Abstract
Hybrid or multi-modality imaging is often applied, in order to take advantage of the unique and complementary strengths of individual imaging modalities. This hybrid non-invasive imaging approach can provide both critical information about anatomical structure in combination with physiological function or targeted molecular signals. While recent advances in software image fusion techniques and hybrid imaging systems have enabled efficient multimodal imaging, accessing the full potential of this technique requires development of a new toolbox of multimodal contrast agents that enhance the imaging process. Toward that goal, we report the development of a hybrid probe for both single photon emission computed tomography (SPECT) and X-ray computed tomography (CT) imaging that facilitates high sensitivity SPECT and high spatial resolution CT imaging. In this work, we report the synthesis and evaluation of a novel intravascular, multimodal dendrimer-based contrast agent for use in pre-clinical SPECT/CT hybrid imaging systems. This multimodal agent offers a long intravascular residence time (t1/2 = 43 min) and sufficient contrast-to-noise for effective serial intravascular and blood pool imaging with both SPECT and CT. The co-localization of the dendritic nuclear and X-ray contrasts offers the potential to facilitate image analysis and quantification by enabling correction for SPECT attenuation and partial volume errors at specified times with the higher resolution anatomic information provided by the circulating CT contrast. This may allow absolute quantification of intramyocardial blood volume and blood flow and may enable the ability to visualize active molecular targeting following clearance from the blood.
Introduction
Hybrid or multi-modality imaging is often applied in the evaluation and management of cardiovascular disease, in order to take advantage of the unique and complementary strengths of individual imaging modalities1-5. This has become particularly critical when combining targeted molecular or physiological imaging with anatomic imaging. Toward this goal, efforts have been directed at combining modalities like magnetic resonance imaging (MRI) or X-ray computed tomography (CT) possessing high spatial resolution for evaluation of anatomical structure with those that possess high sensitivity like single photon emission tomography (SPECT) and positron emission tomography (PET) for detection of molecular or physiological signals. This has been accomplished, both by design and implementation of hybrid imaging systems 6-11 and/or use of software fusion of images collected with multiple independent imaging systems 12-14. Multimodal imaging with hybrid imaging systems combine multiple, independent probes to achieve high sensitivity and high-resolution anatomic visualization with multiple modalities (e.g. SPECT/CT). On the other hand, software image fusion methodologies use a single probe incorporating multiple forms of contrast media along with multiple imaging modalities to adequately obtain desired information (e.g. MRI/optical). With recent advances in both hardware and software, hybrid imaging systems, specifically, PET/CT, PET/MRI, and SPECT/CT have seen widespread implementation in pre-clinical and clinical applications.
Despite these technological advances, the development of multimodal contrast agents has lagged behind, necessitating the use of multiple independent imaging agents and application of software image fusion techniques 12, 13, 15, 16. The limiting factors in the design of multimodal contrast agents for hybrid imaging systems are two-fold: (1) sensitivity differences inherent to the imaging modalities and (2) temporal restrictions related to image acquisition. The main challenge in developing these agents manifests itself within the sensitivities of the modalities17. Because the modalities providing high spatial resolution often exhibit poor sensitivity, and vice versa, it is essential to achieve the appropriate balance required for the selected application. The other significant challenge arises with the selection of the appropriate radionuclide for incorporation in a radiotracer based imaging approach. The decay half-life governs not only the chemistry, but also the imaging protocols.
For targeted molecular imaging, one needs to design a single cardiovascular contrast agent that possess long enough intravascular residence time to allow sufficient binding to the molecular target, with appropriate blood clearance to enable imaging of both the blood pool and the specific molecular target. This might require use of radioisotopes with relatively long decay half-life. Radiotracer based dynamic imaging approaches have remained the mainstay for evaluation of a physiological parameter like absolute regional myocardial blood flow or receptor binding. However, the lower resolution of SPECT and PET approaches require knowledge of anatomical structure in order to correct for attenuation and partial volume errors associated with limited resolution of these radiotracer approaches. To date, assessment of cardiovascular structure and function requires either software image fusion of multiple modalities with single multimodal probes16, 18-20 or hybrid system imaging with multiple probes, for example, Omnipaque and 99mTc-NC100692 for CT and SPECT, respectively21. An integrated imaging approach that employs a hybrid probe, incorporating contrast moieties to achieve both high spatial resolution (e.g. MRI or CT active) and high sensitivity (e.g. PET or SPECT active) with hybrid imaging systems, has the potential to efficiently and non-invasively generate the essential complementary sets of information required for diagnosis with a single dose and one imaging session.
Incorporation of the different contrast enhancing moieties within a single agent is greatly facilitated by core molecules manifesting multifunctional architectures. Satisfying this criterion, dendrimers, a class of hyperbranched polymers22, possess a high degree of surface functionality and have been routinely utilized as macromolecular contrast agents for many pre-clinical imaging modalities, including MRI15, 23-28, CT12, 29-31, PET32 and SPECT33-36. Though multimodal dendrimer-based contrast agents have been developed, their utility has been limited to software image fusion techniques (e.g. MRI/optical).
In this work, we report the synthesis and evaluation of a novel, multimodal blood pool dendrimer-based contrast agent designed for use in pre-clinical hybrid microSPECT/CT imaging systems with direct applicability to hybrid clinical imaging systems. This long-circulating dendrimer, comprising both triiodinated moieties and chelated 99mTc, provides effective, simultaneous contrast enhancement in both CT and SPECT, respectively.
Materials and Methods
Synthesis of G4-[Ac]
Five hundred milligrams of ethylenediamine-core PAMAM generation 4 dendrimers (PAMAM(G4)) [Sigma Aldrich] were desiccated in a 100 mL round bottom flask and subsequently dissolved in 100 mM sodium bicarbonate buffer (pH 9.0) to a final concentration of 10 mg/mL with magnetic stirring. To this dendrimer solution, 36 molar equivalents of sulfosuccinimidyl acetate [Thermo Scientific] were added as a solid and allowed to dissolve. The pH of the reaction mixture was immediately adjusted to 8.5 with 1N NaOH and the reaction was allowed to proceed for 2 hours at 25°C. The partially acetylated PAMAM(G4) product was purified by ultrafiltration with deionized water using 10K MWCO Amicon Ultra-15 filtrators [Millipore] and lyophilized to obtain a white crystalline solid (yield: 97%). The product was characterized by proton nuclear magnetic resonance (1H NMR) spectroscopy (D2O, 400 MHz); δ = 1.91 (105H, COCH3), 2.28-2.42 (248H, C-CH2CONH), 2.50-2.61 (124H, N-CH2CH2NH), 2.68-2.80 (248H, N-CH2CH2CO), 3.14-3.36 (318H, CONH-CH2). Extent of surface acetylation was determined according to the method of Majoros et al.37
Synthesis of G4-[[Ac]-TIBA]
Activation of 2,3,5-triiodobenzoic acid (TIBA)
Two hundred eighty-seven milligrams of 2,3,5-triiodobenzoic acid (18 mol eq.) were added to a 20 mL glass scintillation vial and dissolved in anhydrous dimethyl sulfoxide (DMSO) [Sigma Aldrich] to a final concentration of 50 mg/mL with magnetic stirring. To the solution of TIBA, 2 molar equivalents of both N-hydroxysuccinimide [Thermo Scientific] and N,N-dicyclohexylcarbodiimide [Thermo Scientific] were added and allowed to dissolve. The reaction was allowed to proceed for 1 hour at 25°C in the absence of light. The reaction mixture was then filtered with a 0.22 μm PTFE syringe filter to remove the dicyclohexylurea (DCU) reaction byproduct and the filtrate was retained.
Coupling of G4-[Ac] to TIBA
A three-necked round-bottom flask was charged with 500 milligrams of G4-[Ac] and purged with argon. The G4-[Ac] was then dissolved in 60 mL anhydrous DMSO with magnetic stirring under an argon atmosphere. To this solution, the entire contents of the filtered activated TIBA solution were added via syringe without further purification. The reaction proceeded for 24 hours at 25°C under an argon atmosphere in the absence of light. The reaction mixture was then diluted with 10 volumes of deionized water and subsequently filtered with a 0.22 μm PES vacuum filtration system. The filtrate was purified into deionized water by ultrafiltration using 10K MWCO Amicon Ultra-15 filtrators and lyophilized to obtain a light brown solid (yield: 82%). The product was characterized by 1H NMR spectroscopy (D2O, 400 MHz); δ = 1.91 (105H, COCH3), 2.28-2.42 (248H, C-CH2CONH), 2.50-2.61 (124H, N-CH2CH2NH), 2.68-2.80 (248H, N-CH2CH2CO), 3.14-3.36 (348H, CONH-CH2), 7.49 (15H, aromatic), 8.21 (15H, aromatic).
Synthesis of G4-[[[Ac]-TIBA]-DTPA]
Six hundred milligrams of G4-[[Ac]-TIBA] were added to a 100 mL round bottom flask and subsequently dissolved in 100 mM sodium bicarbonate buffer (pH 9.0) to a final concentration of 10 mg/mL with magnetic stirring. To this dendrimer solution, 4 molar equivalents of 2-(4-isothiocyanatobenzyl)-diethylenetriaminepentaacetic acid [Macrocyclics] dissolved in anhydrous DMSO were added. The pH of the reaction mixture was immediately adjusted to 8.5 with 1N NaOH and the reaction proceeded for 18 hours at 25°C in the absence of light. The product was purified by ultrafiltration with deionized water using 10K MWCO Amicon Ultra-15 filtrators and lyophilized to obtain a pale yellow solid (yield: 89%). The product was characterized by 1H NMR spectroscopy (D2O, 400 MHz); δ = 1.91 (105H, COCH3), 2.28-2.42 (248H, C-CH2CONH), 2.50-2.61 (124H, N-CH2CH2NH), 2.68-2.80 (248H, N-CH2CH2CO), 3.14-3.36 (348H, CONH-CH2), 7.18 (8H, aromatic), 7.27 (8H, aromatic),7.49 (15H, TIBA aromatic), 8.21 (15H, TIBA aromatic).
Synthesis of G4-[[[[Ac]-TIBA]-DTPA]-mPEG12]
Four hundred ninety milligrams of G4-[[[Ac]-TIBA]-DTPA] were added to a 100 mL round bottom flask and subsequently dissolved in 100 mM sodium bicarbonate buffer (pH 9.0) to a final concentration of 10 mg/mL with magnetic stirring. To this dendrimer solution, 8 molar equivalents of succinimidyl-(N-methyl-dodecaethyleneglycol) ester [Thermo Scientific] dissolved in anhydrous DMSO were added. The pH of the reaction mixture was immediately adjusted to 8.5 with 1N NaOH and the reaction proceeded for 12 hours at 25°C in the absence of light. The product was purified by ultrafiltration with deionized water using 10K MWCO Amicon Ultra-15 filtrators, filtered with a 0.22 μm MCE syringe filtered, and lyophilized to obtain a pale yellow solid (yield: 83%). The product was characterized by 1H NMR spectroscopy (D2O, 400 MHz); δ = 1.91 (105H, COCH3), 2.28-2.42 (248H, C-CH2CONH), 2.50-2.61 (124H, N-CH2CH2NH), 2.68-2.80 (248H, N-CH2CH2CO), 3.14-3.36 (360H, CONH-CH2), 3.5-3.76 (336H, -OCH2CH2), 7.18 (8H, aromatic), 7.27 (8H, aromatic), 7.49 (15H, aromatic), 8.21 (15H, aromatic).
1H NMR spectroscopy
All samples were dissolved in D2O (20 mg/mL) for characterization by proton nuclear magnetic resonance (1H NMR) spectroscopy on a 400 MHz Bruker spectrometer with the solvent as a reference. Extent of surface conjugation was determined by analysis of peak integrations.
Dynamic light scattering (DLS)
PAMAM(G4) and G4-[[[[Ac]-TIBA]-DTPA]-mPEG] were dissolved in sterile 1x PBS (pH 7.4) at a final concentration of 5 mg/mL and filtered with a 0.22 μm MCE syringe filter. Following filtration, the hydrodynamic diameter of the solutions was assessed with a Zetasizer Nano ZS-90 (Malvern Instruments) in triplicate sequential runs. The hydrodynamic diameter was obtained from volume distribution fitting averaged over the triplicate runs.
X-ray attenuation measurements
To evaluate the X-ray attenuation properties of G4-[[[[Ac]-TIBA]-DTPA]-mPEG12], 50 milligrams of G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] dissolved in 0.3 mL of deionized water were serially diluted. Nine hundred projections of the serially diluted phantoms were acquired at 90 kVp and 40 mA on a pre-clinical microCT scanner [Explore CT120, GE Healthcare] with 16 msec exposure time in the presence of an external reference containing bone, air and water for subsequent calibration. Three-dimensional (3D) CT images were reconstructed through a filtered back-projection algorithm and transferred to an image processing workstation [Advantage Windows 4.4, GE Healthcare] for analysis. X-ray attenuation values were obtained in Hounsfield units (HU) averaged over the 3D-based region of interest (ROI).
Animal protocol
All animal experiments were performed in accordance with the regulations set by the Yale University Institutional Animal Care and Use Committee (IACUC). Healthy, wild-type C57BL/6 mice fed on a normal diet (n=29, female, 6-8 weeks of age, 19-20 grams, Jackson River Laboratories) were used in these studies. All surgical catheterizations with PE-10 polyethylene tubing and injections were performed under anesthesia (1-2% isoflurane/100% oxygen). All serial in vivo imaging studies were performed under anesthesia (1-2% isoflurane/100%oxygen) with electrocardiographical (ECG) monitoring [AccuSync 71, AccuSync Medical Research Corporation].
Preparation of small-volume reduction ampoules
Small-volume reduction ampoules were prepared according to a previously established method 38 with slight modifications. A 20 mL screw-cap glass scintillation vial was charged with 10 mL deionized water and purged with nitrogen for 30 minutes. To the purged deionized water, sodium bicarbonate (450 mg, 5.36 × 10-3 moles) [Sigma Aldrich], sodium carbonate (60 mg, 5.65 × 10-4 moles) [Sigma Aldrich], and sodium p-aminobenzoate (20 mg, 1.26 × 10-4 moles) were added as solids and mixed with magnetic stirring in a closed system. Tin(II) chloride (1.6 mg, 7 × 10-6 moles) [Sigma Aldrich] and methylenediphosphonic acid (2.5 mg, 1.42 × 10-5 moles) [Sigma Aldrich] were weighed together and added to the buffer salt solution as solids with magnetic stirring. The buffered solution was stirred until a clear solution was obtained. From this solution, either 10, 20, 30 or 40 μL volumes were aliquotted to 1.7 mL Eppendorf ampoules labeled A, B, C or D, respectively, flash frozen, and subsequently lyophilized. Reduction efficiency was assessed by thin layer chromatography (Supporting Information). The assembled reduction ampoules were stored at -20°C.
Radiolabeling for biodistribution and pharmacokinetics
Sodium pertechnetate (Na[99mTcO4]) was eluted from a 99mTc/99Mo generator using 0.9% saline and diluted in deionized water. For each group (n=4 per time point), 500 μCi of [99mTcO4-] in 100 μL deionized water was added to a 40 μL assembled reduction ampoule, vortexed and allowed to equilibrate for 2 minutes at 25°C. The entire contents of the ampoule were then combined with a solution of G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] (20 mg dissolved in 600 μL sterile 1x PBS), vortexed and allowed to equilibrate and chelate for 10 minutes at 25°C. One hundred seventy-five microliters of the final radiolabeled construct (radiopurity >79.7%, Supporting Information) was loaded into 1 mL syringes without further purification.
Radiolabeling for microSPECT/CT imaging
Sodium pertechnetate (Na[99mTcO4]) was eluted from a 99mTc/99Mo generator using 0.9% saline and diluted in deionized water. 500 μCi of [99mTcO4-] in 50 μL deionized water was added to a 20 μL assembled reduction ampoule, vortexed and allowed to equilibrate for 2 minutes at 25°C. The entire contents of the ampoule were then combined with a solution of G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] (50 mg dissolved in 250 μL sterile 1x PBS), vortexed and allowed to equilibrate and chelate for 10 minutes at 25°C. Three hundred microliters of the final radiolabeled construct (radiopurity >80%, Supporting Information) was loaded into 1 mL syringes without further purification.
Biodistribution and pharmacokinetics
The biodistribution and pharmacokinetics of 99mTc-labeled G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] were evaluated in healthy, wild-type C57BL/6 mice (female, 6-8 weeks). Each mouse received a 115 ± 3 μCi dose of the radiolabeled dendrimer construct (99mTc chelated to 5 mg G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] dissolved in 0.175 mL chelation solution) injected intravenously via the left jugular vein (n=4 per time point). Following suturing of the injection site, anesthesia was removed and the mice were euthanized at specific time points (0.5, 1, 2, 4 and 6 hours). Blood samples were collected by cardiac puncture and organs (heart, lungs, liver, spleen and kidneys) were harvested. The blood samples and organs were weighed and counted [Gamma Counter, Cobra Packard] with an appropriate energy window for 99mTc (full width at half-maximum of 140 keV photopeak) to determine their respective radioactivity counts per minute per gram (cpm/g). The obtained radioactivity counts (cpm/g) were decay corrected to the time of injection and normalized to the injected dose.
MicroCT imaging
Serial in vivo imaging of healthy, wild-type C57BL/6 mice (female, 6-8 weeks) was performed on a pre-clinical microCT scanner [Explore CT120, GE Healthcare] using the CT Fast Scan protocol (220 projections, 70 kVp, 32 mA, and a 16 msec exposure time). Following acquisition of a baseline, pre-contrast scan, either 0.15 mL (n=2) or 0.30 mL (n=2) of 166.7 mg/mL solutions of G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] dissolved in sterile 1x PBS were injected via left jugular vein catheter over a 10 minute duration to assess optimal dosage to achieve sufficient contrast-to-noise. Following injection of the contrast, mice were imaged serially from 0-90 minutes. Three-dimensional CT images were reconstructed through a filtered back-projection algorithm and further processed with MicroView software [GE Healthcare] and BioImage Suite 39.
MicroSPECT/CT imaging
The multimodal properties of 99mTc-labeled G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] were evaluated in five healthy, wild-type C57BL/6 mice (female, 6-8 weeks) using a pre-clinical microSPECT/CT hybrid system equipped with a gamma-camera having a 2 mm pinhole collimator [X-SPECT, Gamma Medica-Ideas]. Each mouse received a 440 ± 26 μCi dose of the radiolabeled dendrimer construct (99mTc chelated to 50 mg G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] dissolved in 0.3 mL chelation solution) intravenously via a left jugular vein catheter over a duration of 10 minutes. Following the injection and subsequent 5 minutes of circulation time, the mice were euthanized to eliminate motion artifacts from the chest cavity and imaged in the presence of two porous silica point sources to assist in image fusion. CT scans were acquired with 1024 projections at 75 kVp, 305 μA, 50 μm spot size and magnification factor of 3. Nuclear scans were acquired with 360° counter-clockwise (CCW) rotation, 64 projections, 40 seconds per projection, ROR of 2.7 cm, FOV of 3.6 cm and a 20% energy window centered around 140 keV. CT images were reconstructed with FLEX X-O CT software [Gamma Medica-Ideas] using a filtered back-projection algorithm. Nuclear images were reconstructed using FLEX SPECT software (8 iterations, 4 subsets) [Gamma Medica-Ideas], filtered with a Butterworth filter (Nyquist frequency=0.5, order=6) and subsequently fused with the CT images and visualized using Inveon Research Workstation [Siemens, USA].
Results
Chemistry
Ethylenediamine-core, generation 4 poly(amidoamine) dendrimers (64 terminal amines) exhibit a highly cationic surface with limited water solubility. To increase the water solubility and decrease the overall surface charge arising from residual primary amines, we chose to partially acetylate the surface of PAMAM(G4) dendrimers35, 37, 40 with sulfosuccinimidyl acetate (Figure 1). Partial acetylation was necessary to ensure the aqueous solubility of the subsequent intermediate and final products. 1H NMR characterization of the isolated product G4-[Ac] shows the appearance of a peak at 1.91 ppm corresponding to the methyl protons of the acetyl surface groups (Figure 2a). The degree of acetylation was determined with 1H NMR according to the method of Majoros et al.37, by calculating the ratio of the integrals obtained from the methyl protons of the acetyl groups and the sum of all the methylene protons of the dendrimer (-CH3/-CH2 ratio). The number of acetyl groups coupled to the dendrimer surface was determined to be 35 (Table 1).
Figure 1.
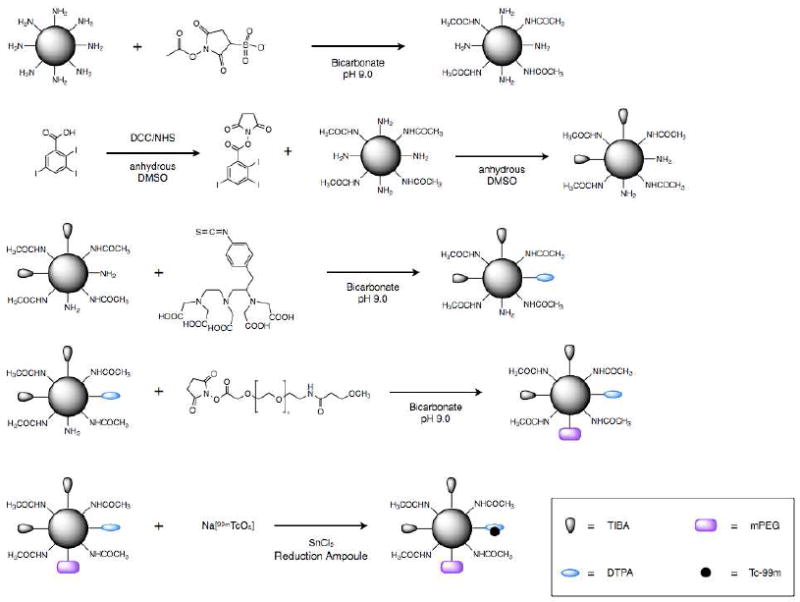
Schematic of the synthesis of 99mTc-labeled G4-[[[[Ac]-TIBA]-DTPA]-mPEG12].
Figure 2.
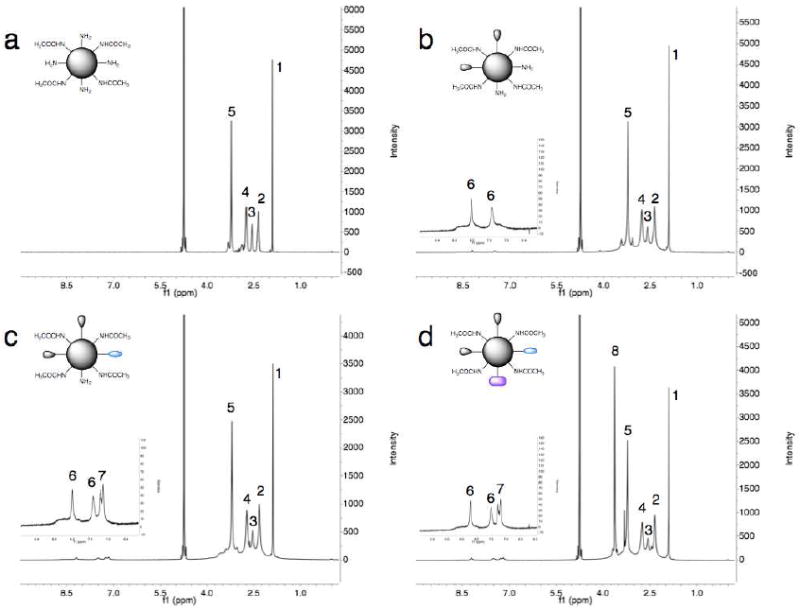
1H NMR characterization of the synthetic route to G4-[[[[Ac]-TIBA]-DTPA]-mPEG12]. 1H NMR spectra were acquired at 400 MHz for 20 mg/mL solutions of (a) G4-[Ac], (b) G4-[[Ac]-TIBA], (c) G4-[[[Ac]-TIBA]-DTPA], and (d) G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] in D2O. Peaks are labeled according to the following: (1) 1.91 (COCH3), (2) 2.28-2.42 (C-CH2CONH), (3) 2.50-2.61 N-CH2CH2NH), (4) 2.68-2.80 (N-CH2CH2CO), (5) 3.14-3.36 (CONH-CH2), (6) 7.49 and 8.21 (TIBA aromatic), (7) 7.18 and 7.27 (DTPA aromatic), and (8) 3.5-3.76 (-OCH2CH2).
Table 1.
Physicochemical properties of G4-[[[[Ac]-TIBA]-DTPA]-mPEG12].
| Compound | NH2 | NHCOCH3 | TIBA | DTPA | mPEG | Molecular Weight | dhyd (nm) |
|---|---|---|---|---|---|---|---|
| G4 | 64 | n/a | n/a | n/a | n/a | 142141 | 4.47 ± 1.45 |
| G4-[[[[Ac]-TIBA]-DTPA]-mPEG12 | 3 | 35 | 15 | 4 | 7 | 297622 | 12.37 ± 6.09 |
Obtained from Sigma Aldrich.
Obtained from 1H NMR.
To transform the partially acetylated dendrimer into a radio-opaque construct, we chose to couple 2,3,5-triiodobenzoic acid to 64% of the remaining dendrimer primary surface amines (Figure 1). To achieve the desired extent of conjugation, a slight molar excess of TIBA was converted to an amine-reactive NHS ester with DCC and NHS. The active NHS ester was retained after filtration to remove insoluble DCU and coupled immediately to the dendrimer surface without further purification. The product G4-[[Ac]-TIBA] showed the appearance of two broad peaks at 7.49 and 8.21 ppm corresponding to the two aromatic protons of TIBA in the 1H NMR spectrum (Figure 2b). Further, the product showed an increase in the CONH-CH2 integral (3.14-3.36 ppm) corresponding to the coupling of ~15 TIBA moieties (Table 1). The degree of acylation was further corroborated by comparing the integral values obtained for the aromatic protons of the TIBA moieties and the core methylene protons of the dendrimer (N-CH2CH2-NH, 2.50-2.61 ppm). The changes in the dendrimer peak integrations and inherent line-broadening of the aromatic protons indicated covalent attachment of TIBA, rather than complexation. Further, no reaction was observed in the absence of DCC and NHS.
Similar to the work of Zhang et al.35, 36, we chose to couple p-SCN-Bn-DTPA, an acyclic chelator, to roughly 28% of the remaining dendrimer primary surface amines for subsequent chelation of the nuclear probe, 99mTc (Figure 1). The product G4-[[[Ac]-TIBA]-DTPA] showed the appearance of two overlapping broad peaks at 7.18 and 7.27 ppm in the 1H NMR spectrum (Figure 2c) corresponding to the aromatic protons of the benzyl linker of p-SCN-Bn-DTPA. By comparing the integral values for the aromatic protons of the benzyl linker and the core methylene protons of the dendrimer (N-CH2CH2-NH, 2.50-2.61 ppm), the extent of conjugation via isothiocyanate linkage was determined to be ~4 (Table 1). Again, the inherent line-broadening of the aromatic protons indicated covalent attachment of the DTPA via isothiocyanate linkage.
To further enhance the water solubility and intravascular residence time, we chose to PEGylate the remaining primary surface amines with mPEG12-NHS (Figure 1). The product G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] showed the presence of a peak 3.5-3.76 ppm in the 1H NMR spectrum (Figure 2d) corresponding to the methylene protons (-OCH2CH2) of the 12 ethylene oxide repeat units. By comparing the integral values for these methylene protons and the core methylene protons of the dendrimer (N-CH2CH2-NH, 2.50-2.61 ppm), the extent of conjugation was determined to be ~7 (Table 1). The final product, G4-[[[[Ac]-TIBA]-DTPA]-mPEG12], was reproducibly attained both in terms of extent of surface conjugation and yield.
In vitro evaluation of G4-[[[[Ac]-TIBA]-DTPA]-mPEG12]
To evaluate the X-ray attenuation properties of the dendrimer construct, we acquired images of serially diluted G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] in deionized water (Figure 3) and subsequently converted them to Hounsfield maps for quantitative analysis. Iodine concentrations of the serially diluted dendrimer construct were extrapolated from the linear attenuation versus iodine concentration calibration curve generated from the serially diluted 20% Omnipaque 350 solution (Figure SI.2) for comparison to theoretical iodine concentrations estimated from 1H NMR. A linear attenuation versus iodine concentration curve, an essential criterion for X-ray contrast agents, was obtained for G4-[[[[Ac]-TIBA]-DTPA]-mPEG12]. The extrapolated iodine concentrations for G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] were observed to be consistent with the estimated iodine weight percent (~19% wt.) determined with 1H NMR.
Figure 3.
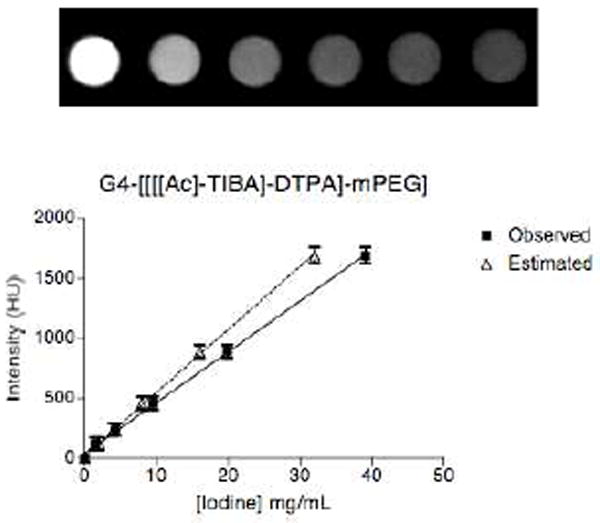
X-ray attenuation measurements. Phantom images of serial dilutions of G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] in deionized H2O acquired at 90 kVp. The intensity values obtained in Hounsfield units averaged over 3D-based ROIs were plotted against iodine concentration. Iodine concentrations for G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] were extrapolated from the linear attenuation calibration curve for Omnipaque 350 (Supporting information) and compared to those estimated from 1H NMR.
Blood clearance and biodistribution
To assess the potential of the multimodal probe for blood pool imaging, we examined both the blood clearance pharmacokinetics and biodistribution of 99mTc-labeled G4-[[[[Ac]-TIBA]-DTPA]-mPEG12]. Following intravenous injection of the radiolabeled dendrimer construct, the blood and organs were collected from healthy, female C57BL/6 mice (6-8 weeks, n=4 per time point) and subsequently analyzed by gamma counting. To determine the circulation half-life of the construct, a blood clearance curve (Figure SI.5) was generated and fit to the mono-exponential decay equation
| (1) |
where k is the rate constant. From the curve-fitting analysis, the circulation half-life was calculated to be 0.72 hours (43 minutes). Analysis of the biodistribution showed that the construct was cleared from the blood pool predominantly by the kidneys (Figure 4), as expected given the small hydrodynamic diameter (12.37 ± 6.09 nm) of the final dendrimer construct (Table 1).
Figure 4.
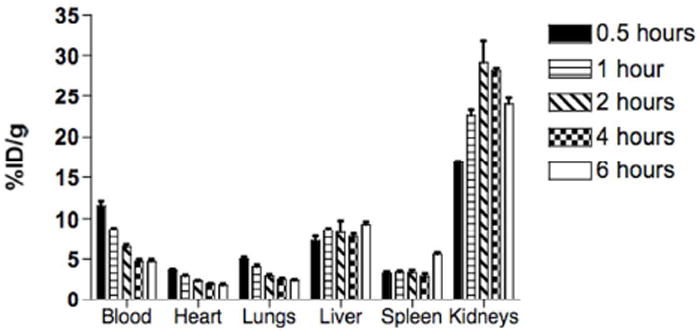
Biodistribution of 99mTc-labeled G4-[[[[Ac]-TIBA]-DTPA]-mPEG12]. The biodistribution of 99mTc-labeled G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] revealed relatively long blood residence with predominant renal clearance and excretion.
Serial blood pool imaging with microCT
The efficacy of G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] as a blood pool contrast agent was assessed in healthy, female C57BL/6 mice (6-8 weeks) with serial cardiac microCT following intravenous injection. We observed intravascular and intraventricular enhancement with an excellent contrast-to-noise ratio in the right and left ventricles of the heart across both doses. This enhancement persisted longer than 90 minutes post-injection (Figure 5a).
Figure 5.
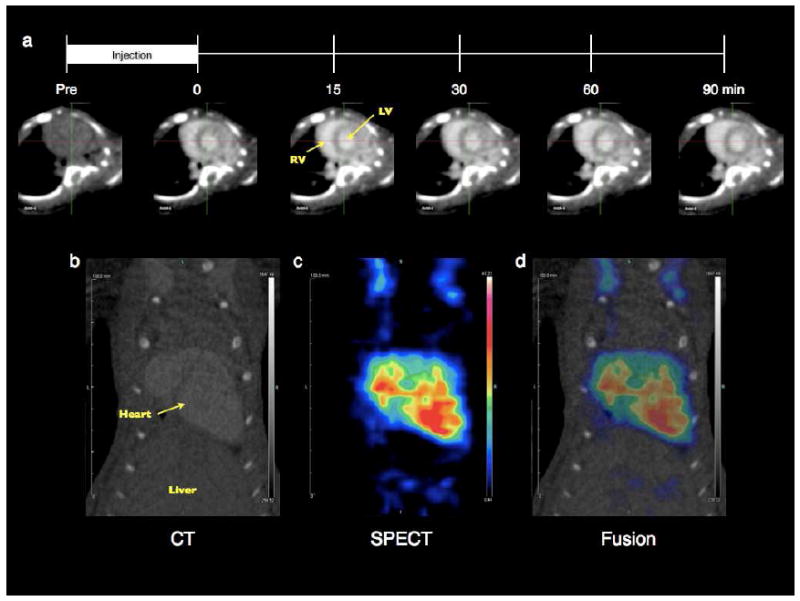
Serial microCT and multimodal microSPECT/CT blood pool imaging. (a) Following acquisition of a pre-contrast image and subsequent intravenous jugular vein injection of 50 mg of G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] dissolved in 0.3 mL sterile 1x PBS (10 minute duration), serial short axis images of the RV/LV blood pool showing significant contrast enhancement were acquired from 0-90 minutes post-injection. Following intravenous jugular vein injection (10 minute duration) of a 440 ± 26 μCi dose of the radiolabeled dendrimer construct (50 mg of 99mTc-labeled G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] dissolved in 0.3 mL chelation solution), post-mortem microSPECT/CT images of the heart were acquired. The fusion of the (b) microCT and (c) microSPECT coronal images presented in (d) shows significant co-localization of the nuclear and X-ray contrast components.
Multimodal blood pool imaging with microSPECT/CT
To assess the multimodal capabilities of the dendrimer construct, 99mTc-labeled G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] was prepared and injected intravenously into healthy, female C57BL/6 mice (6-8 weeks, n=5). Post-mortem microSPECT/CT imaging revealed substantial co-localization of the radioactivity and the dendritic iodinated contrast within the intravascular and intraventricular cavity in the absence of motion artifacts (Figures 5b-d). The intravascular retention, minimal liver accumulation and co-localization of the different contrast media provides evidence for the formation of a sufficiently pure and stable chelate, as either colloidal or free hydrolyzed/reduced 99mTc would be cleared predominantly by the liver41, 42.
Discussion
With increasing desire to extract both structural and functional information with a single probe, the necessity for development of multimodal probes is evident. Along these lines, multimodal probes have been designed for software image fusion techniques, such as, PET-MRI13 CT-MRI12, 43, 44 and optical-MRI15, 16 however, the design of multimodal probes for hybrid imaging systems is relatively unexplored45. Thus, the primary objective of this work was to synthesize a single, multimodal blood pool agent designed to simultaneously offer complementary forms of contrast enhancement for small animal hybrid imaging systems, specifically, SPECT/CT.
We chose generation 4 PAMAM dendrimers as the core structure of the contrast agent for the following reasons: (1) the well-defined, multifunctional surface (64 primary amines) offers the ability to conjugate several different moieties with negligible steric hinderance and (2) dendrimer-based contrast agents have been successfully designed and examined in single, non-invasive imaging modalities, including MRI15, 23-28, CT12, 29-31 and SPECT33-36. Partial acetylation of the PAMAM(G4) surface was performed to enhance the aqueous solubility35, 37, 40 and abrogate the potential of cytotoxicity and hemolysis derived from the inherent positive surface charge40, 46. We chose to couple TIBA to ~25% of the initial surface amines to obtain sufficient iodine weight percent necessary for effective X-ray attenuation without sacrificing aqueous solubility. Further, TIBA was selected because it imparts similar X-ray attenuation properties to the FDA-approved, small-molecule triiodobenzoic acid derivative (Omnipaque 350, GE Healthcare) routinely used in clinical applications. To generate the multimodal character of the agent, the acyclic chelator, DTPA, was chosen to chelate tin(II)-reduced 99mTc because of its established chemistry and stability35, 36, 47 Design of effective macromolecular, polymeric contrast agents for blood pool imaging requires long intravascular residence times. To further enhance the circulation half-life and limit clearance by the reticulo-endothelial system (RES)48, we chose to PEGylate the remainder of the surface with methoxy-terminated PEG.
The final dendrimer construct, G4-[[[[Ac]-TIBA]-DTPA]-mPEG12], offers both sufficient X-ray contrast enhancement and sufficiently long circulation half-life. for blood pool imaging with microCT. Following chelation of 99mTc, demonstration of the multimodal character of 99mTc-labeled G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] was successfully achieved with sequential cardiac microSPECT/CT image acquisition. This showed significant co-localization of nuclear and X-ray contrast in the heart. While others have reported long-circulating iodine-based contrast agents (e.g. Fenestra and liposomal formulations) and nuclear probes (e.g. 99mTc-Sestimibi), as well as, dendrimers, for CT and SPECT imaging, combining these forms of contrast into a single probe for hybrid imaging is virtually unexplored. Thus, this work represents the first report of a multimodal dendrimer-based contrast agent designed specifically for use with hybrid imaging systems to acquire blood pool images that exhibit both high spatial resolution and high sensitivity derived from a single probe. Further, the inherent long intravascular residence time offers the potential to enable assessment of cardiac function with both ECG and respiratory-gated microSPECT and microCT, which often imparts long image acquisition times.
Aside from integrating both the high spatial resolution of CT and the high sensitivity of nuclear imaging, the ability to combine the CT contrast and radionuclide in a single construct offers a great advantage for image processing and quantification. The fusion of sequentially obtained SPECT and CT images to gain information regarding anatomical structure and physiological function, such as absolute myocardial blood flow, relies heavily upon the ability to accurately correct the SPECT images for attenuation and partial volume effects. Specifically, this hybrid intravascular SPECT and CT probe would facilitate determination of intramyocardial blood volume and absolute myocardial blood flow using sequential, hybrid SPECT/CT imaging. Further, the probe would allow correction of SPECT partial volume errors by defining the endocardial and epicardial surfaces with CT imaging, while not compromising the ability to correct non-uniform attenuation within the chest. The multimodal properties of the contrast agent therefore offer the ability to perform these corrections directly with the circulating contrast agent at the precise time of image acquisition.
While the blood residence time is sufficiently long for blood pool imaging applications, simple synthetic modifications to the dendrimer and incorporation of targeting moieties would extend the utility of this construct to targeted imaging applications. For example, changing the acyclic chelator, DTPA, to the cyclic chelator, DOTA, would enable chelation of longer-lived radionuclides like 111In for SPECT and 64Cu for PET. Incorporation of these longer-lived radionuclides would provide sufficiently long nuclear half-lives for detection of the probe at active target sites following clearance from the blood. This would be essential for demonstrating site-specific targeting in vascular models such as angiogenesis and atherosclerosis.
Conclusions
We have successfully demonstrated the synthesis, characterization and application of a radiolabeled, multimodal dendrimer-based contrast agent for pre-clinical microSPECT/CT imaging. The long intravascular residence time and predominant renal clearance of the agent provide optimal characteristics for intravascular and blood pool imaging with microSPECT/CT. The co-localization of the dendritic nuclear and X-ray contrast offers the potential to facilitate image analysis and quantification by enabling correction for SPECT attenuation and partial volume errors at specified times with the higher resolution anatomic information provided by the circulating CT contrast. This may allow absolute quantification of intramyocardial blood volume and blood flow. Finally, the chelation of longer-lived SPECT (e.g. 111In) or PET (e.g. 64Cu) radionuclides might enable the ability to visualize active molecular targeting following sufficient clearance from the blood.
Supplementary Material
Acknowledgments
The authors would like to thank Xiangning Wang and Donald Dione for assistance with data collection and analysis. This work was partially supported by an NIH Autoimmunity Center of Excellence Pilot Award to Award (TMF) and NIH grant HL65662 (AJS).
Footnotes
Experimental details regarding the efficiency of 99mTc reduction and assessment of radiopurity of 99mTc-G4-[[[[Ac]-TIBA]-DTPA]-mPEG12] are presented. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Townsend D. Dual-Modality Imaging: Combining Anatomy and Function. J Nuc Med. 2008;49(6):938–955. doi: 10.2967/jnumed.108.051276. [DOI] [PubMed] [Google Scholar]
- 2.Townsend D. Multimodality imaging of structure and function. Phys Med Biol. 2008;53(4):R1–R39. doi: 10.1088/0031-9155/53/4/R01. [DOI] [PubMed] [Google Scholar]
- 3.Townsend D, Cherry S. Combining anatomy and function: the path to true image fusion. Eur Radiol. 2001;11(10):1968–1974. doi: 10.1007/s003300101007. [DOI] [PubMed] [Google Scholar]
- 4.Judenhofer M, Wehrl H, Newport D, Catana C, Siegel S, Becker M, Thielscher A, Kneilling M, Lichy M, Eichner M, Klingel K, Reischl G, Widmaier S, Röcken M, Nutt R, Machulla H, Uludag K, Cherry S, Claussen C, Pichler B. Simultaneous PET-MRI: a new approach for functional and morphological imaging. Nat Med. 2008;14(4):459–465. doi: 10.1038/nm1700. [DOI] [PubMed] [Google Scholar]
- 5.Schäfers K, Stegger L. Combined imaging of molecular function and morphology with PET/CT and SPECT/CT: Image fusion and motion correction. Basic Res Cardiol. 2008;103(2):191–199. doi: 10.1007/s00395-008-0717-0. [DOI] [PubMed] [Google Scholar]
- 6.Oconnor M, Kemp B. Single-photon emission computed tomography/computed tomography: basic instrumentation and innovations. Sem Nuc Med. 2006;36(4):258–266. doi: 10.1053/j.semnuclmed.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Pichler B, Judenhofer M, Pfannenberg C. Multimodal imaging approaches: PET/CT and PET/MRI. Handb Exp Pharmacol. 2008;185(Pt 1):109–132. doi: 10.1007/978-3-540-72718-7_6. [DOI] [PubMed] [Google Scholar]
- 8.Pichler B, Judenhofer M, Wehrl H. PET/MRI hybrid imaging: devices and initial results. Eur Radiol. 2008;18(6):1077–1086. doi: 10.1007/s00330-008-0857-5. [DOI] [PubMed] [Google Scholar]
- 9.Pichler B, Kolb A, Nagele T, Schlemmer H. PET/MRI: Paving the way for the next generation of clinical multimodality imaging applications. J Nuc Med. 2010;51(3):333–336. doi: 10.2967/jnumed.109.061853. [DOI] [PubMed] [Google Scholar]
- 10.Pichler B, Wehrl H, Kolb A, Judenhofer M. Positron emission tomography/magnetic resonance imaging: the next generation of multimodality imaging? Sem Nuc Med. 2008;38(3):199–208. doi: 10.1053/j.semnuclmed.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maurer AH. Combined imaging modalities: PET/CT and SPECT/CT. Health Phys. 2008;95:571–576. doi: 10.1097/01.HP.0000334064.46217.20. [DOI] [PubMed] [Google Scholar]
- 12.Regino CA, Walbridge S, Bernardo M, Wong KJ, Johnson D, Lonser R, Oldfield EH, Choyke PL, Brechbiel M. A dual CT-MR dendrimer contrast agent as a surrogate marker for convection-enhanced delivery of intracerebral macromolecular therapeutic agents. Contrast Med Mol Imag. 2008;3(1):2–8. doi: 10.1002/cmmi.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi J, Park J, Nah H, Woo S, Oh J, Kim K, Cheon G, Chang Y, Yoo J, Cheon J. A hybrid nanoparticle probe for dual-modality positron emission tomography and magnetic resonance imaging. Angew Chem Int Ed. 2008;47(33):6259–6262. doi: 10.1002/anie.200801369. [DOI] [PubMed] [Google Scholar]
- 14.Zaidi H, Montandon M, Alavi A. The clinical role of fusion imaging using PET, CT, and MR imaging. Mag Res Imag Clinics N A. 2010;18(1):133–149. doi: 10.1016/j.mric.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Talanov VS, Regino CA, Kobayashi H, Bernardo M, Choyke PL, Brechbiel M. Dendrimer-based nanoprobe for dual modality magnetic resonance and fluorescence imaging. Nano Lett. 2006;6(7):1459–1463. doi: 10.1021/nl060765q. [DOI] [PubMed] [Google Scholar]
- 16.Strijkers G, Kluza E, Tilborg G, Schaft D, Griffioen A, Mulder W, Nicolay K. Paramagnetic and fluorescent liposomes for target-specific imaging and therapy of tumor angiogenesis. Angiogenesis. 2010;13(2):161–173. doi: 10.1007/s10456-010-9165-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louie A. Multimodality imaging probes: design and challenges. Chem Rev. 2010;110:3146–3195. doi: 10.1021/cr9003538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skajaa T, Cormode D, Jarzyna P, Delshad A, Blachford C, Barazza A, Fisher E, Gordon R, Fayad Z, Mulder W. The biological properties of iron oxide core high-density lipoprotein in experimental atherosclerosis. Biomaterials. 2011;32(1):206–213. doi: 10.1016/j.biomaterials.2010.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly K. Detection of vascular adhesion molecule-1 expression using a novel multimodal nanoparticle. Circ Res. 2005;96(3):327–336. doi: 10.1161/01.RES.0000155722.17881.dd. [DOI] [PubMed] [Google Scholar]
- 20.McCarthy JR, Patel P, Botnaru I, Haghayeghi P, Weissleder R, Jaffer FA. Multimodal Nanoagents for the Detection of Intravascular Thrombi. Bioconjugate Chem. 2009;20:1251–1255. doi: 10.1021/bc9001163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobrucki LW, Tsutsumi Y, Kalinowski L, Dean J, Gavin M, Sen S, Mendizabal M, Sinusas AJ, Aikawa R. Analysis of angiogenesis induced by local IGF-1 expression after myocardial infarction using microSPECT-CT imaging. J Mol Cell Cardiol. 2010;48(6):1071–1079. doi: 10.1016/j.yjmcc.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomalia DA, Naylor AM, Goddard WA., III Starburst Dendrimers: Molecular-level control of size, shape, surface chemistry, topology, and flexibility from atoms to macroscopic matter. Angew Chem Int Ed. 1990;29:138–175. [Google Scholar]
- 23.Jászberényi Z, Moriggi L, Schmidt P, Weidensteiner C, Kneuer R, Merbach A, Helm L, Tóth É. Physicochemical and MRI characterization of Gd3+-loaded polyamidoamine and hyperbranched dendrimers. J Biol Inorg Chem. 2007;12(3):406–420. doi: 10.1007/s00775-006-0197-3. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi H, Brechbiel M. Nano-sized MRI contrast agents with dendrimer cores. Adv Drug Deliv Rev. 2005;57(15):2271–2286. doi: 10.1016/j.addr.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi H, Brechbiel MW. Dendrimer-based nanosized MRI contrast agents. Curr Pharm Biotechnol. 2004;5:539–549. doi: 10.2174/1389201043376571. [DOI] [PubMed] [Google Scholar]
- 26.Mounzer R, Shkarin P, Papademetris X, Constable T, Ruddle N, Fahmy T. Dynamic imaging of lymphatic vessels and lymph nodes using a bimodal nanoparticulate contrast agent. Lymph Res Biol. 2007;5(3):151–158. doi: 10.1089/lrb.2007.5302. [DOI] [PubMed] [Google Scholar]
- 27.Venditto VJ, Regino CA, Brechbiel M. PAMAM dendrimer based macromolecules as improved contrast agents. Mol Pharm. 2005;2(4):302–311. doi: 10.1021/mp050019e. [DOI] [PubMed] [Google Scholar]
- 28.Wiener EC, Brechbiel MW, Brothers H, Magin RL, Gansow OA, Tomalia DA, Lauterbur PC. Dendrimer-based metal-chelates - a new class of magnetic resonance imaging contrast agents. Magn Reson Med. 1994;31:1–8. doi: 10.1002/mrm.1910310102. [DOI] [PubMed] [Google Scholar]
- 29.Fu Y, Nitecki DE, Maltby D, Simon GH, Berejnoi K, Raatschen HJ, Yeh BM, Shames DM, Brasch RC. Dendritic iodinated contrast agents with PEG-cores for CT imaging: Synthesis and preliminary characterization. Bioconjugate Chem. 2006;17:1043–1056. doi: 10.1021/bc060019c. [DOI] [PubMed] [Google Scholar]
- 30.Yordanov AT, Lodder AL, Woller EK, Cloninger MJ, Patronas N, Milenic D, Brechbiel MW. Novel iodinated dendritic nanoparticles for computed tomography (CT) imaging. Nano Lett. 2002;2:595–599. [Google Scholar]
- 31.Yordanov AT, Mollov N, Lodder AL, Woller E, Cloninger M, Walbridge S, Milenic D, Brechbiel MW. A water-soluble triiodo amino acid and its dendrimer conjugate for computerized tomography (CT) imaging. J Serb Chem Soc. 2005;70:163–170. [Google Scholar]
- 32.Almutairi A, Rossin R, Shokeen M, Hagooly A, Ananth A, Capoccia B, Guillaudeu S, Abendschein D, Anderson CJ, Welch MJ, Fréchet JM. Biodegradable dendritic positron-emitting nanoprobes for the noninvasive imaging of angiogenesis. P Natl Acad Sci U S A. 2009;106(3):685–690. doi: 10.1073/pnas.0811757106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agashe HB, Babbar AK, Jain S, Sharma RK, Mishra AK, Asthana A, Garg M, Dutta T, Jain NK. Investigations on biodistribution of technetium-99m-labeled carbohydrate-coated poly(propylene imine) dendrimers. Nanomed-Nanotechnol. 2007;3:120–127. doi: 10.1016/j.nano.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Parrott MC, Benhabbour SR, Saab C, Lemon JA, Parker S, Valliant JF, Adronov A. Synthesis, Radiolabeling, and Bio-imaging of High-Generation Polyester Dendrimers. J Am Chem Soc. 2009;131:2906–2916. doi: 10.1021/ja8078175. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Sun Y, Xu X, Zhang X, Zhu H, Huang L, Qi Y, Shen Y. Synthesis, Biodistribution, and Microsingle Photon Emission Computed Tomography (SPECT) Imaging Study of Technetium-99m Labeled PEGylated Dendrimer Poly(amidoamine) (PAMAM)-Folic Acid Conjugates. J Med Chem. 2010;53:3262–3272. doi: 10.1021/jm901910j. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Sun Y, Xu X, Zhu H, Huang L, Zhang X, Qi Y, Shen YM. Radiosynthesis and micro-SPECT imaging of (99m)Tc-dendrimer poly(amido)-amine folic acid conjugate. Bioorg Med Chem Lett. 2010;20(3):927–931. doi: 10.1016/j.bmcl.2009.12.075. [DOI] [PubMed] [Google Scholar]
- 37.Majoros IJ, Keszler B, Woehler S, Bull T, Baker JR. Acetylation of poly(amidoamine) dendrimers. Macromolecules. 2003;36:5526–5529. [Google Scholar]
- 38.Marino MECP, Syud FA, Schaffer P, Lee BD, Zhang R, Lindborg M, Gunneriusson E, Lendel C. PDGF-RBeta binders. 20090191124 Patent Application. 2009
- 39.Papademetris X, Jackowski M, Rajeevan N, Constable RT, Staib LH. BioImage Suite: An integrated medical image analysis suite. Section of Bioimaging Sciences Dept. of Diagnostic Radiology. Yale School of Medicine. 2007 Available from URL: http://www.bioimagesuite.org. [PMC free article] [PubMed]
- 40.Kolhatkar R, Kitchens K, Swaan P, Ghandehari H. Surface acetylation of polyamidoamine (PAMAM) dendrimers decreases cytotoxicity while maintaining membrane permeability. Bioconjugate Chem. 2007;18(6):2054–2060. doi: 10.1021/bc0603889. [DOI] [PubMed] [Google Scholar]
- 41.Banerjee S, Samuel G, Kothari K, Unni PR, Sarma HD, Pillai MR. Tc-99m and Re-186 complexes of tetraphosphonate ligands and their biodistribution pattern in animal models. Nucl Med Biol. 2001;28(2):205–213. doi: 10.1016/s0969-8051(00)00173-6. [DOI] [PubMed] [Google Scholar]
- 42.Banerjee T, Singh A, Sharma R, Maitra A. Labeling efficiency and biodistribution of Technetium-99m labeled nanoparticles: interference by colloidal tin oxide particles. Int J Pharm. 2005;289(1-2):189–195. doi: 10.1016/j.ijpharm.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 43.Zheng J, Liu J, Dunne M, Jaffray DA, Allen C. In vivo performance of a liposomal vascular contrast agent for CT and MR-based image guidance applications. Pharm Res. 2007;24(6):1193–1201. doi: 10.1007/s11095-006-9220-1. [DOI] [PubMed] [Google Scholar]
- 44.Zheng J, Perkins G, Kirilova A, Allen C, Jaffray DA. Multimodal contrast agent for combined computed tomography and magnetic resonance imaging applications. Invest Radiol. 2006;41(3):339–348. doi: 10.1097/01.rli.0000186568.50265.64. [DOI] [PubMed] [Google Scholar]
- 45.Seevinck PR, Seppenwoolde JH, de Wit TC, Nijsen JF, Beekman FJ, van Het, Schip AD, Bakker CJ. Factors affecting the sensitivity and detection limits of MRI, CT, and SPECT for multimodal diagnostic and therapeutic agents. Anticancer Agents Med Chem. 2007;7(3):317–334. doi: 10.2174/187152007780618153. [DOI] [PubMed] [Google Scholar]
- 46.Fischer D, Li Y, Ahlemeyer B, Krieglstein J, Kissel T. In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials. 2003;24(7):1121–1131. doi: 10.1016/s0142-9612(02)00445-3. [DOI] [PubMed] [Google Scholar]
- 47.Narasimhan DVS, Vanaja P, Banodkar SM, Mani RS. Studies on the chemistry of technetium-99m radiopharmaceuticals. J Radioanal Nucl Chem. 1984;84:129–136. [Google Scholar]
- 48.Chow T, Lin Y, Hwang J, Wang H, Tseng Y, Pang V, Liu R, Lin W, Yang C, Ting G. Therapeutic efficacy evaluation of 111In-labeled PEGylated liposomal vinorelbine in murine colon carcinoma with multimodalities of molecular imaging. J Nucl Med. 2009;50(12):2073–2081. doi: 10.2967/jnumed.109.063503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


