Abstract
Objective
Elevated serum amylase levels in bulimia nervosa (BN), associated with increased salivary gland size and self-induced vomiting in some patients, provide a possible marker of symptom severity. The goal of this study was to assess whether serum hyperamylasemia in BN is more closely associated with binge eating episodes involving consumption of large amounts of food or with purging behavior.
Method
Participants included women with BN (n=26); women with “purging disorder” (PD), a subtype of EDNOS characterized by recurrent purging in the absence of objectively large binge eating episodes (n=14); and healthy non-eating disorder female controls (n=32). There were no significant differences in age or body mass index (BMI) across groups. The clinical groups reported similar frequency of self-induced vomiting behavior and were free of psychotropic medications. Serum samples were obtained after overnight fast and were assayed for alpha-amylase by enzymatic method.
Results
Serum amylase levels were significantly elevated in BN (60.7 ± 25.4 international units [IU]/liter, mean ± sd) in comparison to PD (44.7 ± 17.1 IU/L, p < 02) and to Controls (49.3 ± 15.8, p < .05).
Conclusion
These findings provide evidence to suggest that it is recurrent binge eating involving large amounts of food, rather than self-induced vomiting, which contributes to elevated serum amylase values in BN.
Keywords: bulimia nervosa, purging disorder, self-induced vomiting, binge eating, serum amylase, hyperamylasemia
1. Introduction
Elevated serum amylase levels have been observed in bulimia nervosa (BN) [1-7], affecting between 25-60% of individuals with this disorder [8]. Although pancreatitis has been observed in BN, this elevation is largely thought to be related to the salivary isoamylase rather than the pancreatic isoamylase [8, 9]. Hyperamylasemia also has been associated with parotid gland enlargement or sialadenosis in persons with BN [10-12]. This may possibly reflect over-stimulation of salivary gland secretion due to binge-eating and / or purging behavior, given preliminary findings suggesting a possible relationship between serum amylase and binge-purge episodes [5].
Subsequent clinical investigations of total serum amylase suggest that it is elevated in persons with BN who experience vomiting [6, 12, 13] or dental enamel erosion suggestive of self-induced vomiting [10], although this has not been observed in all studies [14]. Other investigations have found that serum amylase correlates with frequency of binge eating episodes [5, 12], although this may not hold true for individuals with the non-purging subtype of the disorder [15].
To follow-up on these observations, the goal of this study was to assess whether hyperamylasemia in BN is more closely associated with objective binge eating episodes involving the consumption of large amounts of food than with self-induced vomiting behavior. The study of individuals with “purging disorder,” a subtype of the diagnostic category “Eating Disorder Not Otherwise Specified” (EDNOS) characterized by recurrent purging in the absence of objectively large binge eating episodes [16, 17], provides a new opportunity to examine these relationships.
2. Materials and methods
2.1 Participants
Serum amylase levels were assessed in the context of a follow-up study of a previously reported investigation of symptom patterns and cholecystokinin responses to test meals in individuals with PD [16]. The BN group met current DSM-IV [18] criteria for the purging subtype of the disorder. The current study included those participants who reported episodes of binge eating as well as self-induced vomiting averaging at least twice per week over three months. As previously described [16], PD was characterized by the presence of purging episodes occurring at least twice per week averaged over three months, with no objectively large binge eating episodes and no lifetime history of BN or binge eating disorder. The current study included those participants who reported recurrent self-induced vomiting episodes occurring at least twice per week averaged over three months. The Control group was free of lifetime history of an eating disorder, had no history of dieting for weight loss during the preceding 8 weeks, and scored < 10 on the Cognitive Restraint Scale of the Three Factor Eating Questionnaire [19].
Study participants were recruited from local advertisements. All participants were women between the ages of 18-45 years, in good medical health, and free of known medical conditions or medications with the exception of oral contraceptives. Additionally, participants were within a body mass index (BMI) range of 18.5 to 25.0 kg/m2 and at stable weight (± 5 kg) from screening visit to study day. The BN and PD groups were free of psychotropic medications for at least 8 weeks prior to sample collection. Subjects were instructed to abstain from alcohol ingestion for 72 hours prior to study. Participants provided written informed consent prior to study participation. The study protocol was approved by the Institutional Review Board and participants gave written informed consent prior to participation.
2.2 Procedure
During a screening visit, the Eating Disorders Examination [20] was administered to determine current eating disorder diagnosis, average size of binge eating episodes (kcal/episode), and eating disorder-related symptom patterns. The Structured Clinical Interview for DSM-IV [21] was used to determine lifetime history of eating disorders, and current and lifetime history of other Axis I disorders. Participants were then scheduled for study during an outpatient visit to an NIH-funded General Clinical Research Center (GCRC). Serum samples were collected via an intravenous catheter in the morning following overnight fast and stored at −70 °C prior to batch-analyses. Serum alpha-amylase levels were measured by an enzymatic method (Diagnostic Chemicals Limited/Genzyme Diagnostics, Framingham, MA) [22].
2.3 Data analysis
Group results are presented as mean ± standard deviation (standard error of the mean in figures). Comparisons across the three groups were made using analysis of variance or the Kruskal-Wallis test. Serum amylase values were not normally distributed and were loge-transformed prior to analysis of variance, with subsequent planned testing of the hypothesized elevation of amylase values in BN in comparison to the PD and control groups. The relationship between serum amylase values and other clinical variables was assessed by Pearson or Spearman correlation coefficient. Differences in symptoms between the BN and PD groups were assessed using the independent t-test or Mann-Whitney test. Statistical significance was set at p < .05, two-tailed. Analyses were performed with PASW® software (SPSS/IBM, Chicago, IL).
3. Results
For the BN group (n=26, age 21.6±3.8 years, BMI 21.8±1.3 kg/m2), average weekly frequency of objective binge eating was 4.2±2.5 episodes, self-induced vomiting (SIV) was 5.6±3.0 episodes, and total purging (including SIV and other forms such as misuse of laxatives) was 6.7±3.8 episodes. Average size of objective binge eating episodes was 2,950±2,350 kcal (range 1,530 – 12,145 kcal). For the PD group (n=14, age 20.2±3.4, BMI 21.8±1.7), the average weekly frequency of SIV was 6.7±4.2 episodes and total purging was 6.9±4.4 episodes. For the Control group (n=32), mean age was 22.2±4.3 years and mean BMI was 22.2±1.4 kg/m2. There were no significant differences in mean age or BMI across study groups. Duration of illness did not significantly differ between the BN and PD groups (5.0±3.9 vs. 3.6±3.6 years). The clinical groups did not differ significantly in frequency of self-induced vomiting or total purging episodes per week. Additionally, the interval since the last reported episode of self-induced vomiting prior to the study day did not differ significantly between the BN group (3±3 days) and the PD group (3±3 days).
Serum amylase values were significantly different across study groups (F2,69=3.78; < .05). Amylase values for BN (60.7 ± 25.4 IU/L) were significantly elevated in comparison to values for PD (44.7 ± 17.1 IU/L, p < .02), and in comparison to values for Controls (49.3 ± 15.8, p < .05) (Figure 1). Amylase values for the PD and Control groups were not significantly different.
Figure 1.
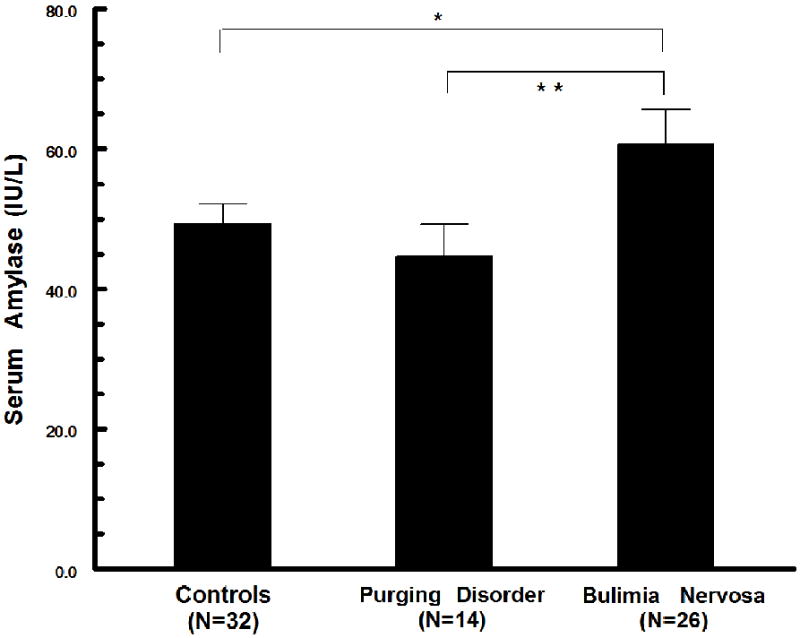
Comparison of fasting serum amylase levels across groups. Amylase levels in participants with BN were significantly elevated in comparison to values for PD (** p<.02) and for Controls (* p<.05).
For the PD group, serum amylase levels were not significantly correlated with average weekly frequency of self-induced vomiting (rho=.39, p=ns), average weekly frequency of total purging (rho=.40, p=ns), number of days since last since last self-induced vomiting episode (rho=-.32, p=ns), or duration of illness (rho=.30, p=ns). For the BN group, amylase values were not significantly correlated with average size of objective binge eating episodes (rho=-.09, p=ns), or with weekly frequency of objective binge eating episodes (rho=.24), self-induced vomiting (rho=.05), or total purging (rho=.15, p=ns). Additionally, amylase values were not significantly correlated with the number of days since last binge eating episode (rho=.06, p=ns) or self-induced vomiting episode (rho=.09; p=ns).
4. Discussion
This is the first study to report on serum amylase levels in persons with PD. While findings of elevated serum amylase levels in the BN group are consistent with previous studies, amylase levels appear to be normal in persons with PD having similar frequency of self-induced vomiting as the BN comparison group. The absence of elevated amylase levels in the PD group suggests the possibility of being more closely related to objective binge eating episodes rather than self-induced vomiting. However, we cannot rule out the possible influence of the volume of emesis in an episode of self-induced vomiting as a potential contributing factor. While this volume is presumably less in the PD group than in BN, this has not been yet studied. Additionally, a previous study of patients with hyperemesis gravidarum (HG) who vomited daily suggests that serum salivary amylase levels may be higher than for controls, although the frequency of elevated levels was lower in HG (24%) than in BN (45%) [23]. This preliminary report did not include statistical comparisons of amylase levels across groups or information on current medication treatment (e.g., antiemetics in the HG group).
It is of note that the current study found no significant relationship between serum amylase levels and available measures of frequency or size of binge eating episodes in the BN group, as might have been expected if binge eating is a major contributor to hyperamylasemia in these individuals. However, self-report of caloric content of binge eating episodes is subject to a wide range of variability within and between individuals [24]. Additionally, previous studies of BN showing a correlation between frequency of binge eating and self-induced vomiting episodes and serum amylase levels have been with individuals more symptomatic than those in the present study [5, 12]. Study of less symptomatic patients with BN (although more severe than in the current study) did not show a relationship between serum salivary amylase and symptom severity, leading the authors to conclude that there would be little argument for routine measurement of serum salivary amylase in diagnostic monitoring in BN [14].
The relatively small sample size and use of a convenience sample of individuals participating in biobehavioral studies may limit generalizability of findings. Future studies may benefit from additional measures including the size of last binge eating and self-induced vomiting episode prior to amylase sample collection. Additionally, examination of serum amylase in clinical populations who experience objective binge eating episodes and who do not purge (e.g., binge eating disorder and bulimia nervosa nonpurging subtype) may help to elucidate the relationships with clinical behaviors observed in persons with eating disorders.
In conclusion, this is the first investigation to report on serum amylase in PD, helping to clarify the relationship of increased serum amylase levels with objective binge eating episodes verses self-induced vomiting. Additional research and the replication of these findings would further suggest the potential utility of serum amylase as marker of moderate to severe symptoms of binge eating activity.
Acknowledgments
Supported in part by grants R01 MH61836, R01 MH057395, M01-RR-59, and M01-RR-0132 from the US Public Health Service, National Institutes of Health, and the Bernice S. Weisman Fund at Beth Israel Deaconess Medical Center.
Role of Funding Sources: Sponsor involvement was limited to providing funding for the study.
Footnotes
Disclosure Statement: Dr. Wolfe, Dr. Jimerson, Ms. Smith, and Dr. Keel report no financial, personal, or other relationships with other people or organizations that could inappropriately influence this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mitchell JE, Pyle RL, Eckert ED, Hatsukami D, Lentz R. Electrolyte and other physiological abnormalities in patients with bulimia. Psychol Med. 1983;13:273–8. doi: 10.1017/s0033291700050881. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs MB, Schneider JA. Medical complications of bulimia: a prospective evaluation. Q J Med. 1985;54:177–82. [PubMed] [Google Scholar]
- 3.Humphries LL, Adams LJ, Eckfeldt JH, Levitt MD, McClain CJ. Hyperamylasemia in patients with eating disorders. Ann Intern Med. 1987;106:50–2. doi: 10.7326/0003-4819-106-1-50. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan AS. Hyperamylasemia in bulimia: a clinical review. Int J Eat Disord. 1987;6:537–43. [Google Scholar]
- 5.Gwirtsman HE, Kaye WH, George DT, Carosella NW, Greene RC, Jimerson DC. Hyperamylasemia and its relationship to binge-purge episodes: development of a clinically relevant laboratory test. J Clin Psychiatry. 1989;50:196–204. [PubMed] [Google Scholar]
- 6.Walsh BT, Wong LM, Pesce MA, Hadigan CM, Bodourian SH. Hyperamylasemia in bulimia nervosa. J Clin Psychiatry. 1990;51:373–7. [PubMed] [Google Scholar]
- 7.Blinder BJ, Hagman J. Serum salivary isoamylase levels in patients with anorexia nervosa, bulimia or bulimia nervosa. Hillside J Clin Psychiatry. 1986;8:152–63. [PubMed] [Google Scholar]
- 8.Levine JM, Walton BE, Franko DL, Jimerson DC. Serum amylase in bulimia nervosa: clinical status and pathophysiology. Int J Eat Disord. 1992;12:431–9. [Google Scholar]
- 9.Kinzl J, Biebl W, Herold M. Significance of vomiting for hyperamylasemia and sialadenosis in patients with eating disorders. Int J Eat Disord. 1993;13:117–24. doi: 10.1002/1098-108x(199301)13:1<117::aid-eat2260130114>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Philipp E, Willershausen-Zonnchen B, Hamm G, Pirke KM. Oral and dental characteristics in bulimic and anorectic patients. Int J Eat Disord. 1991;10:423–31. [Google Scholar]
- 11.Levin PA, Falko JM, Dixon K, Gallup EM, Saunders W. Benign parotid enlargement in bulimia. Ann Intern Med. 1980;93:827–9. doi: 10.7326/0003-4819-93-6-827. [DOI] [PubMed] [Google Scholar]
- 12.Metzger ED, Levine JM, McArdle CR, Wolfe BE, Jimerson DC. Salivary gland enlargement and elevated serum amylase in bulimia nervosa. Biol Psychiatry. 1999;45:1520–2. doi: 10.1016/s0006-3223(98)00221-2. [DOI] [PubMed] [Google Scholar]
- 13.Yasuhara D, Tatebe Y, Nakayama T, Muranaga T, Nozoe S, Naruo T. Insulinogenic index at 15 min as a marker of stable eating behavior in bulimia nervosa. Clin Nutr. 2004;23:711–20. doi: 10.1016/j.clnu.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Kronvall P, Fahy TA, Isaksson A, Theander S, Russell GF. The clinical relevance of salivary amylase monitoring in bulimia nervosa. Biol Psychiatry. 1992;32:156–63. doi: 10.1016/0006-3223(92)90018-u. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka M, Naruo T, Nagai N, Kuroki N, Shiiya T, Nakazato M, et al. Habitual binge/purge behavior influences circulating ghrelin levels in eating disorders. J Psychiatr Res. 2003;37:17–22. doi: 10.1016/s0022-3956(02)00067-5. [DOI] [PubMed] [Google Scholar]
- 16.Keel PK, Wolfe BE, Liddle RA, De Young KP, Jimerson DC. Clinical features and physiological response to a test meal in purging disorder and bulimia nervosa. Arch Gen Psychiatry. 2007;64:1058–66. doi: 10.1001/archpsyc.64.9.1058. [DOI] [PubMed] [Google Scholar]
- 17.Keel PK, Wolfe BE, Gravener JA, Jimerson DC. Co-morbidity and disorder-related distress and impairment in purging disorder. Psychol Med. 2008;38:1435–42. doi: 10.1017/S0033291707001390. [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. fourth edition. Washington, DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- 19.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 20.Fairburn CG, Cooper Z. The eating disorder examination. In: Fairburn CG, Cooper Z, Wilson GT, editors. Binge eating: nature, assessment, and treatment. 12. New York: Guilford Press; 1993. pp. 317–31. [Google Scholar]
- 21.First MB, Gibbon M, Spitzer RL, Williams JBW. User’s guide for the structured clinical interview for DSM-IV axis I disorders-research version-(SCID-I, Version 2.0, February 1996 Final Version) New York: Biometrics Research; 1996. [Google Scholar]
- 22.Winn-Deen ES, David H, Sigler G, Chavez R. Development of a direct assay for alpha-amylase. Clin Chem. 1988;34:2005–8. [PubMed] [Google Scholar]
- 23.Robertson C, Millar H. Hyperamylasemia in bulimia nervosa and hyperemesis gravidarum. Int J Eat Disord. 1999;26:223–7. doi: 10.1002/(sici)1098-108x(199909)26:2<223::aid-eat13>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 24.Wolfe BE, Baker CW, Smith AT, Kelly-Weeder S. Validity and utility of the current definition of binge eating. Int J Eat Disord. 2009;42:674–86. doi: 10.1002/eat.20728. [DOI] [PubMed] [Google Scholar]


