the reconstituted cells be present close to the time of SU5416-mediated vascular injury, athymic-SU5416-treated rats underwent IR at multiple time points relative to SU5416 administration and were evaluated at d21 (Figure 2). IR became progressively less effective the later it was performed relative to vascular injury. These results suggested that the protection conferred by IR involves controlling pathological events soon after the induction of vascular injury by SU5416.
Figure 2.
Immune reconstitution is most effective if administered prior to vascular injury. The hemodynamic analysis of IR was evaluated at multiple time points relative to SU5416 administration (d0). (A) RVSP measurements evaluated at multiple time points. The X-axis represents the day of IR relative to SU5416 administration (e.g. (-7d) athymic IR-SU indicates athymic rats undergoing IR d7 prior to SU5416 administration). All hemodynamic evaluations performed d21 after vehicle or SU5416 administration (n=19 (d-7 through d+10)). (B) RVH as assessed by RV/(LV+S) ratio evaluation at multiple time points. All evaluations performed d21 after vehicle or SU5416 administration (n=19 (d-7 through d+10)). (C,D) Correlations between RVSP and RVH at multiple time points. Data are shown as means with error bars representing SEM. * p<0.05.
Early pulmonary inflammation precedes vascular remodeling and PAH
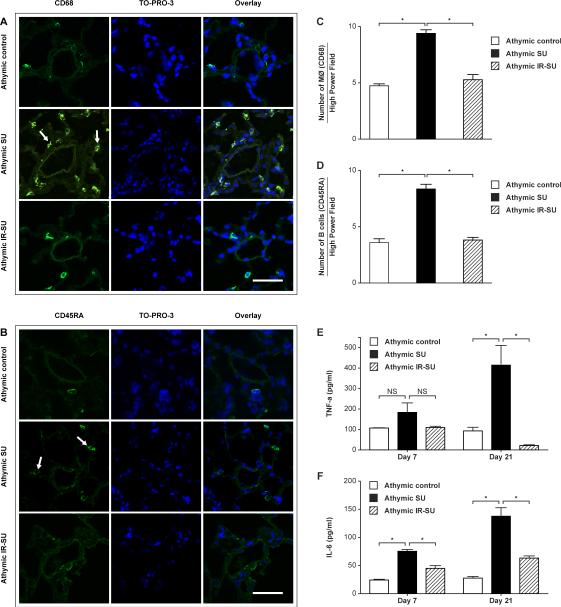
To assess the possibility that regulating early inflammation was responsible for the protective effect of IR, animals were evaluated d7 after SU5416 administration. Inflammation with macrophages and B cells was pronounced by d7 (Figures 3A-D). Notably, PAH was not detected by right heart catheterization or echocardiography prior to 10d nor was there evidence of histological evidence of vascular remodeling at this early time points. To determine whether there was evidence of systemic inflammation, we next evaluated two key cytokines that have been previously implicated in the development of PAH: IL-6 and TNF-α 19, 20. Serum was obtained from animals 7 and 21d after SU5416 or vehicle treatment and evaluated by ELISA. PAH was associated with high serum IL-6 and TNF-α levels, and was suppressed by IR (Figure 3E, F).
Figure 3.
Evidence of anti-inflammatory effect of IR at d7 after SU5416 administration. (A, B) Immunofluorescent images of lung sections from athymic SU animals stained with CD68 (arrows) for macrophages, and with CD45RA (arrows) for B cells at d7 after SU5416 administration (n=8/group). (C,D) Morphometric analysis of macrophages (CD68) and B cells (CD45RA) in lung sections at d7 after SU5416 administration (n=4/group). (E, F) Serum TNF-α and IL-6 evaluated by ELISA at d7 and d21 (n=6-8/group). Data are shown as means with error bars representing SEM. * p<0.05. Scale bars: (A,B)=50 μm.
Early migration of putative Tregs to lungs in IR animals
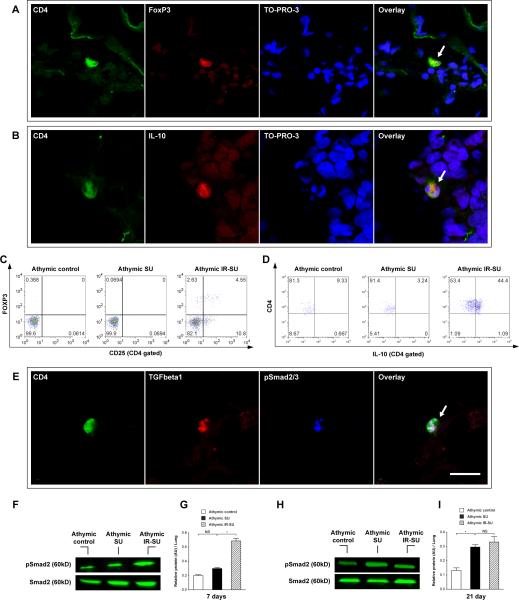
Given that the systemic anti-inflammatory effect that IR had on SU5416-treated animals was detected within 7d of SU5416 administration, we next investigated whether anti-inflammatory Tregs could be detected in the lungs and blood of reconstituted animals at the time when the anti-inflammatory effect was noted. Putative Treg cells (CD4+CD25+FoxP3+; CD4+IL-10+) were readily detected in periarteriolar areas and peripheral blood of IR athymic rats 7 days after IR (Figure 4A-D; Online Figure V). Because TGF-β is an important cofactor expressed by Tregs to maintain self-tolerance following vascular injury 21, 22, we subsequently investigated the expression of TGF-β as well as phosphorylated Smad2 and Smad3 (pSmad2/3), which are cytosolic proteins involved in TGF-β signaling. Lungs of IR-athymic rats demonstrated CD4+TGFβ+pSmad2/3+ cells, and Western blot analysis confirmed increased pSmad2 levels in IR athymic animals (Figure 4E-I). This early accumulation of putative Treg cells expressing the anti-inflammatory cytokines, TGF-β and IL-10, correlated with the suppression of pulmonary and systemic inflammation (Figure 3) observed in reconstituted rats.
Figure 4.
Seven days after SU5416 administration, IR results in migration of TGFβ+ and IL-10+ expressing T cells into the lungs. (A) Immunofluorescent images of lung sections for Tregs cells (CD4+FoxP3+) (arrow) in athymic IR-SU group at d7 (n=7/group). (B) Immunofluorescent images of lung sections for CD4+IL-10+cells (arrow) in athymic IR-SU group at d7 (n=7/group). (C,D) Flow cytometry data of peripheral blood for FoxP3+and IL-10+ detection on d7 in athymic IR-SU group. (E) Immunofluorescent images of lung sections for CD4+TGFβ+ pSmad2/3+ cells (arrow) in athymic IR-SU group at d7 (n=7/group). (F,G,H,I) Western blot analysis from whole lung lysate and protein levels of pSmad2 and Smad2 on d7 and d21(n=8/group). Data are shown as means with error bars representing SEM. * p<0.05. Scale bar = 6.25 μm.
CD4+CD25hi and CD4+CD25- subsets are individually sufficient to confer protection against the development of PAH
We next sought to determine which cellular subset of spleen cells used for IR was essential for the prevention of vascular injury and PAH. Although the athymic rat lacks T cells, it has a normal complement of bone-marrow-derived B cells. While T-dependent responses such as transplant rejection and hypersensitivity reactions are impaired in these rats, the innate immune response is intact, including mast cells, NK cells, macrophages and neutrophils 17. Therefore, the assumption was that a missing T cell subset (absent in athymic animals) was responsible for these rats’ predisposition for disease. We hypothesized that a putative CD4+ Treg population was the essential T cell subset. To rule out that CD8 T cells conferred protection against the development of PAH, we next immune-reconstituted animals with 20 × 106 fractionated CD8+ T cells 7d prior to SU5416 administration and assessed the rats at d21. IR was confirmed by flow cytometry of blood at 7d post SU5416. CD8+ T cell reconstitution was non-protective (Figures 5A, B). Next, to rule out the possibility that other spleen cell populations besides CD4+ T cells were conferring protection, IR was performed with CD4-depleted spleen cells. As with CD8+ T cell reconstitutions, depletion of CD4+ cells from the spleen cell inoculums prior to IR, eliminated the ability of adoptively transferred splenocytes to block PAH and further confirmed that the protective subset of cells was CD4+ (Figures 5C, D).
Figure 5.
Localizing regulatory activity in the CD4 T cell subset. (A, B) RVSP and RVH measurements in athymic rats after SU5416 administration and IR with fractionated CD8+ T cells (n=7-14/group). (C,D) RVSP and RVH measurements in athymic rats after SU5416 administration undergoing IR with CD4-depleted spleen cells (n=8-11/group). (E,F) RVSP and RVH measurements in euthymic rats after SU5416 administration and undergoing CD4 depletion (n=8-13/group). (G,H) RVSP and RVH measurements in athymic rats after SU5416 administration and undergoing reconstitution with CD4+CD25hi cells and CD4+CD25- cells (n=14-16/group). Data are shown as means with error bars representing SEM. * p<0.05.
While we established that it is possible to uniquely generate PAH in rats under normoxic conditions that have a genetic absence of T cells, we thought that the relevance of the general model would be strengthened if an immunologically normal rat exposed to simultaneous vascular injury and CD4 depletion would also develop PAH; the immune system of animals devoid of all T cells from birth may develop in unconsidered ways. For this reason, we investigated, immunologically-intact euthymic rats, which are normally refractory to developing significant PAH with SU5416 therapy in normoxic conditions (Figure 1). Administration of a CD4-depleting monoclonal antibody, which eliminated >98% of circulating CD4+ T cells prior to SU5416 administration, rendered these animals susceptible to the development of PAH (Figure 5E, F; Online Figure V). CD4-depleted animals developing PAH demonstrated increased perivascular inflammation with macrophages and B cells (Online Figure VA, B). These studies cumulatively demonstrated that a population of CD4+ T cells was required to prevent PAH.
To determine if CD4+ cell subsets were differentially protective, athymic rats were reconstituted with either CD4+CD25hi cells or CD4+CD25- cells. Cell subset purity following isolation was ≈98%. CD4+CD25+FoxP3+ cells were detected in the blood 21 days after SU5416 (Online Figure VII) in both CD4+CD25hi-reconstituted athymic rats (4.8% of circulating CD4+ cells) and CD4+CD25--reconstituted animals (1.5% of circulating CD4+ cells); the latter finding being consistent with a peripheral conversion of CD4+CD25- cells into CD4+CD25+ cells 23. Both CD4 T cell subsets attenuated the development of PAH (Figures 5G, H). Therefore, in this rat model, both CD4+CD25hi cells and CD4+CD25- cells exhibited regulatory activity.
IR prevents vascular apoptosis
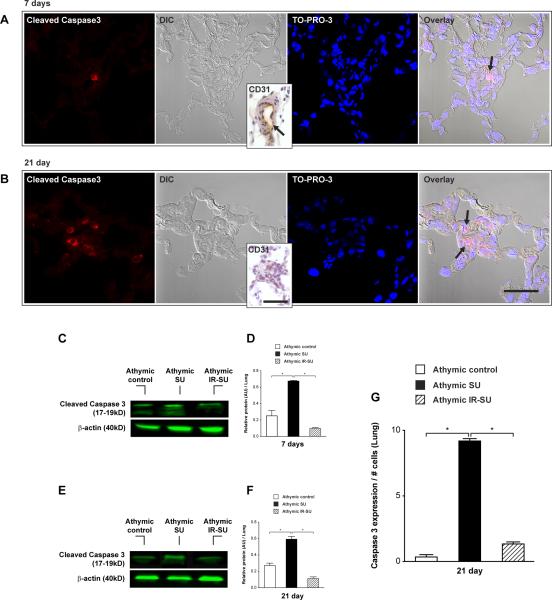
Because PAH induced by SU5416 has been attributed to the death (by apoptosis) of vascular endothelial cells 24, we evaluated whether IR also attenuated this process. The presence of apoptotic cleaved caspase 3+ CD31+ cells was first noted in SU5416-treated animals 7d after SU5416 administration (Figure 6A). Vascular apoptotic cells was more marked by d21 when CD31 staining is minimal presumably due to prior endothelial cell death (Figure 6B). In established PAH, apoptotic cells within the vascular wall were notably CD31-. Evidence of widespread pulmonary apoptosis was also demonstrated by increased lung cleaved caspase 3 protein levels in non-reconstituted SU5416 animals on day 7 (Figure 6C, D) and 21 (Figure 6E, F). A morphometric assessment of cleaved caspase 3+ cells/per vessel demonstrated relatively fewer apoptotic cells/vessel in IR-athymic rats (Figure 6G).
Figure 6.
IR of athymic rats leads to reduced pulmonary vascular endothelial cell apoptosis. (A) Immunofluorescent images of serial lung tissue sections for cleaved caspase 3 expression (arrow) at d7 after SU5416 administration. Differential interference contrast (DIC) represents small lung vessel histology and inset shows CD31 expression in small lung vessel (n=8/group). (B) Immunofluorescent images of serial lung tissue sections for cleaved caspase 3 expression (arrow) at d21 after SU5416 administration. DIC image shows an almost occluded vessel and inset shows absent CD31 staining inside the lung vessel wall (n=8/group). (C,D,E,F) Western blot analysis from whole lung lysate for cleaved caspase 3 protein levels at d7 and d21 (n=8/group). (G) Quantitation of activated caspase 3 positive endothelial cells in precapillary pulmonary arteries in lung tissue sections at d21 (n=4/group). Data are shown as means with error bars representing SEM. * p<0.05. Scale bar: (A,B)=50μm; insets=25μm.
IR results in upregulation of pulmonary BMPR2
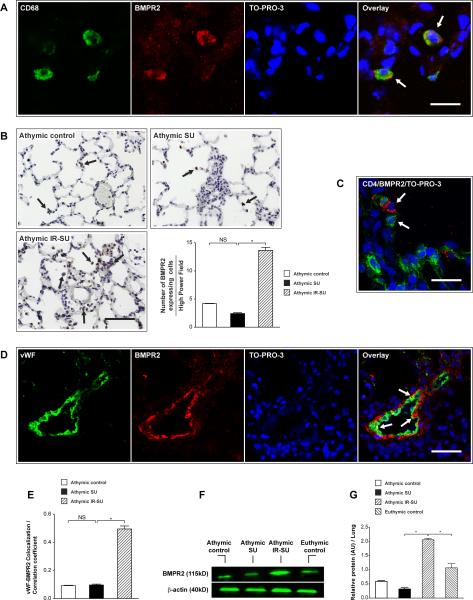
To further evaluate why IR was protective against endothelial apoptosis, we next investigated BMPR2 levels in the three treatment groups. Given that BMPR2 is a protein characterized as vascular-protective in health and vascular-defective in PAH25, we tested whether IR impacted the expression of this receptor in lung tissue. Notably, all three groups exhibited rare BMPR2-expressing macrophages (Figure 7A). Global lung tissue BMPR2 levels appeared elevated in athymic IR-SU by immunochemistry (Figure 7B). BMPR2-expressing cells were frequently noted adjacent to CD4+ cells in immune reconstituted animals (Figure 7C). Vascular BMPR2 expression in athymic IR-SU was notably elevated (Figure 7D, E). By Western blot analysis of whole lysates, BMPR2 expression was significantly increased in athymic IR-SU animals (Figure 7F, G). Thus, the protection conferred by immune reconstitution in this PAH model was strongly associated with upregulation of pulmonary BMPR2.
Figure 7.
IR of athymic rats leads to increased BMPR2 expression in the lung. (A) Immunofluorescent images show colocalization of BMPR2 expressing cells and CD68 in all three animal groups. (B) Immunohistochemistry and quantitation of BMPR2 expressing cells in lungs sections of athymic control, athymic SU and athymic IR-SU rats. (C) Immunofluorescent image of lung tissue shows BMPR2 expressing cells (red) are adjacent to CD4+ cells (green) in athymic IR-SU group. (D) Immunofluorescent images of lung section shows that vWF colocalizes with BMPR2 (arrow) in vascular endothelial cells at d21 in athymic IR-SU rats (n=4/group). (E) The vWF-BMPR2 colocalization coefficient at d21. (F,G) Western blot analysis of BMPR2 protein levels at d21 from whole lung lysates (n=8/group). Data are shown as means with error bars representing SEM. * p<0.05. Scale bar: (A,C)=25 μm; (B,D)=50 μm.
DISCUSSION
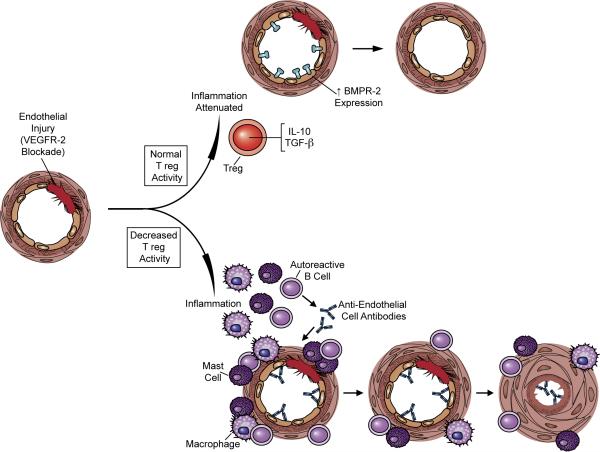
The observation that T cell-deficient athymic rats develop particularly exaggerated PAH 6-8 and that immunodeficiency itself promotes autoimmunity 26 suggested that restoring a missing lymphocyte population could prevent PAH by limiting inflammation. Here we demonstrate for the first time that IR attenuates early inflammation following vascular injury induced with a VEGFR2 inhibitor and is protective against the subsequent development of PAH. IR of athymic animals was only effective if administered prior to SU5416 administration. This finding is consistent with the idea that in normal, immunocompetent animals, the early promotion of injury resolution by Tregs suppresses vascular inflammation and prevents PAH. In PAH development in the SU5416 model, ongoing pulmonary vascular endothelial injury and apoptosis is followed by a progressive proliferative phase of phenotypically abnormal luminal cells; coupled with vascular smooth muscle hypertrophy and vasoconstriction, these pathological changes significantly increase the pulmonary vascular resistance and cause PAH 27. This process is modeled in Figure 8.
Figure 8.
Model of immune regulation contributing to vascular health and immune dysregulation favoring vascular disease following endothelial injury. In this model, endothelial injury causes a local immune response. With normal immune regulation, vascular injury does not culminated in pulmonary vascular remodeling and PAH. The absence of normal immune regulation results in an inappropriately exuberant inflammatory response, accelerated endothelial cell apoptosis, smooth muscle hypertrophy, and increased pulmonary vascular resistance.
Inflammation in T-cell deficient animals was notable for an accumulation of B cells and macrophages within one week of SU5416 administration. This finding is concordant with the fact that the loss of self-tolerance in animals missing normal Treg populations is associated with the appearance of various autoantibodies and autoimmune disease 28, 29. With complete elimination of CD4+CD25hi cells, systemic autoimmunity occurs as manifested by multiorgan inflammation and autoantibody production 16. Thus a loss of Treg-mediated self tolerance leads not only to a loss of T cell tolerance but also to a breakdown in B cell tolerance. Moreover, recent data demonstrate that regulatory T cells are not restricted to regulation of the adaptive immune system but also affect the activation and function of innate immune cells including monocytes, macrophages, dendritic cells and NK cells 30, 31, 32, 33. Exuberant inflammation following vascular injury in our model can therefore be explained by a loss of self-tolerance in the absence of normal immune regulation. We determined that both CD4+CD25hi and CD4+CD25- populations were sufficient for IR-mediated protection from PAH. While CD4+CD25hi are well described as the Tregs in mice, rats exhibit additional Treg populations compared to mice. In rats, coexpression of CD25 on CD4 T cells does not consistently identify all CD4 cells with regulatory function 10. Several studies10-12 demonstrate regulatory activity in the CD4+CD25- population in rats, and this population also appears to have regulatory activity in the current model. We also present evidence that immune reconstituted CD4+CD25- cells may have been converted to CD4+CD25+ Tregs as has been previously described 23. Whether it is this conversion in the periphery or the fundamental nature of rat CD4+CD25- subset, these cells clearly demonstrated regulatory activity. The identification of Tregs as a crucial component of self-tolerance in other diseases has led to major advances in the understanding of how immune injury in autoimmunity and transplantation can be initiated when either insufficient numbers of Tregs or compromised Treg function occurs 34, 35.
TNF-α and IL-6 were elevated in the serum of rats developing PAH. TNF-α over-expression augments PAH19. IL-6, a pro-inflammatory and vasodepressor cytokine, has been shown to have a significant association with mortality in patients with PAH 36. Increased serum and plexiform lesion levels of IL-6 have been found in PAH patients as well as in IL-6 Tg+ mice with replicative pathophysiological changes of PAH 37. IL-6 has also recently been found to negatively regulate the TGF-β/BMP signaling cascade 38. The current study demonstrates that Tregs limit the systemic liberation of IL-6 and TNF-α, a finding that lends credence to the concept that Tregs have therapeutic potential in immune-mediated inflammatory diseases characterized by high levels of these cytokines 39, 40.
While prior studies have described TGF-β in relation to the vascular endothelium in PAH (e.g. 41;), the current study highlights how active TGF-β may also be an important cofactor expressed by Tregs to maintain self-tolerance following vascular injury 21, 22. The proposed mechanisms by which Tregs control inflammation are numerous and involve several basic mechanisms including: 1) secretion of anti-inflammatory cytokines (most notably TGF-β and IL-10), 2) cytolysis of effector T cells, 3) metabolic disruption of effector cells (for example, by serving as an IL-2 ‘sink’ due to high Treg surface concentrations of the IL-2 receptor protein) and 4) modification of dendritic cell function (such as through the induction of the immunosuppressive enzyme indoleamine 2,3-dioxygenase) 42. A striking accumulation of putative Tregs on day 7, including CD4+FoxP3+ and CD4+TGFβ+pSMAD2/3+, CD4+IL-10+cells, were noted in only the IRSU5416 lungs and were concentrated around the arterioles. Whole lung lysate levels of pSMAD2 levels were evaluated and were increased in the IR group on d7. Over time, the diseased athymic-SU5416-treated animals developed increasing pSmad2 levels as well, indicating that increased TGFβ signaling, in isolation, can't explain protection from disease but should be considered in the context of which cells are involved. Tregs noted around the pulmonary arterioles in IR animals also expressed IL-10. IL-10–mediated Treg effects have been particularly evident in antigen-specific responses and represent a normal compensatory mechanism antagonizing the inflammatory response 43, 44. IL-10 is a potent immunomodulator that inhibits the secretion of various proinflammatory cytokines, including IL-6 and IL-8. Adenoviral expression of IL-10 protects against monocrotaline-induced pulmonary hypertension in rats 45. Thus, IL-10 and TGF-β expression by Tregs offer plausible mechanisms explaining how these cells limit inflammatory injury and possibly accelerate pulmonary recovery.
IR resulted in decreased vascular apoptosis following SU5416 administration. Because endothelial apoptosis is the chief mechanism by which SU5416 induces PAH 46, we sought factors associated with IR which could have protected endothelium and found that IR induced vascular BMPR2 expression. BMPR2 mutations are strongly associated with familial PAH 47, and decreased pulmonary vascular BMPR2 expression is associated with idiopathic PAH 48. In experimental models, hypoxia-induced PAH is associated with down-regulation of pulmonary vascular BMPR2, and genetic ablation of pulmonary vascular BMPR2 favors PAH development 25. In the current study, BMPR2-expressing cells in athymic control and athymic SU5416 groups were confined to rare cells that we were not able to characterize except that they were sometimes CD68+ macrophages. By contrast, IR athymic SU5416 animals exhibited large numbers of BMPR2-expressing cells often adjacent to CD4+ cells. Additionally, we demonstrate increased BMPR2-expressing pulmonary vascular cells only in the IR group. The mechanism by which IR could result in such BMPR2 upregulation is not known, nor is it understood if there is a direct causal relationship between immune competency and BMPR2 expression. Of interest, a close (physical) association between BMPR2 expressing cells and T cells was recently observed in idiopathic PAH 49, and future studies will investigate by what mechanism T cells might directly induce endothelial BMPR2.
Treg IR studies in rats are costly, time-consuming, and have limited reagents available compared to mice, but rat models benefit from more robust disease that more closely resembles human PAH pathology 50. While pulmonary inflammation in PAH is a well-established phenomenon 3, 5, it has been unclear whether the inflammation is itself pathogenic or is a secondary immune response to preexisting pulmonary vascular disease. The current study strongly implicates inflammation associated with CD4 immunodeficiency as a primary predisposing factor to experimental PAH development. The clinical relevance of this finding is that certain PAH-associated conditions are characterized by abnormalities in CD4+ T cell number and function. Examples of this association include HIV infection and autoimmune diseases such as systemic sclerosis, systemic lupus erythematosus, polymyositis, Hashimoto's thyroiditis, Sjögren's Syndrome and the antiphospholipid antibody syndrome51-57. The clinical relevance of this issue is that these CD4+ T cell irregularities may render patients susceptible to exaggerated vascular inflammation and subsequent vascular remodeling following vascular injury. By distinction, idiopathic PAH has been characterized by increased Tregs in peripheral blood accompanied by a four-fold elevation of lung CD8+ cells 58, 59. Therefore, in the case of idiopathic PAH, if immune dysregulation contributes to the lung inflammation observed in this condition, current evidence does not link pulmonary injury to abnormal CD4+ T cell populations detectable in the periphery. Correcting immune dysregulation with Treg therapy 60 is an exciting application in development which could prove useful for some PAH conditions 60. Greater recognition that PAH patients have a condition that may begin with impaired resolution of vascular injury could have a significant impact on how this condition is both understood and treated.
Supplementary Material
Novelty and Significance.
What is Known?
Pulmonary arterial hypertension (PAH) is a potentially fatal condition associated with connective tissue diseases and viral infections.
Perivascular inflammation is strongly associated with PAH, but whether lung immunity contributes to disease pathogenesis is controversial.
Regulatory T lymphocytes (Tregs) are important cells that control inflammation and prevent autoimmune injury.
What New Information Does This Article Contribute?
Correcting immune deficiency prevents experimental PAH
The protective effect of IR was localized to Treg populations that prevented the early accumulation of macrophages and B cells, limited pulmonary endothelial apoptosis and upregulated vascular bone morphogenetic protein receptor-II (BMPR2) expression.
Clinical conditions commonly associated with PAH, such as systemic sclerosis and HIV infection, are characterized by abnormal CD4 T lymphocyte function and number - T cell abnormalities that are likely responsible for some of the dysregulated immunity observed in these conditions. Pre-clinical studies have shown that T-cell deficient rats develop lung inflammation and exaggerated PAH following vascular injury. The purpose of the current study was to determine whether restoring T lymphocyte function and number abrogates exuberant inflammatory responses and preventes PAH. We found that lung injury resolution, in the presence of Tregs, is associated with significantly less inflammation and endothelial apoptosis. Inflammation was not merely a consequence of PAH because inflammation and apoptotic cell death occurred prior to the development of vascular remodeling and elevated pulmonary artery pressures. Unexpectedly, the restoration of T cells also led to upregulated vascular BMPR2 in protected rats. This finding is of interest because BMPR2 mutations have been implicated as a cause of familial PAH, and decreased pulmonary vascular BMPR2 expression has been observed in other forms of PAH. These findings suggest that anti-inflammatory Tregs, in association with inducible BMPR2, provide innate protection against PAH following pulmonary vascular injury.
ACKNOWLEDGEMENTS
The authors thank Dr. Marty Zamora and Dr. Joshua Beilke for critically reading the manuscript.
SOURCES OF FUNDING
This study was supported by NIH grant HL082662 to M.R. Nicolls and the Stanford NHLBI Proteomics Center NHLBI-HV-10-05.
Non-Standard Abbreviations and Acronyms
- AECAs
anti-endothelial cell antibodies
- ANP
atrial natriuretic peptide
- AU
arbitrary units
- beta-MHC
beta-myosin heavy chain
- BMPR2
bone morphogenetic protein receptor type 2
- BNP
brain natriuretic peptide
- DIC
differential interference contrast
- dP/dt
maximal rate of pressure increase
- ESPVR
end systolic pulmonary pressure volume relations
- EDPVR
end diastolic pulmonary pressure volume relations
- FoxP3
Forkhead box protein 3
- IL-6
interleukin 6
- IR
immune reconstitution
- LV
left ventricle
- LVEDP
left ventricle end diastolic pressure
- mPAP
mean pulmonary artery pressure
- MCT
monocrotaline
- PAAT
pulmonary artery acceleration time
- PADia
pulmonary artery diameter
- PAH
pulmonary arterial hypertension
- RSVP
right ventricle systolic pressure
- RV
right ventricular
- RVEDP
right ventricle end diastolic pressure
- RVH
right ventricular hypertrophy
- RVU
relative volume units
- S
septum
- SERCA2a
sarco(endo)plasmic reticulum
- Ca2+
ATPase 2a
- SkAct
skeletal α-actin
- TNF - α
tumor necrosis factor α
- Treg
regulatory T cell
- VEGFR2
VEGF receptor 2
Footnotes
In July 2011, the average time from submission to first decision for all original research papers submitted to Circulation Research was 13.5 days.
DISCLOSURES.
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Nicolls MR, Taraseviciene-Stewart L, Rai PR, Badesch DB, Voelkel NF. Autoimmunity and pulmonary hypertension: a perspective. Eur Respir J. 2005;26(6):1110–1118. doi: 10.1183/09031936.05.00045705. [DOI] [PubMed] [Google Scholar]
- 2.Tuder RM, Voelkel NF. Pulmonary hypertension and inflammation. J Lab Clin Med. 1998;132(1):16–24. doi: 10.1016/s0022-2143(98)90020-8. [DOI] [PubMed] [Google Scholar]
- 3.Dorfmuller P, Perros F, Balabanian K, Humbert M. Inflammation in pulmonary arterial hypertension. Eur Respir J. 2003;22(2):358–363. doi: 10.1183/09031936.03.00038903. [DOI] [PubMed] [Google Scholar]
- 4.Rai PR, Cool CD, King JA, Stevens T, Burns N, Winn RA, Kasper M, Voelkel NF. The cancer paradigm of severe pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;178(6):558–564. doi: 10.1164/rccm.200709-1369PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hassoun PM, Mouthon L, Barbera JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, Newman JH, Rabinovitch M, Schermuly R, Stenmark KR, Voelkel NF, Yuan JX, Humbert M. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54(1 Suppl):S10–19. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Miyata M, Sakuma F, Ito M, Ohira H, Sato Y, Kasukawa R. Athymic nude rats develop severe pulmonary hypertension following monocrotaline administration. Int Arch Allergy Immunol. 2000;121(3):246–252. doi: 10.1159/000024324. [DOI] [PubMed] [Google Scholar]
- 7.Taraseviciene-Stewart L, Nicolls MR, Kraskauskas D, Scerbavicius R, Burns N, Cool C, Wood K, Parr JE, Boackle SA, Voelkel NF. Absence of T cells confers increased pulmonary arterial hypertension and vascular remodeling. Am J Respir Crit Care Med. 2007;175(12):1280–1289. doi: 10.1164/rccm.200608-1189OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ormiston ML, Deng Y, Stewart DJ, Courtman DW. Innate Immunity in the Therapeutic Actions of Endothelial Progenitor Cells in Pulmonary Hypertension. Am J Respir Cell Mol Biol. 2009 doi: 10.1165/rcmb.2009-0152OC. [DOI] [PubMed] [Google Scholar]
- 9.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6(4):345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 10.Mizobuchi T, Yasufuku K, Zheng Y, Haque MA, Heidler KM, Woods K, Smith GN, Jr., Cummings OW, Fujisawa T, Blum JS, Wilkes DS. Differential expression of Smad7 transcripts identifies the CD4+CD45RChigh regulatory T cells that mediate type V collagen-induced tolerance to lung allografts. J Immunol. 2003;171(3):1140–1147. doi: 10.4049/jimmunol.171.3.1140. [DOI] [PubMed] [Google Scholar]
- 11.Hillebrands JL, Whalen B, Visser JT, Koning J, Bishop KD, Leif J, Rozing J, Mordes JP, Greiner DL, Rossini AA. A regulatory CD4+ T cell subset in the BB rat model of autoimmune diabetes expresses neither CD25 nor Foxp3. J Immunol. 2006;177(11):7820–7832. doi: 10.4049/jimmunol.177.11.7820. [DOI] [PubMed] [Google Scholar]
- 12.Aiello S, Cassis P, Cassis L, Tomasoni S, Benigni A, Pezzotta A, Cavinato RA, Cugini D, Azzollini N, Mister M, Longaretti L, Thomson AW, Remuzzi G, Noris M. DnIKK2-transfected dendritic cells induce a novel population of inducible nitric oxide synthase-expressing CD4+CD25- cells with tolerogenic properties. Transplantation. 2007;83(4):474–484. doi: 10.1097/01.tp.0000251808.91901.c3. [DOI] [PubMed] [Google Scholar]
- 13.Murphy TJ, Ni Choileain N, Zang Y, Mannick JA, Lederer JA. CD4+CD25+ regulatory T cells control innate immune reactivity after injury. J Immunol. 2005;174(5):2957–2963. doi: 10.4049/jimmunol.174.5.2957. [DOI] [PubMed] [Google Scholar]
- 14.Montagnoli C, Fallarino F, Gaziano R, Bozza S, Bellocchio S, Zelante T, Kurup WP, Pitzurra L, Puccetti P, Romani L. Immunity and tolerance to Aspergillus involve functionally distinct regulatory T cells and tryptophan catabolism. J Immunol. 2006;176(3):1712–1723. doi: 10.4049/jimmunol.176.3.1712. [DOI] [PubMed] [Google Scholar]
- 15.McKinley L, Logar AJ, McAllister F, Zheng M, Steele C, Kolls JK. Regulatory T cells dampen pulmonary inflammation and lung injury in an animal model of pneumocystis pneumonia. J Immunol. 2006;177(9):6215–6226. doi: 10.4049/jimmunol.177.9.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shih FF, Mandik-Nayak L, Wipke BT, Allen PM. Massive thymic deletion results in systemic autoimmunity through elimination of CD4+ CD25+ T regulatory cells. J Exp Med. 2004;199(3):323–335. doi: 10.1084/jem.20031137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rolstad B. The athymic nude rat: an animal experimental model to reveal novel aspects of innate immune responses? Immunol Rev. 2001;184:136–144. doi: 10.1034/j.1600-065x.2001.1840113.x. [DOI] [PubMed] [Google Scholar]
- 18.Sun L, Tran N, Tang F, App H, Hirth P, McMahon G, Tang C. Synthesis and biological evaluations of 3-substituted indolin-2-ones: a novel class of tyrosine kinase inhibitors that exhibit selectivity toward particular receptor tyrosine kinases. J Med Chem. 1998;41(14):2588–2603. doi: 10.1021/jm980123i. [DOI] [PubMed] [Google Scholar]
- 19.Fujita M, Shannon JM, Irvin CG, Fagan KA, Cool C, Augustin A, Mason RJ. Overexpression of tumor necrosis factor-alpha produces an increase in lung volumes and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2001;280(1):L39–49. doi: 10.1152/ajplung.2001.280.1.L39. [DOI] [PubMed] [Google Scholar]
- 20.Humbert M, Monti G, Brenot F, Sitbon O, Portier A, Grangeot-Keros L, Duroux P, Galanaud P, Simonneau G, Emilie D. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med. 1995;151(5):1628–1631. doi: 10.1164/ajrccm.151.5.7735624. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura K, Kitani A, Fuss I, Pedersen A, Harada N, Nawata H, Strober W. TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J Immunol. 2004;172(2):834–842. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- 22.Bommireddy R, Doetschman T. TGFbeta1 and Treg cells: alliance for tolerance. Trends Mol Med. 2007;13(11):492–501. doi: 10.1016/j.molmed.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198(12):1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taraseviciene-Stewart L, Gera L, Hirth P, Voelkel NF, Tuder RM, Stewart JM. A bradykinin antagonist and a caspase inhibitor prevent severe pulmonary hypertension in a rat model. Can J Physiol Pharmacol. 2002;80(4):269–274. doi: 10.1139/y02-047. [DOI] [PubMed] [Google Scholar]
- 25.Hong KH, Lee YJ, Lee E, Park SO, Han C, Beppu H, Li E, Raizada MK, Bloch KD, Oh SP. Genetic ablation of the BMPR2 gene in pulmonary endothelium is sufficient to predispose to pulmonary arterial hypertension. Circulation. 2008;118(7):722–730. doi: 10.1161/CIRCULATIONAHA.107.736801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bach JF. Autoimmune diseases as the loss of active “self-control”. Ann N Y Acad Sci. 2003;998:161–177. doi: 10.1196/annals.1254.017. [DOI] [PubMed] [Google Scholar]
- 27.Sakao S, Taraseviciene-Stewart L, Lee JD, Wood K, Cool CD, Voelkel NF. Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. Faseb J. 2005;19(9):1178–1180. doi: 10.1096/fj.04-3261fje. [DOI] [PubMed] [Google Scholar]
- 28.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 29.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8(2):191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 30.Taams LS, van Amelsfort JM, Tiemessen MM, Jacobs KM, de Jong EC, Akbar AN, Bijlsma JW, Lafeber FP. Modulation of monocyte/macrophage function by human CD4+CD25+ regulatory T cells. Hum Immunol. 2005;66(3):222–230. doi: 10.1016/j.humimm.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A. 2007;104(49):19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahajan D, Wang Y, Qin X, Zheng G, Wang YM, Alexander SI, Harris DC. CD4+CD25+ regulatory T cells protect against injury in an innate murine model of chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2731–2741. doi: 10.1681/ASN.2005080842. [DOI] [PubMed] [Google Scholar]
- 33.Ghiringhelli F, Menard C, Martin F, Zitvogel L. The role of regulatory T cells in the control of natural killer cells: relevance during tumor progression. Immunol Rev. 2006;214:229–238. doi: 10.1111/j.1600-065X.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 34.Chatenoud L, Salomon B, Bluestone JA. Suppressor T cells--they're back and critical for regulation of autoimmunity! Immunol Rev. 2001;182:149–163. doi: 10.1034/j.1600-065x.2001.1820112.x. [DOI] [PubMed] [Google Scholar]
- 35.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3(3):199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 36.Selimovic N, Bergh CH, Andersson B, Sakiniene E, Carlsten H, Rundqvist B. Growth factors and interleukin-6 across the lung circulation in pulmonary hypertension. Eur Respir J. 2009 doi: 10.1183/09031936.00174908. [DOI] [PubMed] [Google Scholar]
- 37.Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA, Waxman AB. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res. 2009;104(2):236–244. 228. doi: 10.1161/CIRCRESAHA.108.182014. following 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hagen M, Fagan K, Steudel W, Carr M, Lane K, Rodman DM, West J. Interaction of interleukin-6 and the BMP pathway in pulmonary smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2007;292(6):L1473–1479. doi: 10.1152/ajplung.00197.2006. [DOI] [PubMed] [Google Scholar]
- 39.Zheng SG, Wang J, Horwitz DA. Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J Immunol. 2008;180(11):7112–7116. doi: 10.4049/jimmunol.180.11.7112. [DOI] [PubMed] [Google Scholar]
- 40.Sethi G, Sung B, Kunnumakkara AB, Aggarwal BB. Targeting TNF for Treatment of Cancer and Autoimmunity. Adv Exp Med Biol. 2009;647:37–51. doi: 10.1007/978-0-387-89520-8_3. [DOI] [PubMed] [Google Scholar]
- 41.Zaiman AL, Podowski M, Medicherla S, Gordy K, Xu F, Zhen L, Shimoda LA, Neptune E, Higgins L, Murphy A, Chakravarty S, Protter A, Sehgal PB, Champion HC, Tuder RM. Role of the TGF-beta/Alk5 signaling pathway in monocrotaline-induced pulmonary hypertension. Am J Respir Crit Care Med. 2008;177(8):896–905. doi: 10.1164/rccm.200707-1083OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8(7):523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr., Muller W, Rudensky AY. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28(4):546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 44.Meiler F, Zumkehr J, Klunker S, Ruckert B, Akdis CA, Akdis M. In vivo switch to IL-10-secreting T regulatory cells in high dose allergen exposure. J Exp Med. 2008;205(12):2887–2898. doi: 10.1084/jem.20080193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ito T, Okada T, Miyashita H, Nomoto T, Nonaka-Sarukawa M, Uchibori R, Maeda Y, Urabe M, Mizukami H, Kume A, Takahashi M, Ikeda U, Shimada K, Ozawa K. Interleukin-10 expression mediated by an adeno-associated virus vector prevents monocrotaline-induced pulmonary arterial hypertension in rats. Circ Res. 2007;101(7):734–741. doi: 10.1161/CIRCRESAHA.107.153023. [DOI] [PubMed] [Google Scholar]
- 46.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF, Tuder RM. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. Faseb J. 2001;15(2):427–438. doi: 10.1096/fj.00-0343com. [DOI] [PubMed] [Google Scholar]
- 47.Newman JH, Wheeler L, Lane KB, Loyd E, Gaddipati R, Phillips JA, 3rd, Loyd JE. Mutation in the gene for bone morphogenetic protein receptor II as a cause of primary pulmonary hypertension in a large kindred. N Engl J Med. 2001;345(5):319–324. doi: 10.1056/NEJM200108023450502. [DOI] [PubMed] [Google Scholar]
- 48.Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, Trembath RC, Morrell NW. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation. 2002;105(14):1672–1678. doi: 10.1161/01.cir.0000012754.72951.3d. [DOI] [PubMed] [Google Scholar]
- 49.Hall S, Brogan P, Haworth SG, Klein N. Contribution of inflammation to the pathology of idiopathic pulmonary arterial hypertension in children. Thorax. 2009;64(9):778–783. doi: 10.1136/thx.2008.106435. [DOI] [PubMed] [Google Scholar]
- 50.Hoshikawa Y, Nana-Sinkam P, Moore MD, Sotto-Santiago S, Phang T, Keith RL, Morris KG, Kondo T, Tuder RM, Voelkel NF, Geraci MW. Hypoxia induces different genes in the lungs of rats compared with mice. Physiol Genomics. 2003;12(3):209–219. doi: 10.1152/physiolgenomics.00081.2001. [DOI] [PubMed] [Google Scholar]
- 51.Speich R, Jenni R, Opravil M, Pfab M, Russi EW. Primary pulmonary hypertension in HIV infection. Chest. 1991;100(5):1268–1271. doi: 10.1378/chest.100.5.1268. [DOI] [PubMed] [Google Scholar]
- 52.Radstake TR, van Bon L, Broen J, Wenink M, Santegoets K, Deng Y, Hussaini A, Simms R, Cruikshank WW, Lafyatis R. Increased frequency and compromised function of T regulatory cells in systemic sclerosis (SSc) is related to a diminished CD69 and TGFbeta expression. PLoS One. 2009;4(6):e5981. doi: 10.1371/journal.pone.0005981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonelli M, Savitskaya A, Steiner CW, Rath E, Smolen JS, Scheinecker C. Phenotypic and functional analysis of CD4+ CD25- Foxp3+ T cells in patients with systemic lupus erythematosus. J Immunol. 2009;182(3):1689–1695. doi: 10.4049/jimmunol.182.3.1689. [DOI] [PubMed] [Google Scholar]
- 54.Benveniste O, Cherin P, Maisonobe T, Merat R, Chosidow O, Mouthon L, Guillevin L, Flahault A, Burland MC, Klatzmann D, Herson S, Boyer O. Severe perturbations of the blood T cell repertoire in polymyositis, but not dermatomyositis patients. J Immunol. 2001;167(6):3521–3529. doi: 10.4049/jimmunol.167.6.3521. [DOI] [PubMed] [Google Scholar]
- 55.Covas MI, Esquerda A, Garcia-Rico A, Mahy N. Peripheral blood T-lymphocyte subsets in autoimmune thyroid disease. J Investig Allergol Clin Immunol. 1992;2(3):131–135. [PubMed] [Google Scholar]
- 56.Mandl T, Bredberg A, Jacobsson LT, Manthorpe R, Henriksson G. CD4+ T-lymphocytopenia--a frequent finding in anti-SSA antibody seropositive patients with primary Sjogren's syndrome. J Rheumatol. 2004;31(4):726–728. [PubMed] [Google Scholar]
- 57.Papo T, Piette JC, Legac E, Frances C, Grenot P, Debre P, Godeau P, Autran B. T lymphocyte subsets in primary antiphospholipid syndrome. J Rheumatol. 1994;21(12):2242–2245. [PubMed] [Google Scholar]
- 58.Ulrich S, Nicolls MR, Taraseviciene L, Speich R, Voelkel N. Increased regulatory and decreased CD8+ cytotoxic T cells in the blood of patients with idiopathic pulmonary arterial hypertension. Respiration. 2008;75(3):272–280. doi: 10.1159/000111548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Austin ED, Rock MT, Mosse CA, Vnencak-Jones CL, Yoder SM, Robbins IM, Loyd JE, Meyrick BO. T lymphocyte subset abnormalities in the blood and lung in pulmonary arterial hypertension. Respir Med. 2009 doi: 10.1016/j.rmed.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008;223:371–390. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.