Abstract
The most important quality for muskmelon (Cucumis melo L.) is their sweetness which is closely related to the soluble sugars content. Leaves are the main photosynthetic organs in plants and thus the source of sugar accumulation in fruits since sugars are translocated from leaves to fruits. The effects of grafting muskmelon on two different inter-specific (Cucurbita maxima×C. moschata) rootstocks was investigated with respect to photosynthesis and carbohydrate metabolism. Grafting Zhongmi1 muskmelon on RibenStrong (GR) or Shengzhen1 (GS) rootstocks increased chlorophyll a, chlorophyll b and chlorophyll a+b content and the leaf area in middle and late developmental stages of the plant compared to the ungrafted Zhongmi1 check (CK). Grafting enhanced the net photosynthesis rate, the stomatal conductance, concentration of intercellular CO2 and transpiration rate. Grafting influenced carbohydrates contents by changing carbohydrate metabolic enzymes activities which was observed as an increase in acid invertase and neutral invertase activity in the functional leaves during the early and middle developmental stages compared to CK. Grafting improved sucrose phosphate synthase and stachyose synthase activities in middle and late developmental stages, thus translocation of sugars (such as sucrose, raffinose and stachyose) in GR and GS leaves were significantly enhanced. However, compared with CK, translocation of more sugars in grafted plants did not exert feedback inhibition on photosynthesis. Our results indicate that grafting muskmelon on inter-specific rootstocks enhances photosynthesis and translocation of sugars in muskmelon leaves.
Keywords: Chlorophyll content, Photosynthesis, Raffinose family oligosaccharides (RFOs), Carbohydrate metabolism, Grafting, Muskmelon
Introduction
Muskmelon is one of the top ten fruit crops grown widely in the world for their delicious sweetness, high nutrient quality and flavor 1, 2. However, owing to large market requirements for off season cucurbits and limited availability of arable land, muskmelons are continuously cultivated under less than ideal conditions worldwide including environments that are too wet, cold and dry, or in cool low-light winter greenhouses such as the conditions prevalent in northern regions of China 3, 4. Successive cropping can also increase salinity, the incidence of cucurbit pests, and serious devastating soil-borne diseases such as muskmelon wilt (Fusarium oxysporum f. melonis) and root-knot nematodes (Meloidogyne spp) that can result in severe crop loss 5-7. Chemical control can be expensive and is not always effective. Furthermore chemical control could also harm the environment 4.
To overcome some of these problems described above, grafting muskmelon on a suitable rootstock is widely being adapted globally, to manage soil-borne diseases of muskmelon and the continuous cultivation barriers 8-10. Grafted vegetables have been cultivated in eastern Asia for decades 5, but their adoption all over the world has only begun since the banning of the fumigant methyl bromide in 2005 by the Montreal Protocol 11. Numerous researchers have confirmed that grafting on rootstocks belonging to a different genera can alter muskmelon fruit quality in various ways 5, 12-14. For instance, total carbohydrate contents in grafted muskmelon and watermelon were reduced significantly compared with self-rooted plants 4, 15, 16 thus, influencing muskmelon fruit flavor and other quality such as sweetness 17, 18. It is well known that leaves, the main photosynthetic organ in plants, are the source of carbohydrates accumulation in fruits and supply carbon for synthesis of sugars and carbohydrate metabolism. Leaf photosynthesis is pivotal for fruit growth and quality. The change in chlorophyll content, photosynthetic rates and carbohydrate partitioning in source leaves can alter photoassimilates export rates, which are directly related to carbohydrate accumulation in fruits that act as the sink 19-21. The plants in Cucurbitaceae family transport stachyose, and some partial sucrose and raffinose in phloem tissue 22-25, thus sucrose and stachyose metabolism in muskmelon leaves play a central role in carbohydrate synthesis. Photosynthesis and sucrose or stachyose metabolic enzymes activities concern carbohydrate synthesis capacity in leaves, while carbohydrate accumulation in leaves will be determined by the carbohydrate synthesis, loading and transportation levels 26. Meanwhile carbohydrate accumulation levels in leaves will feedback regulate photosynthesis and the activities of carbohydrate metabolism related enzymes 27. The objectives of this study were to evaluate the effects of grafting on photosynthesis and carbohydrate metabolism in muskmelon leaves grafted on inter-specific hybrid rootstocks.
Materials and Methods
Plant materials and sampling
Seeds of 'Zhongmi1' a popular muskmelon (Cucumis melo L.), cultivar (commercial maturity 40 days after anthesis [DAA] roughly) were kindly provided by Mr. Huaisong Wang (Chinese Academy of Agricultural Sciences, CAAS) and used as scion in all experiments. Rootstocks (Cucurbita maxima×C. moschata 'RibenStrong' and 'Shengzhen1') were grown in a sand/soil/peat (1:1:1 by volume) mixture. For grafting, rootstock seeds were sown for 7 days to produce seedlings with approximately the same size of hypocotyls as that of muskmelon. Tongue approach grafting was carried out at the two-leaf stage of the muskmelon seedlings. Ungrafted plants (self-rooted control), Zhongmi1/RibenStrong, Zhongmi1/Shengzhen1 graft combinations were named as CK, GR and GS respectively. Approximately 20 days after grafting, the seedlings were transplanted into plastic pots (with upper diameter, base diameter and height of 30, 24 and 25.5 cm, respectively) with NPK (2:1:2) fertilizer in a Liaoshen-2 solar greenhouse (Patent right possessed by High-efficiency Industrialized Agricultural Engineering Technology Research Center of Liaoning Province). All plants were managed as using standard production practices followed in northern regions of China 4. To identify fruit of known age, freshly opened female flowers were tagged on the day of hand-pollination and one fruit per plant was allowed to develop at 12th node. Leaves from fruit-carrying nodes in each treatments were collected on 8, 16, 24, 32, 40 (fruit maturity) and 48 DAA (delayed fruit harvest period), between 10:00 am and 12:00 am, frozen in liquid nitrogen and stored at -80°C for carbohydrates and enzymatic activity assessment. All experiments were repeated three times with three replicates for each analysis.
Photosynthetic measurements
Net photosynthetic rates, stomatal conductance, concentration of intercellular CO2 and transpiration rate of leaves from fruit-carrying nodes in each treatment were measured on 8, 16, 24, 32, 40 and 48 DAA with a portable photosynthesis system (Li-Cor 6400; Li-Cor Inc., Nebraska, USA), between 9:00 am and 11:00 am with constant irradiation (600 μmol photons·m-2·s-1, PAR). Each leaf was equilibrated in the leaf chamber for at least 1 min before a measurement was taken. And leaf area was measured with a LI-3100 A (Li-Cor Inc.). Three plants were examined and the means were calculated across replicates.
After collecting photosynthetic measurements, 0.25 g of fresh leaves were placed in a 100 ml test tube for the determination of the chlorophyll content, then 10-15 ml pure methanol was added, and homogenized with a polytron. The homogenate was then filtered and made up to 100 ml with pure methanol. The chlorophyll concentration in the supernatant was spectrophotometrically determined by measuring the absorbances at 652.0 and 665.2 nm for chlorophyll a and chlorophyll b, respectively, and calculated according to Porra's method. 28. All experiments were repeated three times with three replicates.
Soluble carbohydrate analysis
Soluble carbohydrates in leaves were extracted with 80% (v/v) ethanol (15 ml per 6 g sample FW) at 80 ℃ for 1 h. Ethanol extracts were collected and the pellets re-extracted twice using the same method. The extracts were subsequently cleaned via a Waters Sep-Pak column (C18, Accell Plus QMA and Accell Plus CM), combined and dried in a centrifugal evaporator (MAXI dry Lyo). The dry extracts were dissolved in 500 μL ultra-pure water, filtered through an acetate filter (0.22 μm pore size, Nalgene), and 20 μl samples were analyzed for sugar content by HPLC using a Waters 600 controller fitted with a Luna 5U NH2 100R column (Phenomenex Separation Products, USA), Waters 2410 Refractive Index Detector, Waters In-Line Degasser AF, and Waters 600 pump as previously described 20. The separations were performed at 35 ℃ and eluted with 75/25 (v/v) acetonitrile/H2O at a flow rate of 1 ml·min-1. Fructose (Product No. 31140), glucose (Product No. G5400), sucrose (Product No. 84099), raffinose (Product No. R0250), galactinol (Product No. 79544) and stachyose (Product No. S4001) were determined by co-elution with standards (SIGMA-ALDRICH Co., 3050 Spruce Street, St. Louis, MO63103 USA). According to retention time to distinguish different kinds of sugars, fructose, glucose, sucrose, raffinose, galactinol and stachyose were detected at 4.858 min, 5.315 min, 6.710 min, 11.664 min, 12.885 and 21.923 min in Auto-Scaled Chromatogram of HPLC, respectively. Waters Millennium software was used for controlling and data processing.
Enzyme extractions and activity assays
Leaf samples were finely ground in liquid nitrogen using a chilled mortar and pestle. Approximately 1g of the ground tissue was suspended in 1 ml of ice-cold extraction buffer containing 50 mM HEPES-NaOH (pH 7.5), 10 mM ascorbic acid, 2.5 mM dithiothreitol (DTT), 10 mM MgCl2, 10% (v/v) ethylene glycol (pH 7.5), and 1 mM EDTA. The extracts were filtered through cheesecloth, and centrifuged at 26,000 g (unit of speed) for 20 min at 4°C. The supernatant was desalted by dialyzing for more than 20 h at 4°C.
Acid invertase (AI) and neutral invertase (NI) (EC 3.2.1.26) activities were assayed in a final volume of 25 ml, containing 0.2 ml of dialyzed enzymatic extract, 0.8 ml of reaction solution contained 100 mM Na2HPO4, 100 mM sodium citrate, 100 mM sucrose, and pH 4.8 or 7.2 for acid invertase and neutral invertase, respectively. The activities were measured by the quantity of reducing sugars released in the assay media with dinitrosalicylic acid. The reducing sugars were revealed by incubation at 100°C for 5 min and read at 520 nm in a Cary 100UV-VIS spectrophotometer (Varian, USA) 29.
Sucrose synthase (SS) (EC 2.4.1.13) activity was measured by using 0.4 ml reaction mixture contained 50 mM fructose, 0.82% UDPG, 100 mM Tris, 10 mM MgCl2 adding 0.2 ml enzyme at 37°C for 30 min and bathing for 1 min at 100°C, and a volume of 1 ml reaction products adding 0.1 ml 2 M NaOH was placed in boiling water bath for 10 min and cooled in water, then adding 3.5 ml 30% HCl and 1 ml 0.1% resorcinol. Blank controls were obtained by adding the distilled water to the reaction medium containing resorcinol. The reducing sugars were revealed by incubation at 80°C for 10 min and read at 480 nm in a Cary 100UV-VIS spectrophotometer (Varian, USA). Sucrose phosphate synthase (SPS) (EC 2.4.1.14) was assayed by measurement of sucrose produced from fructose 6-phosphate plus UDP-glucose30.
The alkaline α-galactosidase (AGA) (EC 3.2.1.22) was assayed using p-nitrophenyl-α-galactosidase as substrate. The reaction mixture contained 5 mM p-nitrophenyl-α-galactosidase and 100 mM HEPES buffer (pH 7.5) for alkaline form activity. Reaction was started by adding 30 μl enzyme extract and terminated after 20 min by adding 1 ml 5% (w/v) Na2CO3. The enzyme activity was expressed as micromoles of nitrophenol formation per minute by reading at 410 nm in a Cary 100UV-VIS spectrophotometer (Varian, USA) 31.
Stachyose synthase activity were determined as previously described 32 with minor modifications. Dialyzed 100 ul extract was added to 100 μl reaction buffer containing 50 mM HEPES-NaOH (pH 7.0), 20 mM 2-mercaptoethanol, 10 mM galactinol and 40 mM raffinose. Duplicate measurements were made for each extract sample. Controls were similar but with the omission of galactinol. Reaction mixtures were incubated at 25°C for 90 min and the reactions were terminated by the addition of 100 μl of 0.1 M NaOH. The mixtures were then heated for 30 s in boiling water and cooled to 25°C for 40 min in the presence of 2 mM NAD, 0.1 U myo-inositol dehydrogenase, and 50 mM Na2CO3 (pH 9.5) in a total reaction volume of 1.0 ml. Reduction of NAD was measured spectrophotometrically at 340 nm in a Cary 100UV-VIS spectrophotometer (Varian, USA).
Statistical Analysis
SPSS 13.0 and Excel 2007 were used for data analysis and graphing.
Results and Discussions
One of the most important qualities of fruits is sweetness, which is closely related to the soluble sugar content and therefore carbohydrate metabolism plays a pivotal role in development of muskmelon fruit 4, 18. It is well known that fruit development is dependent largely upon the supply of photoassimilates imported into the fruit via the phloem from leaves 33. Carbohydrate accumulation in leaves is a comprehensive reflection of three respects in carbohydrate synthesis, metabolic transformation, loading and export. The productivity and metabolic capacity of the photoassimilates in leaves will exert a direct influence on the loading and export for the carbohydrate in leaves, critically important to the carbohydrate accumulation in fruits. Photosynthesis and carbohydrate metabolic enzymes activities concern carbohydrate synthesis capacity in leaves, while carbohydrate accumulation in leaves will be determined by the carbohydrate synthesis capacity, loading and transportation level. Meanwhile carbohydrate accumulation levels in leaves will feedback regulate photosynthesis and the activities of carbohydrate metabolism related enzymes 27. Therefore, the present study on the effects of grafting muskmelon on inter-specific hybrid rootstocks on photosynthesis and carbohydrate metabolism in muskmelon leaves is important to help realize high yield and quality.
Effect of grafting on chlorophyll content and photosynthesis level in muskmelon leaves
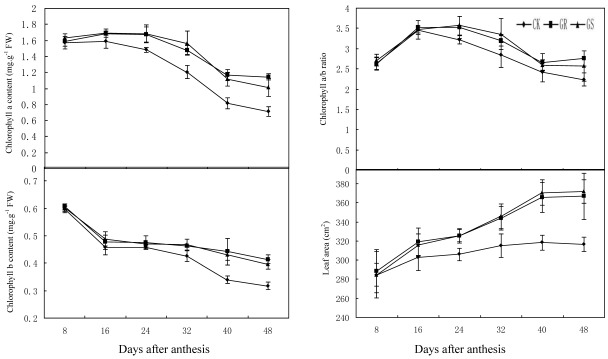
Chloroplast, the fundamental substance for photosynthesis, functions by absorbing and transferring light energy. Chlorophyll content, which displays a direct impact on the photosynthetic efficiency in leaves, is a key index in photosynthetic capacity 2. Compared to most other Suc-translocating plants, photoassimilates in translocation stream of muskmelon is mainly composed of stachyose, along with raffinose and sucrose 34, 35. As a result sucrose from photosynthesis in leaves will have to be converted into stachyose by a series of enzyme catalysis, and then they can be loaded to phloem for long distance transport to the fruits. Since sucrose is the substrate of stachyose synthesis, the level of photosynthesis also will influence stachyose metabolism in leaves. As this experiment indicates, grafting on inter-specific rootstocks significantly increased chlorophyll a content during 24-48 DAA, chlorophyll b content during 32 to 48 DAA and chlorophyll a+b content during 24-48 DAA (Fig. 1). However, grafting did not change the chlorophyll a/b ratio throughout the developmental stage (Fig. 1). In addition, grafting increased leaf area dramatically during 24-48 DAA (Fig. 1).
Fig 1.
Effect of grafting on chlorophyll content, chlorophyll a/b ratio and leaf area in muskmelon leaves.
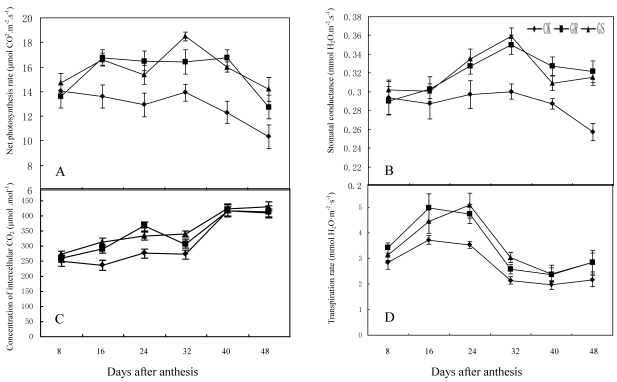
These results were similar to previous studies on grafted cucumber 36, 37. Grafting significantly enhanced net photosynthesis rates during 16-48 DAA, stomatal conductance during 24-48 DAA and concentration of intercellular CO2 or transpiration rate during 16 to 32 DAA (Fig. 2). These findings were similar to the results on grafted oriental melon, watermelon, and citrus 38-40. Grafting can improve net photosynthesis rate and enhance assimilate accumulation and thus enhancing growth potential and dry matter accumulation in roots, stems, leaves and fruits. Grafting, which improves stomatal conductance and intercellular CO2 concentration, will strengthen the transfer capability of photosynthetic substrates and the supply capability of photosynthetic materials to ensure increased photosynthesis efficiency. Grafting on inter-specific rootstocks can boost transpiration rates in the leaves thus improving water and mineral nutrient absorption capacity in plants. Grafting can remarkably increase net photosynthetic rates in leaves of muskmelon, due to increased chlorophyll content and leaf area, similar to what has been observed in grafted cucumbers 41, 42. Further studies to determine the detailed effects of grafting on photosynthesis in muskmelon leaves are still needed.
Fig 2.
Effect of grafting on net photosynthesis rate (A), stomatal conductance (B), concentration of intercellular CO2 (C) and transpiration rate (D) in muskmelon leaves.
Effect of grafting on carbohydrate contents in muskmelon leaves
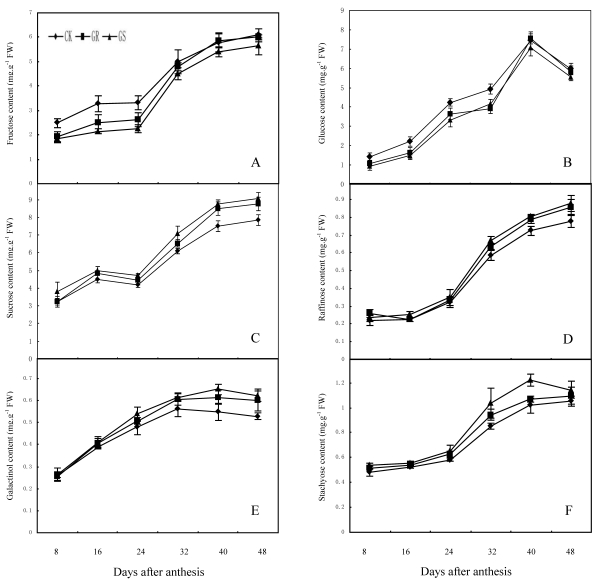
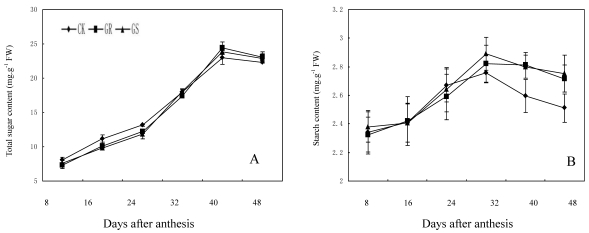
In many plant species, such as arabidopsis, tomato, soybean, maize, sugar beet or tobacco, assimilated CO2 is exported exclusively in the form of sucrose. However, in addition to sucrose, raffinose and stachyose are also used for long distance transport in Cucurbitaceae 26, 35. Carbohydrates from photosynthesis in leaves will generate a large amount of translocation carbohydrates such as stachyose, partial raffinose and sucrose from stachyose metabolism, and then they will be loaded into the phloem for long distance transport to every sink organ in plant 43. Currently available studies have confirmed that for a plant with RFOs-dominant translocation, loading and transportation in its phloem should follow symplastic pathway 44, 45. Among numerous hypotheses supportive for such a pathway, the 'polymer trap model' maintains that sucrose synthesized in mesophyll cells enters the intermediary cells (specialized CCs in minor veins) by diffusion through specialized, highly branched plasmodesmata. Inside the intermediary cells, sucrose and galactinol are used in the synthesis of RFOs. Since the diameter of those RFOs molecules is larger than the pore size of plasmodesmata between mesophyll cells and intermediate cells, carbohydrate backflow to the mesophyll cells may be prevented. However, the single branch plasmodesmata pore units between intermediate cells and sieve element can accommodate the passage of these carbohydrate molecules and their flow into the sieve elements 26, 44. The present study showed that fructose, glucose and sucrose contents in functional leaves were 6.09 mg·g-1, 7.53 mg·g-1 and 9.04 mg·g-1 respectively, while the contents of galactinol, raffinose and stachyose were slightly low and <1.25 mg·g-1 in all treatments (Fig. 3). The lower content of galactinol may be due to its utilization during synthesis of sugars such as stachyose and raffinose. Meanwhile the lower content of raffinose and stachyose may be significantly related to a large amount of raffinose and stachyose loading and export after being synthesized. In addition, grafting reduces the fructose and glucose content in the leaves during the early and middle developmental stages while it accelerates the synthesis and accumulation in terms of sucrose, galactinol, raffinose and stachyose during middle and later developmental stages. Grafting on inter-specific rootstocks can reduce total carbohydrate accumulation during early development and increase starch accumulation in the later developmental stage of leaves. The changes in carbohydrate contents in leaves can be considered as comprehensive reflections of carbohydrate synthesis, metabolic transformation, loading and export in grafted muskmelon plants.
Fig 3.
Effect of grafting on fructose (A), glucose (B), sucrose (C), raffinose (D), galactinol (E) and stachyose (F) contents in muskmelon leaves.
Effect of grafting on carbohydrate-metabolizing enzymes in muskmelon leaves
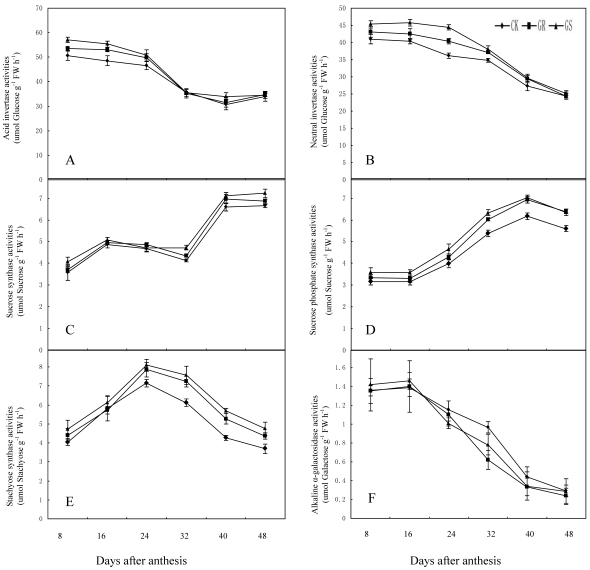
We elucidated the response of enzymes involved in carbohydrate metabolism in muskmelon leaves grafted on inter-specific rootstocks to gain a better understanding on the effects of grafting on carbohydrate metabolism. There have been some reports on sucrose metabolism in leaves so far only 29, 46. The key enzymes in sucrose metabolism include sucrose synthase, sucrose phosphate synthase and invertase 47. Of these, sucrose phosphate synthase is the enzyme necessary for sucrose synthesis, and invertase is involved in sucrose decomposition. Sucrose synthase, the third enzyme can not only catalyze sucrose synthesis, but can decompose sucrose as well 48. The raffinose family oligosaccharides (RFOs) metabolism related enzymes mostly inculde galactinol synthase, raffinose synthase and stachyose synthase. Galactinol synthase is the enzyme in the first step of catalytic synthesis for the raffinose family oligosaccharides 49. Raffinose synthase is a key enzyme in converting sucrose into raffinose 50. And stachyose synthase, a kind of soluble enzyme existing in cytoplasm, will catalyze raffinose into stachyose 51. Therefore, the activities of carbohydrate-metabolizing enzymes in leaves are crucially important to the carbohydrate synthesis, transportation and accumulation in muskmelon 52. Photosynthetic carbon metabolism in muskmelon leaves is thought to be one of the important factors in soluble sugar synthesis, plant growth and fruit yield. However, there are few research reports about the influence of grafting on carbohydrate metabolism in muskmelon leaves. In the present study, grafting muskmelons on inter-specific rootstocks did not change the general trend of carbohydrate metabolism related enzyme activity in leaves during development (Fig. 5A-F). However, grafting muskmelon on inter-specific rootstock increased acid invertase and neutral invertase activities in leaves during the early and middle developmental stages with more pronounced effects on acid invertase than neutral invertase. However, grafting reduced fructose and glucose contents in leaves in the early and middle developmental stages. One explanation for this could possibly be because more glucose and fructose were being utilized for the leaf growth in GR's or GS's (Fig. 3A-B). Grafting increased sucrose synthase activity in the later developmental stage compared to CK (Fig. 5C). Sucrose phosphate synthase and stachyose synthase activity were higher during the middle and later developmental stages in grafted muskmelon leaves compared to CK (Fig. 5D-E). Grafting had slight influence on alkaline α-galactosidase activity (Fig. 5F), a fact favorable for stachyose production in muskmelon leaves (Fig. 3F). During 8 to 40 DAA increased stchyose levels was noted in GS whereas, a similar increase in stachyose levels was only observed on 32 DAA for GR. This indicates that the carbohydrate loading and export levels of GR are higher than those of GS. Experiments are underway to determine if the carbohydrates loading and export capability for GS and GR are remarkably higher than that of CK.
Fig 5.
Effect of grafting on acid invertase (A), neutral invertase (B), sucrose synthase (C), sucrose phosphate synthase (D), stachyose synthase (E) and alkaline α-galactosidase (F) activities in muskmelon leaves.
The accumulation and export of photosynthates in leaves is another major influential element on photosynthesis 53. Our results indicate that the grafted muskmelon leaves generate and accumulate more translocating sugars such as sucrose, raffinose and stachyose than the ungrafted (CK). Though the carbohydrate supply capacity was greater in grafted muskmelons, it did not exert a feedback inhibition on photosynthesis. This could most likely be due to appropriate photoassimilates contents in leaves. Thus it should follow the hypothesis of inhibition threshold of photosynthetic product 54, 55. This hypothesis suggests that though there is photoassimilates feedback inhibition in plants, such an inhibition may only exist when photoassimilates accumulate to very high level. Under stress free conditions, carbohydrates contents in leaves for many plants do not exceed their inhibition threshold of photosynthetic product 54, 56, 57. Grafting increased the translocating sugars content in muskmelon leaves and at the same time also improved photosynthetic capacity in grafted plants without exceeding the inhibition threshold.
In conclusion the increase in photosynthesis and carbohydrate metabolism due to grafting has the potential to improve fruit quality and yield in muskmelon. Breeding and developing appropriate rootstock and scion combinations can significantly help improve fruit quality and yield.
Fig 4.
Effect of grafting on total sugar (A) and starch contents (B) in muskmelon leaves.
Acknowledgments
The studies were funded by National Science Support Item, China (2006BAD07B04), National Natural Science Foundation of China (30972000), Major Scientific and Technological Project of Liaoning Province, China (2006215001) and Natural Science Fund of Liaoning Province, China (20062112). We also wish to acknowledge Mr. Huaisong Wang of the Chinese Academy of Agricultural Sciences (CAAS) for kindly providing the scion cultivar worked with.
References
- 1.Cheng ZJ, Wang HS, Zhang ZB. et al. Genetic diversity of melon (Cucumis melo L.) germplasm based on AFLPs. Acta Bot Boreali-Occidentalia Sinica. 2007;27:244–248. [Google Scholar]
- 2.Keiko O, Akio U, Tomoko T. et al. Enrichment of sugar content in melon fruits by hydrogen peroxide treatment. J Plant Physiol. 2009;166:569–578. doi: 10.1016/j.jplph.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Song WY, Zhang ZB, Shao HB. et al. Relationship between calcium decoding elements and plant abiotic-stress resistance. Int J Biol Sci. 2008;4:116–125. doi: 10.7150/ijbs.4.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu YF, Li TL, Qi HY. et al. Effects of grafting on carbohydrate accumulation and sugar-metabolic enzyme activities in muskmelon. Afr J Biotechnol. 2010;9:25–35. [Google Scholar]
- 5.Lee JM, Oda M. Grafting of herbaceous vegetable and ornamental crops. Hort Rev. 2003;28:61–124. [Google Scholar]
- 6.Pavlou G.C, Vakalonnakis DJ, Ligoxigakis EK. Control of root and stem rot of cucumber, caused by F. oxysporum f. sp radicis cucumerinum, by grafting onto resistant rootstocks. Plant Dis. 2002;86:379–382. doi: 10.1094/PDIS.2002.86.4.379. [DOI] [PubMed] [Google Scholar]
- 7.Shao HB, Chu LY, Lu ZH. et al. Primary antioxidant free radical scavenging and redox signaling pathways in higher plant cells. Int J Biol Sci. 2008;4:8–14. doi: 10.7150/ijbs.4.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angela RD, Penelope PV, Richard H. et al. Grafting effects on vegetable quality. HortScience. 2008;43:1670–1672. [Google Scholar]
- 9.Lee JM. Cultivation of grafted vegetables I: Current status, grafting methods and benefits. HortScienc. 1994;29:235–239. [Google Scholar]
- 10.Zhang JM, Ge ZD. Effects of grafting application on muskmelon. Chin Cucurbit Veg. 2002;1:26–27. [Google Scholar]
- 11.Ristaino JB, Thomas W. Agriculture, methyl bromide and the ozone hole, can we fill the gap? Plant Dis. 1997;81:964–977. doi: 10.1094/PDIS.1997.81.9.964. [DOI] [PubMed] [Google Scholar]
- 12.Angela RD, Penelope PV, Sakata Y. et al. Cucurbit grafting. Crit Rev Plant Sci. 2008;27:50–74. [Google Scholar]
- 13.Jiao ZG, Wang CQ, Dong YM. et al. Effects of grafting on development and quality of cucumber. Shandong Agri Sci. 2000;1:26–30. [Google Scholar]
- 14.Li TL, Liu YF, Qi HY. et al. Grafting effects on root activity and amino acid contents in bleeding sap of muskmelon. Hort Environ Biotechnol. 2009;50:1–6. [Google Scholar]
- 15.Liu HY, Zhu ZJ, Diao M. et al. Characteristic of the sugar metablolism in leaves and fruits of grafted watermelon during fruit development. Plant Physiol Commun. 2006;42:835–840. [Google Scholar]
- 16.Xu CQ, Li TL, Qi HY. Effects of grafting on development, carbohydrate content and sucrose metabolizing enzymes activities of muskmelon fruit. Acta Hort Sinica. 2006;33:773–778. [Google Scholar]
- 17.Yu XY, Wang XF, Fan JD. et al. Cloning and characterization of a sucrose phosphate synthase-encoding gene from muskmelon. J Amer Soc Hort Sci. 2007;132:557–562. [Google Scholar]
- 18.Yu XY, Wang XF, Zhang WQ. et al. Antisense suppression of an acid invertase gene (MAI1) in muskmelon alters plant growth and fruit development. J exper bot. 2008;59:2969–2977. doi: 10.1093/jxb/ern158. [DOI] [PubMed] [Google Scholar]
- 19.Greutert H, Keller F. Further evidence for stachyose and sucrose/H+ antiporters on the tonoplast of Japanese artichoke (Stachys sieboldii) tubers. Plant Physiol. 1993;101:1317–1322. doi: 10.1104/pp.101.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madore MA. Carbohydrate metabolism in photosynthetic and nonphotosynthetic tissues of variegated leaves of coleus blumei Benth. Plant Physiol. 1990;93:617–622. doi: 10.1104/pp.93.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robbins NS, Pharr DM. Regulation of photosynthetic carbon metabolism in cucumber by light intensity and photosynthetic period. Plant Physiol. 1987;85:592–597. doi: 10.1104/pp.85.2.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andreas B, Peterbauer T, Richter A. Inhibition of raffinose oligosaccharide breakdown delays germination of pea seeds. J Plant Physiol. 2007;164:1093–1096. doi: 10.1016/j.jplph.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Henrissat B, Coutinho PM, Davies GJ. A census of carbohydrateactive enzymes in the genome of Arabidopsis thaliana. Plant Mol Biol. 2001;47:55–72. [PubMed] [Google Scholar]
- 24.Micallef BJ, Haskins KA, Vanderveer PJ. Altered photosynthesis, flowering and fruiting in transgenic tomato plants that have incnthesis. Planta. 1995;196:327–334. [Google Scholar]
- 25.Salerno G.L, Curatti L. Origin of sucrose metabolism in higher plants: when, how and why? Trends Plant Sci. 2003;8:63–69. doi: 10.1016/S1360-1385(02)00029-8. [DOI] [PubMed] [Google Scholar]
- 26.Brain GA, Keller F, Robert T. Symplastic continuity between companion cells and the translocation stream: Long-distance transport is controlled by retention and retrieval mechanisms in the phloem. Plant Physiol. 2003;131:1518–1528. doi: 10.1104/pp.012054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pharr DM, Steven CH, Harriet NS. Leaf carbohydrate status and enzymes of translocate synthesis in fruiting and vegetative plants of Cucumis sativus L. Plant Physiol. 1985;77:104–108. doi: 10.1104/pp.77.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porra RJ, Thompson WA, Kriedemann PE. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta. 1989;975:384–394. [Google Scholar]
- 29.Pinheiro C, Rodrigues AP, Chaves MM. Sugar metabolism in developing lupin seeds is affected by a short-term water deficit. J Exp Bot. 2005;56:2705–2712. doi: 10.1093/jxb/eri263. [DOI] [PubMed] [Google Scholar]
- 30.Wardlaw IF, Willenbrink J. Carbohydrate storage and mobilisation by the culm of wheat between heading and grain maturity: the relation to sucrose synthase and sucrose-phosphate synthase. Austra J Plant Physiol. 1994;21:255–271. [Google Scholar]
- 31.Monika ES, James DB, John DW. et al. Galactosyl-sucrose metabolism and UDP-galactose pyrophosphorylase from Cucumis melo L. fruit. Physiol Plant. 1999;106:9–16. [Google Scholar]
- 32.Peterbauer T, Richter A. Galactosylononitol and stachyose synthesis in seeds of adzuki bean: purification and characterization of stachyose synthase. Plant Physiol. 1998;117:165–172. doi: 10.1104/pp.117.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho LC, Shaw AF, Hammond JB. et al. Source-sink relationships and carbon metabolism in tomato leaves 14C assimilate compartmentation. Ann Bot. 1983;52:365–372. [Google Scholar]
- 34.Handley LW, Pharr DM, McFeeters RF. Carbohydrate changes during maturation of cucumber fruit. Plant Physiol. 1983;72:498–502. doi: 10.1104/pp.72.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitchell DE, Michelle VG, Monica AM. Patterns of assimilate production and translocation in muskmelon (Cucumis melo L.) Plant Physiol. 1992;99:959–965. doi: 10.1104/pp.99.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun Y, Huang W, Tian XH. et al. Study on growth situation, photosynthetic characteristics and nutrient absorption characteristics of grafted cucumber seedlings. Plant Nutr Fert Sci. 2002;8:181–185. [Google Scholar]
- 37.Zhang HM, Xie J, Yu JZ. et al. The growth, photosynthesis and fruit quality of different type cucumber varieties grafted on to pumpkin seedlings. Acta Agr Shanghai. 2008;24:40–43. [Google Scholar]
- 38.Qi HY, Li TL, Liu YF. et al. Effects of grafting on photosynthesis characteristics, yield and sugar content in melon. J Shenyang Agr Univ. 2006;37:155–158. [Google Scholar]
- 39.Zhu ZJ, Liu HY, Shi QH. Effects of low temperature stress on characteristics of photosynthesis in leaves of own-rooted and grafted watermelon seedling. Adv Hort. 2004;6:434–440. [Google Scholar]
- 40.Carmen MG, Liosa MJ, Antonio Q. et al. Rootstock effects on leaf photosynthesis in 'Navelina' trees grown in calcareous soil. HortScience. 2009;44:280–283. [Google Scholar]
- 41.Li YJ, Liang GY, Liu XJ. et al. Proteomic study on grafted and non-grafted cucumber (Cucumis sativus L.) Acta Hort Sinica. 2009;36:1147–1152. [Google Scholar]
- 42.Omid A, Keilin T, Glass A. et al. Characterization of phloem sap transcription profile in melon plants. J Exp Bot. 2007;58:3645–3656. doi: 10.1093/jxb/erm214. [DOI] [PubMed] [Google Scholar]
- 43.Hu LP, Meng FZ, Wang SH. et al. Changes in carbohydrate levels and their metabolic enzymes in leaves, phloem sap and mesocarp during cucumber (Cucumis sativus L.) fruit development. Scientia Hort. 2009;121:131–137. [Google Scholar]
- 44.Robert T. Phloem loading and plasmodesmata. Trends Plant Sci. 1996;1:418–423. [Google Scholar]
- 45.McCaskill A, Robert T. Phloem loading in Verbascum phoeniceum L. depends on the synthesis of raffinose family oligosaccharides. Proc Natl Acad Sci USA. 2007;104:19619–19624. doi: 10.1073/pnas.0707368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao Zf, Marina P, Eli Z. et al. Carbohydrate metabolism during early fruit development of sweet melon (Cucumis melo) Physiol Plant. 1999;106:1–8. [Google Scholar]
- 47.Qi HY, Li TL, Liu HT. et al. Studies on carbohydrate content and sucrose metabolizing enzymes activities in different parts of tomato. Acta Hort Sinica. 2005;32:239–243. [Google Scholar]
- 48.Zhao ZZ, Zhang SL, Xu CJ. et al. Roles of sucrose metabolizing enzymes in accumulation of sugars in satsuma mandarin fruit. Acta Hort Sinica. 2001;28:112–118. [Google Scholar]
- 49.Saravitz DM, Pharr DM, Carter TE. Galactinol synthase activity and soluble sugars in developing seeds of four soybean genotypes. Plant Physiol. 1987;83:185–189. doi: 10.1104/pp.83.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peterbauer T, Mucha J, Mach L. et al. Chain elongation of raffinose in pea seeds-isolation, characterization, and molecular cloning of a multifunctional enzyme catalyzing the synthesis of stachyose and verbascose. J Biol Chem. 2002;277:194–200. doi: 10.1074/jbc.M109734200. [DOI] [PubMed] [Google Scholar]
- 51.Holthaus U, Schmitz K. Distribution and immunolocalization of stachyose synthase in Cucumis melo L. Planta. 1991;185:479–486. doi: 10.1007/BF00202956. [DOI] [PubMed] [Google Scholar]
- 52.Emilie AR, Robert T. A comprehensive picture of phloem loading strategies. Proc Natl Acad Sci USA. 2009;106:14162–14167. doi: 10.1073/pnas.0902279106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Christine AR. The calvin cycle revised. Photosyn Res. 2003;75:1–10. doi: 10.1023/A:1022421515027. [DOI] [PubMed] [Google Scholar]
- 54.Proietti P. Effect of fruiting on leaf gas exchange in olive (Olea europaea L.) Photosyn. 2000;38:397–402. [Google Scholar]
- 55.Xu DQ. The relationship between the photosynthate level and the photosynthetic rate. Plant Physiol Commun. 1986;6:1–8. [Google Scholar]
- 56.Gucci R, Grappadelli LC, Tustin S. et al. The effect of defruiting at different stages of fruit development on leaf photosynthesis of “Golden Delicious” apple. Tree Physiol. 1995;15:35–40. doi: 10.1093/treephys/15.1.35. [DOI] [PubMed] [Google Scholar]
- 57.Sharkey TD. Photosynthesis in intact leaves of C3 plants: physics, physiology and rate limitations. Bot Rev. 1985;51:53–105. [Google Scholar]