Abstract
Despite the initial effectiveness of the tyrosine kinase inhibitor lapatinib against HER2 gene-amplified breast cancers, most patients eventually relapse after treatment, implying that tumors acquire mechanisms of drug resistance. To discover these mechanisms, we generated six lapatinib-resistant HER2-overexpressing human breast cancer cell lines. In cells that grew in the presence of lapatinib, HER2 autophosphorylation was undetectable whereas active PI3K-Akt and MAPK were maintained. To identify networks maintaining these signaling pathways, we profiled the tyrosine phosphoproteome of sensitive and resistant cells using an immunoaffinity-enriched mass spectrometry method. We found increased phosphorylation of Src family kinases (SFK) and putative Src substrates in several resistant cell lines. Treatment of these resistant cells with Src kinase inhibitors partially blocked PI3K-Akt signaling and restored lapatinib sensitivity. Further, SFK mRNA expression was upregulated in primary HER2+ tumors treated with lapatinib. Finally, the combination of lapatinib and the Src inhibitor AZD0530 was more effective than lapatinib alone at inhibiting pAkt and growth of established HER2-positive BT-474 xenografts in athymic mice. These data suggest that increased Src kinase activity is a mechanism of lapatinib resistance and support the combination of HER2 antagonists with Src inhibitors early in the treatment of HER2+ breast cancers in order to prevent or overcome resistance to HER2 inhibitors.
Keywords: breast cancer, HER2, lapatinib, Src kinases, tyrosine phosphorylation
INTRODUCTION
HER2 (ErbB2) is a member of the ErbB family of receptor tyrosine kinases that includes the epidermal growth factor receptor (EGFR, HER1), HER3, and HER4. Dimerization of HER2 with ligand-activated EGFR or HER3 activates signaling for growth, differentiation, and survival through multiple downstream effectors including the phosphoinositide-3 kinase (PI3K)-Akt pathway (Yarden and Sliwkowski 2001). Amplification of the HER2 oncogene occurs in approximately 25% of human breast cancers and confers a poor prognosis but also renders tumors susceptible to HER2-targeted therapies (Moasser 2007). Lapatinib, a small-molecule, ATP-competitive tyrosine kinase inhibitor (TKI) of HER2 (Rusnak et al 2001), is an effective therapy for patients with HER2-overexpressing metastatic breast cancer (Geyer et al 2006). However, most patients treated with lapatinib eventually relapse after treatment, suggesting that tumors acquire or intrinsically possess mechanisms for escape from HER2 inhibition.
In HER2-overexpressing cells, the major mechanism of PI3K activation is heterodimerization with kinase-deficient HER3, which when phosphorylated couples to the p85 regulatory subunit of PI3K (Lee-Hoeflich et al 2008, Yakes et al 2002). Treatment of HER2-overexpressing cells with lapatinib blocks HER3 phosphorylation and uncouples p85 from HER3, thus inhibiting PI3K-Akt (Junttila et al 2009, Ritter et al 2007). Sustained inhibition of HER2/HER3 output to PI3K-Akt has been proposed to be essential for the antitumor effect of HER2 inhibitors. Recently, inhibition of HER2 phosphorylation by the EGFR TKI gefitinib in HER2-overexpressing human breast cancer cells was shown to be followed by feedback upregulation of activated HER3 and Akt, thus limiting the inhibitory effect of gefitinib (Sergina et al 2007). Therapeutic doses of lapatinib are also followed by feedback upregulation of phosphorylated HER3 in HER2-dependent breast cancer cells that is only abrogated by pulsed supra-pharmacological doses (Amin et al 2010). Furthermore, aberrant activation of the PI3K pathway has been associated with resistance to the HER2 inhibitors trastuzumab and lapatinib (Berns et al 2007, Eichhorn et al 2008, Nagata et al 2004, Serra et al 2008, Yakes et al 2002).
Src family kinases are intracellular tyrosine kinases implicated in signal transduction downstream of multiple signaling networks including the ErbB receptors. Src association with HER2 has been shown in human breast cancer cell lines and primary tumors (Belsches-Jablonski et al 2001, Sheffield 1998). The interaction is specific for the HER2 kinase domain (Kim et al 2005, Marcotte et al 2009) and results in enhanced Src kinase activity and protein stability (Luttrell et al 1994, Tan et al 2005, Vadlamudi et al 2003). Interestingly, inhibition of a Src-mediated inhibitory phosphorylation of PTEN has been suggested as part of the antitumor mechanism of trastuzumab (Nagata et al 2004). Because of its involvement in multiple signaling cascades, Src has become an attractive therapeutic target with several Src inhibitors in clinical development (Finn 2008).
We generated lapatinib-resistant derivatives of HER2-overexpressing human breast cancer cell lines. All these lines exhibit HER2 amplification and sensitivity to lapatinib with submicromolar IC50s (Konecny et al 2006). Lapatinib-resistant cells exhibited recovery of PI3K-Akt signaling despite continued inhibition of the HER2 tyrosine kinase. Using a mass spectrometry-based phosphoproteomic approach in BT474 cells, we found upregulation of Src family kinase activity in the resistant cells. This upregulation was observed in 3 of 6 lapatinib resistant cell lines. Treatment of these cells with Src inhibitors arrested cell proliferation, partially blocked PI3K-Akt signaling, and reversed lapatinib resistance in these cells. Treatment of HER2-positive xenografts with the combination of lapatinib and a small molecule inhibitor of Src was more effective than either drug alone. Together these data support Src activation as a mechanism of lapatinib resistance, and suggest the combination of HER2 and Src inhibition as a rational therapeutic strategy to prevent and/or overcome lapatinib resistance in HER2-overexpressing breast cancer.
RESULTS
Lapatinib-resistant breast cancer cell lines show reactivation of PI3K-Akt and MAPK signaling
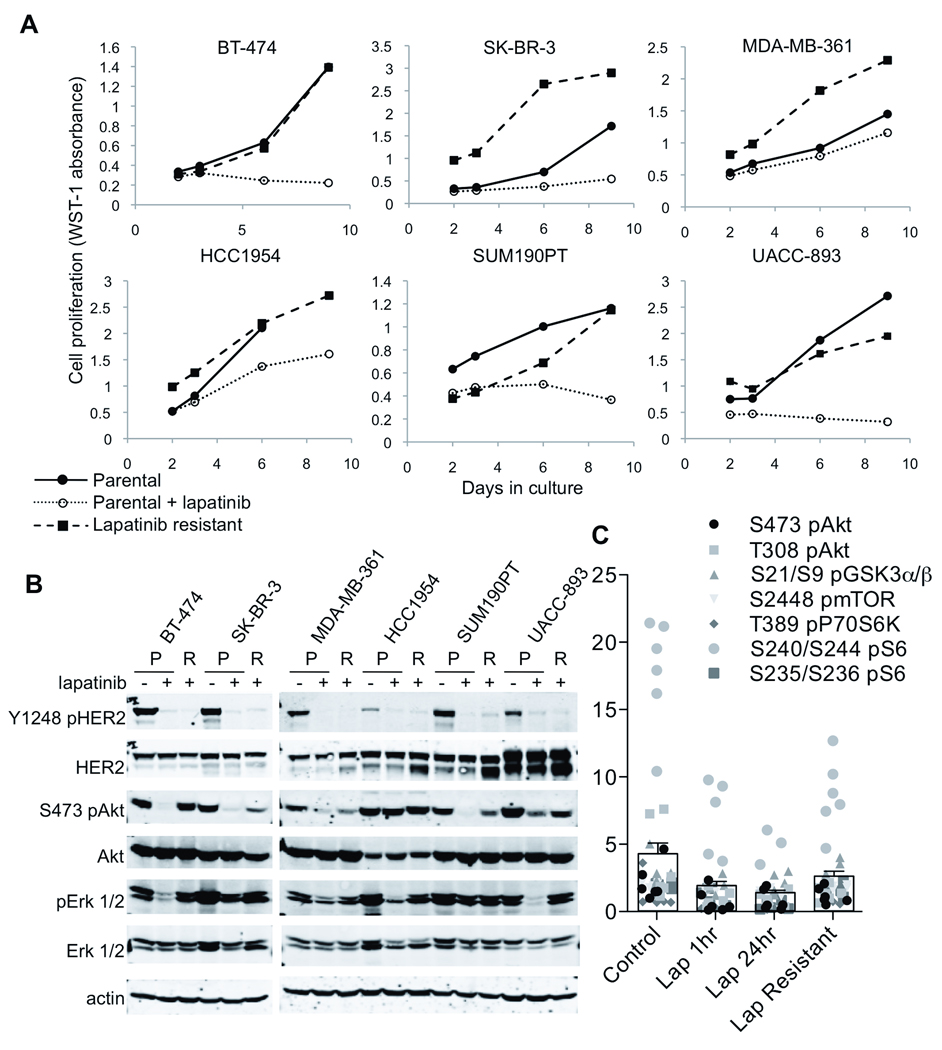
HER2-amplified breast cancer cells (BT-474, SK-BR-3, MDA-MB-361, HCC1954, SUM190PT, and UACC-893) were made drug-resistant by maintenance in gradually increasing concentrations of lapatinib (up 2 µM). Parental cells are highly sensitive with submicromolar IC50 values (Konecny et al 2006), whereas resistant derivatives were maintained at 1 or 2 µM (Figure 1A; 1 µM: SK-BR-3, MDA-MB-361, UACC-893; 2 µM: BT-474, HCC1954, SUM190PT). This concentration is readily achieved in the serum of patients treated with lapatinib (Burris et al 2005). We next investigated activation of HER2 and the downstream PI3K-Akt and MAPK pathways in sensitive and resistant cells by immunoblot. In lapatinib-resistant cells, HER2 Y1248 phosphorylation remained suppressed to levels comparable to lapatinib-treated parental cells. However, despite pHER2 inhibition in resistant cells, PI3K-Akt activity, indicated by S473 pAkt, and Erk activity, indicated by T202/Y204 pErk, were maintained (Figure 1B). The reactivation of these downstream pathways despite continued HER2 inactivation by lapatinib suggested the engagement of alternative compensatory signaling networks to mediate drug resistance.
Figure 1. Lapatinib-resistant cells recover PI3K-Akt and MAPK signaling despite continued HER2 inhibition.
(A) Parental and lapatinib-resistant cells were treated with 1–2 µM lapatinib as follows: 1 µM: SK-BR-3, MDA-MB-361, UACC-893; 2 µM: BT-474, HCC1954, SUM190PT. Cell proliferation was measured by WST-1 assay after 2, 3, 6, and 9 days of treatment. Shown is the mean WST-1 absorbance of 4 replicate wells. (B) Whole cell lysates were prepared from parental cells (P) ± 1 or 2 µM lapatinib or lapatinib-resistant cells (R) maintained in lapatinib as in (A). Lysates were immunoblotted with the indicated antibodies. (C) Lysates were similarly prepared from parental cells after 1 or 24 h lapatinib treatment and from resistant cells and analyzed by RPPA with antibodies to PI3K signaling pathway proteins. The intensity for each protein in each of 6 cell lines is displayed (filled shapes); columns indicate the grand mean of all proteins in all cell lines (bars, S.E.M.). Individual PI3K protein values are in Supplementary Figure 3.
Lapatinib-resistant cells showed levels of HER2 amplification by fluorescence in-situ hybridization comparable to parental lines (Supplementary Figure 1). Reactivation of PI3K-Akt signaling appears causal to lapatinib resistance as all resistant derivatives were sensitive to the PI3K inhibitor BEZ235 (Maira et al 2008) but not to the MEK1/2 inhibitor CI-1040 (Supplementary Figure 2). To identify pathways that could maintain PI3K-Akt signaling, we used reverse-phase protein microarray analysis (RPPA), an approach analogous to high-throughput dot blotting (Tibes et al 2006). We found upregulation of pS6, p70S6K, pmTor, and pGSK3α/β, transducers of PI3K-Akt signaling, in the resistant cells despite continued inhibition of pHER2 (Figure 1C and Supplementary Figure 3).
Global phosphotyrosine profiling identifies upregulation of Src family kinases in lapatinib-resistant cells
To identify upregulated signaling pathways in resistant cells, we used shotgun mass spectrometry (MS) coupled with immunoaffinity enrichment of phosphotyrosine (pTyr)-containing peptides. Mass spectra of phosphopeptides were generated from pTyr pulldowns of tryptic digests of parental ± lapatinib and resistant BT-474 cells. In total, 684 tyrosine phosphopeptide spectra were identified in all three sets of samples. These spectra corresponded to 137 phosphopeptides containing 137 unique phosphotyrosine sites. We focused on pTyr peptides that were more abundant in drug resistant than sensitive cells by filtering for peptides whose spectral counts from resistant cells comprised more than 33% of the total spectral counts recovered from all three sets of samples combined, and for spectra that were obtained more than once from any of the sets of samples. Spectral counting has been shown to correlate with abundance of a peptide species in shotgun proteomics (Old et al 2005, Zhang et al 2006). We found 85 spectra (Table 1, Res column) corresponding to 19 peptides encompassing 20 unique pTyr sites in the resistant cells (Table 1, pTyr site column). These phosphopeptides were mapped to 22 proteins using IDPicker software. Representative spectra for pY877 HER2, pY426 Yes, and pY222 Yes peptides are shown in Figure 2A and Supplementary Figure 4.
Table 1. Tyrosine phosphopeptides identified predominantly in lapatinib-resistant cells by spectral counts.
Phosphotyrosine peptides whose spectra were obtained more than once and more frequently (>33% of the total) from lapatinib-resistant cells are shown. Counts were obtained from 5 replicates for each of 3 types of specimens: parental BT-474 cells ± lapatinib and drug-resistant cells.
| Par | |||||||
|---|---|---|---|---|---|---|---|
| Peptide | Symbol | Protein | pTyr site |
Con | Lap | Res | Tot |
| IGEGTYGVVYK | CDC2 | Cell division control protein 2 homolog | Y15 | 7 | 10 | 10 | 27 |
| CDK2 | cyclin-dependent kinase 2 | Y15 | |||||
| CDK3 | cyclin-dependent kinase 3 | Y15 | |||||
| LIEDNEYTAR | YES1 | Proto-oncogene tyrosine-protein kinase Yes | Y426 | 5 | 5 | 10 | 20 |
| FYN | Tyrosine-protein kinase Fyn | Y420 | |||||
| SRC | Proto-oncogene tyrosine-protein kinase Src | Y416 | |||||
| LCK | Proto-oncogene tyrosine-protein kinase Src | Y394 | |||||
| IYQYIQSR | DYRK1A DYRK1B |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A, 1B | Y321 Y273 |
7 | 8 | 8 | 23 |
| LLDIDETEYHADGGKVPIK | ERBB2 | Receptor tyrosine-protein kinase erbB-2 | Y877 | 1 | 2 | 7 | 10 |
| AVCSTYLQSR | HIPK1 HIPK2 |
Homeodomain-interacting protein kinase 1, 2 | Y352 Y361 |
3 | 4 | 6 | 13 |
| NEEENIYSVPHDSTQGK | GRLF1 | Glucocorticoid receptor DNA-binding factor 1 | Y1105 | 5 | 4 | 5 | 14 |
| KLDNGGYYITTR | YES1 | Proto-oncogene tyrosine-protein kinase Yes | Y222 | 0 | 1 | 5 | 6 |
| FYN | Tyrosine-protein kinase Fyn | Y213 | |||||
| SEDIYADPAAYVMR | PLEKHA6 | Pleckstrin homology domain-containing family A member 6 | Y492 | 4 | 4 | 4 | 12 |
| TVCSTYLQSR | HIPK3 | Homeodomain-interacting protein kinase 3 | Y359 | 3 | 5 | 4 | 12 |
| SESVVYADIR | MPZL1 | myelin protein zero-like 1 | Y263 | 2 | 5 | 4 | 11 |
| IEKIGEGTYGVVYK | CDC2 | Cell division control protein 2 homolog | Y15 | 1 | 0 | 5 | 6 |
| (R)SDSASSEPVGIYQGFEK(K) | PRKCD | Protein kinase C, delta | Y313 | 0 | 3 | 4 | 7 |
| HGHYFVALFDYQAR | FRK | Tyrosine-protein kinase FRK | Y46 | 1 | 3 | 2 | 6 |
| IGEGTYGVVYK | CDC2 | Cell division control protein 2 homolog | Y19 | 1 | 2 | 2 | 5 |
| CDK2 | cyclin-dependent kinase 2 | Y19 | |||||
| CDK3 | cyclin-dependent kinase 3 | Y19 | |||||
| KDPDERPTFEYIQSFLEDYFTATEPQYQPGENL | YES1 | Proto-oncogene tyrosine-protein kinase Yes | Y537 | 0 | 1 | 2 | 3 |
| LSSARPGGLGSSSLYGLGASRPR | KRT7 | Keratin, type II cytoskeletal 7 | Y40 | 1 | 0 | 2 | 3 |
| DKVTIADDYSDPFDAKNDLK | SHB | SH2 domain-containing adapter protein B | Y246 | 1 | 1 | 1 | 3 |
| IEIAQKHPDIYAVPIK | TJP2 | Tight junction protein ZO-2 | Y1118 | 1 | 0 | 1 | 2 |
| SAAASNYV | CLDN4 | Claudin-4 | Y208 | 0 | 1 | 1 | 2 |
| Total | 43 | 60 | 85 | 188 | |||
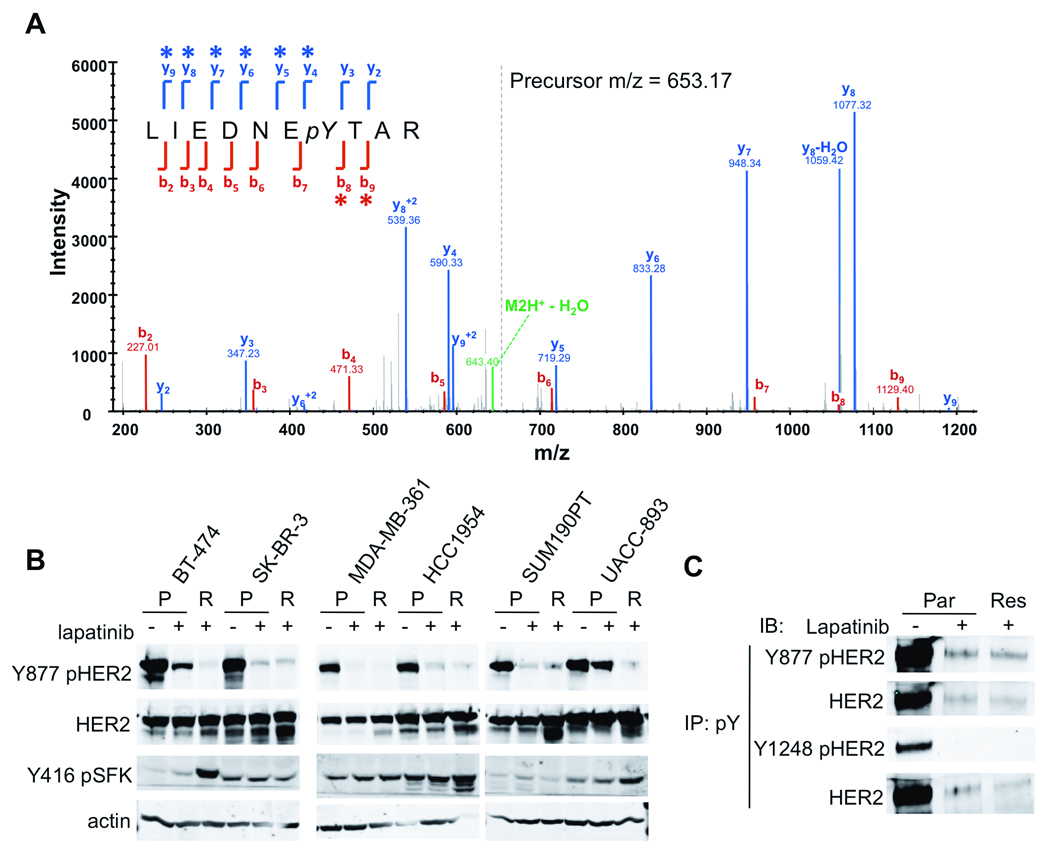
Figure 2. Persistent Y877 HER2 phosphorylation and increased SFK activity in lapatinib-resistant cells.
(A) Representative mass spectrum of the LIEDNEYTAR YES1 tryptic peptide (+2 charge state) containing amino acids 420–429 with a phosphorylation at Y426. This peptide also exists in SRC and FYN. Asterisks denote fragment ions that contain the mass shift as a result of phosphorylation. (B) Lysates of parental cells (P) after 2 h lapatinib treatment or lapatinib-resistant cells (R) were immunoblotted with antibodies as indicated. (C) Lysates from parental cells treated or not with lapatinib, and from resistant cells in the presence of lapatinib were immunoprecipitated with a pTyr antibody. Immune complexes were analyzed by immunoblot with site-specific phosphoantibodies or total HER2 antibody as indicated.
In untreated parental cells, we identified pTyr peptides for several known phosphorylation sites in HER2, EGFR, HER3, and MAPK1/3 (Table 2, Par/Con column). All of these except Y877 HER2 were not recovered or recovered at lower frequency from parental cells treated with lapatinib (Table 2, Par/Lap column), suggesting that Y877 phosphorylation is independent of HER2 tyrosine kinase catalytic activity. Notably, except for the Y877 HER2 peptide, no spectra for HER2 pTyr peptides were recovered from resistant cells (Table 2, Res column), suggesting that HER2 remained inactivated in the resistant cells, consistent with the Y1248 pHER2 immunoblot (Figure 1B).
Table 2. ErbB receptor and MAPK phosphorylation sites identified by MS.
Tyrosine phosphopeptides identified from HER2, EGFR, HER3, and MAPK1/3 are shown. Numbers indicate the spectral counts for each phosphopeptide obtained in total from 5 replicate experiments for each 3 types of specimens: parental BT-474 cells ± lapatinib and drug-resistant cells.
| Par | ||||||
|---|---|---|---|---|---|---|
| Protein | Peptide | pTyr site | Con | Lap | Res | Total |
| HER2 | LLDIDETEYHADGGK(VPIK) | 877 | 5 | 9 | 7 | 21 |
| FVVIQNEDLGPASPLDSTFYR | 1005 | 6 | 0 | 0 | 6 | |
| YSEDPTVPLPSETDGYVAPLTCSPQPEYVNQPDVRPQPPSPR | 1127 | 2 | 0 | 0 | 2 | |
| YSEDPTVPLPSETDGYVAPLTCSPQPEYVNQPDVRPQPPSPR | 1139 | 6 | 2 | 0 | 8 | |
| (GAPPSTFK)GTPTAENPEYLGLDVPV | 1248 | 6 | 4 | 0 | 10 | |
| EGFR | RPAGSVQNPVYHNQPLNPAPSR | 1110 | 1 | 0 | 0 | 1 |
| GSTAENAEYLR | 1197 | 5 | 2 | 0 | 7 | |
| HER3 | HSLLTPVTPLSPPGLEEEDVNGYVMPDTHLK | 1159 | 2 | 0 | 0 | 2 |
| SLEATDSAFDNPDYWHSR | 1328 | 2 | 0 | 0 | 2 | |
| MAPK1 | VADPDHDHTGFLTEYVATR | 187 | 6 | 2 | 2 | 10 |
| MAPK3 | IADPEHDHTGFLTEYVATR | 204 | 7 | 2 | 3 | 12 |
The Src family kinase (SFK) Yes was the protein for which phosphopeptide spectra were most frequently obtained in resistant cells. Seventeen spectra corresponding to three phosphopeptides in Yes (Y426, Y222, and Y537) were observed in resistant cells, more than any other protein (Table 1, rows 2, 7, 15, and Supplementary Table 1). Interestingly, phosphorylation of Y222 in Yes was found predominantly in drug-resistant cells. The homologous site Y216 in Src has been shown to be selectively activated by heregulin and HER2 signaling (Vadlamudi et al 2003). Phosphorylation of Y216 is a potent enhancer of Src kinase activity and can overcome the inhibitory effects of Y527 phosphorylation (Roskoski 2005). These analyses suggested that SFK signaling is associated with acquired resistance to lapatinib.
To identify other signaling pathways associated with escape from lapatinib action, we applied Kinase Enrichment Analysis (KEA) to the 22 phosphoproteins identified in the resistant cells (from Table 1). This approach identifies kinase-substrate interactions by comparing the distribution of kinase substrates occurring in the 22-protein input list to the expected distribution of substrates in databases of known kinase-substrate interactions (Lachmann and Ma'ayan 2009) (amp.pharm.mssm.edu/lib/kea.jsp). KEA ranked the SFKs Lyn and Src as most significantly associated with the 22 phosphoproteins found more abundantly in lapatinib-resistant cells in the initial global phosphoproteomic profiles (p=0.000001, Supplementary Table 2). Notably, four other SFKs, Lck, Fyn, Frk, and Fgr, were also significantly associated with the substrate input list.
Src family kinase expression and phosphorylation is increased in lapatinib-resistant cells
To validate the results of the MS profiling, we analyzed parental, treated, and resistant cell lysates by immunoblot with site-specific phosphoantibodies. Lapatinib treatment largely abolished Y877 pHER2 staining when whole-cell lysates were assayed by immunoblot (Figure 2B). However, after immunoprecipitation with a pTyr antibody, the same ratio of Y877 pHER2/total HER2 was observed in parental cells treated with lapatinib and in resistant cells compared to untreated cells (compare lanes 2 and 3 vs. 1 in Figure 2C, top panels), supporting persistent phosphorylation at this site in cells where the HER2 kinase has been inactivated. Conversely, phosphorylation at Y1248 in the C-terminus, a marker of HER2 kinase-dependent receptor autophosphorylation, was present at baseline but was undetectable in the pTyr pulldowns from lapatinib-treated and drug-resistant cells (Figure 2C, bottom panels). This is consistent with the increase of pY877 HER2 spectral counts (Table 2) using the more sensitive and selective immunoaffinity coupled MS approach.
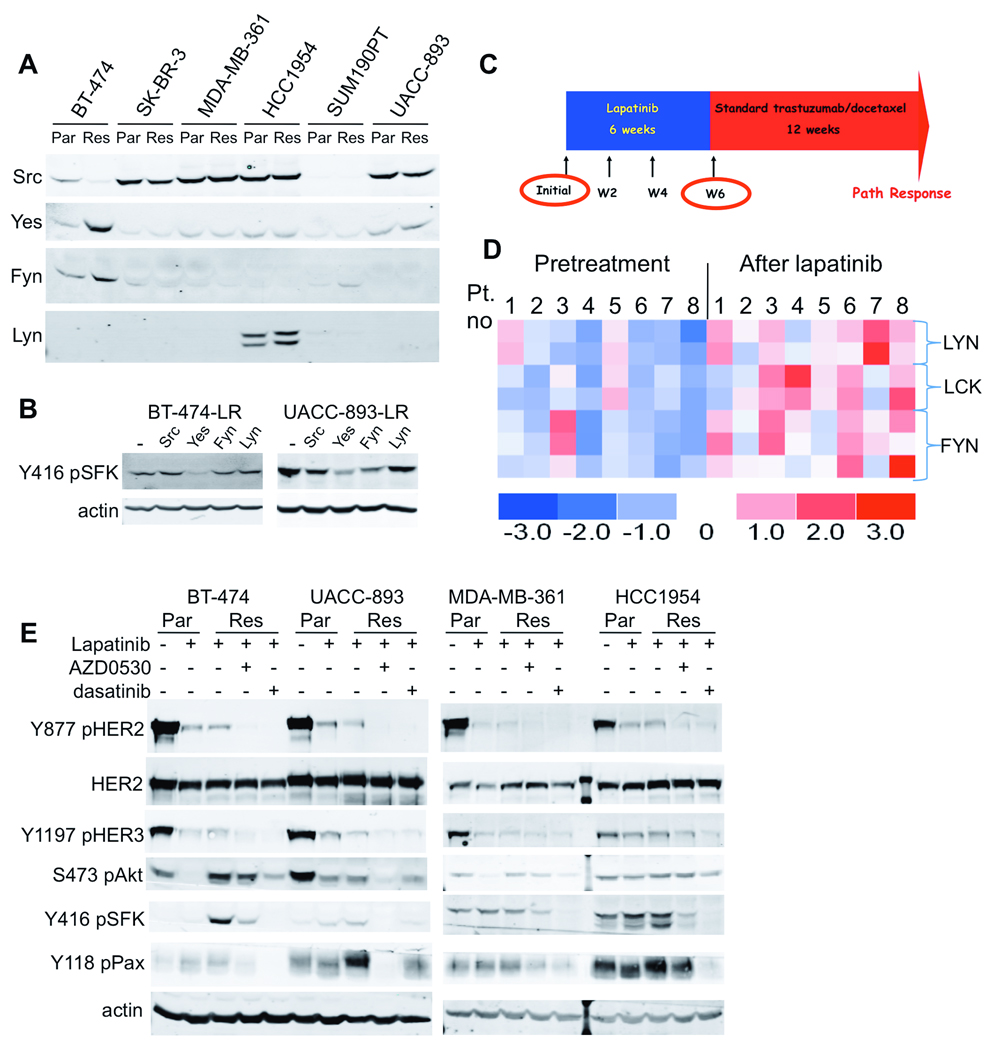
To validate the increase in SFK activity suggested by the kinase enrichment analysis of phosphoproteins in the drug-resistant cells, we immunoblotted cell lysates with an antibody that recognizes Y416 in the activation loop of Src and related SFKs. In three of the lapatinib-resistant cell lines (BT-474, HCC1954, and UACC893), we found increased levels of Y416 pSFK (Figure 2B). One cell line (MDA-MB-361) showed a baseline level of SFK phosphorylation that was modestly increased upon lapatinib treatment, but not further increased in resistant cells. In SKBR3 cells, SFK phosphorylation was present at baseline and did not appear to be affected by lapatinib. In BT-474 cells, global MS pTyr profiling suggested that the upregulated SFK in these cells was Yes (Table 1). However, the most abundant phosphopeptide isolated was LIEDNEpYTAR, which is conserved among Src, Yes, Fyn, Lyn, Lck, and Hck. Using quantitative RT-PCR with primers specific for each kinase, we found that Yes was the predominant SFK in BT-474 and UACC-893 cells while Lyn was most abundant in HCC1954 resistant cells (Supplementary Figure 5). Yes expression was confirmed by immunoblot in BT-474 cells with protein level increased in resistant cells compared to parental cells (Figure 3A). Low levels of Yes were also found in MDA-MB-361, HCC1954, and UACC-893 cells. Src was more ubiquitously expressed in most cell lines tested. Lyn expression was noted only in HCC1954 cells. Interestingly, Yes expression and phosphorylation was increased in resistant vs. parental cells (BT-474 and UACC893), and this was accompanied by a decrease in mRNA level. However, Lyn showed an increased in message level as well as protein expression and phosphorlyation (HCC1954). This highlights the complex regulation of SFK expression and activation that also includes interaction with substrates, phosphatases, and subcellular localization (Finn 2008, Yeatman 2004).
Figure 3. SFK expression is increased in cells and tumors after lapatinib and activity is blocked by SFK inhibitors.
(A) Lysates from parental and resistant cells were immunoblotted with antibodies to Src, Yes, Fyn, and Lyn as indicated. (B) Lysates from BT-474 or UACC-893 cells transfected with siRNA oligonucleotides specific for Src, Yes, Fyn, or Lyn were immunoblotted for Y416 pSFK 72 h after transfection. (C) Schema of the neoadjuvant trial of lapatinib followed by trastuzumab + chemotherapy. Biopsies were obtained pre-treatment and after 6 weeks of lapatinib treatment. (D) Heatmap showing relative expression levels of 7 probesets for Src family kinases (blue = low expression; red = high expression). (E) Lysates from parental (Par) cells ± lapatinib (2 h) and resistant cells (Res) maintained in lapatinib ± the Src inhibitors AZD0530 or dasatinib or (2 h) were analyzed by immunoblot with the indicated antibodies.
To link a particular SFK to the Y416 pSFK band identified by immunoblot, siRNA oligonucleotides for each of the SFKs were transfected into BT-474 and UACC-893 resistant cells and Y416 pSFK assessed by immunoblot. Knockdown of Yes had the more significant inhibitory effect on Y416 pSrc levels in these cells (Figure 3B), further suggesting that Yes the active SFK in lapatinib resistant BT-474 and UACC-893 cells.
Expression of SFKs is increased in primary tumors after treatment with lapatinib
To determine whether lapatinib treatment affected SFK expression in HER2+ cancers, we examined primary tumors from patients with newly diagnosed HER2+ breast cancer treated with lapatinib. Lapatinib was given alone for 6 weeks, before patients were treated with trastuzumab and chemotherapy for 12 weeks prior to surgery (Figure 3C). During the first 6 weeks of lapatinib therapy, tumor volumes overall were decreased (Migliaccio et al 2009). Matched pre- and post-lapatinib treatment biopsies with sufficient tumor material were available from 8 patients for RNA isolation and microarray hybridization to Affymetrix GeneChips. We compared the intensity of expression for probesets corresponding to Src, Yes, Fyn, Lyn, Lck, and Hck before and after lapatinib. We found statistically significant increases in expression of approximately 2-fold (p<0.04) for 7 probesets corresponding to Lyn, Lck, and Fyn (Figure 3D, Supplementary Table 3). Unfortunately, the Y416 pSrc antibody in our hands was inadequate for reliable quantitation of immunohistochemistry in these samples.
Inhibition of SFKs inhibits growth and PI3K-Akt in lapatinib-resistant cells
To determine whether SFK inhibition in drug-resistant cells would restore lapatinib sensitivity, we utilized two small-molecule inhibitors of Src and related kinases: dasatinib and AZD0530. Dasatinib inhibits Src, Lck, and Yes kinases with IC50 of 0.4–0.5 nM (Lombardo et al 2004). AZD0530 inhibits Src, Lck, Yes, Lyn, and Fyn kinases with an IC50 of 2.5–10 nM (Green et al 2009, Hennequin et al 2006). Treatment of lapatinib-resistant cells with either Src inhibitor reduced Y416 pSFK and paxillin phosphorylation (Figure 3E), a downstream target of SFKs that has been evaluated as a biomarker for Src inhibition (Jones et al 2009). Interestingly, there was some cell-line specificity to the relative potency of inhibition of SFKs and downstream targets, with dasatinib being more effective in HCC1954 cells and AZD0530 more effective in UACC-893 cells. Treatment with the Src inhibitors abolished Y877 phosphorylation in the resistant cells, and partially inhibited HER3 phosphorylation. Finally, in four resistant lines, Akt S473 phosphorylation was at least partially inhibited by one of the Src inhibitors in combination with lapatinib. This result suggests that SFK activation at least in part maintains PI3K-Akt in lapatinib-resistant cells.
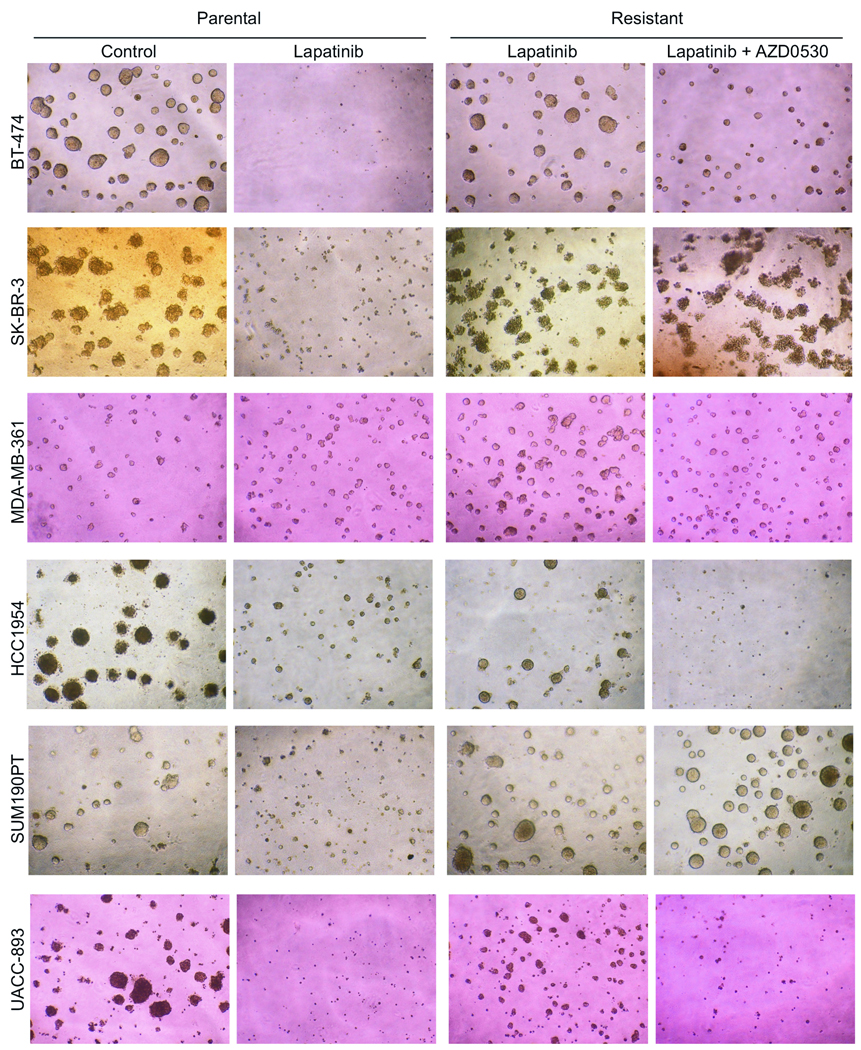
We also tested whether AZD0530 combined with lapatinib would overcome lapatinib resistance in 3D Matrigel growth assays. In the three resistant cell lines with increased SFK activation (BT-474, HCC1954, UACC-893), AZD0530 inhibited 3D acini formation and restored lapatinib sensitivity (Figure 4 and Supplementary Figure 6). In the other lapatinib-resistant cell lines (MDA-MB-361, SK-BR-3 and SUM190PT) where SFKs were not hyperactive compared to drug-sensitive parental cells, the addition of AZD0530 did not enhance lapatinib action. In 2D proliferation assays, Src inhibitors in combination with lapatinib blocked the growth of primarily the lapatinib-resistant cells that exhibited increased SFK activity though in this assay there was moderate inhibition of MDA-MB-361 resistant cell growth (Supplementary Figure 7).
Figure 4. Src inhibitors restore sensitivity to lapatinib.
Parental and lapatinib-resistant cells were seeded in growth-factor reduced Matrigel; parental cells were treated or not with lapatinib while lapatinib-resistant cells were treated with lapatinib with or without AZD0530. Media and inhibitors were replenished every 3 days. Acini were photographed at 400× magnification 10–14 days after plating. Quantification of mean acini area is shown in Supplementary Figure 6.
Lapatinib and the Src inhibitor AZD0530 synergize against HER2-overexpressing xenografts
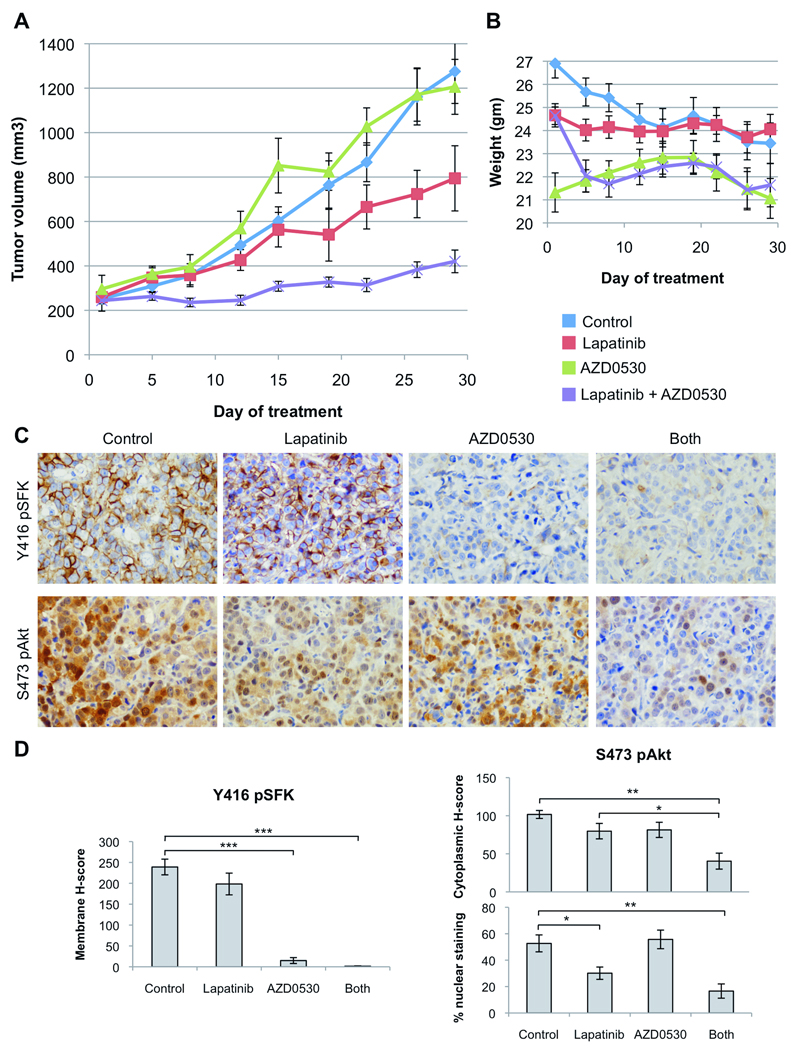
We found that upregulation of SFK activity was acquired as the cells developed resistance to lapatinib. Thus, we hypothesized that the addition of a Src inhibitor to lapatinib would prevent or delay the development of drug resistance and might further suppress tumor growth compared to lapatinib alone. To test this, mice bearing BT-474 xenografts were randomized to therapy with vehicle (controls), lapatinib, AZD0530, or the combination of both drugs for 30 days. Lapatinib inhibited growth of established BT-474 xenografts, while AZD0530 alone had no activity compared to control mice. Tumors treated with the combination exhibited a statistical reduction in tumor volume compared to both lapatinib and control arms starting at 1 week of therapy (Student’s t-test, p<0.05, Figure 5A). The combination was without significant observed toxicity and the weight of mice in the combination arm was maintained throughout the experiment (Figure 5B). Immunohistochemical analysis of tumor sections showed significant inhibition of SFK phosphorylation by AZD0530, alone or in combination with lapatinib. Activation of Akt in situ, as evaluated by nuclear staining for S473 pAkt, was markedly reduced by lapatinib alone or in combination with AZD0530. However, treatment with both lapatinib and AZD0530 inhibited cytoplasmic pAkt more significantly than lapatinib alone (Figure 5C, D). Overall, this immunohistochemical analysis suggested that the combination of lapatinib and AZD0530 more potently inhibited PI3K-Akt in vivo.
Figure 5. Lapatinib and the Src inhibitor AZD0530 synergize against HER2-overexpressing xenografts.
(A) BT-474 xenografts were established in female athymic nude mice. When tumors reached a volume ≥250 mm3, mice were randomly allocated to four treatment groups: vehicle alone, lapatinib (100 mg/kg daily by orogastric gavage), AZD0530 (50 mg/kg daily by orogastric gavage), or the combination. Tumors were measured twice weekly; mean tumor volume (n=10) in mm3 ± SEM for each treatment group is displayed. (B) Mice were weighed twice weekly during treatment and average weight ± SEM of mice in each treatment group is displayed. (C) Tumor fragments were fixed and analyzed by IHC with antibodies to Y416 pSrc and S473 pAkt. Representative sections are shown. (D) IHC was scored as described in the Methods. For pSFKs, columns indicate mean membrane H-score; for pAkt, columns indicate mean cytoplasmic H-score and percent of cells with nuclear staining (bars: mean ± S.E.M.; asterisks: *, p<0.05; **, p<0.005; ***, p<0.000005, Student’s t-test).
DISCUSSION
In this study, we generated lapatinib-resistant HER2-overexpressing human breast cancer cells in order to discover preferential mechanisms of escape from drug-induced inhibition of the HER2 tyrosine kinase. In all resistant cells, HER2 amplification was present and active PI3K-Akt and MAPK were maintained yet HER2 C-terminal autophosphorylation was undetectable. Reactivation of the PI3K-Akt pathway appeared to be causal to lapatinib resistance, as all resistant lines were exquisitely sensitive to PI3K but not MEK inhibition. To identify signaling pathways conferring resistance to lapatinib, we profiled the tyrosine phosphoproteome of resistant cells using an immunoaffinity mass spectrometry approach. The phosphopeptides identified by spectral counts to be more abundant in resistant cells were those corresponding to the Src family kinase Yes (Y222 and Y426) and to HER2 (at the non-autophosphorylation site Y877), suggesting a role for SFKs in mediating resistance.
The Y877 phosphorylation site in the activation loop of the HER2 kinase is analogous to Y426 Yes and Y416 in the activation loop of Src. In other kinases, phosphorylation of this residue allows the activation loop to assume a catalytically competent confirmation and increases kinase activity (Hubbard and Till 2000). Some evidence suggests that Y877 phosphorylation increases the kinase activity of HER2, as mutation of Y877 to phenylalanine in both human HER2 and its rat homolog Neu decreases the kinase’s catalytic activity and transforming activity (Xu et al 2007, Zhang et al 1998). In contrast, mutation of the corresponding Y845 in EGFR, also identified as a Src substrate, disrupts EGFR function but does not decrease the catalytic activity of the kinase (Biscardi et al 1999, Tice et al 1999). Since C-terminal autophosphorylation depends on the catalytic activity of HER2, the lack of phosphorylation in Y1248 in the C-terminus of HER2 in drug-resistant cells (Figure 2C) suggests that maintenance of Y877 phosphorylation does not overcome lapatinib-induced inhibition of the receptor’s kinase activity. Another possible role for Y877 phosphorylation in enhancing HER2/HER3 heterodimer formation has been proposed (Ishizawar et al 2007). Maintenance of HER2/HER3 heterodimers would be a mechanism for partial maintenance of PI3K activity in light of the six p85 binding sites in HER3. This would support a role for persistent Y877 phosphorylation in engaging the HER3-PI3K-Akt axis in order to circumvent drug action.
We also identified increased phosphorylation of the corresponding activation loop residue of Yes, Y426, in resistant cells. In addition, we found phosphorylation at Y222 Yes (Y216 Src) exclusively in lapatinib-resistant cells. Phosphorylation at Y216 Src can significantly increase the kinase activity of Src and can overcome the inhibitory effects of phosphorylation at the regulatory Y527 site (Roskoski 2005). Of note, heregulin, a HER3 ligand that activates HER2/HER3 signaling, has been shown to induce phosphorylation of Y216 in Src in MCF-7 breast cancer cells. Further, higher levels of phosphorylation at Y216 correlates with increased HER2 expression in breast tumors (Vadlamudi et al 2003). As with Y877 HER2, the phosphorylation at Y222 in Yes was limited to lapatinib-resistant cells where the catalytic activity of HER2 remained inhibited, suggesting that the HER2 kinase is not involved in phosphorylation of Y216 Yes.
The correlation of increased Yes activity indicated by Y222 and Y426 phosphorylation with persistent Y877 HER2 phosphorylation in resistant cells suggested that Y877 in HER2 is a Src kinase substrate. This is supported by our observation that Src inhibitors decreased Y877 pHER2 (Figure 3E), and by other observations where treatment with PP1 or PP2 (Ishizawar et al 2007, Xu et al 2007) or expression of kinase-dead or dominant-negative Src (Marcotte et al 2009) abrogated phosphorylation at this site. Fyn and Yes can also mediate Y877 HER2 phosphorylation (Xu et al 2007). In contrast, an earlier report found that Y877 phosphorylation was decreased by treatment with PD168393, a HER2 TKI, leading to the conclusion that Y877 was an autophosphorylation site (Bose et al 2006). While we observed a similar result in immunoblots of whole cell lysates after lapatinib treatment, these observations contrast with the level of phosphorylation at this site detected with immunoaffinity enrichment for pTyr prior to analysis by immunoblot (Figure 2C) or by MS (Table 2). Using the more sensitive and specific MS-based approach, we found that the relative level of phosphorylation of Y877 HER2 is not decreased whatsoever by lapatinib. This implies that HER2 is not the kinase that phosphorylates Y877 HER2, and further underscores the importance of persistent Y877 phosphorylation in lapatinib-resistant cells.
While Yes was the predominant SFK in two of the cell lines we tested, Lyn was also overexpressed and phosphorylated in lapatinib-resistant HCC1954 cells. This is in agreement with the findings of Hochgrafe et al., who employed a phosphoproteomic approach to identify signaling networks in basal-like breast cancer (Hochgrafe et al 2010). In their study, they found higher levels of total and phosphorylated Lyn in breast cancer cells with a basal-like gene expression signature, including HCC1954. They further noted that combining a Src inhibitor to block Lyn with the inhibitor of EGFR/HER2 AG1478 (Lenferink et al 2001) was more effective than either alone in inhibiting proliferation of HCC1954 cells. We have extended this previous report and show herein that dasatinib inhibited the proliferation of lapatinib-resistant HCC1954 cells.
Finally, we showed that the combination of HER2 and SFK inhibitors is more effective than either agent alone at preventing and/or overcoming escape from lapatinib. There is the potential to use this combination clinically; recently the combination of lapatinib and dasatinib was found to be well-tolerated in a phase I trial (Erlichman et al 2009). However, it will be important to identify predictors of sensitivity to Src inhibition or biomarkers of Src activation for appropriate patient selection. In this study, we observed increased Src activity only after the development of resistance to lapatinib and, second, Src inhibitors inhibited cell growth only in combination with lapatinib. These results should be contrasted from data in two prior reports (Finn et al 2007, Huang et al 2007), where the three cell lines exhibiting upregulated SFK activity upon development of resistance to lapatinib in our study were classified as modestly sensitive or resistant to dasatinib alone. Taken together, these data imply that biomarkers predictive of sensitivity to Src inhibitors may be different for tumors prior to vs. after the onset of resistance to HER2 inhibitors. This also implies the need to rebiopsy tumors at the time of progression following primary anti-HER2 therapy to assess the status of Src activation. Finally, these results suggest that, at least for HER2+ tumors, Src antagonists will only be effective as part of combinations with anti-HER2 therapy.
MATERIALS AND METHODS
Detailed methods are in the Supplementary Information online.
Cell lines and reagents
All cells were from the American Type Culture Collection except SUM190PT (Asterand). The following inhibitors were used at the indicated concentrations: lapatinib ditosylate (GW-572016, LC Laboratories), 1 µM; BEZ235 (Novartis), 0.25 µM; AZD0530 (AstraZeneca Pharmaceuticals), 1 µM; and dasatinib (Bristol-Myers Squibb), 1 µM; CI-1040 (Pfizer), 1 µM.
Cell proliferation and 3D culture assays
Cell proliferation was measured with the WST-1 reagent after drug treatment for the indicated times. For 3D assays, cells were grown in Matrigel (BD Biosciences) with inhibitors for 10–14 days.
Immunoblot and immunoprecipitation
Cells were lysed in NP-40 lysis buffer and quantitated by BCA assay (Pierce). Lysates were separated by SDS-PAGE, transferred to PVDF-FL (Millipore), and blotted with the indicated antibodies.
Reverse phase protein lysate microarray (RPPA)
RPPA was performed as described (Tibes et al 2006) using lysates from untreated parental cells, cells treated with lapatinib for 1 or 24 h, or lapatinib-treated resistant cells. Lysates were analyzed with the indicated antibodies.
Immunoaffinity mass-spectrometry phosphotyrosine profiling
pTyr peptides were enriched from tryptic digests of cell lysates as described (Rush et al 2005) except that lysates were subjected to brief PAGE and in-gel trypsin digestion. LC-MS-MS analysis of immunoaffinity-purified peptides was performed as described (Luo et al 2008) with modifications described in Supplementary Methods. MS/MS peptide spectra were acquired using data-dependent scanning in which one full MS spectrum was followed by 5 MS/MS spectra. A data-dependent scan for the neutral loss of phosphoric acid or phosphate resulted in acquisition of an MS/MS/MS of the neutral loss ion. Proteins were identified from mass spectra using the Myrimatch algorithm and the human IPI database (v. 3.56) (Tabb et al 2007). Data were filtered using a 2% FDR for all peptides using the IDPicker algorithm (Zhang et al 2007) allowing for a single peptide/spectrum match.
Real-time quantitative PCR
RNA isolated with the RNeasy kit (Qiagen) was converted to cDNA and used as template for SYBR Green qPCR (SABiosciences). Fold-change in gene expression was calculated using the ΔΔCt (threshold cycle) method with normalization to levels of actin expression in each template.
siRNA-mediated SFK knockdown
Lapatinib-resistant cells were transiently transfected with siRNA oligos for Src, Yes, Fyn, or Lyn (Qiagen) for 72h before lysis and analysis by immunoblot.
Human tumor biopsies and microarray analyses
After informed consent, patients with newly diagnosed HER2-overexpressing breast cancer were enrolled in an IRB-approved trial at Baylor College of Medicine (Houston, TX). Lapatinib was administered at 1,500 mg/day p.o. for 6 weeks. Core biopsies were obtained at baseline before treatment and after 42 days of therapy. There were 8 paired samples with sufficient material for RNA isolation and microarray hybridization. Microarray analysis was performed as described in Supplementary Methods. Expression levels of probes for Src family kinases were analyzed with dCHIP and expression levels displayed in a heatmap.
Fluorescent in situ hybridization (FISH)
HER2 gene copy number was determined by FISH as described (Ritter et al 2007). HER2 and CEP17 signals were quantified from 50 consecutive cells, and ratios of HER2 to CEP17 for each cell line were calculated.
Studies with xenografts
Animal studies were approved by the Vanderbilt University Medical Center Institutional Animal Care and Use Committee. BT-474 cells were injected into female athymic nude mice bearing slow-release estrogen pellets (Innovative Research of America) as described (Wang et al 2006). After tumors reached 250 mm3, treatment was begun with lapatinib (100 mg/kg) and/or AZD0530 (50 mg/kg) daily by oral gavage.
Immunohistochemistry (IHC)
Tumor sections were analyzed by IHC with the indicated antibodies. Staining was evaluated by a pathologist blinded to treatment groups (N.M.G-I.) and an H-score was calculated as described in Supplementary Methods. H-scores and percent of positive nuclear staining were compared between sections from different treatment groups with the Student’s t-test.
Supplementary Material
Acknowledgements
This work was supported by NIH R01 CA80195 (CLA), Dinah Armstrong Kukes Fund/Phi Mu foundation (BNR), T32 CA119910 (BNR), Department of Defense BC087465 Post-Doctoral Fellowship (BNR), ASCO Young Investigator Award (BNR), Kleberg Center for Molecular Markers at MD Anderson Cancer Center, ASCO Career Development Award (AMG), NCI 1K23CA121994-01 (AMG), Susan G. Komen Foundation FAS0703849 (AMG, GBM), ACS Clinical Research Professorship CRP-07-234 (CLA), Lee Jeans Translational Breast Cancer Research Program (CLA), Breast Cancer SPORE P50 CA98131, and Vanderbilt-Ingram Cancer Center Support Grant P30 CA68485.
Footnotes
Conflict of interest:
The authors declare no conflict of interest.
Supplementary information is available at the Oncogene website.
REFERENCES
- Amin DN, Sergina N, Ahuja D, McMahon M, Blair A, Wang D, et al. Resiliency and Vulnerability in the HER2–HER3 Tumorigenic Driver. Sci Transl Med. 2010;2 doi: 10.1126/scitranslmed.3000389. 16ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsches-Jablonski AP, Biscardi JS, Peavy DR, Tice DA, Romney DA, Parsons SJ. Src family kinases and HER2 interactions in human breast cancer cell growth and survival. Oncogene. 2001;20:1465–1475. doi: 10.1038/sj.onc.1204205. [DOI] [PubMed] [Google Scholar]
- Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- Biscardi JS, Maa MC, Tice DA, Cox ME, Leu TH, Parsons SJ. c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J Biol Chem. 1999;274:8335–8343. doi: 10.1074/jbc.274.12.8335. [DOI] [PubMed] [Google Scholar]
- Bose R, Molina H, Patterson AS, Bitok JK, Periaswamy B, Bader JS, et al. Phosphoproteomic analysis of Her2/neu signaling and inhibition. Proc Natl Acad Sci USA. 2006;103:9773–9778. doi: 10.1073/pnas.0603948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris HA, Hurwitz HI, Dees EC, Dowlati A, Blackwell KL, O'Neil B, et al. Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J Clin Oncol. 2005;23:5305–5313. doi: 10.1200/JCO.2005.16.584. [DOI] [PubMed] [Google Scholar]
- Eichhorn PJA, Gili M, Scaltriti M, Serra V, Guzman M, Nijkamp W, et al. Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer Res. 2008;68:9221–9230. doi: 10.1158/0008-5472.CAN-08-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlichman C, Menefee ME, Northfelt DW, Qin R, Reid JM, Lingle WL, et al. Abstract B56: A phase I trial of the combination of dasatinib and lapatinib -- Erlichman et al. 8 (1001): B56 -- Molecular Cancer Therapeutics. Mol Cancer Ther. 2009;8:B56. [Google Scholar]
- Finn R. Targeting Src in breast cancer. Annals of Oncology. 2008;19:1379. doi: 10.1093/annonc/mdn291. [DOI] [PubMed] [Google Scholar]
- Finn RS, Dering J, Ginther C, Wilson CA, Glaspy P, Tchekmedyian N, et al. Dasatinib, an orally active small molecule inhibitor of both the src and abl kinases, selectively inhibits growth of basal-type/"triple-negative" breast cancer cell lines growing in vitro. Breast Cancer Res Treat. 2007;105:319–326. doi: 10.1007/s10549-006-9463-x. [DOI] [PubMed] [Google Scholar]
- Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- Green T, Fennell M, Whittaker R, Curwen J, Jacobs V, Allen J, et al. Preclinical anticancer activity of the potent, oral Src inhibitor AZD0530. Mol Oncol. 2009;3:248–261. doi: 10.1016/j.molonc.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennequin LF, Allen J, Breed J, Curwen J, Fennell M, Green TP, et al. N-(5-chloro-1,3-benzodioxol-4-yl)-7-[2-(4-methylpiperazin-1-yl)ethoxy]-5- (tetrahydro-2H-pyran-4-yloxy)quinazolin-4-amine, a novel, highly selective, orally available, dual-specific c-Src/Abl kinase inhibitor. J Med Chem. 2006;49:6465–6488. doi: 10.1021/jm060434q. [DOI] [PubMed] [Google Scholar]
- Hochgrafe F, Zhang L, O'Toole SA, Browne BC, Pinese M, Porta Cubas A, et al. Tyrosine phosphorylation profiling reveals the signaling network characteristics of Basal breast cancer cells. Cancer Res. 2010;70:9391–9401. doi: 10.1158/0008-5472.CAN-10-0911. [DOI] [PubMed] [Google Scholar]
- Huang F, Reeves K, Han X, Fairchild C, Platero S, Wong TW, et al. Identification of candidate molecular markers predicting sensitivity in solid tumors to dasatinib: rationale for patient selection. Cancer Res. 2007;67:2226–2238. doi: 10.1158/0008-5472.CAN-06-3633. [DOI] [PubMed] [Google Scholar]
- Hubbard SR, Till JH. Protein tyrosine kinase structure and function. Annu Rev Biochem. 2000;69:373–398. doi: 10.1146/annurev.biochem.69.1.373. [DOI] [PubMed] [Google Scholar]
- Ishizawar RC, Miyake T, Parsons SJ. c-Src modulates ErbB2 and ErbB3 heterocomplex formation and function. Oncogene. 2007;26:3503–3510. doi: 10.1038/sj.onc.1210138. [DOI] [PubMed] [Google Scholar]
- Jones RJ, Young O, Renshaw L, Jacobs V, Fennell M, Marshall A, et al. Src inhibitors in early breast cancer: a methodology, feasibility and variability study. Breast Cancer Res Treat. 2009;114:211–221. doi: 10.1007/s10549-008-9997-1. [DOI] [PubMed] [Google Scholar]
- Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15:429–440. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Kim H, Chan R, Dankort DL, Zuo D, Najoukas M, Park M, et al. The c-Src tyrosine kinase associates with the catalytic domain of ErbB-2: implications for ErbB-2 mediated signaling and transformation. Oncogene. 2005;24:7599–7607. doi: 10.1038/sj.onc.1208898. [DOI] [PubMed] [Google Scholar]
- Konecny GE, Pegram MD, Venkatesan N, Finn R, Yang G, Rahmeh M, et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66:1630–1639. doi: 10.1158/0008-5472.CAN-05-1182. [DOI] [PubMed] [Google Scholar]
- Lachmann A, Ma'ayan A. KEA: kinase enrichment analysis. Bioinformatics. 2009;25:684–686. doi: 10.1093/bioinformatics/btp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP, et al. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2008;68:5878–5887. doi: 10.1158/0008-5472.CAN-08-0380. [DOI] [PubMed] [Google Scholar]
- Lenferink AE, Busse D, Flanagan WM, Yakes FM, Arteaga CL. ErbB2/neu kinase modulates cellular p27(Kip1) and cyclin D1 through multiple signaling pathways. Cancer Res. 2001;61:6583–6591. [PubMed] [Google Scholar]
- Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, et al. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658–6661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- Luo W, Slebos RJ, Hill S, Li M, Brábek J, Amanchy R, et al. Global impact of oncogenic Src on a phosphotyrosine proteome. J Proteome Res. 2008;7:3447–3460. doi: 10.1021/pr800187n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell DK, Lee A, Lansing TJ, Crosby RM, Jung KD, Willard D, et al. Involvement of pp60c-src with two major signaling pathways in human breast cancer. Proc Natl Acad Sci USA. 1994;91:83. doi: 10.1073/pnas.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maira S-M, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- Marcotte R, Zhou L, Kim H, Roskelly CD, Muller WJ. c-Src associates with ErbB2 through an interaction between catalytic domains and confers enhanced transforming potential. Mol Cell Biol. 2009;29:5858–5871. doi: 10.1128/MCB.01731-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio I, Gutierrez MC, Wu M-F, et al. P13 Kinase activation and response to trastuzumab or lapatinib in HER-2 overexpressing locally advanced breast cancer (LABC) Cancer Res. 2009;69(supplt) doi: 10.1200/JCO.2009.27.7814. abstr 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moasser MM. Targeting the function of the HER2 oncogene in human cancer therapeutics. Oncogene. 2007;26:6577–6592. doi: 10.1038/sj.onc.1210478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata Y, Lan K-H, Zhou X, Tan M, Esteva FJ, Sahin AA, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Old WM, Meyer-Arendt K, Aveline-Wolf L, Pierce KG, Mendoza A, Sevinsky JR, et al. Comparison of label-free methods for quantifying human proteins by shotgun proteomics. Mol Cell Proteomics. 2005;4:1487–1502. doi: 10.1074/mcp.M500084-MCP200. [DOI] [PubMed] [Google Scholar]
- Ritter CA, Perez-Torres M, Rinehart C, Guix M, Dugger T, Engelman JA, et al. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res. 2007;13:4909–4919. doi: 10.1158/1078-0432.CCR-07-0701. [DOI] [PubMed] [Google Scholar]
- Roskoski R. Src kinase regulation by phosphorylation and dephosphorylation. Biochem Biophys Res Commun. 2005;331:1–14. doi: 10.1016/j.bbrc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Rush J, Moritz A, Lee KA, Guo A, Goss VL, Spek EJ, et al. Immunoaffinity profiling of tyrosine phosphorylation in cancer cells. Nat Biotechnol. 2005;23:94–101. doi: 10.1038/nbt1046. [DOI] [PubMed] [Google Scholar]
- Rusnak DW, Lackey K, Affleck K, Wood ER, Alligood KJ, Rhodes N, et al. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther. 2001;1:85–94. [PubMed] [Google Scholar]
- Sergina NV, Rausch M, Wang D, Blair J, Hann B, Shokat KM, et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445:437–441. doi: 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra V, Markman B, Scaltriti M, Eichhorn PJA, Valero V, Guzman M, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68:8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- Sheffield LG. C-Src activation by ErbB2 leads to attachment-independent growth of human breast epithelial cells. Biochem Biophys Res Commun. 1998;250:27–31. doi: 10.1006/bbrc.1998.9214. [DOI] [PubMed] [Google Scholar]
- Tabb DL, Fernando CG, Chambers MC. MyriMatch: highly accurate tandem mass spectral peptide identification by multivariate hypergeometric analysis. J Proteome Res. 2007;6:654–661. doi: 10.1021/pr0604054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M, Li P, Klos KS, Lu J, Lan KH, Nagata Y, et al. ErbB2 promotes Src synthesis and stability: novel mechanisms of Src activation that confer breast cancer metastasis. Cancer Res. 2005;65:1858–1867. doi: 10.1158/0008-5472.CAN-04-2353. [DOI] [PubMed] [Google Scholar]
- Tibes R, Qiu Y, Lu Y, Hennessy B, Andreeff M, Mills GB, et al. Reverse phase protein array: validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Mol Cancer Ther. 2006;5:2512–2521. doi: 10.1158/1535-7163.MCT-06-0334. [DOI] [PubMed] [Google Scholar]
- Tice DA, Biscardi JS, Nickles AL, Parsons SJ. Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc Natl Acad Sci USA. 1999;96:1415–1420. doi: 10.1073/pnas.96.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadlamudi RK, Sahin AA, Adam L, Wang RA, Kumar R. Heregulin and HER2 signaling selectively activates c-Src phosphorylation at tyrosine 215. FEBS Lett. 2003;543:76–80. doi: 10.1016/s0014-5793(03)00404-6. [DOI] [PubMed] [Google Scholar]
- Wang SE, Narasanna A, Perez-Torres M, Xiang B, Wu FY, Yang S, et al. HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell. 2006;10:25–38. doi: 10.1016/j.ccr.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Xu W, Yuan X, Beebe K, Xiang Z, Neckers L. Loss of Hsp90 association up-regulates Src-dependent ErbB2 activity. Mol Cell Biol. 2007;27:220–228. doi: 10.1128/MCB.00899-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002;62:4132–4141. [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4:470–480. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- Zhang B, VerBerkmoes NC, Langston MA, Uberbacher E, Hettich RL, Samatova NF. Detecting differential and correlated protein expression in label-free shotgun proteomics. J Proteome Res. 2006;5:2909–2918. doi: 10.1021/pr0600273. [DOI] [PubMed] [Google Scholar]
- Zhang B, Chambers MC, Tabb DL. Proteomic parsimony through bipartite graph analysis improves accuracy and transparency. J Proteome Res. 2007;6:3549–3557. doi: 10.1021/pr070230d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HT, O'Rourke DM, Zhao H, Murali R, Mikami Y, Davis JG, et al. Absence of autophosphorylation site Y882 in the p185neu oncogene product correlates with a reduction of transforming potential. Oncogene. 1998;16:2835–2842. doi: 10.1038/sj.onc.1201820. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.