Abstract
Most estrogen receptor α (ER)-positive breast cancers initially respond to antiestrogens, but many eventually become estrogen-independent and recur. We identified an estrogen-independent role for ER and the CDK4/Rb/E2F transcriptional axis in the hormone-independent growth of breast cancer cells. ER downregulation with fulvestrant or siRNA inhibited estrogen-independent growth. Chromatin immunoprecipitation identified ER genomic binding activity in estrogen-deprived cells and primary breast tumors treated with aromatase inhibitors. Gene expression profiling revealed an estrogen-independent, ER/E2F-directed transcriptional program. An E2F activation gene signature correlated with a lesser response to aromatase inhibitors in patients' tumors. siRNA screening showed that CDK4, an activator of E2F, is required for estrogen-independent cell growth. Long-term estrogen-deprived cells hyperactivate phosphatidylinositol 3-kinase (PI3K) independently of ER/E2F. Fulvestrant combined with the pan-PI3K inhibitor BKM120 induced regression of ER+ xenografts. These data support further development of ER downregulators and CDK4 inhibitors, and their combination with PI3K inhibitors for treatment of antiestrogen-resistant breast cancers.
Keywords: Estrogen receptor, breast, aromatase inhibitor, resistance, CDK4
Introduction
Approximately 70% of breast cancers express estrogen receptor α (ER) and/or progesterone receptor (PR), biomarkers indicative of hormone dependence. Therapies for such patients inhibit ER signaling by 1) antagonizing ligand binding to ER (tamoxifen), 2) downregulating ER (fulvestrant), or 3) blocking estrogen biosynthesis (aromatase inhibitors, AIs). Although endocrine therapies have changed the natural history of hormone-dependent breast cancer, many tumors develop drug resistance (1). The only mechanism of resistance to endocrine therapy for which clinical data exist is overexpression of the ErbB2/HER2 protooncogene (2, 3). However, <10% of hormone receptor-positive breast cancers express high HER2 levels, suggesting that for the majority of ER+ breast cancers, mechanisms of escape from endocrine therapy remain undefined. We and others have shown that activation of the phosphatidylinositol 3-kinase (PI3K) signaling pathway also promotes resistance to endocrine therapy (4, 5), although demonstration of such a mechanism in the clinic awaits confirmation.
Tamoxifen has been the standard treatment for patients with hormone receptor-positive breast cancer. This drug exhibits dual agonistic/antagonistic effects on ER transcriptional activity and cancer cell growth (6). In contrast, AIs suppress estrogen biosynthesis and thus ligand-induced ER activity with no known agonistic effects. In post-menopausal patients, AIs are modestly superior to tamoxifen in preventing disease recurrence. However, a significant fraction of patients with early ER+ breast cancer receiving adjuvant AIs recur within the first decade of follow-up (1). Interestingly, a recent comparison of high-dose fulvestrant to the AI anastrozole as first-line treatment for advanced breast cancer revealed that fulvestrant provided a longer time-to-progression (7). In other studies, ~30% of patients who progressed on an AI responded to second-line fulvestrant (8, 9). These data suggest that in some clinical situations, downregulation of ER may be superior or add to estrogen deprivation.
Fulvestrant has been shown to inhibit the growth of (ER+) MCF-7 human breast cancer cells and xenografts under estrogen-depleted conditions (10, 11). However, the role of ER in estrogen-independent growth remains unclear. In order to model the low estrogen levels seen in patients treated with an AI [≤3 pM plasma 17β-estradiol, E2 (12)], we generated long-term estrogen-deprived (LTED) derivatives of four ER+ human breast cancer cell lines. These LTED lines exhibit hyperactivation of the PI3K pathway, and variable changes in ER levels and E2 sensitivity (4). Herein, we report that the unliganded ER frequently plays a role in the hormone-independent growth of LTED cells by modulating a transcriptional program directed by E2F proteins. Concurrently, a siRNA screen identified that CDK4, a kinase that activates E2F transcription, is required for hormone-independent cell growth. CDK4 inhibition suppressed the hormone-independent growth of both fulvestrant-sensitive and -insensitive ER+ cell lines. Furthermore, post-menopausal ER+ tumors with a gene expression signature of E2F activation following neoadjuvant therapy with an AI exhibited a lesser response, suggesting that estrogen-independent E2F activation promotes resistance to estrogen deprivation. Finally, fulvestrant in combination with a PI3K inhibitor induced marked regression of ER+ breast cancer xenografts in mice devoid of estrogen supplementation. These results provide a basis for 1) further development of potent ER downregulators and CDK4 inhibitors, particularly in AI-resistant ER+ tumors with a gene expression signature of E2F activation, and 2) their combination with PI3K inhibitors for the treatment of ER+ breast cancers that escape estrogen deprivation.
Results
Unliganded ER is required for the hormone-independent growth of ER+ breast cancer cells
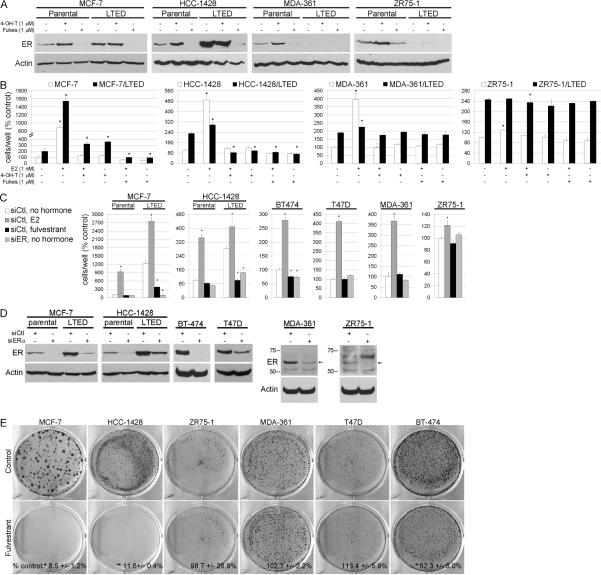
We previously reported that LTED cells show variable changes in ER levels after selection in hormone-depleted medium (10% dextran-charcoal-treated FBS, DCC-FBS). MCF-7/LTED and HCC-1428/LTED cells exhibit higher ER levels compared to parental controls, while ZR75-1/LTED and MDA-361/LTED cells show the opposite [Fig. 1A and ref. (4)]. We sought to determine whether ER plays a role in hormone-independent growth. Treatment with the ER downregulator fulvestrant reduced growth of MCF-7/LTED and HCC-1428/LTED cells in estrogen-depleted medium (p<0.05 vs. controls), but not MDA-361 or ZR75-1 parental or LTED cells (Fig. 1B). These results suggest that the (upregulated) ER in MCF-7/LTED and HCC-1428/LTED cells drives estrogen-independent growth. Conversely, cells which did not require ER for hormone-independent growth (MDA-361, ZR75-1) downregulated ER during prolonged estrogen deprivation (Fig. 1A). Treatment of both parental and LTED cells with 4-hydroxy-tamoxifen (4-OH-T) or fulvestrant suppressed E2-induced growth (Fig. 1B) and E2-induced ER transcriptional reporter activity (Fig. S1). In agreement with a prior report (11), prolonged hormone deprivation of MCF-7/LTED cells (>6 months) generated an estrogen-insensitive line (MCF-7/LTED-I). MCF-7/LTED-I cells maintained high ER levels. Their growth was inhibited by fulvestrant (data not shown), suggesting that dependence upon ER for hormone-independent growth is not a transient phenotype. To confirm that the effects of fulvestrant were through action on ER, we knocked-down ESR1 (ER) expression using siRNA. siER-transfected MCF-7/LTED, HCC-1428/LTED, and BT-474 cells exhibited reduced growth in hormone-depleted medium compared to siControl. In contrast, ER knockdown did not alter the hormone-independent growth of MDA-361, ZR75-1, or T47D cells (Fig. 1C). ER knockdown was confirmed by immunoblot (Fig. 1D); results were confirmed using a second siRNA targeting ER (data not shown).
Fig. 1.
ER is required for acquired hormone-independent breast cancer cell growth. A) Lysates from cells treated with 10% DCC-FBS ± fulvestrant or 4-OH-T × 24 h were analyzed by immunoblotting using ER and actin antibodies. B) Cells were treated as in (A) ± E2, 4-OH-T, or fulvestrant. Media and drugs were replenished every 2–3 days. Adherent cells were counted after 5–10 days. Data are presented as % parental control, mean of triplicates ± SD. C) Cells transfected with siRNA targeting ER or non-silencing control (siCtl) were treated as in (B), and adherent cells were counted after 6–9 days. Data are presented as % parental siCtl/no hormone, mean of triplicates ± SD. D) Immunoblots of lysates from the same batches of cells used in (C). E) Parental cells were treated as in (B). When control wells reached 30–50% confluence (18–60 days), cells were stained with crystal violet. Representative images are shown. Quantification of staining intensity is noted at bottom as mean of triplicates ± SD (% control). *p<0.05 by Bonferroni post-hoc test compared to control [or siCtl/no hormone in (C)] of each cell line.
We next assessed whether the lines in which growth was inhibited by ER downregulation were the same in which ER was required for acquired resistance to estrogen deprivation. Parental cells were reselected for hormone-independent growth in the presence of fulvestrant. Fulvestrant suppressed the emergence of hormone-independent MCF-7, HCC-1428, and BT-474 cells (p<0.05 vs. respective controls), but not ZR75-1, MDA-361, or T47D cells (Fig. 1E), implying that the requirement for ER in estrogen-independent growth is intrinsic to the parental cells.
ER promotes an estrogen-independent, E2F-mediated transcriptional program
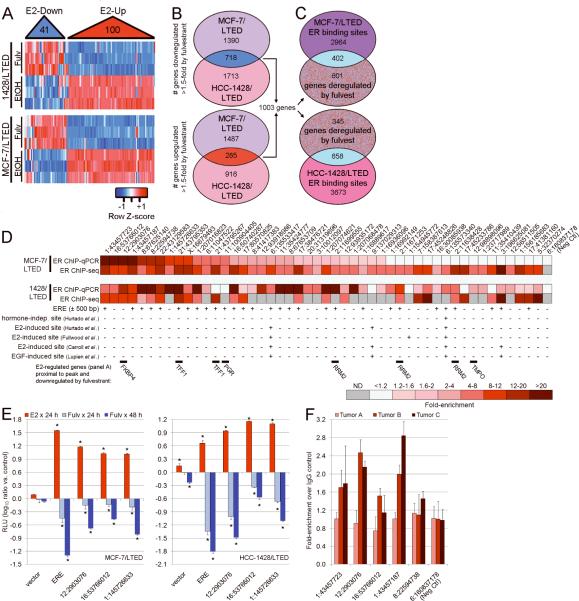
To determine the role of ER in hormone-independent growth, we performed gene expression analysis of “ER-dependent” MCF-7/LTED and HCC-1428/LTED cells treated ± fulvestrant. To assess whether ER regulates similar gene sets in the presence and absence of estrogen, we derived a signature of 141 genes altered by exogenous E2 in MCF-7 cells from two prior datasets (13, 14). Hierarchical clustering of these 141 genes in LTED cells ± fulvestrant (in the absence of estrogen) showed that fulvestrant induced a gene expression pattern opposite to E2 (Fig. 2A). We next determined which genes were commonly altered by fulvestrant in both MCF-7/LTED and HCC-1428/LTED cells (≥1.5-fold, FDR-adjusted p≤0.05). This analysis yielded 718 genes downregulated and 285 genes upregulated by fulvestrant (Fig. 2B). Of these 1,003 genes, 671 were available to query the Connectivity Map, a collection of gene signatures induced by the treatment of cells with a panel of 1,309 small molecules (15). Treatment of MCF-7 cells with fulvestrant in 10% FBS induced gene signatures (data from Connectivity Map) that were directly correlated with the 671-gene signature derived from fulvestrant-treated LTED cells in the absence of hormones (p<0.00001). Therefore, fulvestrant induced similar gene expression profiles in the presence and absence of hormones, implying that ER regulates overlapping sets of genes under E2-stimulated as well as hormone-depleted conditions.
Fig. 2.
Gene expression and genomic profiling reveal estrogen-independent ER transcriptional activity. A) RNA from MCF-7/LTED and HCC-1428/LTED cells treated ± fulvestrant × 48 h was analyzed using gene expression microarrays. We derived a signature of 141 genes commonly up- or down-regulated by E2 stimulation in MCF-7 cells. Using LTED cell gene expression data, we hierarchically clustered these 141 genes (x-axis). Fulvestrant induced expression changes diametrically opposed to those induced by E2. B) We determined probe sets up- or down-regulated by fulvestrant in each LTED line (≥1.5-fold, p≤0.05), and used Venn diagrams to identify commonly deregulated probe sets (yielded 1,434 probe sets for 718 down- and 285 up-regulated genes). C) ChIP-seq analysis to identify ER genomic binding regions. To focus on functional ER binding sites, we determined which sites were within 60 kb of the transcription start site of a fulvestrant-deregulated gene. D) Forty-eight ER binding regions identified in (C) were tested by ChIP-qPCR. Data presented as heatmap of fold-enrichment in ER-ChIP over IgG-ChIP control, or fold-enrichment score from ChIP-seq. ND- not detected. E2-regulated genes deregulated by fulvestrant in (A), presence of a proximal estrogen-response-element (ERE) within 500 bp of a peak, and overlap of peaks with published ER-ChIP datasets are noted below heatmap. E) ER-bound regions verified in (D) were subcloned into a luciferase reporter vector. Cells transfected with reporter plasmids were treated with 10% DCC-FBS ± fulvestrant or E2. Luciferase activities were measured after 24 or 48 h. ERE- contains two consensus EREs. RLU- relative light units (firefly/Renilla). Data are presented as log10(ratio vs. each untreated control), mean of triplicates ± SD. *p<0.05 by Bonferroni post-hoc test compared to each control. F) ChIP-qPCR as in (D) using human ER+ breast tumor samples acquired from patients following 10–21 days of neoadjuvant letrozole. Data presented as fold-enrichment in ER-ChIP over IgG-ChIP control.
In order to further evaluate the estrogen-independent genomic function of ER, we performed chromatin immunoprecipitation followed by next-generation DNA sequencing (ChIP-seq). We identified 3,366 and 4,331 regions (peaks) that were significantly enriched following ER-ChIP-seq in MCF-7/LTED and HCC-1428/LTED cells, respectively (Fig. 2C). A comparison of these data with published hormone-independent, E2-induced, and EGF-induced ER binding regions in MCF-7 cells (14, 16–18) showed 18.1% (hormone-independent), 23–52.4% (E2-induced), and 46.1–53.3% (EGF-induced) overlap with MCF-7/LTED cells, and 4.9% (hormone-independent), 9.3–26.6% (E2), and 11.8–18.1% (EGF) overlap with HCC-1428/LTED cells (Fig. S2). Since a significant fraction of E2-induced ER binding sites are distal to transcription start sites of E2-regulated genes, some of which may be non-functional (14, 16), we focused on ER binding sites within 60 kb of transcription start sites of the 1,003 genes commonly deregulated by fulvestrant (Figs. 2C, S3). This analysis revealed 402 and 658 ER binding regions in MCF-7/LTED and HCC-1428/LTED cells, respectively. Using ChIP-qPCR, we tested 48 of these sites and found that most were enriched in ER-ChIP compared to IgG-ChIP control (Fig. 2D). Interestingly, only approximately half of these ER binding regions contained a proximal estrogen-response-element (ERE); many had not been previously identified in genome-wide ER-ChIP studies (14, 16–18). Transcription factor motif analysis revealed that ER-bound regions were enriched for motifs which bind ER, FoxA1, RARα, and C/EBP, among others (Fig. S4–S5 & Tables S1–S4); such proteins are known to functionally cooperate with ER (18–21). We next subcloned three ER-bound regions (Fig. S6) into a transcriptional reporter vector. In MCF-7/LTED and HCC-1428/LTED cells, reporter activity increased with E2 and decreased with fulvestrant (Fig. 2E). The latter results suggest that these ER binding sites regulate transcription in the absence of ER ligands.
We next examined whether estrogen-independent ER genomic signaling occurs in primary ER+ cancers in patients subjected to estrogen deprivation. Breast tumors in patients treated with aromatase inhibitors (AIs) can be considered estrogen-deprived since these drugs maximally suppresses plasma estrogen levels within two weeks (22), providing a setting in which to test estrogen-independent ER activity. Hence, we performed ChIP-qPCR for ER binding events using three ER+ breast tumors acquired from patients following 10–21 days of neoadjuvant therapy with the AI letrozole (clinical trial NCT00651976). In 2/3 tumors, ER-ChIP samples showed significant enrichment for ER-bound genomic regions identified in cell lines compared to IgG controls (Fig. 2F), supporting the presence of estrogen-independent ER-DNA binding activity in primary human tumors deprived of estrogens.
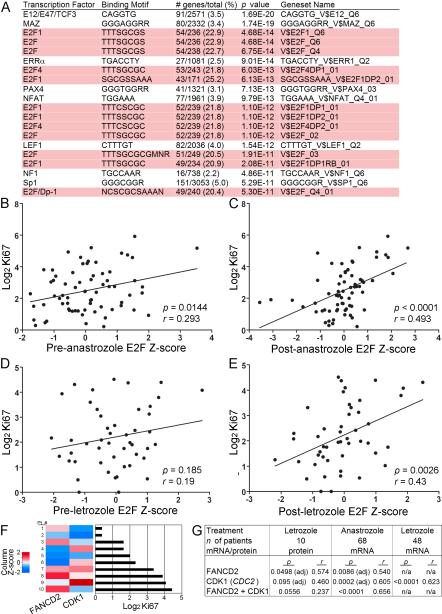
To identify the functional output of estrogen-independent ER activity, we classified the proteins encoded by the 1,003 fulvestrant-deregulated genes (Fig. 2B) using Gene Ontology (GO) analysis (23). The top enriched biological processes were Cell Cycle, Cell Proliferation, M Phase, Mitotic Cell Cycle, and DNA Replication and Chromosome Cycle (all p<0.0001). These data suggest that in the absence of hormones, ER modulates the expression of genes encoding proteins that regulate the cell cycle. We then asked whether there was an underlying transcriptional program directed by ligand-independent ER within this large set of modulated genes. We computed the overlap between 1) the 1,003 genes altered by fulvestrant in LTED cells, and 2) sets of genes that share a transcription factor binding site within 2 kb of transcription start sites. Twelve of the top 20 gene sets that showed significant overlap with the fulvestrant-induced signature contained E2F binding sites (Fig. 3A). E2F1 binds predominantly within 2 kb of transcription start sites (24). The E2F family contains eight proteins (E2F1-8) which modulate the expression of genes that coordinate cell cycle progression, DNA replication, mitosis, and apoptosis (25). Included in the set of fulvestrant-suppressed genes were E2F1, -2, -7, and -8. Prior work has shown that E2 stimulates E2F1 expression (26) and phosphorylation/inactivation of the E2F repressor Rb (27). These findings suggest that ER also promotes E2F expression and activation in an estrogen-independent manner in MCF-7/LTED and HCC-1428/LTED cells. In contrast, fulvestrant did not alter estrogen-independent E2F1, -2, -7, or -8 expression in MDA-361 or ZR75-1 cells (data not shown), suggesting that these ER+ cell lines harbor an ER-independent mechanism(s) to promote E2F transcription.
Fig. 3.
A gene expression signature of E2F activation correlates with poor tumor response to aromatase inhibitors in patients. A) The set of 1,003 genes commonly deregulated by fulvestrant was used to query TRANSFAC database. Top 20 Genesets are shown, 12 of which contain E2F motifs (highlighted). The number of fulvestrant-deregulated genes as a percentage of the total genes in each set is shown. We derived a set of 24 genes containing E2F motifs but lacking a Gene Ontology cell cycle annotation. B–E) Expression values of these 24 genes were used to generate E2F activation scores for ER+ primary breast tumors from patients treated with an AI using pre- and post-treatment gene expression data. E2F scores were standardized to generate Z-scores. Patients were treated with neoadjuvant anastrozole for 14 days (B–C), or with letrozole for 10–14 days (D–E). The associations of the pre-anastrozole (B), post-anastrozole (C), pre-letrozole (D), and post-letrozole (E) E2F Z-scores with log2-transformed post-AI Ki67 score were analyzed by linear regression. Pearson correlation coefficients (r) and ANOVA p values were calculated. F) In a third cohort, protein levels of FANCD2 and CDK1 were measured by RPPA in ER+ primary breast tumors following 10–21 days of neoadjuvant letrozole. Log2-transformed signal values were standardized into Z-scores, which were compared to post-letrozole Ki67 score. G) The protein (Z-score) and mRNA levels (from post-treatment microarrays) of FANCD2 and CDK1 (CDC2) were compared to Ki67 score in all 3 datasets using linear regression. adj = adjusted p-value from multiple regression analysis.
E2F activation signature correlates with resistance to estrogen deprivation in ER+ primary tumors
Since fulvestrant suppressed hormone-independent growth in cells with ER-dependent E2F activity, we postulated that an estrogen-independent, E2F-induced gene expression profile would predict for resistance to estrogen deprivation in human tumors. Thus, we generated a signature of E2F activation from LTED cells and assessed its ability to predict response to neoadjuvant therapy with an AI in patients with ER+ breast cancer. Primary tumor biopsies were obtained from 68 patients with newly diagnosed ER+ breast cancer before and after 14 days of therapy with anastrozole (28). High tumor cell proliferation measured by immunohistochemistry (IHC) for the proliferation marker Ki67 after short-term endocrine therapy has been shown to predict a worse long-term disease outcome (29). Hence, we used the post-treatment Ki67 score as a surrogate of clinical response.
GO analysis showed that 37/61 genes containing an E2F motif that were deregulated by fulvestrant in LTED cells were associated with cell cycle regulation. Tumor Ki67 score has been shown to correlate with the expression of genes encoding proteins involved in the cell cycle (30). This analysis suggested that the remaining 24 genes, which did not have a cell cycle-related GO annotation, could be used to assess E2F activity independent of the cell cycle (Fig. S7A–B, Table S5). In order to distinguish between cell cycle-associated and -independent effects of E2F activation, we evaluated both the 61-gene E2F signature, and the 24-gene E2F signature devoid of cell cycle-associated genes.
Hierarchical clustering revealed that tumors did not group into distinct clusters (Fig. S7C–D), suggesting that E2F activation may be better represented as a continuous variable. Hence, we generated E2F activation scores for each tumor using both pre- and post-anastrozole gene expression data, and compared them to the post-anastrozole (2 weeks) Ki67 score. As expected, the pre- and post-anastrozole 61-gene E2F scores were significantly associated with the Ki67 score (ANOVA p=0.0004, r=0.412, and p<10−4, r=0.641, respectively). Importantly, both the pre- and post-anastrozole non-cell-cycle 24-gene E2F scores also correlated significantly with the Ki67 score (ANOVA p=0.0144, r=0.293, and p<10−4, r=0.493, respectively; Fig. 3B–C). These data suggest that an expression signature of E2F activation in ER+ tumors may be linked with resistance to AI-induced estrogen deprivation.
We confirmed this correlation in a second cohort of 48 patients with ER+ breast cancer treated with neoadjuvant letrozole (31). Again, the pre- and post-two-week-letrozole 61-gene E2F scores correlated significantly with the Ki67 score (ANOVA p=0.043, r=0.293, and p<10−4, r=0.558, respectively). In this second set, however, the post-treatment but not the pre-treatment 24-gene E2F score was significantly associated with Ki67 score (ANOVA p=0.0026, r=0.43; Fig. 3D–E). Variable estrogen levels in the pre-treatment tumor samples may have increased noise and confounded the analysis of estrogen-independent E2F activation. Considering the E2F signature was derived from LTED cells, and the consistent statistical correlations between the post-treatment E2F score and Ki67 score, we speculate the contribution of E2F signaling to endocrine resistance may be best evaluated under conditions of estrogen deprivation.
We finally determined whether the Ki67 score following neoadjuvant therapy with an AI correlated with E2F-regulated expression at the protein level. The promoters of FANCD2 and CDC2 contain E2F binding sites (32, 33) and were part of the 24-gene and 61-gene E2F signatures, respectively. Antibodies were validated for analysis of these proteins using reverse-phase protein arrays (RPPA). In a third cohort of 10 patients with ER+ breast cancer, we quantified levels of FANCD2 and CDK1 (CDC2) by RPPA analysis of tumor samples obtained following 10–21 days of neoadjuvant letrozole (Fig. 3F). FANCD2 levels were significantly correlated with post-letrozole Ki67 score, while CDK1 levels showed a correlative trend (Fig. 3G). In addition to FANCD2 and CDC2, mRNA levels of another 8/21 and 8/20 available genes in the 24-gene E2F signature showed significant univariate correlations with Ki67 score in the post-anastrozole and post-letrozole microarray datasets, respectively (Fig. 3C/E, p<0.05).
CDK4 is required for hormone-independent ER+ breast cancer cell growth
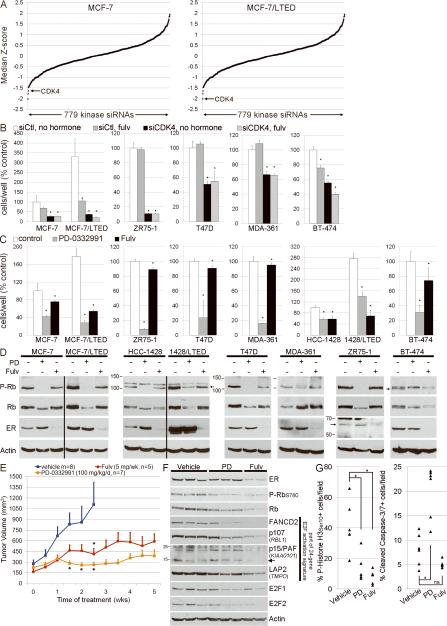
In a complementary experiment, we screened a siRNA library targeting 779 kinases to identify pathways required for hormone-independent growth. siCDK4 ranked as the overall 1st and 3rd strongest hits which inhibited growth of MCF-7/LTED and MCF-7 cells, respectively (Fig. 4A). CDK4 and CDK6 phosphorylate Rb to derepress E2F signaling which, in turn, promotes cell cycle progression (25). Hence, CDK4 knockdown may phenocopy the effects of ER downregulation since both pathways converge on E2F. Verification showed that CDK4 knockdown inhibited hormone-independent growth and reduced P-Rb levels in 6/6 ER+ breast cancer cell lines (Figs. 4B, S8; confirmed using an independent CDK4 siRNA, data not shown). This is consistent with recent reports showing that ER+ breast cancer cell lines were more sensitive to the CDK4/6 inhibitor PD-0332991 (34) than ER-negative lines (35), and that PD-0332991 inhibits the growth of antiestrogen-resistant MCF-7 cells (36). In support of a role of CDK4 in hormone-independent growth, PD-0332991 treatment reduced growth and P-Rb levels in 6/6 parental and 2/2 LTED ER+ cell lines in estrogen-depleted conditions (Fig. 4CD). These findings suggest that CDK4/6-mediated phosphorylation of Rb (and E2F activation) is required for both hormone-independent and ER-independent growth of ER+ cells.
Fig. 4.
CDK4 is required for the hormone-independent growth of ER+ breast cancer cells. A) MCF-7 and MCF-7/LTED cells transiently transfected with a siRNA library targeting 779 kinases were reseeded in 10% DCC-FBS. Cell viability was measured 4–5 days later. Cell growth for each kinase siRNA relative to non-silencing controls (siCtl) was transformed to a Z-score, and the median Z-score across 3–4 independent experiments was calculated. B) Cells transfected with an independent siRNA targeting CDK4 or siCtl were treated with 10% DCC-FBS ± 1 μM fulvestrant. Media and drugs were replenished every 2–3 days. Adherent cells were counted after 6–9 days. Data are presented as % parental siCtl/no hormone, mean of triplicates ± SD. We were unable to achieve CDK4 knockdown in HCC-1428 lines. C) Cells were treated with 10% DCC-FBS ± fulvestrant or 0.2 μM PD-0332991. Adherent cells were counted after 6–16 days. Data are presented as % parental control, mean of triplicates ± SD. *p<0.05 by Bonferroni post-hoc test compared to control [or siCtl/no hormone in (B)] for each cell line. D) Immunoblots of lysates from cell treated as in (C) × 24 h. E) Mice bearing established MCF-7 xenografts were randomized to the indicated treatments. Mean tumor volumes ± SEM are shown. *p<0.05 by general linear model compared to vehicle control at the indicated time point. F) Immunoblot analysis of lysates from tumors from (E) using the indicated antibodies. G) IHC scoring for phospho-Histone H3Ser10 and cleaved caspase-3/7 was performed on tumors from (E). *p<0.05 by Bonferroni post-hoc test. ns-not significant.
To validate these findings in vivo, we established MCF-7 xenografts in athymic ovariectomized female mice supplemented with a 14-day-release E2 pellet. Fifteen to twenty-six days after implantation, tumor-bearing mice were randomized to treatment with vehicle, PD-0332991, or fulvestrant. Both therapies significantly inhibited estrogen-independent tumor growth compared to vehicle (Fig. 4E). Biomarkers of response were assessed by immunoblot and IHC after five weeks of therapy. Fulvestrant decreased ER levels, and both agents decreased P-Rb, FANCD2, p107 (RBL1), LAP2 (TMPO), E2F1, and E2F2 (Fig. 4F). FANCD2, RBL1, and TMPO contain proximal E2F motifs and are part of the 24-gene E2F signature (Fig. S7). These data imply that fulvestrant reduces estrogen-independent, ER-regulated E2F activity in vivo. While both drugs decreased tumor cell proliferation (assessed by P-Histone H3Ser10 IHC), PD-0332991 also induced apoptosis [assessed by IHC using an antibody for cleaved caspase-3 which may cross-react with cleaved caspase-7 (37), Figs. 4G, S9].
Combined targeting of ER and PI3K induces regression of ER+ xenografts
We previously reported that in LTED cells, hyperactivation of the PI3K pathway is required for estrogen-independent growth (4). Crosstalk between the PI3K and ER pathways has been suggested as a mechanism of endocrine resistance (38). PI3K activation was shown to induce ER phosphorylation at Ser167 and estrogen-independent transcriptional activity (39, 40); thus, we examined whether PI3K activates ER in LTED cells. While we detected higher levels of PERSer167 in MCF-7/LTED and HCC-1428/LTED cells compared to parental controls in hormone-depleted conditions, treatment with the PI3K inhibitor BKM120 (41) or the TORC1 inhibitor RAD001 (42) only modestly increased and decreased P-ERSer167 levels in MCF-7/LTED and HCC-1428/LTED cells, respectively (Fig. S10A). Treatment with BKM120 did not alter ER-DNA binding (Fig. S10B) or ER transcriptional reporter activities (data not shown). Conversely, fulvestrant did not alter P-AKT levels (Fig. S10C), suggesting that ER does not activate PI3K under hormone-depleted conditions. In addition, estrogen deprivation has been shown to induce synthetic lethality in ER+ breast cancer cells treated with a PI3K inhibitor or a siRNA targeting PI3K (5). These data collectively suggest that PI3K and ER modulate non-overlapping pathways in the absence of estrogens. We thus reasoned that combined inhibition of both pathways may be synergistic.
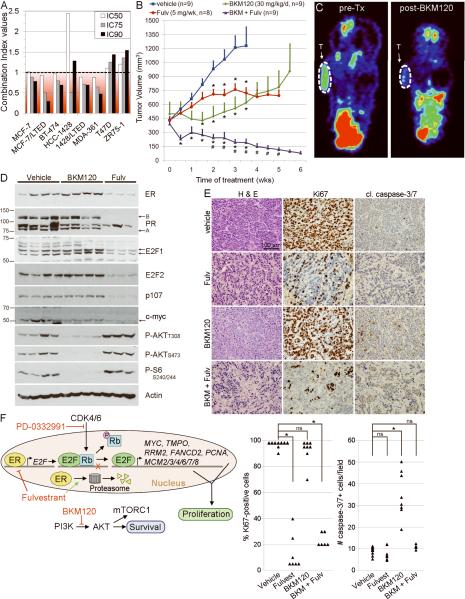
Analysis of the effects of BKM120 and fulvestrant on hormone-independent cell growth showed synergy in 6/8 ER+ lines (Figs. 5A, S11). To assess whether these drugs inhibit estrogen-independent growth, mice bearing MCF-7 xenografts (as in Fig. 4E) were randomized to vehicle, BKM120, fulvestrant, or the combination. While the single-agent therapies significantly inhibited tumor growth compared to vehicle, the drug combination induced near-complete tumor regression and was significantly more effective than each single agent (Fig. 5B). We used [18F]FDG-PET as a biomarker of PI3K/AKT inhibition. Mice were imaged before and after nine days of treatment. BKM120 but not fulvestrant significantly decreased tumor [18F]FDG uptake compared to baseline (p=0.013; Figs. 5C, S12). We also observed a significant correlation between the percent change in tumor [18F]FDG uptake and the percent change in tumor volume after 9–10 days of treatment (r=0.805, p=0.0028), suggesting that a reduction in FDG uptake correlates with early tumor response.
Fig. 5.
Combined downregulation of ER and inhibition of PI3K induces regression of ER+ xenografts. A) Cells were treated with 10% DCC-FBS ± fulvestrant, BKM120, or both. Combination index (CI) values were calculated using the Median Effect method at the IC50, IC75, and IC90 for both drugs. CI<1 is indicative of synergy. B) Mice bearing MCF-7 tumors were randomized to the indicated treatments. Mean tumor volumes ± SEM are shown. *p<0.05 by general linear model compared to vehicle control at the indicated time point.#p<0.05 compared to both single-agent treatment arms. C) Tumor-bearing mice were imaged before and after nine days of treatment by [18F]FDG-PET. Representative images show [18F]FDG uptake pre- and post-BKM120 (T- tumor). Quantified in Fig. S12. D) Immunoblot analysis of lysates of tumors from (B) using the indicated antibodies. E) H&E staining and IHC for Ki67 and cleaved caspase-3/7 of tumors from (B). Quantification is shown below. *p<0.001 by Bonferroni post-hoc test. ns- not significant. F) Model depicting the synergistic effects of fulvestrant and BKM120.
Molecular analyses of tumor lysates showed that fulvestrant decreased ER levels, whereas BKM120 decreased P-AKT and P-S6 (Fig. 5D). Fulvestrant also decreased the levels of PR, E2F1, E2F2, p107 (RBL1), and c-myc (MYC), while BKM120 did not. Of note, RBL1 and MYC contain proximal E2F binding motifs and are downregulated by fulvestrant in LTED cells. These data imply that ER and PI3K signal independently in estrogen-depleted conditions, and that ER but not PI3K modulates E2F activity.
Analysis of tumors treated with BKM120 plus fulvestrant for >6 weeks revealed decreased tumor cell density and increased fibrosis (Fig. 5E, H&E). BKM120-treated tumors showed an increase in cleaved caspase-3/7+ tumor cells compared to controls but no change in Ki67+ tumor cells, suggesting that PI3K inhibition predominantly induced apoptosis. In contrast, tumors treated with fulvestrant and the combination showed a reduction in Ki67+ cells but no change in cleaved-caspase-3/7+ cells. Whether fulvestrant inhibited the pro-apoptotic effect of BKM120 by blocking tumor cell proliferation, and/or there was an early wave of cell death not captured by the time of tumor collection is unclear. However, the marked drug synergy observed and the late timing of this assessment in small residual tumors does not support the former speculation. Thus, the combination of fulvestrant and BKM120 likely induced tumor regression by both inhibition of tumor cell proliferation and induction of apoptosis (Fig. 5F).
Discussion
We identified a role for ER in the acquired hormone-independent growth of human breast cancer cells. Fulvestrant treatment suppressed the emergence of hormone-independent cells from 3/6 ER+ breast cancer lines. Gene expression profiling revealed that ER was required for the estrogen-independent modulation of an E2F transcriptional program in “ER-dependent” but not in “ER-independent” cells. A kinome-wide siRNA screen showed that CDK4, which derepresses E2F signaling, is required for hormone-independent growth. Expression of an LTED-derived E2F activation signature in primary breast cancers following short-term therapy with an AI correlated with high levels of Ki67, a predictive biomarker of worse treatment outcome, suggesting that estrogen-independent E2F signaling may confer resistance to estrogen deprivation. Finally, fulvestrant synergized with a PI3K inhibitor to suppress hormone-independent growth in vitro and induce marked tumor regressions in vivo.
In parallel to the role of ER in estrogen-independent breast cancer cell growth, androgen receptor (AR) has been implicated in androgen-independent prostate cancer growth. AR is analogous to ER and conventionally acts as a ligand-inducible transcription factor. The majority of prostate cancers are androgen-dependent at the time of diagnosis as most patients initially respond to androgen-ablation therapy. However, recurrent cancers are typically androgen (ligand)-independent and AR+ (43). AR gene amplification has been observed in some recurrent prostate cancers following androgen deprivation therapy (44). Wang et al. reported that ligand-independent AR upregulates M-phase cell cycle genes in androgen-independent prostate cancer cells (45). Similarly, we observed that gene sets downregulated following fulvestrant-induced ER downregulation were significantly enriched for cell cycle genes, suggesting that some breast cancers utilize ER in a ligand-independent fashion to drive cell proliferation. We should note that ESR1 (ER) amplification has also been detected in primary breast cancers (46); however, its association with disease outcome remains unclear.
The involvement of ER in estrogen-independent breast cancer progression is being assessed in a randomized clinical trial [SoFEA (47)] in postmenopausal patients with advanced ER+ breast cancer who progressed on an AI. This trial compares treatment with fulvestrant (250 mg), an alternative AI, or the combination. If this study were to be enriched with patients harboring tumors that remain ER-dependent under conditions of estrogen suppression, we predict that the combination arm will show the best clinical outcome. While high-dose fulvestrant (500 mg) induces only partial ER downregulation (48), a 500-mg dose was shown to increase time-to-progression more effectively than a 250-mg dose in patients with advanced ER+ breast cancer (49). Therefore, the optimal therapeutic dose of fulvestrant remains undefined, and there is a need for discovery of strong ER downregulators to completely neutralize ER activity in breast cancers that adapt to estrogen deprivation but continue to utilize ER for growth.
Data shown herein suggest that some ER+ cells utilize the unliganded ER to drive an E2F transcriptional program and cell cycle progression, while other ER+ cells utilize ER-independent means. In effect, the latter cells behave as “ER-negative” under estrogen-depleted conditions. We therefore predict that, in patients treated with AIs, only a fraction of estrogen-independent cancers will continue to rely on the ER for growth and, thus, will respond clinically to therapeutic downregulation of the ER. At this time, short of testing an ER downregulator and/or analyzing biopsies of drug-resistant recurrent metastases to assess ER levels and activity, there is no clinical assay that would infer continued reliance on ER in tumors that progress in patients where estrogen production is suppressed. In this study, a gene expression signature of E2F activation in ER+ tumors after short-term estrogen deprivation with an AI correlated with high post-AI tumor cell proliferation (Fig. 3). This suggests that an E2F activation signature may identify those ER+ tumors that are resistant to estrogen deprivation but may respond to an ER downregulator or E2F/Rb-directed therapy.
Estrogen-independent, ER-dependent E2F transcription may promote endocrine resistance but this can be abrogated by an ER downregulator (Figs. 2–3). In contrast, both hormone-independent and ER-independent growth of all ER+ cells was suppressed by CDK4/6 inhibition (Fig. 4). Therefore, we speculate that a CDK4/6 inhibitor may be more broadly applicable than an ER downregulator for the treatment of breast cancers progressing after an AI. The benefit of CDK4/6-directed therapy is being tested in a clinical trial where patients with advanced ER+ breast cancer are randomized to treatment with letrozole ± PD-0332991 (50).
We previously reported that LTED cells exhibit hyperactivation of the PI3K pathway, which is required for estrogen-independent growth (4). Despite reports suggestive of crosstalk between PI3K and ER signaling (39, 40), we were unable to detect such interactions in LTED cells, implying that both ER and PI3K signaling are required for hormone-independent growth. Therefore, we examined the effects of combined inhibition of ER and PI3K in ER+ xenografts. While single-agent therapies targeting these pathways slowed tumor growth, the combination of fulvestrant and BKM120 induced regression of established tumors in mice devoid of estrogen supplementation. These data collectively suggest that ER and PI3K modulate independent pathways critical for tumor maintenance, growth, and resistance to estrogen deprivation in ER+ tumors. Hence, in patients with ER+ breast cancer which progresses on AI therapy, particularly in those that harbor an E2F activation signature, combined treatment with an ER downregulator (or a CDK4 inhibitor) and a PI3K inhibitor is a strategy worthy of clinical investigation.
Methods
Cell lines
Parental lines (ATCC) were maintained in IMEM/10% FBS (Gibco), and were authenticated on March 15, 2011 by short tandem repeat profiling using Sanger sequencing. LTED cells were generated upon long-term culture in phenol red-free IMEM/10% DCC-FBS (Hyclone) as described (4).
Cell proliferation assays
Cells were treated with 10% DCC-FBS ± E2, 4-OH-T, fulvestrant, or PD-0332991 (gift from Pfizer) for 5–10 days before being trypsinized and counted using a Coulter counter, or fixed and stained with crystal violet. In siRNA transfection experiments, cells were reverse-transfected, then reseeded and treated as above.
Statistical analyses
In the above assays, significant differences (p<0.05) were determined by ANOVA and Bonferroni post-hoc tests (multiple testing-corrected).
Gene expression analyses
MCF-7/LTED and HCC-1428/LTED cells were treated ± 1 μM fulvestrant for 48 h. RNA was analyzed using gene expression arrays (GEO #GSE22533). Breast tumor gene expression data were obtained from GEO (GSE5462) and Array Express (E-MTAB-520).
Chromatin immunoprecipitation
ChIP was performed using MCF-7/LTED and HCC-1428/LTED cells, and primary human breast tumors. ChIP DNA was analyzed by high-throughput sequencing (GEO #GSE27300) and real-time PCR.
Reverse-phase protein array analysis (RPPA)
Pre- and post-treatment primary tumor biopsies were obtained from 10 post-menopausal patients with operable ER+/HER2-negative breast cancer treated with neoadjuvant letrozole (2.5 mg/d) for 10–21 days. The study was approved by the Vanderbilt University IRB (NCT00651976). Post-letrozole tumor lysates were analyzed by RPPA using antibodies against FANCD2 (Abcam) and CDK1 (Calbiochem).
siRNA library screen
MCF-7 and MCF-7/LTED cells were screened using the Dharmacon RTF Protein Kinase siRNA library.
Mouse xenograft experiments were approved by the Vanderbilt IACUC. Ovariectomized female athymic mice were implanted subcutaneously with a 14-day-release E2 pellet (0.17 mg; Innovative Research of America) and 0.5–1×107 MCF-7 cells. After 15–26 days, mice bearing tumors >100 mm3 were randomized to vehicle, PD-0332991 (100 mg/kg/d), or fulvestrant (5 mg/wk). In a separate experiment, tumor-bearing mice received vehicle, BKM120 (30 mg/kg/d), fulvestrant, or BKM120/fulvestrant. Tumor diameters were measured twice per week. General linear modeling was used to evaluate differences between treatment groups at each time point. Tumors were harvested and snap-frozen in liquid N2 or fixed in 10% formalin prior to paraffin embedding and IHC.
[18F]FDG-PET imaging was performed before and 9 days after treatment with vehicle, BKM120 (60 mg/kg/d), or fulvestrant (5 mg on days 0 and 7).
Supplementary Material
Statement of significance.
ERα retains genomic activity and drives a CDK4/E2F-dependent transcriptional program despite estrogen deprivation therapy. Combined inhibition of ER and PI3K induced regression of ER+ xenografts, supporting further development of strong ER downregulators and CDK4 inhibitors, and their combination with PI3K inhibitors for the treatment of antiestrogen-resistant breast cancers.
Acknowledgments
Financial Support: This work was supported by NIH F32CA121900 (TWM), K99CA142899 (TWM), Breast Cancer Specialized Program of Research Excellence (SPORE) grant P50CA98131, Vanderbilt-Ingram Cancer Center Support Grant P30CA68485, U24CA126588 (South-Eastern Center for Small-Animal Imaging), R01CA140628 (HCM), RC1CA145138 (HCM), K25CA127349 (HCM); a Vanderbilt Institute for Clinical and Translational Research grant (TWM); a grant from the Breast Cancer Research Foundation (CLA); ACS Clinical Research Professorship Grant CRP-07-234 (CLA); the Lee Jeans Translational Breast Cancer Research Program (CLA); Stand Up to Cancer/AACR Dream Team Translational Cancer Research Grant SU2C-AACR-DT0209 (CLA,GBM).
Footnotes
Conflicts of interest: M.D. receives research grant funds and has received honoraria for consulting and advisory board work from AstraZeneca and Novartis. S.-M.M. is an employee of Novartis.
Further details are provided in Supplemental Experimental Procedures.
References
- 1.Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 2.De Laurentiis M, Arpino G, Massarelli E, Ruggiero A, Carlomagno C, Ciardiello F, et al. A meta-analysis on the interaction between HER-2 expression and response to endocrine treatment in advanced breast cancer. Clin Cancer Res. 2005;11:4741–8. doi: 10.1158/1078-0432.CCR-04-2569. [DOI] [PubMed] [Google Scholar]
- 3.Ellis MJ, Tao Y, Young O, White S, Proia AD, Murray J, et al. Estrogen-independent proliferation is present in estrogen-receptor HER2-positive primary breast cancer after neoadjuvant letrozole. J Clin Oncol. 2006;24:3019–25. doi: 10.1200/JCO.2005.04.3034. [DOI] [PubMed] [Google Scholar]
- 4.Miller TW, Hennessy BT, Gonzalez-Angulo AM, Fox EM, Mills GB, Chen H, et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Invest. 2010;120:2406–13. doi: 10.1172/JCI41680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowder RJ, Phommaly C, Tao Y, Hoog J, Luo J, Perou CM, et al. PIK3CA and PIK3CB inhibition produce synthetic lethality when combined with estrogen deprivation in estrogen receptor-positive breast cancer. Cancer Res. 2009;69:3955–62. doi: 10.1158/0008-5472.CAN-08-4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, et al. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96:926–35. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 7.Robertson JF, Llombart-Cussac A, Rolski J, Feltl D, Dewar J, Macpherson E, et al. Activity of fulvestrant 500 mg versus anastrozole 1 mg as first-line treatment for advanced breast cancer: results from the FIRST study. J Clin Oncol. 2009;27:4530–5. doi: 10.1200/JCO.2008.21.1136. [DOI] [PubMed] [Google Scholar]
- 8.Perey L, Paridaens R, Hawle H, Zaman K, Nole F, Wildiers H, et al. Clinical benefit of fulvestrant in postmenopausal women with advanced breast cancer and primary or acquired resistance to aromatase inhibitors: final results of phase II Swiss Group for Clinical Cancer Research Trial (SAKK 21/00) Ann Oncol. 2007;18:64–9. doi: 10.1093/annonc/mdl341. [DOI] [PubMed] [Google Scholar]
- 9.Ingle JN, Suman VJ, Rowland KM, Mirchandani D, Bernath AM, Camoriano JK, et al. Fulvestrant in women with advanced breast cancer after progression on prior aromatase inhibitor therapy: North Central Cancer Treatment Group Trial N0032. J Clin Oncol. 2006;24:1052–6. doi: 10.1200/JCO.2005.04.1053. [DOI] [PubMed] [Google Scholar]
- 10.Jelovac D, Macedo L, Goloubeva OG, Handratta V, Brodie AM. Additive antitumor effect of aromatase inhibitor letrozole and antiestrogen fulvestrant in a postmenopausal breast cancer model. Cancer Res. 2005;65:5439–44. doi: 10.1158/0008-5472.CAN-04-2782. [DOI] [PubMed] [Google Scholar]
- 11.Chan CM, Martin LA, Johnston SR, Ali S, Dowsett M. Molecular changes associated with the acquisition of oestrogen hypersensitivity in MCF-7 breast cancer cells on long-term oestrogen deprivation. J Steroid Biochem Mol Biol. 2002;81:333–41. doi: 10.1016/s0960-0760(02)00074-2. [DOI] [PubMed] [Google Scholar]
- 12.Dixon JM, Renshaw L, Young O, Murray J, Macaskill EJ, McHugh M, et al. Letrozole suppresses plasma estradiol and estrone sulphate more completely than anastrozole in postmenopausal women with breast cancer. J Clin Oncol. 2008;26:1671–6. doi: 10.1200/JCO.2007.13.9279. [DOI] [PubMed] [Google Scholar]
- 13.Bourdeau V, Deschenes J, Laperriere D, Aid M, White JH, Mader S. Mechanisms of primary and secondary estrogen target gene regulation in breast cancer cells. Nucleic Acids Res. 2008;36:76–93. doi: 10.1093/nar/gkm945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, et al. Genome-wide analysis of estrogen receptor binding sites. Nat Genet. 2006;38:1289–97. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- 15.Lamb J, Crawford ED, Peck D, Modell JW, Blat IC, Wrobel MJ, et al. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science. 2006;313:1929–35. doi: 10.1126/science.1132939. [DOI] [PubMed] [Google Scholar]
- 16.Lupien M, Meyer CA, Bailey ST, Eeckhoute J, Cook J, Westerling T, et al. Growth factor stimulation induces a distinct ER(alpha) cistrome underlying breast cancer endocrine resistance. Genes Dev. 2010;24:2219–27. doi: 10.1101/gad.1944810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fullwood MJ, Liu MH, Pan YF, Liu J, Xu H, Mohamed YB, et al. An oestrogen-receptor-alpha-bound human chromatin interactome. Nature. 2009;462:58–64. doi: 10.1038/nature08497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurtado A, Holmes KA, Ross-Innes CS, Schmidt D, Carroll JS. FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nat Genet. 2011;43:27–33. doi: 10.1038/ng.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross-Innes CS, Stark R, Holmes KA, Schmidt D, Spyrou C, Russell R, et al. Cooperative interaction between retinoic acid receptor-alpha and estrogen receptor in breast cancer. Genes Dev. 2010;24:171–82. doi: 10.1101/gad.552910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hua S, Kittler R, White KP. Genomic antagonism between retinoic acid and estrogen signaling in breast cancer. Cell. 2009;137:1259–71. doi: 10.1016/j.cell.2009.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stein B, Yang MX. Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-kappa B and C/EBP beta. Mol Cell Biol. 1995;15:4971–9. doi: 10.1128/mcb.15.9.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bajetta E, Zilembo N, Dowsett M, Guillevin L, Di Leo A, Celio L, et al. Double-blind, randomised, multicentre endocrine trial comparing two letrozole doses, in postmenopausal breast cancer patients. Eur J Cancer. 1999;35:208–13. doi: 10.1016/s0959-8049(98)00392-x. [DOI] [PubMed] [Google Scholar]
- 23.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–9. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bieda M, Xu X, Singer MA, Green R, Farnham PJ. Unbiased location analysis of E2F1-binding sites suggests a widespread role for E2F1 in the human genome. Genome Res. 2006;16:595–605. doi: 10.1101/gr.4887606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knudsen ES, Knudsen KE. Tailoring to RB: tumour suppressor status and therapeutic response. Nat Rev Cancer. 2008;8:714–24. doi: 10.1038/nrc2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang W, Dong L, Saville B, Safe S. Transcriptional activation of E2F1 gene expression by 17beta-estradiol in MCF-7 cells is regulated by NF-Y-Sp1/estrogen receptor interactions. Mol Endocrinol. 1999;13:1373–87. doi: 10.1210/mend.13.8.0323. [DOI] [PubMed] [Google Scholar]
- 27.Foster JS, Wimalasena J. Estrogen regulates activity of cyclin-dependent kinases and retinoblastoma protein phosphorylation in breast cancer cells. Mol Endocrinol. 1996;10:488–98. doi: 10.1210/mend.10.5.8732680. [DOI] [PubMed] [Google Scholar]
- 28.Ghazoui Z, Buffa FM, Dunbier AK, Anderson H, Dexter T, Detre S, et al. Close and stable relationship between proliferation and a hypoxia metagene in aromatase inhibitor treated ER-positive breast cancer. Clin Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-10-1704. [DOI] [PubMed] [Google Scholar]
- 29.Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, A'Hern R, et al. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst. 2007;99:167–70. doi: 10.1093/jnci/djk020. [DOI] [PubMed] [Google Scholar]
- 30.Tan PH, Bay BH, Yip G, Selvarajan S, Tan P, Wu J, et al. Immunohistochemical detection of Ki67 in breast cancer correlates with transcriptional regulation of genes related to apoptosis and cell death. Mod Pathol. 2005;18:374–81. doi: 10.1038/modpathol.3800254. [DOI] [PubMed] [Google Scholar]
- 31.Miller WR, Larionov AA, Renshaw L, Anderson TJ, White S, Murray J, et al. Changes in breast cancer transcriptional profiles after treatment with the aromatase inhibitor, letrozole. Pharmacogenet Genomics. 2007;17:813–26. doi: 10.1097/FPC.0b013e32820b853a. [DOI] [PubMed] [Google Scholar]
- 32.Dalton S. Cell cycle regulation of the human cdc2 gene. EMBO J. 1992;11:1797–804. doi: 10.1002/j.1460-2075.1992.tb05231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoskins EE, Gunawardena RW, Habash KB, Wise-Draper TM, Jansen M, Knudsen ES, et al. Coordinate regulation of Fanconi anemia gene expression occurs through the Rb/E2F pathway. Oncogene. 2008;27:4798–808. doi: 10.1038/onc.2008.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, Trachet E, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3:1427–38. [PubMed] [Google Scholar]
- 35.Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11:R77. doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thangavel C, Dean JL, Ertel A, Knudsen KE, Aldaz CM, Witkiewicz AK, et al. Therapeutically activating RB: reestablishing cell cycle control in endocrine therapy-resistant breast cancer. Endocr Relat Cancer. 2011;18:333–45. doi: 10.1530/ERC-10-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arpino G, Gutierrez C, Weiss H, Rimawi M, Massarweh S, Bharwani L, et al. Treatment of human epidermal growth factor receptor 2-overexpressing breast cancer xenografts with multiagent HER-targeted therapy. J Natl Cancer Inst. 2007;99:694–705. doi: 10.1093/jnci/djk151. [DOI] [PubMed] [Google Scholar]
- 38.Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009;9:631–43. doi: 10.1038/nrc2713. [DOI] [PubMed] [Google Scholar]
- 39.Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem. 2001;276:9817–24. doi: 10.1074/jbc.M010840200. [DOI] [PubMed] [Google Scholar]
- 40.Yamnik RL, Digilova A, Davis DC, Brodt ZN, Murphy CJ, Holz MK. S6 kinase 1 regulates estrogen receptor alpha in control of breast cancer cell proliferation. J Biol Chem. 2009;284:6361–9. doi: 10.1074/jbc.M807532200. [DOI] [PubMed] [Google Scholar]
- 41.Voliva CF, Pecchi S, Burger M, Nagel T, Schnell C, Fritsch C, Brachmann S, Menezes D, Knapp M, Shoemaker K, Wiesmann M, Huh K, Zaror I, Dorsch M, Sellers WR, Garcia-Echeverria C, Maira M. Biological characterization of NVP-BKM120, a novel inhibitor of phosphoinosotide 3-kinase in Phase I/II clinical trials. American Association for Cancer Research Annual Meeting: Proceedings; 2010. Abstract 4498. [Google Scholar]
- 42.Sedrani R, Cottens S, Kallen J, Schuler W. Chemical modification of rapamycin: the discovery of SDZ RAD. Transplant Proc. 1998;30:2192–4. doi: 10.1016/s0041-1345(98)00587-9. [DOI] [PubMed] [Google Scholar]
- 43.Debes JD, Tindall DJ. Mechanisms of androgen-refractory prostate cancer. N Engl J Med. 2004;351:1488–90. doi: 10.1056/NEJMp048178. [DOI] [PubMed] [Google Scholar]
- 44.Visakorpi T, Hyytinen E, Koivisto P, Tanner M, Keinanen R, Palmberg C, et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9:401–6. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- 45.Wang Q, Li W, Zhang Y, Yuan X, Xu K, Yu J, et al. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell. 2009;138:245–56. doi: 10.1016/j.cell.2009.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holst F, Stahl PR, Ruiz C, Hellwinkel O, Jehan Z, Wendland M, et al. Estrogen receptor alpha (ESR1) gene amplification is frequent in breast cancer. Nat Genet. 2007;39:655–60. doi: 10.1038/ng2006. [DOI] [PubMed] [Google Scholar]
- 47.Dodwell D, Coombes G, Bliss JM, Kilburn LS, Johnston S. Combining fulvestrant (Faslodex) with continued oestrogen suppression in endocrine-sensitive advanced breast cancer: the SoFEA trial. Clin Oncol (R Coll Radiol) 2008;20:321–4. doi: 10.1016/j.clon.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 48.Robertson JFR, Dixon JM, Sibbering DM, Jahan A, Ellis IO, Channon EJ, Nicholson RI, Gee JMW. Tumor Biomarker Changes Following Pre-Surgical Treatment with 500 mg Fulvestrant Plus Anastrozole Versus 500 mg Fulvestrant Alone and 1 mg Anastrozole Alone. Cancer Res. 2009;69 abstract 24. [Google Scholar]
- 49.Di Leo A, Jerusalem G, Petruzelka L, Torres R, Bondarenko I, Khasanov R, Verhoeven D, Pedrini J, Lichinitser M, Pendergrass K, Garnett S, Lindemann JPO, Sapunar F, Martin M. Results of the CONFIRM Phase III Trial Comparing Fulvestrant 250 mg With Fulvestrant 500 mg in Postmenopausal Women With Estrogen Receptor-Positive Advanced Breast Cancer. J Clin Oncol. 2010 doi: 10.1200/JCO.2010.28.8415. Epub. [DOI] [PubMed] [Google Scholar]
- 50.Finn RS, Boer K, Lang I, Parikh RJ, Patel R, Schmidt M, Hagenstad CT, Lim HJ, Pinter T, Amadori D, Chan D, Dichmann R, Kim ST, Randolph S, Slamon DJ, Crown JP. A randomized phase II study of PD 0332991, cyclin-dependent kinase (CDK) 4/6 inhibitor, in combination with letrozole for first-line treatment of patients with postmenopausal, estrogen receptor (ER)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced breast cancer. J Clin Oncol. 2011;29 abstr. TPS100. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.