Abstract
Background: Bisphosphonate-induced osteonecrosis of the jaw (BONJ) is a frequently reported complication. The aim of this study was to investigate the clinical and histopathological presentation of BONJ with the Hounsfield score and to evaluate the reliability of the score for determining necrosis in an animal model.
Material/Methods: Eighty rats were prospectively and randomly divided into two groups of 40 each: a control group and an experimental group. Half of the animals from each group underwent extraction of the left mandibular molars, and the other half underwent extraction of the left maxillary molars under pentobarbital-induced general anesthesia. All animals were euthanized 28 days after tooth extraction. Maxillae and mandibles were extracted, cone beam computed tomography (CBCT) was performed, and Hounsfield scores were evaluated.
Results: The Hounsfield scores of the experimental group were found to be compatible with chronic osteomyelitis and periosteal reactions. The Hounsfield scores of the control group were compatible with a healthy healing period.
Conclusion: In light of these results, both cone beam computed tomography (CBCT) and the Hounsfield Units (HU) evaluations together are thought to be efficient in the diagnosis of BONJ.
Keywords: Bisphosphonate, Osteonecrosis, Jaws, Hounsfield unit
Introduction
Bisphosphonates (BPs) are a group of drugs that are effective in inhibiting bone resorption and that have been used in clinical settings for over 30 years 1. They are used in the management of patients with multiple myeloma, bone metastasis, and hypercalcemia of malignancy, and they are also effective in patients with Paget's disease of bone and osteoporosis 2,3.
Starting in 2003, a growing number of case reports and case series have been published that link BP administration, particularly intravenously, with the previously rare condition of osteonecrosis of the jaw and facial bones 3.
The mechanism that underlies the association between intravenous BP treatment and jaw osteonecrosis is not understood. Current explanations include an infectious etiology, the loss of blood supply, or suppression of bone turnover 4,5,6. There is also a lack of information on the epidemiology of the toxicity. There is no information from prospective trials and only limited information from observational cohorts on incidence, time course, or other risk factors 7.
The lesions usually occur after dental extraction or oral trauma, but also spontaneously, with no history of surgical procedures, trauma, or radiotherapy 8,9.
The dental literature has many studies about the usefulness of computed tomography (CT) for assesing bone volume and morphology using the Hounsfield score. The Hounsfield unit (HU) is a linear transformation of the original linear attenuation coefficient such that the radiodensity of distilled water at standard pressure and temperature (STP) is defined as 0 HU and radiodensity of air at STP is defined as -1000 HU 10,11.
Cone beam computed tomography (CBCT) is a CT technique that uses far less radiation than conventional CT and captures multiple views of the patient in a single 360° rotation. The information thus obtained is then processed and reconstructed in different planes (multiplanar reconstruction) and, if necessary, three-dimensionally as well 12.
The aim of the present study was to investigate the clinical and histological presentation of BP-induced osteonecrosis of the jaw (BONJ) with the CBCT and to evaluate the reliability of the Hounsfield score for the early diagnosis of necrosis in an animal model.
Material and Methods
The study was carried out in the Istanbul University, Faculty of Dentistry, Department of Oral & Maxillofacial Surgery and Institute of Oncology, Department of Tumor Pathology & Cytology.
Experimental animals
Eighty male Wistar Albino rats (body weight 110-120 g, approximately 5 weeks old) were used in this study. Animals were assigned to either the BP group or the control group (n = 40 for each). Animals were acclimatized prior to study start and any animal observed to be in poor condition was rejected from study inclusion. All animals in experimental group given at a dose of 1 mg/kg dexamethasone (DX) and 7.5 µg/kg zoledronic acid (ZA) subcutaneously at 0, 7, or 14 days; In the control group, physiological saline was injected into the same area at the same days. Rats were anesthetized before injection using diethyl ether. Extractions were performed the day following the last injections. Half of the animals from each group underwent extraction of the left mandibular molars and remaining animals underwent extraction of the left maxillary molars with a dental explorer under pentobarbital-induced general anesthesia. Rats were housed in cages provided with filtered air at a temperature of 70° F ± 5° and 50 ± 20% relative humidity. A 12 h light/dark cycle was maintained. Animals were fed with a standard diet of rat chow and watered ad libitum. All animals were euthanized twenty-eight 28 days following tooth extractions. The maxillae and mandibles were resected.
All experiments were performed in accordance with the guidelines for Animal Experiments of the Faculty of Medicine, Istanbul University, Istanbul, TURKEY.
Tissue Preparation and Histopathological Examination
The specimens were fixed in 10% neutral buffered formalin overnight at 4ºC, rinsed in phosphate buffered saline and decalcified in 20% formic acid solution (Merck, Darmstadt, Germany) for 10 days. The decalcified specimens were embedded in paraffin and cut into 20 semi-serial sections using a microtome (Leica Microsystemic, Germany), and routine hematoxylin and eosin (HE) staining and Mallory Trichrome staining were performed. The sections were examined with light microscope under 40, 100 and 200x magnification (Eclipse E600; Nikon, Tokyo, Japan). A histomorphological review was performed by a single blinded oral pathologist to evaluate the presence of infection, necrosis, fibrosis, new bone formation, and foreign body reaction. The scores for infection, necrosis, fibrosis and new bone formation scores were determined by counting the associated cells and their ratio to the total cell count in a standardized area at 40x magnification. Inflammation and fibrosis have been given +(%1-30), ++(%30-60) or +++(>%60) scores according to their surface covering. Necrosis of tissue samples was noted as yes or no and was used to determine the percentage of animals with necrosis. For new bone regeneration if there's no bone formation (-), %1-30 bone formation (+), %30-60 bone formation (++) and for >%60 (+++) scores are given. Additionally foreign body reaction noted as present or absent.
Radiographic analysis
The CBCT scans were obtained and reviewed by an oral radiologist and both oral medicine specialists at Harvard School of Dental Medicine, Boston. Images were acquired using an ILUMA Ultra CBCT (IMTEC, 3M Company) with the following technical parameters: 120 kV, 5 mA, 18.54 mAs, resolution 0.4 voxel, and field size 8.0 cm. Images were processed and analyzed using ILUMA 3D software (Materialize, Glen Burnie, MD).
Hounsfield Unit evaluation
Hounsfield Unit scores were analyzed using ILUMA 3D software (Materialize, Glen Burnie, MD). Regarding the extraction socket 1 mm cross-sectional views were evaluated. Each corner of the extraction socket was marked and a 2 dimensional rectangular bone area with approximately 5 mm perimeter for each animal was created. Afterwards minimum, maximum, average and standard deviation parameters for the region of interest were collected.
Statistical analysis
We used + score for data between 1 and 30%, ++ score for data between 31 and 60%, and +++ score for data >60%. We transformed the continuous data to non-continuous data. For that reason, we preferred the χ2 test, which was used to evaluate the significance of differences between the groups. A p-value of 0.05 was considered statistically significant for all tests.
Results
Gross observations
In general, animals tolerated the procedure well and demonstrated good hemostasis and rapid recovery from anesthesia. Gross evidence of extraction-site healing was seen in the majority of both groups by 28 days. In contrast, open wounds and exposed bone were noted in ZA/dexamethasone (DX)-treated group.
Histopathological findings
At 28 days after tooth extraction, resorption of residual interdental bone septum by osteoclasts and formation of new bone trabeculae were viewed in sockets of both groups. However, the amount of new bone trabeculae was significantly decreased in the ZA/DX-treated sockets. This difference between groups was significant (p = 0.0001).
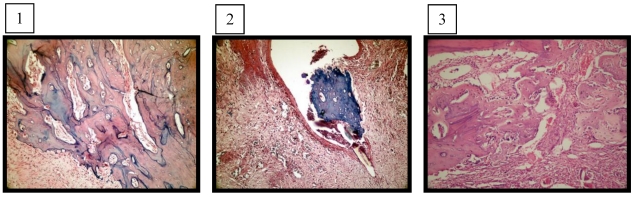
There was no foreign body reaction in the sockets of both groups, and no significant difference in fibrosis was observed. The necrosis scores were significantly higher in the ZA/DX-treated sockets (p = 0.015) (Figure A.1, Figure A.2). Normal bone healing was present in the control group (Figure A.3). The inflammation scores were also significantly higher in the ZA/DX-treated group (p = 0.0001) (Table I).
Figure A.
1: The amount of new bone trabeculae was significantly decreased in BP/DX-treated sockets (experimental group, H&E, X200). 2: Fistula formation over the necrotic bone area and granulation tissue under the epithelium (experimental group, H&E, X400). 3: Bone trabeculae among the connective tissue rich in newly formed vessels filling the extraction socket (control group, H&E, X200).
Table I.
Histopathological findings of the experimental and control group: - (0%), + (1-30%), ++ (30-60%), +++ (>60%).
| Experimental Group | Control Group | |||||
|---|---|---|---|---|---|---|
|
New Bone Formation |
(-) | 10 | 28.60% | 0 | 0.00% | |
| (+) | 16 | 45.70% | 0 | 0.00% | ||
| (++) | 9 | 25.70% | 18 | 47.40% | χ2=48.96 | |
| (+++) | 0 | 0.00% | 20 | 52.60% | P=0.0001 | |
| Inflammation | (-) | 1 | 2.90% | 16 | 42.10% | |
| (+) | 16 | 45.70% | 18 | 47.40% | ||
| (++) | 15 | 42.90% | 4 | 10.50% | χ2=22.64 | |
| (+++) | 3 | 8.60% | 0 | 0.00% | р=0.0001 | |
| Necrosis | No | 14 | 40.00% | 26 | 68.40% | χ2=5.94 |
| Yes | 21 | 60.00;% | 12 | 31.60% | р=0.015 | |
| Fibrosis | (+) | 17 | 48.60% | 24 | 63.20% | |
| (++) | 17 | 48.60% | 14 | 36.80% | χ2=2.37 | |
| (+++) | 1 | 2.90% | 0 | 0.00% | р = 0.306 | |
| Foreign-body Reaction | (-) | 35 | 100.00% | 38 | 100.00% | |
Radiological findings
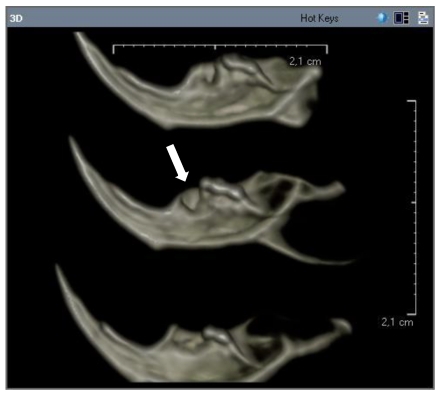
In the experimental group, radiolucency extended from the first molar to almost the midline in the mandible, with destruction of the inferior and lingual cortex in that area. Irregularity of the alveolar crest of the extraction socket was observed, as well as disruption of the lingual cortex in that area (Figure B).
Figure B.
Radiolucency extended from the first molar to almost the midline in the mandible, with destruction of the inferior and lingual cortex in that area (experimental group).
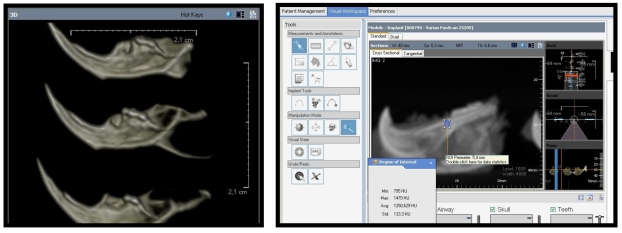
The body of the right mandible was sclerotic, with the trabecular bone being almost as dense as the cortical bone. Hounsfield scores of the control group were significantly higher for maxilla and mandible (p = 0.0001) (Figure C, Table II).
Figure C.
CBCT images of dissected mandibles (experimental group) and detection of the Hounsfield score of the extraction sockets in ILUMA 3D software (Materialize, Glen Burnie, MD).
Table II.
Hounsfield units for experimental and control groups
| Experimental group | Control group | p | |
|---|---|---|---|
| Mandible | 249.22 ± 80.21 | 827.1 ± 20.32 | 0.0001 |
| Maxilla | 435.4 ± 61,13 | 868.3 ± 49.19 | 0.0001 |
Discussion
There have been several recent reports of BONJ occurring after oral surgery (e.g., tooth extraction) in patients (especially cancer patients) undergoing long-term BP treatment. However, there has been no clear demonstration of a relationship between BP treatment and BONJ 4,5.
The aim of the present study was to investigate the clinical and histological presentation of BONJ and the associated Hounsfield scores and to evaluate the reliability of the Hounsfield score for measuring necrosis in an animal model. We first had to replicate both the clinical and histological presentation of BONJ in an animal model of the disease state.
In previous histological studies of healing in extraction sockets in normal rats, it has been found that new bone begins to form on the floor of the extraction socket about 2 days after extraction and that the extraction socket becomes filled with newly formed bone 14 days after extraction 13,14. In contrast, in our study, clinical and histological evidence of delayed healing, as manifested by exposed bone or ulceration, was seen in most of the zoledronic acid (ZA)/DX-treated animals.
Sonis et al. reported an animal model for BONJ. They used ZA/DX to treat a BONJ-like indication in an animal model 15. We first duplicated this study on 80 Wistar albino rats and found similar histopathological results.
Radiological investigations are the first routine tool. Several reports have presented various radiographic findings in BONJ using intraoral plain radiographs, panoramic tomography, CT, magnetic resonance imaging, and positron emission tomography (PET). Conventional orthopantomography provides an excellent general assessment of the entire jaw, but mineral loss must be as high as 30-50% to be visible. Orthopantomography shows bone destruction but is not able to differentiate necrotic from normal bone or osteolytic lesions from metastases 16.
Hematologically, almost all cases of osteonecrosis of the jaw occur in patients affected by multiple myeloma after exposure to BPs with or without chemotherapy. In this context, the most frequent diagnostic question is whether a painful area with radiographic signs of osteolysis in the maxillary region, be it swollen or not, is caused by a neoplastic focus or by an inflammatory or necrotic process. In this setting, 99mTc-MDP-based techniques of bone imaging are not helpful for the differential diagnosis. 99mTc-MDP binds to hydroxyapatite crystals in sites of active osteoblastic activity, which is erratic in osteolysis caused by neoplastic plasma cells 17. The comparison between an imaging technique based on a compound taken up by neoplastic cells and not by inflammatory ones, such as sestamibi scintigraphy, and an imaging procedure exploiting a tracer taken up also by inflammation, such as 18F-FDG PET, has recently been shown to be helpful in differentiating osteonecrosis of the jaw from myeloma osteolysis 18,19.
Data from previous studies have shown that MRI has a high detectability for BONJ lesions. The extent of the BONJ lesions assessed from MRI scans does not differ significantly from the intraoperative situation. However, there was no significant correlation between MRI measurements and intraoperative measurements. Therefore, it seems that MRI sometimes has limitations in assessing the extent of BONJ in the individual patient preoperatively 20,21.
It has been shown previously that CT scans are useful in providing detailed information about cortical and trabecular bone and allow estimation of the real extent of osteonecrosis and distinguishing BONJ from malignant diseases like plasmocytoma or bone metastases 22. Although preliminary evidence has pointed toward greater ability of CT to detect bone changes in BONJ, there is also significantly increased cost and radiation exposure 23.
Bone quality and bone density are one of the major problems for detecting the BONJ before it can be visually seen as necrotic bone. Bone mineral density (BMD) and micro architecture both together serve as the most important factor for determining the mechanical properties of bone and it can only be evaluated by using histomorphometric procedures 24.
There are some different opinions in literature on using Hounsfield scale in CBCT. In some papers it has been advised that CBCT can be used to assess bone density and to determine HU 25. This leads to some concern, since scanned regions of the same density in the skull can have a different grayscale value in the reconstructed CBCT dataset. Swennen and Schutyser stated that with CBCT, the image value of a voxel of an organ depends on the position in the image volume. This means that the X-ray attenuation of CBCT acquisition systems currently produces different HU values for similar bony and soft tissue structures in different areas of the scanned volume (e.g. dense bone has a specific image value at the level of the menton, but the same bone has a significantly different image value at the level of the cranial base) 26. Because we all worked on the same region of the maxillae and mandible we have used CBCT to evaluate the average HU among the extraction sockets.
The application of CBCT technology, which uses far less radiation than conventional CT, has recently been reported in BONJ and is well suited for bone evaluation of the maxillofacial region 27,28. We think that it would be very useful for researchers to access for an opinion of bone quality before BONJ occurs. The data from the present study show that CBCT has a high detectability for BONJ lesions. It is possible to assess bone mineral distribution or bone density by using CBCT which can give us an opinion of impaired bone quality. The extent of the BONJ lesions assessed from CBCT scans did not differ significantly from the intraoperative situation, and a significant correlation between CBCT measurements and intraoperative measurements was found.
Conclusion
The pathophysiology of BONJ continues to be defined, and radiographic evaluation will likely play an increasing role in the diagnosis and management of this condition. In our study, we found that the CBCT findings supported the histopathological findings. To evaluate risk factors, detection, biological correlates, and management outcomes, CBCT imaging and Hounsfield Unit evaluations both together should be strongly considered because it provides the greatest level of detail with a lower dose of radiation compared with other options.
Acknowledgments
The authors would like to thank Kutay Eryılmaz, for his help with the radiographic evaluation.
References
- 1.Rogers MJ, Watts DJ, Russel RG. Overview of bisphosphonates. Cancer. 1997;80:1652–60. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1652::aid-cncr15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 2.Coleman RE. Future directions in the treatment and prevention of bone metastases. Am J Clin Oncol. 2002;25:2–8. doi: 10.1097/00000421-200212001-00006. [DOI] [PubMed] [Google Scholar]
- 3.Holen I, Coleman RE. Bisphosphonates as treatment of bone metastases. Curr Pharm Des. 2010;16(11):262–7. doi: 10.2174/138161210791034003. [DOI] [PubMed] [Google Scholar]
- 4.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003 Sep;61(9):1115–7. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 5.Hansen T, Kunkel M, Weber A, James Kirkpatrick C. Osteonecrosis of the jaws in patients treated with bisphosphonates - histomorphologic analysis in comparison with infected osteoradionecrosis. J Oral Pathol Med. 2006;35:155–60. doi: 10.1111/j.1600-0714.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- 6.Flichy-Fernández AJ, Balaguer-Martínez J, Peñarrocha-Diago M, Bagán JV. Bisphosphonates and dental implants: current problems. Med Oral Pathol Oral Cir Bucal. 2009;14:355–60. [PubMed] [Google Scholar]
- 7.Marx RE, Cillo JE Jr, Ulloa JJ. Oral bisphosphonate-induced osteonecrosis: risk factors, prediction of risk using serum CTX testing, prevention and treatment. J Oral Maxillofac Surg. 2007;65:2397–410. doi: 10.1016/j.joms.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Pérez SB, Barrero MV, Hernández MS, Knezevic M, Navarro JM, Millares JR. Bisphosphonate-associated osteonecrosis of the jaw. A proposal for conservative treatment. Med Oral Patol Oral Cir Bucal. 2008;13:770–3. [PubMed] [Google Scholar]
- 9.Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004;62:527–34. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Fanuscu MI, Chang TL. Three-dimensional morphometric analysis of human cadaver bone: microstructural data from maxilla and mandible. Clin Oral Implants Res. 2004 Apr;15(2):213–8. doi: 10.1111/j.1600-0501.2004.00969.x. [DOI] [PubMed] [Google Scholar]
- 11.Hanazawa T, Sano T, Seki K, Okano T. Radiologic measurements of the mandible: a comparison between CT-reformatted and conventional tomographic images. Clin Oral Implants Res. 2004 Apr;15(2):226–32. doi: 10.1111/j.1600-0501.2004.00991.x. [DOI] [PubMed] [Google Scholar]
- 12.Fullmer JM, Scarfe WC, Kushner GM, Alpert B, Farman AG. Cone beam computed tomographic findings in refractory chronic suppurative osteomyelitis of the mandible. Br J Oral Maxillofac Surg. 2007;45:364–71. doi: 10.1016/j.bjoms.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Pietrokovski J, Massler M. Ridge remodeling after tooth extraction in rats. J Dent Res. 1967;46:222–31. doi: 10.1177/00220345670460011501. [DOI] [PubMed] [Google Scholar]
- 14.Johansen JR. Repair of the post-extraction alveolus in the Wistar rats. A histologic and autoradiographic study. Acta Odontol Scand.1. 1970;28:441–61. doi: 10.3109/00016357009028237. [DOI] [PubMed] [Google Scholar]
- 15.Sonis ST, Watkins BA, Lyng GD, Lerman MA, Anderson KC. Bony changes in the jaws of rats treated with zoledronic acid and dexamethasone before dental extractions mimic bisphosphonate-related osteonecrosis in cancer patients. Oral Oncol. 2009;45:164–72. doi: 10.1016/j.oraloncology.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Dore F, Filippi L, Biasotto M, Chiandussi S, Cavalli F, Di Lenarda R. Bone scintigraphy and SPECT/CT of bisphosphonate-induced osteonecrosis of the jaw. J Nucl Med. 2009;50:30–35. doi: 10.2967/jnumed.107.048785. [DOI] [PubMed] [Google Scholar]
- 17.Scutellari PN, Spanedda R, Feggi LM, Cervi PM. The value and limitation of total body scan in the diagnosis of multiple myeloma: a comparison with conventional skeletal radiography. Haematologica. 1985;70:136–142. [PubMed] [Google Scholar]
- 18.Fonti R, Del Vecchio S, Zanetti A. et al. Bone marrow uptake of 99mTc-MIBI in patients with multiple myeloma. Eur J Nucl Med. 2001;28:214–220. doi: 10.1007/s002590000434. [DOI] [PubMed] [Google Scholar]
- 19.Catalano L, Del Vecchio S, Petruzziello F. et al. Sestamibi and FDG-PET scans to support diagnosis of jaw osteonecrosis. Ann Hematol. 2007;86:415–423. doi: 10.1007/s00277-007-0263-0. [DOI] [PubMed] [Google Scholar]
- 20.Stockmann P, Hinkmann FM, Lell MM, Fenner M, Vairaktaris E, Neukam FW, Nkenke E. Panoramic radiograph, computed tomography or magnetic resonance imaging. Which imaging technique should be preferred in bisphosphonate-associated osteonecrosis of the jaw? A prospective clinical study. Clin Oral Investig. 2010 Jun;14(3):311–7. doi: 10.1007/s00784-009-0293-1. [DOI] [PubMed] [Google Scholar]
- 21.Krishnan A, Arslanoglu A, Yildirm N, Silbergleit R, Aygun N. Imaging findings of bisphosphonate-related osteonecrosis of the jaw with emphasis on early magnetic resonance imaging findings. J Comput Assist Tomogr. 2009;33(2):298–304. doi: 10.1097/RCT.0b013e31817e4986. [DOI] [PubMed] [Google Scholar]
- 22.Wutzl A, Biedermann E, Wanschitz F, Seemann R, Klug C, Baumann A, Watzinger F, Schicho K, Ewers R, Millesi G. Treatment results of bisphosphonate-related osteonecrosis of the jaws. Head Neck. 2008;30:1224–1230. doi: 10.1002/hed.20864. [DOI] [PubMed] [Google Scholar]
- 23.Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–84. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 24.Hohlweg-Majert B, Metzger MC, Kummer T, Schulze D. Morphometric analysis - Cone beam computed tomography to predict bone quality and quantity. J Craniomaxillofac Surg. 2011 Jul;39(5):330–4. doi: 10.1016/j.jcms.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 25.De Vos W, Casselman J, Swennen GR. Cone-beam computerized tomography (CBCT) imaging of the oral and maxillofacial region: a systematic review of the literature. Int J Oral Maxillofac Surg. 2009 Jun;38(6):609–25. doi: 10.1016/j.ijom.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 26.Swennen GR, Schutyser F. Three-dimensional cephalometry: spiral multi-slice vs cone-beam computed tomography. Am J Orthod Dentofacial Orthop. 2006 Sep;130(3):410–6. doi: 10.1016/j.ajodo.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 27.Fullmer JM, Scarfe WC, Kushner GM, Alpert B, Farman AG. Cone beam computed tomographic findings in refractory chronic suppurative osteomyelitis of the mandible. Br J Oral Maxillofac Surg. 2007;45:364–71. doi: 10.1016/j.bjoms.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Tsiklakis K, Donta C, Gavala S, Karayianni K, Kamenopoulou V, Hourdakis CJ. Dose reduction in maxillofacial imaging using low dose cone beam CT. Eur J Radiol. 2005;56:413–417. doi: 10.1016/j.ejrad.2005.05.011. [DOI] [PubMed] [Google Scholar]