Abstract
Objective
This study examined the moderating effect of caregiver burden on the relationship between the health status of Alzheimer’s disease (AD) patients and their use of institutional services (i.e., hospitalization, nursing home, and residential care).
Methods
Data were obtained at baseline and 3-, 6-, and 9-months following study entry on 421 community-dwelling patients with AD in the CATIE-AD trial. The outcome variable includes use of any institutional services. Logistic regression was employed to estimate the interaction of Health Utility Index (HUI)-III score (a general health status measure) at outcome and four concurrent caregiver burden measures. Marginal effects were calculated and plotted using random effects models for observations at multiple time points per individual. Average effects were calculated across all observations using models without random effects.
Results
Random effects results suggest that caregiver burden weakens the inverse relationship between health utilities and institutional service use, leading to greater likelihood of institutional use than would be expected at a given level of health. This is indicated by positive, significant signs on the HUI-III*caregiver burden interaction when burden is measured using the Caregiver Distress Scale, Beck Depression Inventory (BDI), and Caregiver Assessment Survey (all p<.05). It is reinforced by positive, significant average effects deriving from Caregiver Distress and BDI models without random effects (both p<.10). Results deriving from the Burden Interview Scale, though positive, were non-significant and weak by comparison.
Conclusion
Caregiver support interventions should be offered to individuals caring for less advanced AD patients. Otherwise healthy patients may be at increased risk for institutionalization when caregivers experience high levels of burden.
Keywords: Alzheimer’s disease, service use, caregiver burden, Health Utilities Index, health status, correlates, institutions
INTRODUCTION
Alzheimer’s disease (AD) is among the top ten leading causes of death with approximately 5 million afflicted individuals in the United States alone (Alzheimer’s Association 2008). AD is characterized by progressive memory loss and decline in other cognitive functioning. It also is associated with mood and behavioral symptoms, including aggression, agitation, anxiety, depression, psychosis, irritability, and wandering (Grossberg, 2003). Family members, friends, and other unpaid caregivers experience considerable psychological and physical comorbidity and reduced quality of life as well (Bell, Araki, and Neumann 2001; Dunkin and Anderson-Hanley 1998). It estimated that in 2007 unpaid caregivers provided nearly 8.5 billion hours in AD/dementia-related care (Alzheimer’s Association 2008).
A large number of studies have examined the correlates of institutional service use associated with AD. These include models predicting inpatient medical use (Balardy, et al. 2005; Fillenbaum et al., 2000; Miller, Schneider, and Rosenheck 2009; Small et al., 2002; Porell and Carter 2005), nursing home use (Chan et al., 2003; Cohen, et al. 1993; Gaugler et al., 2000, 2004; Hebert, et al,. 2001; Hill, et al. 2006; Hux, et al. 1998; Mausbach, et al. 2004; Phillips and Diwan 2003), and residential care use(Cox 1998; Miller, Schneider, and Rosenheck 2009). One rarely studied correlate is health utilities which are measured using instruments that assign patients a quality of life state based on responses to a health status questionnaire weighted using previously determined weights from other populations (Miller, et al. 2009; Naglie, et al. 2006). Higher utilities imply better adaptation and functioning, which, in turn, should reduce the probability of using particular services. This assumption is supported by two recent studies (Miller, Rosenheck, and Schneider 2009; Miller, Schneider, and Rosenheck 2009).
In contrast to patient-level correlates there has been little research examining caregiver burden as a potentially important determinant of institutional service use. This is an important gap because what few studies do exist suggest that patients with more burdened caregivers are at greater risk of entering a hospital (Balardy, et al. 2005), residential care facility (Cox 1998), or nursing home (Cohen, et al. 1993; Gaugler, et al. 2004; Hebert, et al. 2001). Caregivers face substantial stress due to physical, mental and emotional strain, financial hardship, and occupational insecurity (Haley 1998; Miller, Allen and Mor 2009). They are at greater risk for poor health, depression, and use of psychotropic drugs and health services (Draper, et al. 1992; Grafstrom, et al. 1992; Song, Biegel, and Milligan 1997; Vitaliano, Zhang, and Scanlan 2003).
It has been demonstrated that caregiver burden is related to care recipient health, suggesting that the two may reinforce one another (Coen et et al. 1997; Cummings, et al. 2004; Mohamed, et al. 2009). Our aim is to assess whether the negative association between patient’s general health status (that is, higher health utility scores) and institutional service use is moderated by caregiver burden. It is expected that the protective effects of health on decreasing the likelihood of institutionalization will be dampened among patients with more burdened caregivers as caregiver depression, distress, and time commitments make it more difficult for caregivers to keep AD patients at home. Data derive from the Clinical Antipsychotic Trial of Intervention Effectiveness—AD (CATIE-AD), a 9-month randomized trial designed to evaluate the performance of second generation antipsychotics (SGAs) and placebo vis-à-vis behavioral and psychological symptoms in dementia (Rosenheck, et al. 2007; Schneider, et al. 2006).
METHODS
Sample and source of data
The CATIE-AD trial was conducted at 42 sites in the US and included 421 ambulatory outpatients who fulfilled criteria for dementia of the Alzheimer’s type (DSM-IV) and probable AD. Participants also had to be living at home or in assisted living, in addition to having clinically severe delusions, hallucinations, aggression, or agitation. A caregiver who lived with or visited the participant for at least 3 days/week for an accumulative 8 hours was required to contribute to assessments. The study was approved by the institutional review board for each site. Additional details can be found elsewhere (Rosenheck, et al. 2007; Schneider, et al. 2001, 2006). In general, data on service use, health utilities, clinical status, and caregiver burden were collected simultaneously; for those who dropped out of the study, however, clinical data may have been collected from 4 to 8 weeks earlier.
Measures
Service use
At baseline and 3-, 6-, and 9-months post-random assignment it was determined whether or not study participants had received each of three institutional services during the previous month: inpatient hospital, nursing home, and residential care. Inpatient hospital care refers to hospitalization for medical, surgical, psychiatric, or substance abuse problems across six different types of facilities. Nursing home use refers to nights spent in nursing facilities at a skilled or intermediate level of care. Residential care use refers to placement in a halfway house or board & care home, or in respite care programs. A dichotomous measure was developed which indicated use of one or more of these services.
Health utilities
Health utilities were assessed using the HUI-III, which consists of a classification system describing 927,000 unique health states, and a preference or utility function (Feeny, et al. 2002). The HUI-III assesses capacity in eight dimensions—vision, hearing, speech, ambulation, dexterity, emotion, pain/discomfort, and cognition. Preference measures collected from a population based in Hamilton, Ontario are used to score each state on a scale from −0.36 (worse than death) to 1 (perfect health), with 0 representing death. The original HUI-III scores were multiplied by 10 to facilitate interpretation, so that a unit difference on the transformed HUI-III corresponds to a 0.10 difference on the original (Lima and Kopec 2005).
Caregiver burden
Caregiver burden is measured using four separate indicators. The Caregiver Distress Scale is based on the distress items of the Neuropsychiatric Inventory (NPI) (Cummings, et al. 1994). It asks caregivers to rate the level of distress stemming from 12 psychiatric symptoms. Each item is rated on a scale from 0 (“not distressing at all”) to 5 (“extremely distressing”). Total scores range from 0 to 60.
The Beck Depression Inventory (BDI) asks respondents 21 items assessing their experience over the previous week with mood, pessimism, self-hate, crying-spells, social withdrawal, body image, sleep disturbance, libido, and other behaviors/attitudes (Beck, et al. 1961). Each item is rated from 0 (e.g., “I do not feel sad’) to 3 (e.g., “I can’t stand it”). Total scores range from 0 to 63.
The Caregiver Burden Interview asks respondents 22 items assessing how they feel about taking care of another person (Zarit, Reever, and Bach-Peterson 1980). Items cover perceived burden in a variety of areas, including physical health, psychological well-being, finances, and relationships. Each item is rated on a scale from 0 (“never”) to 4 (“nearly always”). Total scores can range from 0 to 88.
The Caregiver Activity Survey (CAS) is a five-item questionnaire that measures the amount of time spent supervising and assisting AD patients during the previous 24 hours (Davis, et al. 1997). Items include: using transportation, eating, dressing, appearance, and supervision. Total CAS scores equal the number of hours performing these activities.
Control variables
Sociodemographic factors were represented by age, gender, race, marital status, and education. Global cognitive functioning was measured on a 0–30 scale using the MMSE, which consists of 11 questions testing cognition in five areas: orientation, registration, attention and calculation, recall, and language (Folstein, et al. 1975). Psychiatric symptoms were assessed using the neuropsychiatric inventory (NPI), which evaluates 12 neuropsychiatric disturbances, including delusions, hallucinations, agitation, dysophoria, anxiety, apathy, irritability, etc. (Cummings, et al. 1994). Physical functioning was measured using the AD Cooperative Study Activities of Daily Living Scale (ADCS-ADL), which assesses performance in 5 basic ADLs (eating, walking, toileting, bathing, grooming) and 18 instrumental ADLs (telephoning, shopping, reading, writing, conversing, etc.) (Galasko, et al. 1997). Quality of life was assessed using the AD Related Quality of Life scale (ADRQoL), which assesses quality of life in five domains: social interaction, awareness of self, feelings and mood, enjoyment of activities, and response to surroundings (Rabins 1999). We also considered controlling for caregiver demographics, including age, gender, education, and relationship to the care recipient (spouse, child, other) but there were too many missing values.
Analysis
To examine the moderating effect of caregiver burden on the relationship between health utilities and institutional service use, we estimated four logistic regression models interacting HUI-III score in separate models with each of the four caregiver burden measures described net of the HUI-III and caregiver burden main effects. Each model included HUI-III score, caregiver burden, and the HUI-III*caregiver burden interaction, controlling for other sociodemographic and clinical/disease-specific indicators. All analyses were conducted using the pooled dataset of all observations across all four time periods and included fixed effects accounting for observation period (baseline and 3-, 6-, and 9-months). Random effects models were estimated using the XTLOGIT command in Stata 11.0 to account for clustering of observations across individuals over time. Following the UCLA Statistical Consulting Group (2010) the marginal effect of a 0.10 increase in HUI-III score on the probability of institutional service use when caregiver burden is held constant at different values was produced by Stata’s MARGINS command. The size and significance of the marginal effect was calculated at 5 unit intervals. Following Jaccard (2001) we also report the percent change in odds of institutional use for each 0.10 increment in HUI-III score at different levels of burden.
Since the main effect sign of the HUI-III is negative in baseline models without interactions (Miller, Schneider, and Rosenheck 2009), the sign on each HUI-III*caregiver burden interaction term is hypothesized to be positive, indicating that it has a dampening effect on the inverse relationship between HUI-III score and institutional service use identified previously, i.e. leading to greater use than would be expected at a given level of health. Basing conclusions about interactions exclusively on the signs, significance, and magnitude of the effects reported in logistic regression models is problematic, however, because they do reflect the full interactions. Unlike the case with linear models calculating the full interaction effect in non-linear models requires computing the cross-partial derivative which, in turn, has several implications. First, it implies that the magnitude of an interaction effect is conditional on all independent variables in a model. Second, it implies that an interaction effect may have different signs for different values of the covariates. The INTEFF command developed by Norton and colleagues employs the appropriate methodology for computing the interaction effect, standard error, and z-statistic across all observations (Ai and Norton 2003; Norton, Wang, and Ai 2004). Because additional, limiting assumptions would be required, this command is not available for random effects models. We therefore apply it to fixed-effects only models with robust standard errors instead.
RESULTS
Descriptive statistics of study participants at baseline are reported in Table 1. On average, 13.0% of patients were admitted to an institution monthly, including average monthly hospital, nursing home, and residential care rates of 4.5%, 5.6%, and 3.9%, respectively. Respondents spent an average of 2.4 days per month in institutions. Of those using institutional services, an average of 18.4 days per month was spent.
TABLE 1.
Characteristics of Study Sample at Baseline (n=409–421)
| Mean | Standard Deviation | Minimum | Maximum | |
|---|---|---|---|---|
| Age | 77.9 | 7.5 | 51.0 | 103.0 |
| Female | 0.56 | 0.50 | 0.00 | 1.00 |
| Non-Hispanic White | 0.79 | 0.41 | 0.00 | 1.00 |
| Married | 0.59 | 0.49 | 0.00 | 1.00 |
| Some College or More | 0.29 | 0.45 | 0.00 | 1.00 |
| ADCS-ADL Scale | 39.44 | 17.11 | 2.00 | 76.00 |
| Neuropsychiatric Inventory | 36.88 | 18.29 | 3.00 | 104.00 |
| Mini-Mental State Exam | 15.00 | 5.80 | 4.00 | 29.00 |
| AD-Related Quality of Life | 67.31 | 14.66 | 18.71 | 100.00 |
| Health Utilities Index | 0.18 | 0.25 | −0.29 | 1.00 |
| Caregiver Distress Scale | 16.47 | 8.56 | 0.00 | 45.00 |
| Beck Depression Inventory | 8.40 | 7.33 | 0.00 | 42.00 |
| Burden Interview Score | 34.42 | 15.99 | 0.00 | 76.00 |
| Caregiver Activity Survey | 16.34 | 11.91 | 0.00 | 54.00 |
n varies depending on the number of missing values
Table 2 reports the logistic regression results. Each model reveals a significant negative relationship between HUI-III score and institutional service use (all p<.05). Two models—Caregiver Distress and Burden interview—reveal an inverse relationship between caregiver burden and institutional service use as well (both p<.01). All models display a positive sign on the interaction term, thereby supporting the expectation that caregiver burden would dampen the inverse relationship between HUI-III score and utilization. Interactions in the Caregiver Distress, Beck Depression Inventory or BDI, and Caregiver Activity models were statistically significant at the .05 level. The interaction in the Burden Interview model did not achieve statistical significance.
TABLE 2.
Results of Logistic Regression Models of Institutional Service Use1
| Caregiver Distress 95% CI |
Beck Depression Inv 95% CI |
Burden Interview 95% CI |
Caregiver Activity 95% CI |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | β | Lower | Upper | β | Lower | Upper | β | Lower | Upper | β | Lower | Upper |
| Health Utilities Index-III(transf.) | −.298** | −.492 | −.104 | −.279** | −.483 | −.074 | −.292* | −.557 | −.027 | −.273* | −.482 | −.064 |
| Caregiver Burden Measure | −.065** | −.106 | −.024 | −.018 | −.070 | .034 | −.031** | −.054 | −.008 | .016 | −.012 | .044 |
| HUI *Caregiver Burden Measure | .015* | .001 | .030 | .024* | .004 | .043 | .004 | −.004 | .012 | .010* | .000 | .019 |
| (Constant) | −5.05 | −10.45 | .356 | −7.54** | −12.78 | −2.31 | −6.26* | −11.62 | −.895 | −7.70* | −13.06 | −2.33 |
| −2 Log Likelihood: | −2 Log Likelihood: | −2 Log Likelihood: | −2 Log Likelihood: | |||||||||
| 842.8*** | 785.85*** | 797.2*** | 784.5*** | |||||||||
| n=1,355 | n=1,307 | n=1,331 | n=1,313 | |||||||||
Control variables include: age, female, non-Hispanic White, Married, Some College or More, ADCS-ADL Scale, Mini-Mental State Exam, Neuropsychiatric Inventory, AD-Related Quality of Life, and Time Fixed Effects (Three Months, Six Months, Nine Months)
|Wald χ2|
p<.10,
p<.05,
p<.01,
p<.001
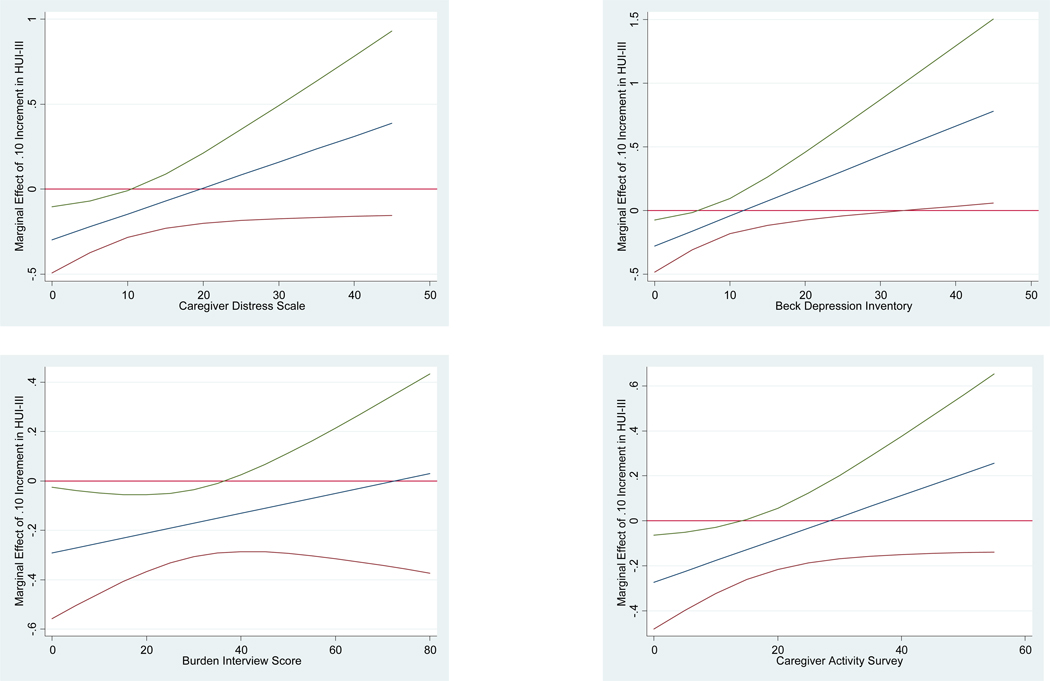
Table 3 reports the marginal effects of HUI-III score on institutional service use when caregiver burden is held constant at different values. These are displayed graphically in Figure 1 along with the 95% confidence intervals. Results deriving from all four models indicate that as caregiver burden increases the probability of institutional service use associated with a .10 increase in HUI-III score rises. In three models—Caregiver Distress, Burden Interview, and Caregiver Activity—the marginal effect of HUI-III score achieved statistical significance at the lower ends of the scales used (p<.10). The marginal effect of HUI-III score achieved statistical significance with the Beck Depression Inventory model but at higher values.
TABLE 3.
Marginal Effects of a .10 Increment in HUI-III Score on Institutional Service Use When Caregiver Burden Scores Are Held Constant at Different Values1
| Marginal Effect | Marginal Effect | ||||
|---|---|---|---|---|---|
| Caregiver Distress Score | Beck Depression Inventory | ||||
| Value | 0 | −.298** | Value | 0 | −.279** |
| 5 | −.222** | 5 | −.161* | ||
| 10 | −.146* | 10 | −.043 | ||
| 15 | −.069 | 15 | .074 | ||
| 20 | .007 | 20 | .192 | ||
| 25 | .083 | 25 | .309# | ||
| 30 | .158 | 30 | .427# | ||
| 35 | .235 | 35 | .545* | ||
| 40 | .311 | 40 | .662* | ||
| 45 | .388 | 45 | .780* | ||
| Burden Interview Score | Caregiver Activity Survey | ||||
| Value | 0 | −.292* | Value | 0 | −.273* |
| 5 | −.272* | 5 | −.224* | ||
| 10 | −.252* | 10 | −.177* | ||
| 15 | −.231* | 15 | −.128# | ||
| 20 | −.211* | 20 | −.080 | ||
| 25 | −.191* | 25 | −.032 | ||
| 30 | −.171* | 30 | .016 | ||
| 35 | −.151* | 35 | .064 | ||
| 40 | −.131# | 40 | .112 | ||
| 45 | −.111 | 45 | .160 | ||
| 50 | −.091 | 50 | .209 | ||
| 55 | −.071 | 55 | .257 | ||
| 60 | −.050 | ||||
| 65 | −.030 | ||||
| 70 | −.101 | ||||
| 75 | .010 | ||||
| 80 | .030 | ||||
Derived using the MARGINS command in Stata 11.0 based on random effects regressions reported in Table 2. Marginal effects were computed at 5 unit intervals for each caregiver burden measure used.
|z|
p<.10,
p<.05,
p<.01,
p<.001
Figure 1. Marginal Effects of HUI-III Score on Institutional Service Use at Caregiver Burden Scores with 95% Confidence Intervals*.
*Derived using the MARGINS command in Stata 11.0 based on logistic regressions with random effects. The x-axis is caregiver burden score; the y-axis is the marginal effect of a .10 increment of HUI-III score. The middle line represents the marginal effects of HUI-III score on institutional service use when caregiver burden is held constant at different values. The outer two curvilinear lines represent the 95% confidence interval for the values reported.
Table 4 illustrates the moderating effect of caregiver burden on the health utilities-institutional service use relationship. It shows that an increase in Caregiver Distress, Beck Depression Inventory, Burden Interview, and Caregiver Activity score from zero to one standard deviation below the mean raises the odds of institutional service use for each 0.10 increment on the HUI-III scale slightly, from 1.8 to 9.4 percentage points. Further increasing each score, however, raises the odds considerably in three of the four models. Thus, whereas each 0.10 increment in HUI-III score is associated with a 26.0% decrease in the likelihood of institutionalization when Caregiver Distress is zero; it is associated with an 16.6% decrease when Caregiver Distress is low, one standard deviation below the mean (8.0); a 5.9% percent decrease when it is at its mean (16.0); and a 7.7% and 45.3% greater likelihood of institutional use when it is high, one standard deviation above the mean (25) and at its maximum (45), respectively.
TABLE 4.
Moderating Effect of Caregiver Burden on the Health Utilities-Institutional Service Use Relationship1
| Burden Measure Score | 0 | −1 St.Dev. | Mean | +1 St.Dev | Max. |
|---|---|---|---|---|---|
| Caregiver Distress | (0)2 | (8) | (16) | (25) | (45) |
| −26.03 | −16.6 | −5.9 | 7.7 | 45.3 | |
| Beck Depression | (0) | (1) | (8) | (16) | (42) |
| −24.0 | −22.2 | −7.9 | 11.6 | 108.2 | |
| Burden Interview | (0) | (18) | (34) | (50) | (76) |
| −25.0 | −22.6 | −14.1 | −8.4 | 1.6 | |
| Caregiver Assessment | (0) | (4) | (16) | (28) | (54) |
| −24.0 | −20.9 | −10.8 | 0.6 | 30.4 |
The table shows the moderating effect of burden measure score on the odds of institutional service use for each 0.10 increment on the HUIIII scale.
Numbers in parentheses represent corresponding scores on each caregiver burden measure.
Odds of utilization for each .10 increment in HUI-III score based on regression coefficients reported in Table 2
Similar patterns can be observed with the other models, though, perhaps most dramatically in the case of the Beck Depression Inventory where the likelihood of institutional service use associated with a .10 increment in HUI-III score ranges from a 24% decrease when Inventory score is 0 to a 108.2% increase when it is at its maximum. This is contrast to the Burden Interview model, the only one in which the HUI-III*caregiver burden interaction term was not significant, where the increase in the odds of institutional service use associated with a .10 increment in HUI-III score is comparatively muted, rising by just 26.6% percentage points from a Burden Interview score of 0 to 76, its maximum.
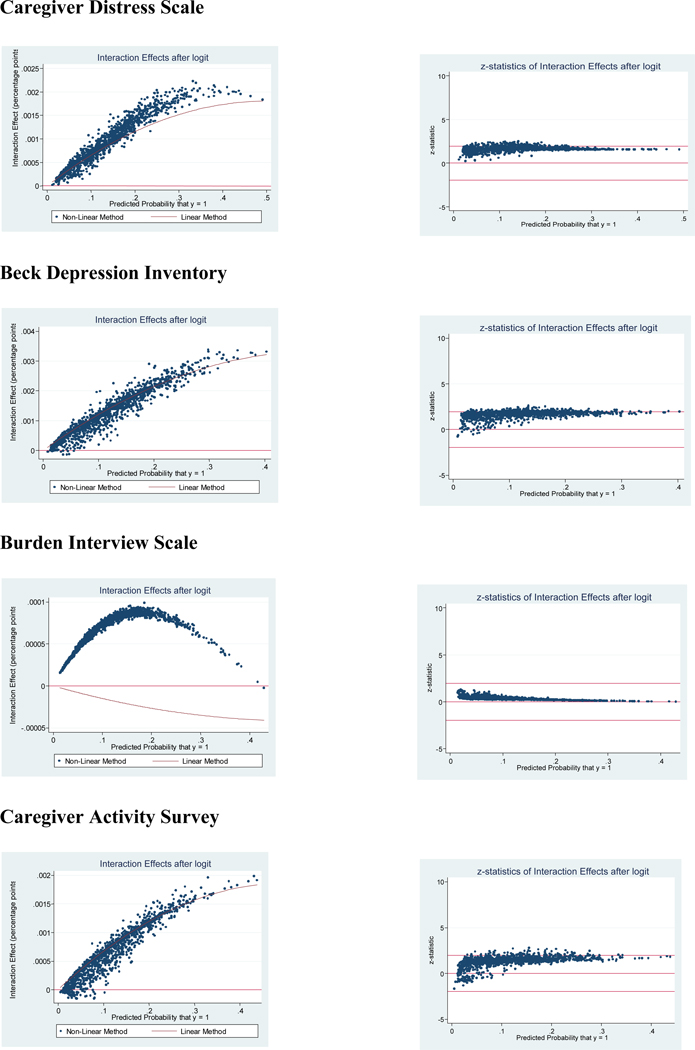
Table 5 reports interaction effects, standard errors, and z statistics across the average of all observations based on logistic regression models without random effects. Individual values are displayed in Figure 2 which reports two graphs for each of the four HUI-III*caregiver burden interaction models generated. Both plot the predicted probabilities for each observation on the x axis. The first compares the interaction effects calculated using the non-linear method to those calculated using the linear method employed above. The second plots the z-statistics along with the 95% confidence interval. In each model the average interaction term across all observations is positive, as expected (see Table 5). Furthermore, although there are some negative values, almost all of the interaction effects reported across all observations are positive; in three of the four models—Caregiver Distress, Beck Depression Inventory, and Caregiver Activity—interaction effects calculated using the non-linear approach tracks very closely with those calculated using the linear approach (see Figure 2). Based on the average z-statistic the average interaction effect deriving from the Caregiver Distress and Beck Inventory models are statistically significant at the .10 level (both z>1.64) (see Table 5). In contrast, those deriving from the Burden Interview and Caregiver Activity models are not statistically significant, though the former is much further from being so than the latter (z=0.34 and 1.34, respectively). Whereas none of the interaction effects in the Burden Interview model achieved statistical significance, a non-negligible number either achieve significance or approach doing so in the Caregiver Distress and Beck Depression inventory models, somewhat fewer in the Caregiver Activity model (see Figure 2).
TABLE 5.
Average Interaction Effects, Standard Errors, and Z-Statistics across All Observations*
| Mean | Std. Dev. | Minimum | Maximum | |
|---|---|---|---|---|
| Caregiver Distress Scale (n=1,355) | ||||
| Interaction Effect | .0008811 | .0005236 | −.0000196 | .002229 |
| Standard Error | .0005328 | .0003206 | .000046 | .00146 |
| Z statistic | 1.650879 | .2848747 | .2064624 | 2.52781 |
| Beck Depression Inventory (n=1,307) | ||||
| Interaction Effect | .0012551 | .0007612 | −.0001396 | .0033812 |
| Standard Error | .0007363 | .0004364 | .0000597 | .0026942 |
| Z statistic | 1.668147 | .3968817 | −.7687988 | 2.619478 |
| Burden Interview Scale (n=1,331) | ||||
| Interaction Effect | .000066 | .0000215 | −2.51e−06 | .000099 |
| Standard Error | .0002656 | .0001672 | .0000147 | .0007416 |
| Z statistic | .3368954 | .1763186 | −.0034622 | 1.314903 |
| Caregiver Activity Survey (n=1,313) | ||||
| Interaction Effect | .0006652 | .0004562 | −.0001555 | .001986 |
| Standard Error | .0004511 | .0002729 | .0000244 | .0013635 |
| Z statistic | 1.340527 | .5741552 | −1.678575 | 2.795955 |
Derived using the INTEFF command in Stata 11.0 based on logistic regressions without random effects
Figure 2. Interaction Effects and Z-Statistics with 95% CIs across All Observations*.
* Derived using the INTEFF command in Stata 11.0 based on logistic regressions without random effects. Two graphs are reported for each of the four HUI-III*caregiver burden interaction models generated. Both plot the predicted probabilities for each observation on the x axis. The first compares the interaction effects calculated using the non-linear method (scatter plot) to those calculated using the linear method (line). The second plots the z-statistics calculated for each observation along with the 95% confidence interval. Those z-statistics falling between −1.96 and +1.96 (the outer two lines) are statistically significant the .05 level.
DISCUSSION
We know of no previous study that has examined the moderating effect of caregiver burden on the relationship between general health status and institutional service use in AD. Findings support the hypothesis that caregiver burden would dampen the inverse relationship between health utilities and institutional service use identified previously, i.e. that at a given level of health, higher burden would lead to increased use of institutional services than might be expected based on the health utilities-institutional service use relationship alone. This conclusion is supported by positive, significant signs on the HUI-III*caregiver burden interaction when burden is measured using the Caregiver Distress Scale, Beck Depression Inventory, and Caregiver Assessment Survey (all p<.05). These models imply that the odds of institutional use increases from approximately −25.0% for each .10 increment in HUI-III score when caregiver burden is zero to −14.1% to −5.9% when caregiver burden is at its mean and up 11.6% when caregiver burden is at one standard deviation above.
Conclusions regarding the health utility, caregiver burden, and institutional service relationship are further reinforced by findings deriving from interactions calculated using the non-linear method. This is reflected in positive, significant average interactions in the Caregiver Distress and Beck Depression Inventory models (both p<.10). It also is reflected in predominately positive interactions across observations in all models. With the exception of the Burden Interview model there was close approximation between interaction effects calculated using both the linear and non-linear approaches as well. Taken together our findings provide evidence suggesting that caregiver burden moderates that relationship between patients’ general health status and institutional service use whereby the inverse association between HUI-III score and the likelihood of entering a nursing home, hospital, or residential care facility becomes less negative (i.e., more positive) as caregiver burden increases. This is consistent with previous research documenting similar relationships with respect to institutional service costs in AD (Miller, Schneider, and Rosenheck 2010).
Overall, the moderating effect of caregiver burden is most consistent when measured using the Beck Depression Inventory and Caregiver Distress Scale, followed by the Caregiver Assessment Survey. It is least consistent when measured using the Burden Interview Scale which although positive is non-significant and weak compared to the others. It is unclear why some burden measures might be stronger moderators than others. However, each indicator is intended to measure a somewhat different underlying construct, including subjective burden, caregiver depressive symptoms, distress with patient’s psychiatric symptoms, and time devoted to caregiving. That the Burden Interview Scale proved to be the weakest moderator suggests that it is caregivers’ clinical psychopathology – especially depression and distress -- and objective time demands, rather than subjective burden that influence the relationship between patient health and institutional service use the most.
Family caregivers play a critical role in keeping patients with AD and other dementia in the community (Alzheimer’s Association 2008). Greater caregiver burden is associated with increased probability of institutional placement (Balardy, et al. 2005; Cohen, et al. 1993; Cox 1998; Gaugler, et al. 2004; Hebert, et al. 2001). It also is associated with reduced use of preventive services that might preclude institutionalization down-the-road (Thorpe 2006). Yet despite their importance most interventions aimed at slowing down the trajectory toward hospital or nursing home use focus on the needs of patients themselves rather than the people who care for them (Miller, Allen and Mor 2009). Our findings suggest that additional interventions be directed toward informal caregivers because even patients with comparatively high health utilities may be at increased risk for institutionalization if caregivers are sufficiently depressed, distressed, or burdened. This lends further credence to the view that efforts to direct care away from institutions toward home- and community-based services will fail unless the informal caregiving network becomes engaged. It will require improving access to interventions developed to support informal caregivers caring for Alzheimer’s disease patients and other chronically ill and disabled individuals. Specific strategies include facilitating access to requisite information and supports through expansion of adult day, respite and in-home support programs, caregiver education, and training, case management, counseling and care coordination, consumer direction, service integration, chronic disease management, and as importantly, evaluation and improvement of programs such as these (Martin-Carrasco, et al. 2009; Miller, Allen and Mor 2009; Olazaran, et al. 2010; Pusey and Richards 2001; Thompson, et al. 2007; Tompkins and Bell 2009).
This is the first study to examine the moderating effect of caregiver burden on the relationship between HUI-III score and institutional service use. Several limitations are worth noting. First, although we used a longitudinal dataset, the results are associational, not causal, since the key variables in our study—caregiver burden, health utiliies, and service utilization—were collected simultaneously while we cannot be sure about the relative timing of other data collection which may have occurred earlier depending on the case.
Second, the findings may not be generalizeable to other populations of AD patients. All CATIE-AD participants had an active caregiver who were provided basic information and counseling by program staff. CATIE-AD also relied on a cohort of AD cases with substantial psychiatric or behavioral symptoms thought to potentially benefit from antipsychotic therapy.
Third, we employ a single institutional measure rather than separate measures for nursing home, inpatient hospital, and residential care use. Our general conclusions, however, would have been the same no matter whether separate measures were used or not. Furthermore, all three specific outcomes represent the same underlying concept—the inability to live at home due to comparatively high levels of care needs—thereby supporting our decision substantively.
Fourth, service use was measured during the month preceding assessment, leaving the previous two months unmeasured. We do not believe this to be a concern, however. This is because each key independent variable—caregiver burden and health utilities—was measured simultaneously with service use. It also is because it is more reliable to ask about service use over a 4 week period than a 12 week period.
Fifth, the health utilities data used in this study derived from proxy raters rather than from patients themselves. As a consequence, both the HUI-III and caregiver burden questionnaires are filled out by the same individuals, i.e., the caregivers. Though most AD studies use proxy raters, it remains uncertain whether caregiver proxy or patient provided quality of life information is most appropriate, especially since proxies routinely rate impairments higher than patients do, including when measured using health utilities (Naglie 2006). It is unlikely, however, that patient assessment would have been possible or appropriate among the advanced AD patients recruited for CATIE. Furthermore, even though caregivers are used to assess both caregiver burden and patient health, they are assessing very different things, one of which is inward looking (self-perceived burden, depression, distress, time commitments), the other of which is outward looking (patient’s health utilities). While not ideal, doing so is generally accepted in studies such as this where cognitive impairment limits patients’ ability to make judgments about their own experiences and to comprehend and respond to questionnaires.
CONCLUSION
Findings suggest that support and interventions should be provided to informal caregivers regardless of patient health status. This highlights the importance of broadening eligibility criteria for caregiver support to include individuals caring for people with less advanced AD or other dementia for even seemingly manageable patients may be at heightened risk for institutional placement depending on caregiver burden. Based on our results particularly salient criteria for basing eligibility decisions include depression, distress, and amount of time devoted to caregiving activities. Future research should examine whether caregiver burden moderates the relationship between institutional service use and other known correlates such as ADLs, MMSE score, and prior service use (Miller and Weissert 2000).
Key Points.
(1) Results suggest that caregiver burden weakens the inverse relationship between health utilities and institutional service use (i.e., hospitalization, nursing home, and residential care), leading to greater likelihood of institutionalization among patients with Alzheimer’s disease than would be expected at a given level of health; (2) The moderating effect of caregiver burden on the relationship between the Health Utilities Index (HUI)-III (a general health status measure) and caregiver burden is strongest when measured using the Beck Depression Inventory and Caregiver Distress Scale, followed by the Caregiver Assessment Survey, and weakest when measured using the Burden Interview Scale; (3) The odds of institutional placement increases from approximately −25.0% for each .10 increment in HUI-III score when caregiver burden is 0 to −5.9% to −14.1% when caregiver burden is at its mean, and up to 11.6% when caregiver burden is at one standard deviation above; and (4) Even patients with strong health utilities may be at increased risk for institutionalization if caregivers experience high enough levels of burden, suggesting that caregiver supports should be offered to those caring for AD patients regardless of patient health status.
Acknowledgments
Funding Sources: This study was supported by a grant (N01 MH9001) from the NIMH.
Footnotes
Disclosures: EAM: Has no disclosures to report. RAR: Has received research support from Eli Lilly, Janssen Pharmaceutica, Astra-Zeneca and Wyeth Pharmaceuticals. He has been a consultant to GlaxoSmithKline, Bristol Myers Squibb, Organon and Janssen Pharmaceutica. He provided expert testimony for the plaintiffs in UFCW Local 1776 and Participating Employers Health and Welfare Fund, et al. v. Eli Lilly and Company; for the respondent in Eli Lilly Canada Inc vs Novapharm Ltd and Minister of Health, respondent; and for the Patent Medicines Prices Review Board. Canada, in the matter of Janssen Ortho Inc. and “Risperdal Consta.” LSS: Has received research support from Astra-Zeneca, Bristol Myers Squibb, Eli Lilly, Johnson and Johnson, and Pfizer; and has been a consultant to Astra-Zeneca, Bristol Myers Squibb, Forest Laboratories, Johnson and Johnson, Lundbeck, Merz, and Pfizer, all manufacturers of antipsychotics and antidepressants related to CATIE-AD. He has served as an expert witness in litigation involving these drugs. He has been a consultant to Wyeth.
REFERENCES
- Ai C, Norton EC. Interaction terms in logit and probit models. Economic Letters. 2003;80(1):123–129. [Google Scholar]
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimers Demen. 2008;4(2):110–133. doi: 10.1016/j.jalz.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Bell CM, Araki SS, Neumann The association between caregiver burden and caregiver health-related quality of life in Alzheimer’s disease. Alzheimer Dis Assoc Disord. 2001;15(3):129–136. doi: 10.1097/00002093-200107000-00004. [DOI] [PubMed] [Google Scholar]
- Balardy L, Voisin T, Cantet C, Vellas B. Predictive factors of emergency hospitalization in Alzheimer’s patients: Results of one-year follow-up in the REAL.FR Cohort. J Nutr Health Aging. 2005;9(2):112–116. [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory of measuring depression. Arch Gen Psychiatry. 1961 June;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Chan DC, Kasper JD, Black BS, et al. Presence of behavioral and psychological symptoms predicts nursing home placement in community-dwelling elders with cognitive impairment in univariate but not multivariate analysis. J Gerontol: Med Sci. 2003;58A:548–554. doi: 10.1093/gerona/58.6.m548. [DOI] [PubMed] [Google Scholar]
- Cohen CA, Gold DP, Shulman KI, Wortley JT, McDonald G, Wargon M. Factors determining the decision to institutionalize dementing individuals: A prospective study. Gerontologist. 1993;33(6):714–720. doi: 10.1093/geront/33.6.714. [DOI] [PubMed] [Google Scholar]
- Cox C. Findings from a statewide program of respite care: A comparison of service users, stoppers, and nonusers. Gerontologist. 1998;37(4):511–517. doi: 10.1093/geront/37.4.511. [DOI] [PubMed] [Google Scholar]
- Coen RF, Swanwick GR, et al. Behaviour disturbance and other predictors of carer burden in Alzheimer's disease. Int J Geriatr Psychiatry. 1997;12(3):331–336. [PubMed] [Google Scholar]
- Cummings JL, Schneider L, et al. Reduction of behavioral disturbances and caregiver distress by galantamine in patients with Alzheimer's disease. Am J Psychiatry. 2004;161(3):532–538. doi: 10.1176/appi.ajp.161.3.532. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;144(12):2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Davis KL, Marin DB, Kane R, Patrick D, Peskind ER, Raskind MA, et al. The Caregiver Activity Survey (CAS): Development and validation of a new measure for caregivers of persons with Alzheimer’s disease. Int J Geriatr Psychiatry. 1997;12(10):978–988. doi: 10.1002/(sici)1099-1166(199710)12:10<978::aid-gps659>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Dunkin JJ, Anderson-Hanley A. Dementia caregiver burden: a review of the literature and guidelines for assessment and intervention. Neurology. 1998;51 Suppl. 1:S53–S60. doi: 10.1212/wnl.51.1_suppl_1.s53. [DOI] [PubMed] [Google Scholar]
- Draper BM, Poulos CJ, Cole AM, Poulos RG, Ehrlich F. A comparison of caregivers for elderly stroke and dementia victims. J Am Geriatr Soc. 1992;40(9):896–901. doi: 10.1111/j.1532-5415.1992.tb01986.x. [DOI] [PubMed] [Google Scholar]
- Feeny D, Furlong W, Torrance GW, et al. Multiattribute and single attribute utility functions for the Health Utilities Index Mark 3 system. Med Care. 2002;40(2):113–128. doi: 10.1097/00005650-200202000-00006. [DOI] [PubMed] [Google Scholar]
- Fillenbaum G, Heyman A, Peterson B, et al. Frequency and duration of hospitalization of patients with AD based on Medicare data: CERAD XX. Neurology. 2000;54(3):740–743. doi: 10.1212/wnl.54.3.740. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Galasko D, Bennett D, Sano S, et al. An inventory to assess Activities of Daily Living for clinical trials in Alzheimer’s disease. Alzheimer Dis Assoc Disord. 1997;22 Suppl:S33–S39. [PubMed] [Google Scholar]
- Gaugler JE, Edward AB, Femia EE, et al. Predictors of institutionalization of cognitively impaired elders: Family help and the timing of placement. J Gerontology: Pysch Sci. 2000;55B(4):P247–P255. doi: 10.1093/geronb/55.4.p247. [DOI] [PubMed] [Google Scholar]
- Gaugler JE, Leach CR, Clay T, et al. Predictors of nursing home placement in African Americans with dementia. J Am Geriatr Soc. 2004;52(3):445–452. doi: 10.1111/j.1532-5415.2004.52120.x. [DOI] [PubMed] [Google Scholar]
- Grafstrom M, Fratiglioni L, Sandman PO, Winblad B. Health and social consequences for relatives of demented and non-demented elderly. A population-based study. J Clin Epidemiol. 1992;45(8):861–870. doi: 10.1016/0895-4356(92)90069-y. [DOI] [PubMed] [Google Scholar]
- Grossberg GT. Diagnosis and treatment of Alzheimer’s disease. J Clin Psychiatry. 2003;64 suppl. 9:3–6. [PubMed] [Google Scholar]
- Haley WE. The family caregiver's role in Alzheimer's disease. Neurology. 1998;48(5 Suppl 6):S25–S29. doi: 10.1212/wnl.48.5_suppl_6.25s. [DOI] [PubMed] [Google Scholar]
- Hebert R, Dubois MF, Wolfson C, Chambers L, Cohen C. Factors associated with long-term institutionalization of older people with dementia: Data from the Canadian study of health and aging. J Gerontologly Med Sci. 2001;56A(11):M693–M699. doi: 10.1093/gerona/56.11.m693. [DOI] [PubMed] [Google Scholar]
- Hill J, Fillit H, Thomas SK, et al. Functional impairment, healthcare costs and the prevalence of institutionalization in patients with Alzheimer’s Disease and other dementias. Pharmacoeconomics. 2006;24(3):265–280. doi: 10.2165/00019053-200624030-00006. [DOI] [PubMed] [Google Scholar]
- Hux MJ, O’Brien BJ, Iskedjian M, et al. Relation between severity of Alzheimer’s disease and costs of caring. Canadian Medical Association Journal. 1998;159(5):457–465. Alzheimer’s disease. Journal of Mental Health and Aging 1999;5:33–48. [PMC free article] [PubMed] [Google Scholar]
- Jaccard J. Interaction seffects in logistic regression (Series/Number 07–135) Thousand Oaks, California: Sage Publications; 2001. [Google Scholar]
- Lima VD, Kopec JA. Quantifying the effect of health status on health care utilization using a preference-based health measure. Soc Sci Med. 2005;60(3):515–524. doi: 10.1016/j.socscimed.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Martín-Carrasco M, Martín MF, Valero CP. Effectiveness of a psychoeducational intervention program in the reduction of caregiver burden in Alzheimer's disease patients' caregivers. Int J Geriatr Psychiatry. 2009;24(5):489–499. doi: 10.1002/gps.2142. [DOI] [PubMed] [Google Scholar]
- Mausbach BT, Coon DW, Depp C, et al. Ethnicity and time to institutionalization of dementia patients: A comparison of Latina and Caucasian female caregivers. J Am Geriatr Soc. 2004;52(7):1077–1084. doi: 10.1111/j.1532-5415.2004.52306.x. [DOI] [PubMed] [Google Scholar]
- Miller EA, Allen SM, Mor V. Navigating the Labyrinth of Long-Term Care: Shoring Up Informal Caregiving in a Home- and Community-Based World. J Aging Soc Policy. 2009;21(1):1–16. doi: 10.1080/08959420802473474. [DOI] [PubMed] [Google Scholar]
- Miller EA, Rosenheck RA, Schneider LS. Assessing the relationship between health utilities, quality of life, and health care costs in Alzheimer’s Disease: The CATIE-AD Study. Curr Alzheimer Res. 2009 doi: 10.2174/156720510791162386. Epub ahead of pring. [DOI] [PubMed] [Google Scholar]
- Miller EA, Rosenheck RA, Schneider LS. Caregiver burden, health utilities, and institutional service costs among community-dwelling patients with Alzheimer’s Disease. Alzheimer Dis Assoc Disor. 24(4):280–289. doi: 10.1097/WAD.0b013e3181eb2f2e. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EA, Schneider LS, Rosenheck RA. Assessing the relationship between health utilities, quality of life, and health services use in Alzheimer’s disease. Int J Geriatr Psychiatry. 2009;24(1):96–105. doi: 10.1002/gps.2160. [DOI] [PubMed] [Google Scholar]
- Miller EA, Schneider LS, Zbrozek A, Rosenheck R. Sociodemographic and clinical correlates of utility scores in Alzheimer’s Disease. Value Health. 2009;11(7):1120–1130. doi: 10.1111/j.1524-4733.2008.00351.x. [DOI] [PubMed] [Google Scholar]
- Miller EA, Weissert WG. Predicting elderly people’s risk for nursing home placement, hospitalization, functional impairment, and mortality: A synthesis. Med Care Res Rev. 2000;57(3):259–297. doi: 10.1177/107755870005700301. [DOI] [PubMed] [Google Scholar]
- Naglie G, Tomlinson G, Tansey C, et al. Utility-based quality of life measures in Alzheimer’s disease. Qual Life Res. 2006;15(4):631–643. doi: 10.1007/s11136-005-4364-8. [DOI] [PubMed] [Google Scholar]
- Norton EC, Wang H, Ai C. Computing interaction effects and standard errors in logit and probit models. The Stata Journal. 2004;4(2):154–167. [Google Scholar]
- Olazarán J, Reisberg B, Clare L, et al. Nonpharmacological therapies in Alzheimer's disease: a systematic review of efficacy. Dement Geriatr Cogn Disord. 2010;30(2):161–178. doi: 10.1159/000316119. [DOI] [PubMed] [Google Scholar]
- Phillips VL, Diwan S. The incremental effect of dementia-related problem behaviors on the time to nursing home placement in poor, frail, demented older people. J Am Geriatr Soc. 2003;51(2):188–193. doi: 10.1046/j.1532-5415.2003.51057.x. [DOI] [PubMed] [Google Scholar]
- Porell FW, Carter M. Discretionary hospitalization of nursing home residents with and without Alzheimer’s Disease: A multilevel analysis. J Aging Health. 2005;17(2):207–238. doi: 10.1177/0898264304274302. [DOI] [PubMed] [Google Scholar]
- Pusey H, Richards D. A systematic review of the effectiveness of psychosocial interventions for carers of people with dementia. Aging Ment Health. 2001;5(2):107–119. doi: 10.1080/13607860120038302. [DOI] [PubMed] [Google Scholar]
- Rabins PV, Kasper JD, Kleinman L, et al. Concepts and methods in the development of the ADRQL: An instrument for assessing health-related quality of life in persons with Alzheimer’s disease. Journal of Mental Health and Aging. 1999;5:33–48. [Google Scholar]
- Rosenheck RA, Leslie DL, Sindelar J, Miller EA, Tariot PN, Dagerman KS, et al. Cost-benefit analysis of second-generation antipsychotics and placebo in a randomized trial of the treatment of psychosis and aggression in Alzheimer Disease. Arch Gen Psychiatry. 2007;64(11):1259–1268. doi: 10.1001/archpsyc.64.11.1259. [DOI] [PubMed] [Google Scholar]
- Schneider LS, Tariot PN, Lyketsos CG, et al. National Institute of Mental Health Clinical Antipsychotic Trials in Intervention Effectiveness (CATIE): Alzheimer’s disease trial methodology. Am J Geriatr Psychiatry. 2001;9(4):1–14. [PubMed] [Google Scholar]
- Schneider LS, Tariot PN, Dagerman KS, Davis SM, Hsiao JK, Ismail MS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. New Engl J Med. 2006;355(15):1525–1538. doi: 10.1056/NEJMoa061240. [DOI] [PubMed] [Google Scholar]
- Small GW, McDonnell DD, Brooks RL, et al. The impact of symptom severity on the cost of Alzheimer’s Disease. J Am Geriatr Soc. 2002;50(2):321–327. doi: 10.1046/j.1532-5415.2002.50065.x. [DOI] [PubMed] [Google Scholar]
- Song LY, Biegel DE, Milligan SE. Predictors of depressive symptomatology among lower social class caregivers of persons with chronic mental illness. Community Ment Health J. 1997;33(4):269–286. doi: 10.1023/a:1025090906696. [DOI] [PubMed] [Google Scholar]
- Thorpe J, Sleath BL, Thorpe K, Van Houtven CH, Blalock SJ, Landerman LR, et al. Caregiving psychological distress as a barrier to influenza vaccination among community-dwelling elderly with dementia. Med Care. 2006;44(8):713–721. doi: 10.1097/01.mlr.0000215905.36968.76. [DOI] [PubMed] [Google Scholar]
- Tompkins SA, Bell PA. Examination of a psychoeducational intervention and a respite grant in relieving psychosocial stressors associated with being an Alzheimer's caregiver. J Gerontol Soc Work. 2009;52(2):89–104. doi: 10.1080/01634370802561877. [DOI] [PubMed] [Google Scholar]
- Thompson CA, Spilsbury K, Hall J, et al. Systematic review of information and support interventions for caregivers of people with dementia. BMC Geriatr. 2007 July 27;7:18. doi: 10.1186/1471-2318-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UCLA Statistical Consulting Group. [(accessed July 10, 2010)];How Can I Understand a Continuous by Continuous Interaction in Logistic Regression ? (Stata 11) 2010 Available at: http://www.ats.ucla.edu/stat/stata/faq/logitconcon.htm. [Google Scholar]
- Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one’s physical health? A meta-analysis. Psychol Bull. 2003;129(6):946–972. doi: 10.1037/0033-2909.129.6.946. [DOI] [PubMed] [Google Scholar]
- Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20(6):649–655. doi: 10.1093/geront/20.6.649. [DOI] [PubMed] [Google Scholar]