Abstract
Global environmental change is expected to affect profoundly the transmission of the parasites that cause human malaria. Amongst the anthropogenic drivers of change, deforestation is arguably the most conspicuous, and its rate is projected to increase in the coming decades. The canonical epidemiological understanding is that deforestation increases malaria risk in Africa and the Americas and diminishes it in South–east Asia. Partial support for this position is provided here, through a systematic review of the published literature on deforestation, malaria and the relevant vector bionomics. By using recently updated boundaries for the spatial limits of malaria and remotely-sensed estimates of tree cover, it has been possible to determine the population at risk of malaria in closed forest, at least for those malaria-endemic countries that lie within the main blocks of tropical forest. Closed forests within areas of malaria risk cover approximately 1.5 million km2 in the Amazon region, 1.4 million km2 in Central Africa, 1.2 million km2 in the Western Pacific, and 0.7 million km2 in South–east Asia. The corresponding human populations at risk of malaria within these forests total 11.7 million, 18.7 million, 35.1 million and 70.1 million, respectively. By coupling these numbers with the country-specific rates of deforestation, it has been possible to rank malaria-endemic countries according to their potential for change in the population at risk of malaria, as the result of deforestation. The on-going research aimed at evaluating these relationships more quantitatively, through the Malaria Atlas Project (MAP), is highlighted.
Human malaria is a disease of global extent that has been eradicated from most temperate areas relatively recently and is now predominantly restricted to tropical zones (Hay et al., 2004). This substantial geographical reduction has not been followed by a similar decrease in the population at risk of malaria (PARM). The PARM has, in fact, increased exponentially because of population growth and a failure to control the disease within its restricted range (Hay et al., 2004). It has been estimated that the PARM currently exceeds 3000 million people (Guerra et al., 2006) and that, each year, there are >500 million cases of Plasmodium falciparum malaria globally (Snow et al., 2005) and 1 million malaria-attributable deaths in Africa (Snow et al., 2003; Hay et al., 2005). Several environmental factors are known to affect the transmission of the parasites that cause human malaria (Walsh et al., 1993; Robert et al., 2003; Hay et al., 2005, 2006a; Keiser et al., 2005a; Snow et al., 2006). One such factor, deforestation, is of particular concern because of its scale and ubiquity in tropical areas. The size of the PARM in forested areas is not known with precision (Keiser et al., 2005b), however, and this hampers reliable quantification of the effects of deforestation on the burden of malaria.
Although estimates of the global extent of humid tropical forest vary greatly, from 11.16 million to 15.71 million km2, the largest surviving areas of such forest are to be found in Latin America (6.53 million–7.80 million km2), chiefly in the Amazon region, followed by Africa (1.93 million–5.19 million km2) and South–east Asia (2.70 million–2.72 million km2) (Anon., 2001b; Achard et al., 2002; Mayaux et al., 2005). Deforestation in these areas is extensive, with global estimates of its rate ranging from 36,000–69,000 km2/year. The mean annual rate of deforestation in South–east Asia (0.71%–0.79%) is higher than that in Latin America (0.33%–0.51%) or Africa (0.34%–0.36%) (Anon., 2001b; Achard et al., 2002; Mayaux et al., 2005). Tropical deforestation not only has obvious environmental and socio–economic impacts, including loss of biodiversity, loss of agricultural productivity, and alteration of the carbon and water cycles (Fearnside, 2005), but also detrimental effects on vector-borne diseases (Walsh et al., 1993).
In this article, the relevant literature relating to deforestation and human malaria is systematically reviewed, to elucidate the relationships between the disease and forest cover and any regional variation in these links. Articles dealing directly with these issues and those on the bionomics of relevant vectors were made the focus of the literature review. Geographical information systems (GIS) were used, with maps of forest cover and the distribution of human populations, to determine the areas of forest cover within the spatial limits of malaria transmission, and then to derive estimates of the PARM in these areas. These values were then combined with country-specific estimates of deforestation rates, to identify those countries in which the epidemiological impact of deforestation on malaria is likely to be of most concern. Finally, the results of the literature review and data analysis were used to fuel a discussion of the probable implications of deforestation on the future risks of malaria transmission across the world.
DEFINING FOREST EXTENTS AND REGIONS
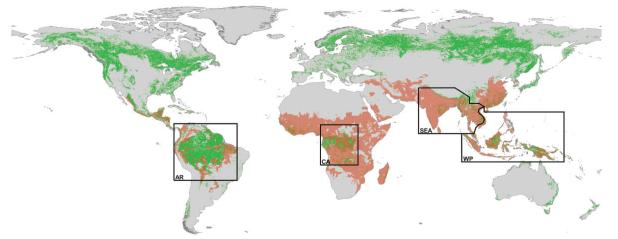
In order to quantify the relationship between malaria transmission and forests, ‘forest’ must be defined. In 1973, the United Nations Educational, Scientific and Cultural Organization’s Standing Committee on Classification and Mapping of Vegetation on a World Basis established a vegetation classification (Anon., 1973). Eighteen years later, the forest component of this classification was revised and extended by the Food and Agricultural Organization (FAO). In this revision, forest is defined as ‘land with a tree canopy cover of more than 10% and an area of more than 0.5 ha’, including natural forests and forest plantations but excluding tree stands specifically established for agricultural production (Anon., 2001b). Natural forests (i.e. those not planted by humans) are subdivided in the revision, as ‘closed’ (>40% canopy cover) or ‘open’ (>10%–40% canopy cover). In this review, the definition of forests has been limited to the closed (or ‘deep’) types, since these represent a biological barrier for the development of many vectors of the parasites that cause human malaria, and the discussion is restricted to the remaining tracts of tropical forest in the world (i.e. those in Amazonia, Central Africa and Asia). Asia is split, according to malaria epidemiological zones (Macdonald, 1957) and vector distribution (Service, 1993), into South–east Asia [corresponding to the eastern half of the Indo–Iranian epidemiological zone and the whole Indo–Chinese epidemiological zone (Macdonald, 1957)] and the Western Pacific [corresponding to tropical forests in the Malaysian and Australasian epidemiological zones (Macdonald, 1957)]. The present analyses are therefore focused on the 30 malaria-endemic countries encompassed by these regions. Thailand, Myanmar and Cambodia fall mostly, but not exclusively, in South–east Asia, and Vietnam mostly in the Western Pacific (Macdonald, 1957), and are allocated accordingly (Fig. 1).
FIG. 1.
The global spatial limits of malaria in 2005 ( ) overlaid on the areas of closed forests [
) overlaid on the areas of closed forests [ ; as indicated on the Global Forest Cover map (Anon., 2001a)]. The Amazon region (AR), Central Africa (CA), South–east Asia (SEA) and the Western Pacific (WP) are roughly indicated; the sinuous limit between SEA and WP is based on the map of malaria zones developed by Macdonald (1957).
; as indicated on the Global Forest Cover map (Anon., 2001a)]. The Amazon region (AR), Central Africa (CA), South–east Asia (SEA) and the Western Pacific (WP) are roughly indicated; the sinuous limit between SEA and WP is based on the map of malaria zones developed by Macdonald (1957).
THE LINKS BETWEEN MALARIA TRANSMISSION, FOREST COVER AND DEFORESTATION
The relationship between malaria transmission, forest cover and deforestation is complex. Aspects related to microclimate and/or the chemical composition of soils can be important (Smith, 1981). Ecological factors can regulate the species composition of the mosquito populations, and thus the numbers and types of malaria vector, by, for example, changes in host-preference and predation patterns (Deane, 1986). Human population migrations to and from forests (usually driven by economic and social pressures) and the associated changes in land cover are often critical (Walsh et al., 1993). Such migrations often bring human populations closer to the forest. The direction of land-use that follows forest clearing — usually towards grasslands or crops — is also important but its influence will be mediated by the local ecology and vectors (Kondrashin et al., 1991). The replacement of forest with rice cultivation, for example, may provide more favourable conditions for Anopheles gambiae s.s. or An. albitarsis s.s. (Forattini et al., 1993a, b; Briet et al., 2003) but can reduce transmission in areas where An. dirus is the main vector (Kondrashin et al., 1991). In contrast, An. dirus can find tree-crop plantations suitable for breeding since such plantations provide conditions that are similar to this vector’s natural habitat (Kondrashin et al., 1991). As the result of such links, the effects of deforestation on malaria transmission are spatially variable and largely dependent on vector distribution, since the vector species have adapted to different types of land cover, including forests and near-forest habitats. This makes the effects of deforestation on malaria transmission regionally distinctive and even locally specific. A basic knowledge of vector bionomics leads to the generally accepted (though largely qualitative) opinion that deforestation increases the risk of malaria transmission in Africa and tropical America but decreases it in Asia (Mouchet and Brengues, 1990; Walsh et al., 1993; Anon., 2005).
In the following sections, an overview of the relationships between deforestation and malaria is presented, and the epidemiologically important issues are highlighted by region. The vectors that are most important when studying malaria transmission and forest cover are listed in Table 1. Table 2 provides a comprehensive listing of studies that have, directly or indirectly, investigated the relationship between forest cover and malaria risk, grouped into the dominant themes.
TABLE 1.
The most relevant regional vectors that merit consideration when studying the links between malaria transmission and forests
| Region |
||||
|---|---|---|---|---|
| Vector type | Amazonia | Central Africa | South–east Asia | Western Pacific |
| Deep or closed forest* | Anopheles nili | An. dirus † | An. balabacensis † | |
| An. dirus † | ||||
| An. donaldi † | ||||
| An. flavirostris † | ||||
| An. leucosphyrus † | ||||
| Near- or non-forest‡ | An. albitarsis † | An. funestus † | An. culicifacies † | An. farauti † |
| An. darlingi † | An. gambiae s.s.† | An. fluviatilis † | An. koliensis † | |
| An. marajoara | An. moucheti | An. minimus † | An. letifer † | |
| An. nuneztovari | An. maculatus † | |||
| An. pseudopunctipennis † | An. minimus † | |||
| An. punctulatus † | ||||
Deep-forest vectors are considered as those for which deep shade is a requisite for breeding. This categorization is not absolute and vectors considered as deep-forest species are sometimes responsible for malaria transmission on forest fringes or in anthropic environments.
Considered a main vector (dominant and wide-spread) throughout its range (Service, 1993).
Vectors considered non-forest or forest-fringe species are seldom, if ever, implicated in deep-forest transmission.
TABLE 2.
Compendium of published studies (excluding review articles) related to malaria transmission and forests
Three examples from West Africa (Coz et al., 1966; Bockarie et al., 1955; Nzeyimana et al., 2002) are included because the vector ecology described is similar to that in Central Africa.
There is a striking lack of primary research directly measuring the impact of deforestation on malaria.
Malaria and Forests in the Amazon Region
Amazonia holds the highest risk of malaria transmission in the Americas, with 80% of all cases reported in 2002 coming from the nine countries that share the Amazon basin (PAHO, 2003). Despite its large area, the Amazon region has a relative low diversity of competent malaria vectors (Rubio-Palis and Zimmerman, 1997; Tadei and Dutary Thatcher, 2000). Of the 54 Anopheles species described in Brazil, for example, only 10 have been reported to be naturally infected with parasites that cause human malaria (Rosa-Freitas et al., 1998; Tadei et al., 1998). Nine of these 10 species (i.e. all except An. darlingi) are zoophilic and/or exophilic and therefore possibly of limited epidemiological significance (Deane, 1986).
There are no known closed-canopy vectors in Amazonia (Table 1) and forests support a lower density and diversity of potential vectors than deforested areas (Tadei and Dutary Thatcher, 2000). Given its anthropophilia, endophagy and common endophilia, An. darlingi is by far the most important malaria vector in the region (Forattini, 1962; Deane, 1986). It breeds in partly shaded pools found in flooded areas of forests and in forest creeks, river edges and pools left after river-level recession during the dry season (Forattini, 1962; Rozendaal, 1990). The human colonization of forest or near-forest areas in the Amazon typically promote the establishment and expansion of An. darlingi populations, by increasing human exposure to this species’ natural breeding habitats and by the generation of new breeding habitats on the forest fringes. By eliminating deep shade and changing the acidity and chemical composition of the soil, slash-and-burn techniques often create favourable conditions for the breeding of An. darlingi and so increase the local risk of human malaria (Singer and Caldas de Castro, 2001). This phenomenon has led to the expression ‘frontier malaria’ (Sawyer, 1993; Singer and Caldas de Castro, 2001). In the longer-term, however, the establishment of agriculture and urbanization after forest clearance eventually tend to decrease malaria transmission, through classic mechanisms (Hay et al., 2005), and render it largely dependent upon human behaviour (Caldas de Castro et al., 2006; Table 2).
Malaria and Forests in Central Africa
The most competent malaria vectors in Africa are An. gambiae s.s., An. funestus, An. moucheti and An. nili (Mouchet et al., 2004). Importantly, the geographical range of all of these species encompasses the Central African forest block (Rogers et al., 2002; Mouchet et al., 2004). Anopheles gambiae s.s. and An. funestus are considered ‘main’ vectors (being both dominant and wide-spread) throughout their ranges (Service, 1993). Anopheles nili and An. moucheti, which are more incidental or localized in their distribution, are usually considered to be ‘subsidiary’ vectors but can be locally important. Three of these Central African vectors are mainly non-forest species (Table 1). Anopheles nili is the exception because it can breed in shaded streams (Gillies and de Meillon, 1968) but its role in transmission is generally restricted to localised forested areas (Carnevale et al., 1992). The wide-spread, main vectors, An. gambiae s.s. and An. funestus, are generally absent from deep forests since their larvae require sunlit pools (Gillies and de Meillon, 1968). They can, however, play an important role in transmission after deforestation or forest degradation. Although An. moucheti has a more localised range than An. gambiae s.s. and An. funestus, its sporozoite ‘rates’ are high enough for it to be considered a main vector in specific areas (Mouchet et al., 2004). It is confined to Central Africa and is described as a forest species (Gillies and De Meillon, 1968). The penetration of sunlight into its breeding sites is an obligate requirement, however, so canopy discontinuities, such as those made by rivers or human intervention, are essential.
In summary, deforestation in Africa tends to increase malaria transmission by creating habitats that are suitable for the breeding of the very efficient, non-forest vectors, although a modest reduction in transmission might be expected following deforestation in the localised settings where An. nili is the main vector (Table 2).
Malaria and Forests in South–east Asia and the Western Pacific
The transmission of malaria in forests is particularly prominent in South–east Asia and the Western Pacific. Clusters of malaria cases in the Mekong region, for example, are closely associated with dense forest cover, with cultivated areas supporting relatively low levels of transmission (Singhasivanon, 1999). In 1990, when forest covered only 20% of the land area of the malarious countries in the World Health Organization’s South–east Asian region, 40% of all the malaria cases in the region and 60% of the cases of P. falciparum malaria were reported from forest areas (Kondrashin, 1992). In 1989, 87% of the malaria cases and almost all (99%) of the P. falciparum cases recorded in Bangladesh occurred in forests (Sharma et al., 1991). In India in 1987, tribal communities living in forested areas represented only 7% of the country’s population but contributed 30% of the country’s malaria cases, 60% of the P. falciparum cases, and 60% of the malaria-attributable deaths (Narasimham, 1991). One of the main risk factors for malaria in these areas is the movement of humans to and from the forest (Kondrashin, 1992), which not only exposes immunologically naïve individuals to high levels of transmission (Rosenberg and Maheswary, 1982) but also provides a constant flow of malarial parasites from the forest to rural communities (Verdrager, 1995).
A crucial reason for the high levels of malaria transmission seen in and near many forested areas of South–east Asia and the Western Pacific is the existence of many species of highly efficient vectors that have adapted to forest habitats (Table 1). For some of these species, closed forests provide favourable ecological conditions that result in long adult-mosquito life-spans and an abundance of breeding sites. Moreover, most of these species, including An. dirus, An. balabacensis, An. donaldi, An. flavirostris and An. leucosphyrus, are considered main vectors throughout their ranges (Service, 1993). Anopheles dirus is probably the most important because of its wide geographical range and its efficiency and ecological plasticity as a vector. In addition, forest-fringe and deforested areas create adequate breeding habitats for several main vectors, including An. minimus, An. maculatus, An. culicifacies, An. fluviatilis, An. farauti, An. koliensis, An. letifer and An. punctulatus. The wide diversity of both the deep-forest and near-forest main vectors, as well as their great potential to adapt to habitat changes, mean that the consequences of deforestation on malaria transmission in South–east Asia and the Western Pacific are difficult to predict and unlikely to be unidirectional. Although deforestation may deplete the populations of deep-forest vectors and so initially reduce malaria transmission, in some localities this depletion may be followed by the invasion of the deforested areas by other efficient vectors and an increase in transmission. The position is therefore more complex than generally considered (Table 2).
CONVERGENCE OF POPULATIONS AT RISK OF MALARIA AND CLOSED FORESTS
GIS platforms allow the study and quantification of the spatial associations between forest cover, malaria and patterns of human settlement. For the present investigation, the spatial limits of malaria were defined using a contemporary map of the disease, which has already been described in detail (Guerra et al., 2006; Fig. 1). Briefly, this map was generated using existing information, retrieved from international travel and health guidelines, to identify areas at risk of malaria transmission at sub-national level. The administrative areas of malaria-endemic countries that were categorized as ‘no risk’ in these guidelines were excluded first. The maximum altitudinal limits of recorded malaria were then used to generate an altitudinal mask that excluded highland areas. A population-density mask, derived from a contemporary global population grid (Balk et al., 2006), was used to exclude areas with human population densities that were considered too low or too high for malaria transmission. Areas with less than one person/km2 were deemed free of malaria risk, because human–vector contact in such areas would be sufficiently low to interrupt transmission. Population-density thresholds were then defined, by region, as a proxy of urban agglomerations, to allow for the effect of urbanization on malaria transmission (Hay et al., 2005).
A land-cover map developed by the FAO as part of the Forest Resources Assessment (FRA) for the year 2000 (Anon., 2001a) was used to identify areas of closed forest. This map classifies land cover into ‘closed forests’, ‘open forests’, ‘other wooded land’, ‘other land cover’, and ‘water’. The first two classes were derived by applying a mixture-analysis model to normalised-difference-vegetation-index (NDVI) imagery derived from the Advanced Very High Resolution Radiometer for the year 1995; the use of this imagery in epidemiology has been reviewed by Hay et al. (1996, 2006b) and Hay (2000). The other three classes were adapted from an existing land-cover classification developed by the United States Geological Survey (Loveland et al., 1999). The rationale of using the FRA 2000 map is its explicit differentiation between closed and open forests, in accordance with the standards set by the FAO (Anon., 2001b) and the definitions adopted here.
Values for the PARM were derived from the human-population map created by the Global Rural–Urban Mapping Project for the year 2000 (Balk et al., 2006). This surface was developed, on a 30-arc-second grid, from the areal weighting of census data and the re-allocation of population according to urban-area proxy data. Since this data-set was generated for the year 2000, country-specific medium-variant rates of intercensal population growth (http://esa.un.org/unpp) were used to project the population totals to 2005, for consistency with the map of the spatial limits of malaria that was used (Hay et al., 2005).
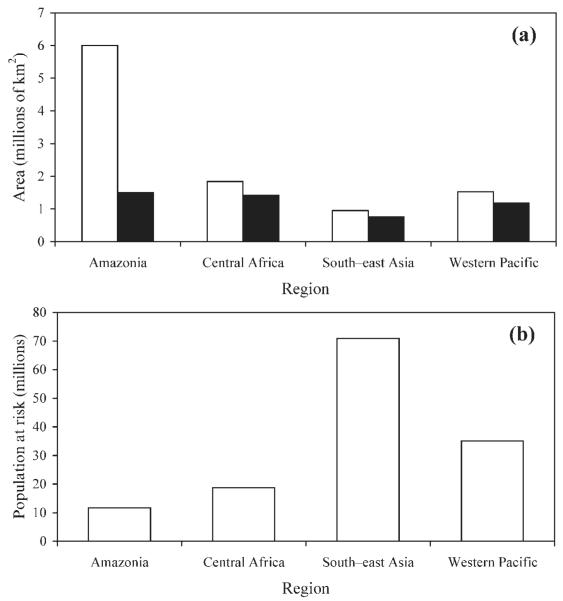
By overlaying the malaria-distribution map on the FAO’s delimitations of ‘closed forest’, it was possible to identify areas of closed forest that are malarious (Fig. 1). An equal-area projection and GIS software (ArcView 3.2; ESRI, Redlands, CA) were then used to evaluate the areas (in km2) of the malarious closed forests and the numbers of individuals (i.e. the PARM) living in such forests. The largest extent of closed tropical forests is that of the Amazon region, which, according to the FAO map (Anon., 2001a), covers >6 million km2 and accounts for about 60% of the estimated global area of closed humid forests. [Table 3 and Figure 2(a)]. Only 25.1% of the area covered by closed Amazonian forest is deemed malarious, however, mainly because of extremely low human-population densities (Guerra et al., 2006). In contrast, the total areas covered by closed forest in Central Africa, South–east Asia and the Western Pacific are much smaller (1.83 million, 0.95 million, and 1.53 million km2, respectively) but mostly malarious (77.1%, 79.9% and 77.8%, respectively). The regional differences in the estimated sizes of the PARM living in areas of closed forest are even more striking [Table 3 and Figure 2(b)]. Although about 71 million and 35 million people are estimated to be at risk of malaria in areas of closed forest within South–east Asia and the Western Pacific, respectively, the corresponding numbers for the Amazon region and Central Africa are markedly lower (11.65 million and 18.71 million, respectively). The differences are largely attributable to regional variation in human population densities, which are, in general, substantially higher in the forested areas of Asia than in the corresponding areas of Amazonia and Central Africa. In addition, South–east Asia, the Western Pacific and Central Africa have higher rates of forest degradation than Amazonia (Achard et al., 2002). In Amazonia, therefore, the level of forest fragmentation is probably relatively low, and so relatively few people are driven close to the deep forests.
TABLE 3.
Regional estimates of the total areas of closed forest and malarious closed forest and of the populations at risk of malaria (PARM) living in closed forests
| Region | Area of closed forest (km2) |
% of area of closed forest that is malarious |
PARM in 2005 | |
|---|---|---|---|---|
| Total | Within malaria limits | |||
| Amazonia | 6,004,864 | 1,507,395 | 25.1 | 11,654,151 |
| Central Africa | 1,838,338 | 1,417,118 | 77.1 | 18,713,936 |
| South–east Asia | 951,356 | 760,352 | 79.9 | 70,879,923 |
| Western Pacific | 1,528,344 | 1,188,253 | 77.8 | 35,093,490 |
| All four | 10,322,902 | 4,873,118 | 47.2 | 136,341,500 |
FIG. 2.
Regional comparisons of (a) the areas of closed forest (□) and malarious closed forest (■), and (b) the populations at risk of malaria within areas of closed forest.
Country Estimates and Ranking
Table 4 shows the estimated area of closed forest and the estimated PARM for each country of interest. In order to identify those countries where the problem of deforestation might have the greater impact on the epidemiology of malaria, the countries investigated were ranked in terms of three variables: the total areas of malarious closed forest; the PARM living in these areas; and the annual rates of deforestation between 1990 and 2000. [The latter were derived by the FAO as part of FRA 2000 (Anon., 2001b) and, although based on all-forest surveys of national inventories and mapping reports, were assumed to be applicable to the ‘closed forest’ class.] Ranking scores were assigned for each of these variables and then totalled to yield a final country score. Countries that rank high in the list, such as Indonesia and Myanmar, are therefore those with a combination of relatively large extents of closed forest in malarious areas, high numbers of people living in these areas, and high rates of deforestation.
TABLE 4.
The country-specific data, showing the areas of closed forest and malarious closed forest, the populations at risk of malaria (PARM) living in closed forests, deforestation rates, and ranking according to the size of the malaria problem that deforestation is likely to pose
| Area of closed forest (km2) |
|||||||
|---|---|---|---|---|---|---|---|
| Region* | Country | Total | Within malaria limits |
PARM in 2005 |
Deforestation rate† (%) |
Rank‡ | Deep-forest vectors? |
| WP | Indonesia | 917,003 | 629,179 | 19,035,489 | −1.2 | 1 | Present |
| SEA | Myanmar | 267,609 | 175,667 | 5,387,273 | −1.4 | 2 | Present |
| CA | Democratic Republic of the Congo |
1,161,386 | 1,018,804 | 15,113,330 | −0.4 | 3 | |
| AR | Brazil | 3,613,076 | 783,221 | 5,448,638 | −0.4 | 4 | |
| SEA | India | 330,681 | 302,441 | 46,756,606 | +0.1 | 6 | Present |
| SEA | Nepal | 52,233 | 26,075 | 5,938,885 | −1.8 | 6 | |
| WP | Malaysia | 160,405 | 144,192 | 2,089,900 | −1.2 | 7 | Present |
| CA | Cameroon | 161,570 | 160,739 | 1,802,808 | −0.9 | 9 | |
| WP | Papua New Guinea | 319,490 | 300,115 | 2,745,244 | −0.4 | 9 | |
| WP | Philippines | 42,607 | 28,209 | 3,574,096 | −1.4 | 10 | Present |
| SEA | Thailand | 61,989 | 56,128 | 3,446,919 | −0.7 | 11 | Present |
| AR | Colombia | 494,133 | 164,125 | 1,558,926 | −0.4 | 13 | |
| SEA | Laos | 118,906 | 115,931 | 2,372,844 | −0.4 | 13 | Present |
| AR | Peru | 593,014 | 223,020 | 1,262,409 | −0.4 | 13 | |
| AR | Ecuador | 127,174 | 51,618 | 1,352,002 | −1.2 | 16 | |
| SEA | Sri Lanka | 13,817 | 12,213 | 1,653,265 | −1.6 | 16 | |
| WP | Viet Nam | 87,151 | 84,941 | 7,563,063 | +0.5 | 16 | Present |
| SEA | Cambodia | 66,959 | 55,570 | 1,304,546 | −0.6 | 18 | Present |
| AR | Bolivia | 419,198 | 172,149 | 721,302 | −0.3 | 19 | |
| AR | Venezuela | 371,158 | 36,408 | 964,271 | −0.4 | 20 | |
| CA | Congo | 203,479 | 124,141 | 524,650 | −0.1 | 21 | |
| SEA | Bangladesh | 10,913 | 10,224 | 3,243,854 | +1.3 | 22 | Present |
| AR | Guyana | 173,933 | 66,238 | 304,571 | −0.3 | 23 | |
| CA | Equatorial Guinea | 17,920 | 12,582 | 260,588 | −0.6 | 24 | |
| CA | Central African Republic | 97,951 | 59,967 | 488,335 | −0.1 | 25 | |
| WP | Timor-Leste | 1687 | 1618 | 85,698 | −0.6 | 26 | |
| SEA | Bhutan | 28,248 | 6103 | 775,732 | |||
| AR | French Guiana | 80,977 | 124 | 21,960 | |||
| CA | Gabon | 196,031 | 40,886 | 524,225 | |||
| AR | Suriname | 132,200 | 10,491 | 20,070 | |||
AR, Amazonian region; CA, Central Africa; SEA, South–east Asia; WP, Western Pacific.
The mean percentage change in forest cover/year, between 1990 and 2000 (Anon., 2001b).
Countries with the same relative ranking score are ordered alphabetically. Bhutan, French Guiana, Gabon and Suriname could not be ranked because deforestation rates for these countries were not available.
Seven of the 10 highest scoring countries are in South–east Asia (three) or the Western Pacific (four). These seven rank highly because of their high deforestation rates and the large numbers of people at risk of malaria in their closed forests. Brazil is ranked fourth on the list, largely because it has a very large area of malarious closed forest. The Democratic Republic of the Congo (DRC) and Cameroon are the only African countries ranked in the top 10. The DRC has a greater area of malarious closed forest than any other country, whereas Cameroon has higher rates of deforestation and larger extents of malarious closed forests than Congo, the Central African Republic or Equatorial Guinea. Unfortunately, Bhutan, French Guiana, Gabon and Suriname could not be ranked because there have been no estimates of the deforestation rates in these countries.
An important consideration when ranking countries in this way is that of vector competence. In the present study, the lack of a consistent classification of vector competence hindered its inclusion as a ranking criterion. To compensate, Table 4 includes an indication of the countries in which there is at least one, main, deep-forest vector. The transmission of malaria in the forests of such countries, which are all in South–east Asia or the Western Pacific (Table 1), is more severe than that in the other countries considered.
DISCUSSION
By using the best knowledge available on the distribution of malaria, closed forests and human populations, it has been possible to identify regional differences in the sizes of the human populations and areas at risk of malaria within closed forests (Fig. 2). The results indicate that, in South–east Asia and the Western Pacific, high population densities in or near areas of malarious closed forest expose large numbers of people to malarial parasites transmitted by highly efficient forest vectors. The prevalence of such vectors has historically represented a challenge for malaria control and stimulated environmental-management approaches such as vegetation clearing — including deforestation (Mouchet and Brengues, 1990; Arbani, 1992). Because of the complexity of interactions that may involve populations of closed-forest, near-forest and/or non-forest vectors, it is not easy to predict the impact of deforestation on malaria transmission in South–east Asia and the Western Pacific. A different set of circumstances shape the relationships between forests and human malaria in Amazonia and Central Africa. The PARM living in closed forests in these regions are much smaller (almost an order of magnitude lower) than those in South–east Asia and the Western Pacific combined. It is the vast area of the closed forests in Amazonia that is the most important contributor to the estimates of the PARM in the region [Fig. 2(a)]. Most of the Amazonian rainforests harbour such low numbers of humans that there is no or only a negligible risk of human malaria (Fig. 2). These rainforests are being felled and degraded at an increasing rate (Fearnside, 2005), however, and, in the current absence of a deep-forest vector, malaria is more likely to get worse than to get better after deforestation, with the generation of new habitats for heliophilic vectors such as An. darlingi. As Central Africa resembles the Amazon region, in terms of vector ecology within its closed forest, deforestation will probably have similar malaria-related outcomes in the two regions. In Central Africa, however, a much larger proportion of the area covered by closed forest is categorized as malarious (77.1% v. 25.1% in Amazonia) and the PARM is about 50% greater. The presence of extremely efficient vectors such as An. gambiae s.s. and An. funestus, which both generally benefit by the clearing of forests, means that deforestation in Central Africa may dramatically increase the incidence of human malaria in the region.
A country-level analysis has allowed those territories where the problem of deforestation is highest and its impact on malaria transmission would be most significant to be identified (Table 4). The ranking of countries supports the results of the regional comparisons, with Asian countries generally ranking higher on the list than African or South American ones. If predictions of the effects of future deforestation on human malaria are to be made more accurate, the precise distributions of the vector mosquitoes, with respect to the deforestation, need to be mapped. Unfortunately, there are currently few, if any, relevant and reliable maps of vector distribution and deforestation. A better knowledge of the relative competence of each Anopheles species as a vector of the parasites causing human malaria is also needed, to allow more accurate predictability and comparability between countries. In those countries that have deep-forest and near-forest Anopheles species as main vectors (i.e. most South–east Asian and Western Pacific countries; Table 4) there is a particular uncertainty about the consequences of deforestation on malaria transmission.
CONCLUSIONS
An understanding of the relationships between forests and malaria transmission is important to guide strategies designed to reduce malaria burden in endemic forested areas. This review article provides a global overview of these relationships, highlighting the regional differences, and assessing the extent to which qualitative assertions about malaria and deforestation are supported by hard data. The numbers of people at risk of malaria within closed forests are estimated here, for the first time. South–east Asia and the Western Pacific have the highest PARM in forests but assessing future trends within these areas is particularly problematic because of the complex interaction of influences on the forest and non-forest vectors. It is more straightforward to predict the impacts of deforestation in Central Africa and Amazonia. Deforestation in Central Africa is of more concern globally, because of the efficacy of the non-forest vectors and the high densities of the human populations in this region’s forests. ‘Frontier’ malaria remains the greatest concern for malaria epidemiology in South America, because of the significance of malaria transmission in Amazonia.
This review forms part of the Malaria Atlas Project (www.map.ox.ac.uk), which aims to build up a comprehensive, global, spatial and epidemiological framework for mapping malaria. The core of this project is the development of a map of malaria endemicity based on a global database of malaria prevalence. In conjunction with current land-cover data-sets, this database will eventually allow a more detailed examination of the links between malaria and deforestation, and contribute to improving our knowledge in this neglected area.
ACKNOWLEDGEMENTS
C.A.G. is supported by the Wellcome Trust (via project grant 076951) and also acknowledges the support of the Fundación para la Ciencia y Tecnología (FUNDACYT). S.I.H. is funded by a Research Career Development Fellowship from the Wellcome Trust (069045). R.W.S. is a Wellcome Trust Senior Research Fellow (058992) and acknowledges the support of the Kenyan Medical Research Institute (KEMRI). This paper is published with the permission of the director of KEMRI. This work forms part of the output of the Malaria Atlas Project, which is funded by the Wellcome Trust, U.K.
REFERENCES
- Achard F, Eva HD, Stibig HJ, Mayaux P, Gallego J, Richards T, Malingreau JP. Determination of deforestation rates of the world’s humid tropical forests. Science. 2002;297:999–1002. doi: 10.1126/science.1070656. [DOI] [PubMed] [Google Scholar]
- Anon . International Classification and Mapping of Vegetation. United Nations Educational, Scientific and Cultural Organization; Paris: 1973. [Google Scholar]
- Anon . FRA 2000 — Global Forest Cover Mapping Final Report. Food and Agriculture Organization of the United Nations; Rome: 2001a. [Google Scholar]
- Anon . Global Forest Resources Assessment 2000 — Main Report. Food and Agriculture Organization of the United Nations; Rome: 2001b. [Google Scholar]
- Anon . Ecosystems and Human Well-being: Synthesis. World Resources Institute; Washington, DC: 2005. [Google Scholar]
- Arbani PR. Malaria control program in Indonesia. Southeast Asian Journal of Tropical Medicine and Public Health. 1992;23(Suppl. 4):29–38. [PubMed] [Google Scholar]
- Balk D, Deichmann U, Yetman G, Pozzi F, Hay SI, Nelson A. Determining global population distribution: methods, applications and data. Advances in Parasitology. 2006;62:120–156. doi: 10.1016/S0065-308X(05)62004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee MK, Palikhe N, Shrestha BL, Vaidya RG. Persistent malaria transmission in forests of central Nepal. In: Sharma VP, Kondrashin AV, editors. Forest Malaria in Southeast Asia — Proceedings of an Informal Consultative Meeting; 18–22 February, 1991; New Delhi: World Health Organization; 1991. pp. 155–169. [Google Scholar]
- Bockarie MJ, Service MW, Barnish G, Touré YT. Vectorial capacity and entomological inoculation rates of Anopheles gambiae in a high rainfall forested area of southern Sierra Leone. Tropical Medicine and Parasitology. 1995;46:164–171. [PubMed] [Google Scholar]
- Briet OJ, Dossou Yovo J, Akodo E, van de Giesen N, Teuscher TM. The relationship between Anopheles gambiae density and rice cultivation in the savannah zone and forest zone of Côte d’Ivoire. Tropical Medicine and International Health. 2003;8:439–448. doi: 10.1046/j.1365-3156.2003.01054.x. [DOI] [PubMed] [Google Scholar]
- Butraporn P, Sornmani S, Hungsapruek T. Social, behavioural, housing factors and their interactive effects associated with malaria occurrence in east Thailand. Southeast Asian Journal of Tropical Medicine and Public Health. 1986;17:386–392. [PubMed] [Google Scholar]
- Butraporn P, Prasittisuk C, Krachaiklin S, Chareonjai P. Behaviors in self-prevention of malaria among mobile population in east Thailand. Southeast Asian Journal of Tropical Medicine and Public Health. 1995;26:213–218. [PubMed] [Google Scholar]
- Caldas de Castro M, Monte-Mor RL, Sawyer DO, Singer BH. Malaria risk on the Amazon frontier. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2452–2457. doi: 10.1073/pnas.0510576103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevale P, Le Goff G, Toto JC, Robert V. Anopheles nili as the main vector of human malaria in villages of southern Cameroon. Medical and Veterinary Entomology. 1992;6:135–138. doi: 10.1111/j.1365-2915.1992.tb00590.x. [DOI] [PubMed] [Google Scholar]
- Chang MS, Hii J, Buttner P, Mansoor F. Changes in abundance and behaviour of vector mosquitoes induced by land use during the development of an oil palm plantation in Sarawak. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1997;91:382–386. doi: 10.1016/s0035-9203(97)90248-0. [DOI] [PubMed] [Google Scholar]
- Coene J. Malaria in urban and rural Kinshasa: the entomological input. Medical and Veterinary Entomology. 1993;7:127–137. doi: 10.1111/j.1365-2915.1993.tb00665.x. [DOI] [PubMed] [Google Scholar]
- Cohuet A, Simard F, Wondji CS, Nkondjio C. Antonio, Awono Ambene P, Fontenille D. High malaria transmission intensity due to Anopheles funestus (Diptera: Culicidae) in a village of savannah–forest transition area in Cameroon. Journal of Medical Entomology. 2004;41:901–905. doi: 10.1603/0022-2585-41.5.901. [DOI] [PubMed] [Google Scholar]
- Conn JE, Wilkerson RC, Segura MN, de Souza RT, Schlichting CD, Wirtz RA, Povoa MM. Emergence of a new Neotropical malaria vector facilitated by human migration and changes in land use. American Journal of Tropical Medicine and Hygiene. 2002;66:18–22. doi: 10.4269/ajtmh.2002.66.18. [DOI] [PubMed] [Google Scholar]
- Coz J, Hamon J, Sales S, Eyraud M, Brengues J, Subra R, Accrombessi R. Études entomologiques sur la transmission du paludisme humain dans une zone de forêt humide dense, la région de Sassandra, République de Côte d’Ivoire. Cahiers O.R.S.T.O.M., Série Entomologie Médicale et Parasitologie. 1966;4:13–42. [Google Scholar]
- Deane LM. Malaria vectors in Brazil. Memórias do Instituto Oswaldo Cruz. 1986;81(Suppl. 2):5–14. doi: 10.1590/s0074-02761986000400015. [DOI] [PubMed] [Google Scholar]
- Erhart A, Thang ND, Hung NQ, Le VT, Le XH, Tuy TQ, Le DC, Speybroeck N, Coosemans M, d’Alessandro U. Forest malaria in Vietnam: a challenge for control. American Journal of Tropical Medicine and Hygiene. 2004;70:110–118. [PubMed] [Google Scholar]
- Erhart A, Ngo TD, Phan KV, Ta TT, van Overmeir C, Speybroeck N, Obsomer V, Le HX, Le KT, Coosemans M, d’Alessandro U. Epidemiology of forest malaria in central Vietnam: a large scale cross-sectional survey. Malaria Journal. 2005;4:58. doi: 10.1186/1475-2875-4-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearnside PM. Deforestation in Brazilian Amazonia: history, rates, and consequences. Conservation Biology. 2005;19:680–688. [Google Scholar]
- Forattini OP. Entomologia Médica: Parte Geral, Diptera, Anophelini. Departamento de Parasitologia, Faculdade de Higiene e Saúde Pública; São Paulo, Brazil: 1962. [Google Scholar]
- Forattini OP, Kakitani I, Massad E, Marucci D. Studies on mosquitoes (Diptera: Culicidae) and anthropic environment. 2. Immature stages research at a rice irrigation system location in south–eastern Brazil. Revista de Saúde Pública. 1993a;27:227–236. doi: 10.1590/s0034-89101993000400001. [DOI] [PubMed] [Google Scholar]
- Forattini OP, Kakitani I, Massad E, Marucci D. Studies on mosquitoes (Diptera: Culicidae) and anthropic environment. 3. Survey of adult stages at the rice irrigation system and the emergence of Anopheles albitarsis in south–eastern, Brazil. Revista de Saúde Pública. 1993b;27:313–325. doi: 10.1590/s0034-89101993000500001. [DOI] [PubMed] [Google Scholar]
- Gillies MT, de Meillon B. The Anophelinae of Africa South of the Sahara (Ethiopian Zoogeographical Region) South African Institute for Medical Research; Johannesburg, South Africa: 1968. [Google Scholar]
- Guerra CA, Snow RW, Hay SI. Defining the global spatial limits of malaria transmission in 2005. Advances in Parasitology. 2006;62:157–179. doi: 10.1016/S0065-308X(05)62005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbach RE, Baimai V, Sukowati S. Some observations on sympatric populations of the malaria vectors Anopheles leucosphyrus and Anopheles balabacensis in a village-forest setting in South Kalimantan. Southeast Asian Journal of Tropical Medicine and Public Health. 1987;18:241–247. [PubMed] [Google Scholar]
- Hay SI. An overview of remote sensing and geodesy for epidemiology and public health application. Advances in Parasitology. 2000;47:1–35. doi: 10.1016/s0065-308x(00)47005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay SI, Tucker CJ, Rogers DJ, Packer MJ. Remotely sensed surrogates of meteorological data for the study of the distribution and abundance of arthropod vectors of disease. Annals of Tropical Medicine and Parasitology. 1996;90:1–19. doi: 10.1080/00034983.1996.11813021. [DOI] [PubMed] [Google Scholar]
- Hay SI, Guerra CA, Tatem A, Noor AM, Snow RW. The global distribution and population at risk of malaria: past, present, and future. Lancet Infectious Diseases. 2004;4:327–336. doi: 10.1016/S1473-3099(04)01043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay SI, Guerra CA, Tatem A, Atkinson P, Snow RW. Urbanization, malaria transmission and disease burden in Africa. Nature Reviews Microbiology. 2005;3:81–90. doi: 10.1038/nrmicro1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay SI, Tatem AJ, Guerra CA, Snow RW. Foresight on Population at Malaria Risk in Africa: 2005, 2015 & 2030. Foresight Project, Office of Science and Technology; London: 2006a. [Google Scholar]
- Hay SI, Tatem AJ, Graham AJ, Goetz SJ, Rogers DJ. Global environmental data for mapping infectious disease distribution. Advances in Parasitology. 2006b;62:38–77. doi: 10.1016/S0065-308X(05)62002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser J, de Castro MC, Maltese MF, Bos R, Tanner M, Singer BH, Utzinger J. Effect of irrigation and large dams on the burden of malaria on a global and regional scale. American Journal of Tropical Medicine and Hygiene. 2005a;72:392–406. [PubMed] [Google Scholar]
- Keiser J, Singer BH, Utzinger J. Reducing the burden of malaria in different eco–epidemiological settings with environmental management: a systematic review. Lancet Infectious Diseases. 2005b;5:695–708. doi: 10.1016/S1473-3099(05)70268-1. [DOI] [PubMed] [Google Scholar]
- Kobayashi J, Nambanya S, Miyagi I, Vanachone B, Manivong K, Koubouchan T, Amano H, Nozaki H, Inthakone S, Sato Y. Collection of anopheline mosquitos in three villages endemic for malaria in Khammouane, Lao PDR. Southeast Asian Journal of Tropical Medicine and Public Health. 1997;28:20. [PubMed] [Google Scholar]
- Kobayashi J, Vannachone B, Sato Y, Sinjo M, Nambanya S, Manivang K, Inthakone S. Current status of malaria infection in a southeastern province of Lao PDR. Southeast Asian Journal of Tropical Medicine and Public Health. 1998;29:236–241. [PubMed] [Google Scholar]
- Kondrashin AV. Malaria in the WHO Southeast Asia region. Indian Journal of Malariology. 1992;29:129–160. [PubMed] [Google Scholar]
- Kondrashin AV, Jung RK, Akiyama J. Ecological aspects of forest malaria in Southeast Asia. In: Sharma VP, Kondrashin AV, editors. Forest Malaria in Southeast Asia — Proceedings of an Informal Consultative Meeting; 18–22 February, 1991; New Delhi: World Health Organization; 1991. pp. 1–22. [Google Scholar]
- Lansang MA, Belizario VY, Bustos MD, Saul A, Aguirre A. Risk factors for infection with malaria in a low endemic community in Bataan, The Philippines. Acta Tropica. 1997;63:257–265. doi: 10.1016/s0001-706x(96)00625-0. [DOI] [PubMed] [Google Scholar]
- Loveland TR, Zhu ZL, Ohlen DO, Brown JF, Reed BC, Yang LM. An analysis of the IGBP global land-cover characterization process. Photogrammetric Engineering and Remote Sensing. 1999;65:1021–1032. [Google Scholar]
- Lwin M, Htut Y. Study of the malaria situation in forested foothill and nearby plain areas of Myanmar. Southeast Asian Journal of Tropical Medicine and Public Health. 1991;22:509–514. [PubMed] [Google Scholar]
- Macdonald G. The Epidemiology and Control of Malaria. Oxford University Press; London: 1957. [Google Scholar]
- Manga L, Toto JC, Carnevale P. Malaria vectors and transmission in an area deforested for a new international airport in southern Cameroon. Annales de la Société Belge de Médecine Tropicale. 1995;75:43–49. [PubMed] [Google Scholar]
- Manga L, Bouchite B, Toto JC, Froment A. La faune anophélienne et la transmission du paludisme dans une zone de transition forêt/savane au centre du Cameroun. Bulletin de la Société de Pathologie Éxotique. 1997a;90:128–130. [PubMed] [Google Scholar]
- Manga L, Toto JC, Le Goff G, Brunhes J. The bionomics of Anopheles funestus and its role in malaria transmission in a forested area of southern Cameroon. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1997b;91:387–388. doi: 10.1016/s0035-9203(97)90249-2. [DOI] [PubMed] [Google Scholar]
- Mayaux P, Holmgren P, Achard F, Eva H, Stibig H, Branthomme A. Tropical forest cover change in the 1990s and options for future monitoring. Philosophical Transactions of the Royal Society, Series B. 2005;360:373–384. doi: 10.1098/rstb.2004.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGreevy PB, Dietze R, Prata A, Hembree SC. Effects of immigration on the prevalence of malaria in rural areas of the Amazon basin of Brazil. Memórias do Instituto Oswaldo Cruz. 1989;84:485–491. doi: 10.1590/s0074-02761989000400005. [DOI] [PubMed] [Google Scholar]
- Meunier JY, Safeukui I, Fontenille D, Boudin C. Étude de la transmission du paludisme dans une future zone d’essai vaccinal en forêt équatoriale du sud Cameroun. Bulletin de la Société de Pathologie Éxotique. 1999;92:309–312. [PubMed] [Google Scholar]
- Mouchet J, Brengues J. Les interfaces agriculture–santé dans les domaines de l’épidemiologie des maladies à vecteurs et de la lutte antivectorielle. Bulletin de la Société de Pathologie Éxotique. 1990;83:376–393. [PubMed] [Google Scholar]
- Mouchet J, Carnevale P, Coosemans M, Julvez J, Manguin S, Richards-Lenoble D, Sircoulon J. Biodiversité du Paludisme dans le Monde. John Libbey Eurotext; Paris: 2004. [Google Scholar]
- Narasimham MVVL. Perspectives of forest malaria in India. In: Sharma VP, Kondrashin AV, editors. Forest Malaria in Southeast Asia — Proceedings of an Informal Consultative Meeting; 18–22 February, 1991; New Delhi: World Health Organization; 1991. pp. 81–91. [Google Scholar]
- Nzeyimana I, Henry MC, Yovo J. Dossou, Doannio JM, Diawara L, Carnevale P. Épidemiologie du paludisme dans le sud–ouest forestier de la Côte d’Ivoire (région de Tai) Bulletin de la Société de Pathologie Éxotique. 2002;95:89–94. [PubMed] [Google Scholar]
- Oo TT, Storch V, Becker N. Anopheles dirus and its role in malaria transmission in Myanmar. Journal of Vector Ecology. 2003;28:175–183. [PubMed] [Google Scholar]
- Pan American Health Organization . Report on the Status of Malaria Programs in the Americas (Based on 2002 Data) Pan American Health Organization; Washington, DC: 2003. Document CD44/INF/3. [PubMed] [Google Scholar]
- Pichainarong N, Chaveepojnkamjorn W. Malaria infection and life-style factors among hill-tribes along the Thai–Myanmar border area, northern Thailand. Southeast Asian Journal of Tropical Medicine and Public Health. 2004;35:834–839. [PubMed] [Google Scholar]
- Prakash A, Bhattacharyya DR, Mohapatra PK, Mahanta J. Seasonal prevalence of Anopheles dirus and malaria transmission in a forest fringed village of Assam, India. Indian Journal of Malariology. 1997;34:117–125. [PubMed] [Google Scholar]
- Robert V, Macintyre K, Keating J, Trape JF, Duchemin JB, Warren M, Beier JC. Malaria transmission in urban sub-Saharan Africa. American Journal of Tropical Medicine and Hygiene. 2003;68:169–176. [PubMed] [Google Scholar]
- Rogers DJ, Randolph SE, Snow RW, Hay SI. Satellite imagery in the study and forecast of malaria. Nature. 2002;415:710–715. doi: 10.1038/415710a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa-Freitas MG, Lourenco-de-Oliveira R, de Carvalho-Pinto CJ, Flores-Mendoza C, Silva-do-Nascimento TF. Anopheline species complexes in Brazil. Current knowledge of those related to malaria transmission. Memórias do Instituto Oswaldo Cruz. 1998;93:651–655. doi: 10.1590/s0074-02761998000500016. [DOI] [PubMed] [Google Scholar]
- Rosenberg R, Maheswary NP. Forest malaria in Bangladesh. II. Transmission by Anopheles dirus. American Journal of Tropical Medicine and Hygiene. 1982;31:183–191. doi: 10.4269/ajtmh.1982.31.183. [DOI] [PubMed] [Google Scholar]
- Rozendaal JA. Observations on the distribution of anophelines in Suriname with particular reference to the malaria vector Anopheles darlingi. Memórias do Instituto Oswaldo Cruz. 1990;85:221–234. doi: 10.1590/s0074-02761990000200014. [DOI] [PubMed] [Google Scholar]
- Rubio-Palis Y, Zimmerman RH. Ecoregional classification of malaria vectors in the Neotropics. Journal of Medical Entomology. 1997;34:499–510. doi: 10.1093/jmedent/34.5.499. [DOI] [PubMed] [Google Scholar]
- Sawyer D. Economic and social consequences of malaria in new colonization projects in Brazil. Social Science and Medicine. 1993;37:1131–1136. doi: 10.1016/0277-9536(93)90252-y. [DOI] [PubMed] [Google Scholar]
- Seng CM, Matusop A, Sen FK. Differences in Anopheles composition and malaria transmission in the village settlements and cultivated farming zone in Sarawak, Malaysia. Southeast Asian Journal of Tropical Medicine and Public Health. 1999;30:454–459. [PubMed] [Google Scholar]
- Service MW. Mosquitoes (Culicidae) In: Lane RP, Crosskey RW, editors. Medical Insects and Arachnids. Chapman & Hall; London: 1993. pp. 120–240. [Google Scholar]
- Sharma SK, Tyagi PK, Padhan K, Adak T, Subbarao SK. Malarial morbidity in tribal communities living in the forest and plain ecotypes of Orissa, India. Annals of Tropical Medicine and Parasitology. 2004;98:459–468. doi: 10.1179/000349804225003569. [DOI] [PubMed] [Google Scholar]
- Sharma VP, Prasittisuk C, Kondrashin AV. Magnitude of forest related malaria in the WHO Southeast Asia region. In: Sharma VP, Kondrashin AV, editors. Forest Malaria in Southeast Asia — Proceedings of an Informal Consultative Meeting; 18–22 February, 1991; New Delhi: World Health Organization; 1991. pp. 29–53. [Google Scholar]
- Shrestha JPB, Banerjee MK, Vaidya RG, Shrestha BL. Malaria situation in forested areas of Nepal. In: Sharma VP, Kondrashin AV, editors. Forest Malaria in Southeast Asia — Proceedings of an Informal Consultative Meeting; 18–22 February, 1991; New Delhi: World Health Organization; 1991. pp. 141–154. [Google Scholar]
- Shukla RP, Sharma SN, Kohli VK, Nanda N, Sharma VP, Subbarao SK. Dynamics of malaria transmission under changing ecological scenario in and around Nanak Matta dam, Uttaranchal, India. Indian Journal of Malariology. 2001;38:91–98. [PubMed] [Google Scholar]
- Singer BH, Caldas de Castro M. Agricultural colonization and malaria on the Amazon frontier. Annals of the New York Academy of Sciences. 2001;954:184–222. doi: 10.1111/j.1749-6632.2001.tb02753.x. [DOI] [PubMed] [Google Scholar]
- Singh N, Mishra AK, Chand SK, Sharma VP. Population dynamics of Anopheles culicifacies and malaria in the tribal area of central India. Journal of the American Mosquito Control Association. 1999;15:283–290. [PubMed] [Google Scholar]
- Singh N, Mishra AK, Shukla MM, Chand SK. Forest malaria in Chhindwara, Madhya Pradesh, central India: a case study in a tribal community. American Journal of Tropical Medicine and Hygiene. 2003;68:602–607. doi: 10.4269/ajtmh.2003.68.602. [DOI] [PubMed] [Google Scholar]
- Singhanetra Renard A. Population movement, socio–economic behavior and the transmission of malaria in northern Thailand. Southeast Asian Journal of Tropical Medicine and Public Health. 1986;17:396–405. [PubMed] [Google Scholar]
- Singhanetra Renard A. Malaria and mobility in Thailand. Social Science and Medicine. 1993;37:1147–1154. doi: 10.1016/0277-9536(93)90254-2. [DOI] [PubMed] [Google Scholar]
- Singhasivanon P. Mekong malaria. Malaria, multi-drug resistance and economic development in the greater Mekong subregion of Southeast Asia. Southeast Asian Journal of Tropical Medicine and Public Health. 1999;30(Suppl. 4):1–101. [PubMed] [Google Scholar]
- Smith NJH. Colonization lessons from a tropical forest. Science. 1981;214:755–761. doi: 10.1126/science.214.4522.755. [DOI] [PubMed] [Google Scholar]
- Snow RW, Craig MH, Newton CRJC, Steketee RW. The Public Health Burden of Plasmodium falciparum Malaria in Africa: Deriving the Numbers. National Institutes of Health; Bethesda, MD: 2003. Working Paper No. 11, Disease Control Priorities Project. [Google Scholar]
- Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow RW, Hay SI, Marsh K. Malaria in Africa: Sources, Risks, Drivers and Disease Burden 2005–2030. Foresight Project, Office of Science and Technology; London: 2006. [Google Scholar]
- Somboon P, Aramrattana A, Lines J, Webber R. Entomological and epidemiological investigations of malaria transmission in relation to population movements in forest areas of north–west Thailand. Southeast Asian Journal of Tropical Medicine and Public Health. 1998;29:3–9. [PubMed] [Google Scholar]
- Tadei WP, Dutary Thatcher B. Malaria vectors in the Brazilian Amazon: Anopheles of the subgenus Nyssorhynchus. Revista do Instituto de Medicina Tropical de São Paulo. 2000;42:87–94. doi: 10.1590/s0036-46652000000200005. [DOI] [PubMed] [Google Scholar]
- Tadei WP, Thatcher BD, Santos JM, Scarpassa VM, Rodrigues IB, Rafael MS. Ecologic observations on anopheline vectors of malaria in the Brazilian Amazon. American Journal of Tropical Medicine and Hygiene. 1998;59:325–335. doi: 10.4269/ajtmh.1998.59.325. [DOI] [PubMed] [Google Scholar]
- Verdrager J. Localized permanent epidemics: the genesis of chloroquine resistance in Plasmodium falciparum. Southeast Asian Journal of Tropical Medicine and Public Health. 1995;26:23–28. [PubMed] [Google Scholar]
- Vittor AY, Gilman RH, Tielsch J, Glass G, Shields T, Lozano WS, Pinedo-Cancino V, Patz JA. The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of falciparum malaria in the Peruvian Amazon. American Journal of Tropical Medicine and Hygiene. 2006;74:3–11. [PubMed] [Google Scholar]
- Walsh JF, Molyneux DH, Birley MH. Deforestation: effects on vector-borne disease. Parasitology. 1993;106(Suppl.):S55–S75. doi: 10.1017/s0031182000086121. [DOI] [PubMed] [Google Scholar]