Abstract
PURPOSE
This randomized clinical trial was conducted to assess the safety and effectiveness of the ErhBMP-2 in alveolar bone regeneration as well as preservation of the β-TCP bone graft material that contains ErhBMP-2.
MATERIALS AND METHODS
This study involved 72 patients at the 3 study centers. The patients, who were divided into 2 groups: the experiment group who had ErhBMP-2 coated TCP/HA and the control group who had TCP/HA graft material alone transplanted immediately after tooth extraction. CT was taken before and 3 months after the transplantation and healing status was compared between the two groups. The efficacy endpoints that were used to measure the degree of bone induction included alveolar bone height and 3 measurements of bone width. The paired t test was used to determine the significance of the changes (P<.05).
RESULTS
Changes in alveolar bone height were -1.087 ± 1.413 mm in the control group and -.059 ± 0.960 mm in the experimental group (P<.01). At 25% extraction socket length [ESL], the changes were 0.006 ± 1.149 mm in the control group and 1.279 ± 1.387 mm in the experimental group. At 50% ESL, the changes were 0.542 ± 1.157 mm and 1.239 ± 1.249 mm, respectively (P<.01 for 25% ESL, and P<.05 for 50% ESL). During the experiment, no adverse reactions to the graft material were observed.
CONCLUSION
ErhBMP-2 coated β-TCP/HA were found to be more effective in preserving alveolar bone than conventional β-TCP/HA alloplastic bone graft materials.
Keywords: ErhBMP-2, β-TCP/HA, Tooth extraction, Alveolar bone regeneration, Randomized clinical trial
INTRODUCTION
When tooth extraction is performed for the management of diseases or trauma, complete hemostasis should be achieved in order to prevent infection at the extraction site and sutures may be used to allow natural healing. After extraction, the bone resolves and remodels itself due to the nature of the alveolar bone. Tallgren et al.1 observed alveolar bone loss for 25 years and reported that most bone loss occurred during the first year after extraction and that the bone continues to resolve afterwards at a slow rate. Therefore, bone graft is performed at the extraction site or at the resolved alveolar ridge in order to prepare the site for an implant, to prevent bone loss for aesthetic reasons, or to preserve the extraction socket.2,3
In the treatment of periodontal defects, current practice includes the use of alloplastic materials, such as β-Tricalcium phosphate (β-TCP) and hydroxyapatite (HA), which are synthetic osteoconductive materials. A recent systematic review has also indicated that bone replacement grafts lead to significant clinical improvements in periodontal osseous defects.4 Although β-TCP/HA does not form new connective tissue, it provides not only a scaffold for new bone formation but also facilitates the stabilization of blood clot.5 As β-TCP is porous, it entraps growth factors within its micropores, thereby prolonging their activity.6
BMP, a protein derived from a subgroup of the transforming growth factor β family,7 accelerates ossification by controlling the essential factors of the bone induction cascade, resulting in the proliferation of osteoblasts from mesenchymal stem cells and the biosynthesis of bone matrices.8,9 Recombinant human BMPs are currently produced by BMP gene-transfected mammalian cell (CHO) cultures,10,11 and rhBMP-2 and BMP-7 are commercially available for the treatment of bony defects.12,13 One of the problems associated with clinical application of CHO-cell-derived rhBMP-2 (CrhBMP-2) is its high costs due to high dose requirements. One possible way of solving this problem is to produce monomer rhBMPs from BMP-gene-transfected Escherichia coli (E. coli) with a high efficiency and low costs. Bessho et al.14 examined the bone-inducing ability of an E. coli-derived rhBMP-2 (ErhBMP-2) variant with an N-terminal sequence and compared it with CrhBMP-2. Quantitative analysis indicated that the activity of ErhBMP-2 is similar to that of CrhBMP-2. However, it is unclear whether the characteristics of ErhBMP-2 are appropriate for clinical application. In particular, there have been few studies about the efficacy and safety of ErhBMP-2 and β-TCP graft materials in osseous defects, such as tooth extraction sockets. Recently, ErhBMP-2 has been developed in the Republic of Korea. Therefore, this randomized clinical trial was conducted to assess the safety and effectiveness of the ErhBMP-2 in alveolar bone regeneration as well as preservation of the β-TCP bone graft material that contains ErhBMP-2.
MATERIALS AND METHODS
Study design
A double-blind, active-controlled, randomized, parallel, multicenter, prospective, phase III study was conducted with the approval of the Korean Food and Drug Association at 3 centers in the Republic of Korea from April 2009 to March 2010, in order to assess the efficacy of ErhBMP-2 + β-TCP/HA in comparison with β-TCP/HA alone for the treatment of tooth extracted sockets. The Institutional Review Board at each of the 3 study centers approved the study protocol.
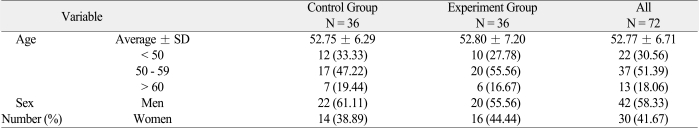
This study initially involved 72 patients aged from 35 to 65 years at the 3 study centers, whose premolars or molars were indicated for extraction with less than 50% of localized alveolar vertical bone loss (Table 1). However, patients with the following conditions were excluded from the study: (1) those who had severe periodontitis with localized alveolar vertical bone loss of more than 50%, (2) those who were currently pregnant or planned to get pregnant within 1 year of the experiment, (3) those who were older than 65 years, (4) those who had recent myocardial infarction or uncontrolled bleeding disorders, (5) those who were contraindicated to minor surgeries, (6) those who had mental illness or suspected mental illness or hypersensitivity to bone graft materials, and (7) those who were classified as inappropriate for clinical trial participation by the clinician due to ethical reasons or other possible impacts on the results of clinical trials.
Table 1.
Age and sex distribution of the control and experiment groups
Patients were divided into 2 groups: the experiment group who had ErhBMP-2 coated TCP/HA (Cowellmedi Co, Pusan, Korea; 1.5 mg/ml) and the control group who had TCP/HA graft material alone transplanted immediately into the socket of tooth extraction.
Study protocol
At the first visit, the purpose of the study was explained, written consent was obtained, and patient blood samples were taken to check pre-existing antibodies against ErhBMP-2. At the second visit, the indicated tooth of the patients, who provided written consent, was extracted and the socket was filled with the bone graft and sutured. The first CT scan was then taken to determine baseline characteristics. At the third and fourth visits, sutures were removed and the patient's intraoral condition was checked. The fifth visit was made 1 month after the second visit, and a blood sample was drawn and an intraoral examination was performed. The final visit was made 3 months after the second visit, and a computed tomography (CT) and an intraoral examination were performed.
Efficacy parameter
To verify the effectiveness of these bone grafts, CT was performed before the transplantation and 3 months after the transplantation and healing status was compared between the two. The efficacy endpoints that were used to measure the degree of bone induction included alveolar bone height (1 measurement) and 3 measurements of bone width (1 measurement each at 25%, 50% and 75% of the extraction socket length [ESL]). This method followed a previous similar study.15 In addition, maxillary teeth extraction sockets were measured by the same way as in a previous study.15 Mandibular tooth extraction sockets were measured by the method depicted in Fig. 1.
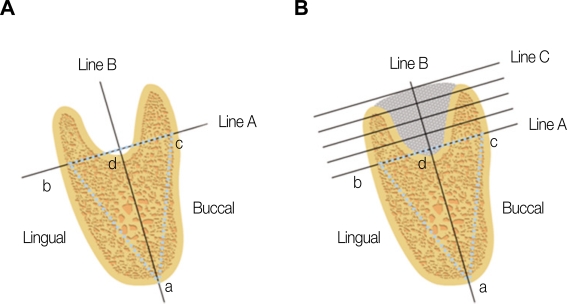
Fig. 1.
Computed tomography (CT) height and width measurements at baseline before tooth extraction (A) and 3 months after transplantation (B).
(1) Point a was marked on the same point of the lower edge of the mandible on representative CT scans taken before and after transplantation. (2) Points b and c were marked at the most prominent points on the buccal and lingual aspects, respectively. (3) Line A connected points b and c. (4) Line B, the "axial line", connected the midpoint between points b and c, which was called (5) Line C was drawn perpendicular to the "axial line"from the most superior point of the alveolar bone. (6) Bone height was defined as the distance between point d and line C. (7) The distance between point d and line C was divided into 4 aliquot portions and bone width was measured on each line before and after transplantation.
Statistical analysis
In order to assess the major effects of the bone graft materials, alveolar bone heights at baseline and 3 months post-transplantation were compared between the control and experiment groups. The mean and standard deviation of the test parameters were calculated using SPSS (Ver. 12.0, SPSS, Chicago, IL, USA). The paired t test was used to determine the significance of the changes. To assess the minor effects of the bone graft materials, changes in alveolar bone width at 25% ESL, 50% ESL and 75% ESL at the baseline and 3 months post-treatment were compared between control and experiment groups. The paired t test was used to determine the significance of the changes. A P value of <.05 was considered statistically significant.
RESULTS
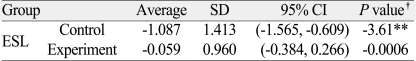
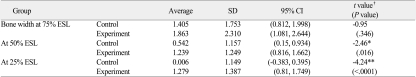
Changes in alveolar bone height were examined using CT scans taken before and 3 months after treatment, which turned out to be -1.087±1.413 mm in the control group and -0.059±0.960 mm in the experimental group. The paired Student t test was used to compare the mean change between the 2 groups in alveolar bone height preservation, and the difference was statistically significant (P<.01) (Table 2). Changes in alveolar bone width were also measured to determine the minor effects of bone grafts on the preservation of alveolar bone. At 25% ESL, the changes were 0.006±1.149 mm in the control group and 1.279±1.387 mm in the experimental group. At 50% ESL, the changes were 0.542±1.157 mm and 1.239±1.249 mm, respectively, and at 75% ESL, the changes were 1.405±1.753 mm and 1.863±2.310 mm, respectively. The paired Student t-test was used to compare the changes between the 2 groups, and the differences were statistically significant (P<.01 for 25% ESL, and P<.05 for 50% ESL) (Table 3).
Table 2.
Evaluation of the efficacy in maintaining alveolar bone height
ESL: extraction socket level
*: P<.05, **: P<.01, †: Student t-test
Table 3.
Evaluation of the efficacy in maintaining alveolar bone width
ESL: Extraction Socket Level
*: P<.05, **: P<.01, †: Student t-test
DISCUSSION
The potential therapeutic efficacy of rhBMP-2 in orthopedic and craniofacial reconstruction has been investigated. Preclinical studies have evaluated induction and repair of bony defects in a variety of indications.16,17 A previous study of utilizing rhBMP-2 in humans showed the safety and technical feasibility. Howell et al.18 reported that in local ridge preservation and augmentation, 0.43 mg/ml rhBMP/absorbable collagen sponge (ACS) was well tolerated locally and systemically, with no adverse events. In a pivotal study by Fiorellini et al.15 assessment of alveolar bone indicated that patients treated with 1.50 mg/ml CrhBMP-2/ACS had significantly better results after bone augmentation than control patients (P≤.05). The adequacy of bone for the placement of a dental implant was approximately twice greater in the rhBMP-2/ACS group than in the non-treatment or placebo group.
This randomized and double-blind clinical trial was designed to assess the safety and bone regenerative ability of the ErhBMP-2 coated β-TCP/HA bone graft material, which is coated with ErhBMP-2. After the completion of the clinical trial, the ANOVA test was performed to evaluate whether the evaluation parameters had homogeneity according to institutional and demographic data. Since there were no significant variations in these parameters, the results of this study on the safety and effectiveness of the bone graft materials are considered valid.
In this study, to assess the major effects of the bone graft material in preserving the alveolar bone, alveolar bone height at baseline and 3 months post-treatment were compared by measuring bone height in the cross sectional CT images. To assess the minor effects of the bone graft material, changes in alveolar bone width at 25% ESL, 50% ESL and 75% ESL were compared using cross-sectional CT images at baseline and 3 months post-treatment. In addition, clinical observation was performed to evaluate the safety of the graft material, and antibody against rhBMP-2 was tested to evaluate its immunological safety. No clinical adverse reactions or even inflammatory responses were observed in patients who received the bone graft. Basedon the immunological evaluations of the blood tests at the fifth visit of 72 patients (36 in the control group and 36 in the experiment group), none of the patients were suspected of having developed antibodies against the bone graft material. CT images were analyzed using the same software (Ondemend, Cybermed Inc, LA, CA, USA) at the same site immediately after transplantation and 3 months post-transplantation.
Changes in alveolar bone height were considered as a major evaluation variable in determining the effectiveness of the bone graft for alveolar bone regeneration. Based on CT scans at baseline and 3 months post-transplantation, the changing rate of the alveolar bone height, was higher in the control group than in the experimental group (P<.05). Changes in alveolar bone width were considered a minor evaluation variable in determining the effectiveness of the bone graft in alveolar bone regeneration.
In this study, materials other than the subject's gingiva (e.g., collagen membrane and others) were excluded in order to eliminate the effects of other factors. However, one crucial factor that affects the outcome of the conventional guided-bone regeneration (GBR) procedure is the fact that the treatment area must be protected from the surrounding soft tissue. Therefore, because of chewing, brushing and other oral habits, as well as differences in remaining gingival levels, the tested bone graft material did not survive at the treatment site over a sufficient time period. Therefore, it is conceivable that if the bone graft material is transplanted after allowing the gingival tissue to heal to a certain extent after the extraction, rather than being transplanted immediately after extraction, the alveolar bone preservation effect of the bone graft material may increase.
CONCLUSION
In this study, β-TCP/HA bone grafts coated with ErhBMP-2 were found to be more effective in preserving alveolar bone than conventional β-TCP/HA alloplastic bone grafts. Furthermore, during the experiment, no adverse reactions to the graft material were observed. Thus, this alloplastic bone graft coated with ErhBMP-2 is considered to be an effective bone graft material.
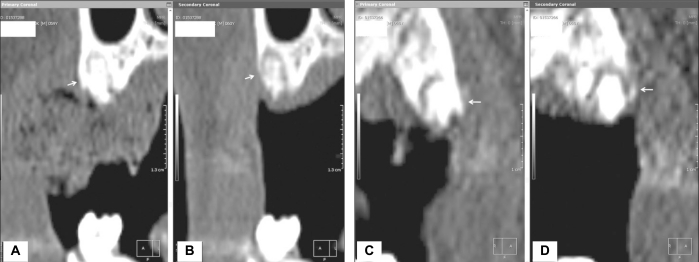
Fig. 2.
Computed tomography views at baseline following tooth extraction (A, C) and 3 months post-treatment (B, D). In the control group, there was resorption of the buccal plate and reduction in bone width. However, in the experimental group, the buccal plate was maintained and bone width increased (white arrow). ((A) at post-treatment following tooth extraction in the control group, (B) after 3 months in the control group (C) at post-treatment following tooth extraction in the experiment group, (D) after 3 months in the experiment group).
Footnotes
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A090958).
References
- 1.Tallgren A, Lang BR, Walker GF, Ash MM., Jr Roentgen cephalometric analysis of ridge resorption and changes in jaw and occlusal relationships in immediate complete denture wearers. J Oral Rehabil. 1980;7:77–94. doi: 10.1111/j.1365-2842.1980.tb01466.x. [DOI] [PubMed] [Google Scholar]
- 2.Jackson BJ, Morcos I. Socket grafting: a predictable technique for site preservation. J Oral Implantol. 2007;33:353–364. doi: 10.1563/1548-1336(2007)33[353:SGAPTF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.John V, De Poi R, Blanchard S. Socket preservation as a precursor of future implant placement: review of the literature and case reports. Compend Contin Educ Dent. 2007;28:646–653. [PubMed] [Google Scholar]
- 4.Reynolds MA, Aichelmann-Reidy ME, Branch-Mays GL, Gunsolley JC. The efficacy of bone replacement grafts in the treatment of periodontal osseous defects. A systematic review. Ann Periodontol. 2003;8:227–265. doi: 10.1902/annals.2003.8.1.227. [DOI] [PubMed] [Google Scholar]
- 5.Szpalski M, Gunzburg R. Applications of calcium phosphate-based cancellous bone void fillers in trauma surgery. Orthopedics. 2002;25:s601–s609. doi: 10.3928/0147-7447-20020502-10. [DOI] [PubMed] [Google Scholar]
- 6.Urist MR, Lietze A, Dawson E. Beta-tricalcium phosphate delivery system for bone morphogenetic protein. Clin Orthop Relat Res. 1984;187:277–280. [PubMed] [Google Scholar]
- 7.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 8.Bessa PC, Casal M, Reis RL. Bone morphogenetic proteins in tissue engineering: the road from the laboratory to the clinic, part I (basic concepts) J Tissue Eng Regen Med. 2008;2:1–13. doi: 10.1002/term.63. [DOI] [PubMed] [Google Scholar]
- 9.Bessa PC, Casal M, Reis RL. Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery) J Tissue Eng Regen Med. 2008;2:81–96. doi: 10.1002/term.74. [DOI] [PubMed] [Google Scholar]
- 10.Wang EA, Rosen V, D'Alessandro JS, Bauduy M, Cordes P, Harada T, Israel DI, Hewick RM, Kerns KM, LaPan P, Luxenberg DP, McQuaid D, Moutsatsos IK, Nove J, Wozney JM. Recombinant human bone morphogenetic protein induces bone formation. Proc Natl Acad Sci USA. 1990;87:2220–2224. doi: 10.1073/pnas.87.6.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Israel DI, Nove J, Kerns KM, Kaufman RJ, Rosen V, Cox KA, Wozney JM. Heterodimeric bone morphogenetic proteins show enhanced activity in vitro and in vivo. Growth Factors. 1996;13:291–300. doi: 10.3109/08977199609003229. [DOI] [PubMed] [Google Scholar]
- 12.Friedlaender GE, Perry CR, Cole JD, Cook SD, Cierny G, Muschler GF, Zych GA, Calhoun JH, LaForte AJ, Yin S. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am. 2001;83-A:S151–S158. [PMC free article] [PubMed] [Google Scholar]
- 13.Burkus JK, Heim SE, Gornet MF, Zdeblick TA. Is INFUSE bone graft superior to autograft bone? An integrated analysis of clinical trials using the LT-CAGE lumbar tapered fusion device. J Spinal Disord Tech. 2003;16:113–122. doi: 10.1097/00024720-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Bessho K, Konishi Y, Kaihara S, Fujimura K, Okubo Y, Iizuka T. Bone induction by Escherichia coli -derived recombinant human bone morphogenetic protein-2 compared with Chinese hamster ovary cell-derived recombinant human bone morphogenetic protein-2. Br J Oral Maxillofac Surg. 2000;38:645–649. doi: 10.1054/bjom.2000.0533. [DOI] [PubMed] [Google Scholar]
- 15.Fiorellini JP, Howell TH, Cochran D, Malmquist J, Lilly LC, Spagnoli D, Toljanic J, Jones A, Nevins M. Randomized study evaluating recombinant human bone morphogenetic protein-2 for extraction socket augmentation. J Periodontol. 2005;76:605–613. doi: 10.1902/jop.2005.76.4.605. [DOI] [PubMed] [Google Scholar]
- 16.Einhorn TA, Majeska RJ, Mohaideen A, Kagel EM, Bouxsein ML, Turek TJ, Wozney JM. A single percutaneous injection of recombinant human bone morphogenetic protein-2 accelerates fracture repair. J Bone Joint Surg Am. 2003;85-A:1425–1435. doi: 10.2106/00004623-200308000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Cochran DL, Jones AA, Lilly LC, Fiorellini JP, Howell H. Evaluation of recombinant human bone morphogenetic protein-2 in oral applications including the use of endosseous implants: 3-year results of a pilot study in humans. J Periodontol. 2000;71:1241–1257. doi: 10.1902/jop.2000.71.8.1241. [DOI] [PubMed] [Google Scholar]
- 18.Howell TH, Fiorellini J, Jones A, Alder M, Nummikoski P, Lazaro M, Lilly L, Cochran D. A feasibility study evaluating rhBMP-2/absorbable collagen sponge device for local alveolar ridge preservation or augmentation. Int J Periodontics Restorative Dent. 1997;17:124–139. [PubMed] [Google Scholar]