Abstract
Although the role of Langerhans cells (LC) in skin immune responses is still a matter of debate, it is known that LC require the chemokine receptor CCR7 for migrating to skin-draining LN. A report in the current issue of the European Journal of Immunology unfolds some of the intricacies of LC migration, showing that LC need CXCR4, but not CCR7, for their migration from the epidermis to the dermis. Thus, LC migration to skin-draining LN occurs in two distinct phases: a first step from the epidermis to the dermis regulated by CXCR4 and a second CCR7-dependent step from the dermis to LN. Here we discuss the potential implications of this new two-step LC migration paradigm.
Keywords: CCR7, CXCR4, DC, Langerhans cells, Skin
Role of Langerhans cells (LC) in skin-associated immune responses
LC are a special type of DC found in the stratified epithelium of the epidermis, cornea, oral cavity, esophagus, vagina and uterine cervix. Even though LC were described more than 100 years ago, the immunological function of LC remains enigmatic [1–4]. The study of LC was initially hindered by a lack of good LC markers that are able to specifically target these cells in vivo. Therefore, insights about LC function were originally provided by in vitro experiments in which it was shown that ex vivo-differentiated LC primed T cells much more efficiently than dermal DC (dDC) or monocyte-derived DC [5–8]. These results [5–8] led to the classical view that LC play a prominent role in skin immunity by capturing and processing antigens in the epidermis in order to activate T cells in the skin-draining LN; however, the advent of new LC markers made it possible to engineer mice in which LC could be depleted in vivo and recent results using these new mouse models have challenged our traditional view of the role of LC in skin immunity [1, 3].
Two proteins are currently used as LC markers, the C-type lectin langerin (which contributes the formation of LC’s characteristic Birbeck granules) and epithelial cell adhesion molecule (EpCAM) [1]. EpCAM is expressed in LC, but not in other DC subsets [9], whereas langerin is also expressed in a subset of dDC and in some CD8α+ DC in LN [1, 3, 10]. Based on the expression of these markers, at least three DC subsets can be found in the skin: LC (EpCAM+langerin+) and two subsets of dDC (EpCAM−langerin+ or EpCAM−langerin−). Given the initial belief that langerin was specific for LC, different groups independently created mice in which it was possible to deplete langerin+ LC either constitutively [11] or in a transient and inducible manner [12, 13]. Surprisingly, these studies gave some unexpected and even contradictory findings, with reports suggesting an important role of LC in skin immunity [12, 14–16], whereas other studies found that LC were dispensable for inducing skin-associated immune responses [13, 15]. These disparate observations may be explained, at least in part, by the variable degree of deletion of other DC subsets that also express langerin (including some dDC) and also by the timing of LC depletion and the protocols used for antigen dose/administration [3, 4]. More recent studies [10, 15] in which LC were selectively depleted, while other langerin+ DC subsets (including dDC) were preserved, did not show an essential role for LC in inducing contact hypersensitivity responses to either haptens or peptide antigens [10, 15] or in a model of skin allograft rejection [17]. Nonetheless, although LC may not be strictly required for skin immune responses in some settings, they might still be sufficient to trigger effective protective or pathogenic skin immune responses. Consistent with this possibility, allogeneic LC are sufficient to trigger skin graft-versus-host disease in the absence of host-derived dDC [18].
Studies involving infection with skin-tropic viruses have also generated some intriguing results. During infection with HSV, dDC or CD8α+ DC in LN, but not LC, were involved in presenting viral antigens and inducing HSV-specific T-cell responses, suggesting that LC are not required for mounting anti-viral immune responses [19, 20]. A caveat for the interpretation of these studies [19, 20] is that HSV are cytolytic viruses that can either kill and/or affect LC maturation [4]. In fact, impairment of LC function may represent a mechanism by which these viruses evade protective immune responses. Other viruses, such as HIV, can infect LC without inducing cell death, but instead use LC as “Trojan horses” for spreading the infection to other DC and T cells [4].
DC maturation changes the expression of chemokine receptors
Immature DC (iDC) express CCR1, CCR2, CCR5, CCR6 and CXCR1, endowing iDC with the capacity to migrate to inflamed areas where they capture and process antigens [21]. On the other hand, CXCR4 and CCR7 are expressed at very low levels on these cells [22, 23]. Upon exposure to inflammatory stimuli, iDC undergo maturation and markedly upregulate the expression of CXCR4 and CCR7 [22]. Although CXCR4 and CCR7 have been considered as “late genes” due to their expression on DC 24 h after exposure to maturation stimuli [24], Ouwehand et al. [25] show in this issue of the European Journal of Immunology that, in LC, the expression of CXCR4 and CCR7 can be temporally dissociated, with CXCR4 being expressed within 24 h after hapten exposure, whereas CCR7 is highly induced only after 48 h [25]. These data suggested that LC may require CXCR4 during the early stages of their migratory journey from the epidermis to the LN [25].
LC maturation and migration from the epidermis
LC exhibit some distinctive and unique properties compared with other DC, for example, LC absolutely require TGF-β for their differentiation [1, 26]. LC also exhibit a very slow turnover under steady-state conditions compared with other DC subsets (including dDC), which undergo renewal at a much faster rate [1, 27]. During skin inflammation, LC turnover is rapidly and markedly increased [18, 27]. The increased LC turnover allows the recruitment of new bone-marrow-derived LC precursors to the epidermis, a process that requires the expression of CCR2 and CCR6 on LC precursors [18, 27].
In order to leave the epidermis, LC need to cross the basement membrane at the dermo-epidermal junction [21, 28]. IL-1β and TNF-α play a central role in the process of LC migration across the basement membrane [21]. Upon initiation of the maturation process, LC produce IL-1β, which induces TNF-α secretion from adjacent keratinocytes [21, 28]. TNF-α contributes to decreasing the attachment between LC and keratinocytes by downregulating E-cadherin and by inhibiting the expression of CCR6, which renders LC insensitive to CCL20 produced by keratinocytes [21, 28]. TNF-α also induces the expression of α6β1 integrin on LC [29], which is important for their interaction with extra-cellular matrix proteins such as laminin that is present in the basement membrane of the epidermis [28, 30]. The integrin LFA-1 (leukocyte function-associated antigen-1) is also implicated in skin DC migration to LN and the LFA-1 ligand ICAM-1 is expressed by lymphatic endothelial cells [28].
Role of CCR7 and CXCR4 in skin DC migration to LN
It has been demonstrated that CCR7 is crucial for DC migration from peripheral tissues to the draining LN at all major surfaces exposed to the external environment, including the lungs, the intestinal mucosa and the skin [31–33]. In fact, CCR7-deficient mice or plt/plt mutant mice (which lack the CCR7 ligands CCL19 and CCL21-Ser) have a severe defect in LC migration to the skin-draining LN [31, 34–36]; however, this defect is not complete and these mice show some residual DC migration to this lymphoid compartment [31, 34, 36], suggesting the existence of a CCR7-independent mechanism of DC migration to LN.
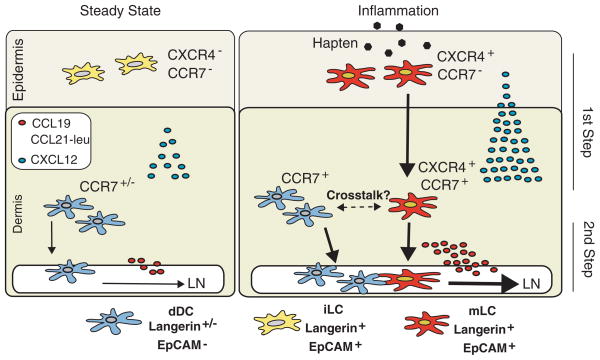
More recently, Kabashima et al. [37] showed that CXCR4, which is induced on DC upon maturation, also plays a role in the migration of skin DC to LN. Consistent with this finding, the CXCR4 ligand CXCL12 is expressed by lymphatic endothelial cells in the murine skin [37]. Importantly, contact hypersensitivity was impaired when blocking CXCR4 with a selective pharmacological antagonist [37], demonstrating that this receptor is required for an effective cutaneous immune response in this setting. However, until the current report [25], the precise role of CXCR4–CXCL12 in skin DC migration was unknown. Ouwehand et al. [25] demonstrate that dermal fibroblasts exposed to TNF-α produced CXCL12 and that human stromal cells in the dermis markedly increased their expression of CXCL12 under inflammatory conditions. An analogous increase in CXCL12 mRNA was previously observed in murine skin upon hapten exposure [37]. Moreover, using human skin explants, Ouwehand et al. [25] demonstrate that CXCR4 and CXCL12 are crucial for LC migration from the epidermis to the dermis (Fig. 1). Migration of LC to the dermis is abrogated by CXCR4 or CXCL12 blocking antibodies, whereas antagonizing the CCR7 ligands CCL19 and CCL21 does not affect this process, indicating that CCR7 is not required for LC migration to the dermis [25]. Ouwehand et al.’s data [25] are consistent with previous work [31] showing that LC mobilization from the epidermis to the dermis was not altered in CCR7-deficient mice, whereas DC entry into the dermal lymphatics was abrogated in the absence of this receptor. All together, these data support a model in which LC migrate to LN in two phases: following initial exposure to inflammatory stimuli, LC upregulate CXCR4 and migrate to the dermis in a CXCR4–CXCL12-dependent and CCR7-independent manner. Once LC are in the dermis, they increase their expression of CCR7 and enter into the dermal lymphatics in order to migrate to LN (Fig. 1). This additional step involving CXCR4 upregulation and migration to the dermis in addition to the delayed expression of CCR7 may contribute to explaining as to why the migration of dDC into the skin-draining LN occurs within 24–48 h, whereas the peak in LC accumulation is only observed at day 4 [2].
Figure 1.
Two-step Langerhans cell migration to LN. Dermal DC (dDC, EpCAM− and either Langerin+ or Langerin−) constitutively migrate to the LN in a process dependent on CCR7 and its ligands CCL19 and CCL21-leu (leucine isoform of CCL21), which are expressed by lymphatic endothelial cells. On the other hand, during steady-state non-inflammatory conditions, immature Langerhans cells (iLC, Langerin+EpCAM+) do not express CXCR4 or CCR7 and remain mostly restricted to the epidermal compartment (left panel). Upon exposure to inflammatory agents (e.g. haptens), iLC undergo maturation and upregulate CXCR4, whose ligand CXCL12 is also increased in the dermis during inflammation (right panel). CXCR4 expression allows maturing LC (mLC, Langerin+EpCAM+) to cross the dermo-epidermal junction and reach the dermis (first step). In the dermis, mLC acquire high levels of CCR7, endowing these cells with the capacity to migrate to skin-draining LN (second step). It is also possible that mLC functionally interact with dDC during their time in the dermis.
Potential implications of a two-step LC migration mechanism
Why do LC need to upregulate a chemokine receptor other than CCR7 to exit from the epidermis? CCR7 is induced only at later stages of LC maturation and its ligands CCL19 and CCL21 are expressed in lymphatic endothelial cells that are not immediately underneath the epidermal layer, making them not readily available for LC that are still in the epidermis [25]. Therefore, earlier CXCR4 expression confers upon LC the capacity to migrate faster to the dermis where the CXCR4 ligand CXCL12 is increased during inflammation [25]. Once in the dermis, LC complete their maturation and upregulate CCR7, allowing their final migration to LN [25].
The temporal dissociation of CXCR4 and CCR7 expression would also permit LC to transiently dwell in the dermis before continuing their transit to the LN, potentially allowing these cells to interact with and deliver antigens to resident dDC [25]. The latter could be envisioned as a mechanism for amplifying an immune response by recruiting more DC to present antigens in the LN. On the other hand, infectious agents, such as HIV, may subvert this mechanism and use it to spread the infection to other DC and T cells [4]. A temporary stay in the dermis may also serve as a checkpoint, allowing LC to integrate other environmental cues that may influence their function and also determine whether they will continue their transit to LN. Among those potential “licensing” signals are some eicosanoids produced during inflammation, such as PGE2 and cysteinyl leukotrienes, which have been shown to enhance CCR7 expression and functionality [38, 39] and increase MMP expression [40] in DC.
Although the results presented by Ouwehand et al. [25] confirm the notion that CCR7 is not necessary for LC emigration from the epidermis [31], they do not exclude that, in the absence of CXCR4, this receptor may be able to compensate and trigger LC exit from the epidermis in a CCR7-dependent manner. In this regard, it would be interesting to assess whether, upon exposure to contact sensitizers, CCL19 or CCL21 can induce LC exit from epidermal sheets after blocking CXCR4–CXCL12. Another approach would be to specifically delete CXCR4 in langerin+ DC (e.g. through the use of langerin-driven Cre expression) and assess whether LC are impaired to exit the epidermis in the absence of CXCR4 when compared with wild-type or CCR7-deficient LC.
It is worth mentioning that CXCR4–CXCL12 interaction might also have other effects on LC, such as enhancing their survival and maturation [41]. In addition, CXCR4–CXCL12 interaction has been shown to upregulate MMP in some tumors [42, 43]. Since LC need MMP in order to cross the basement membrane and exit the epidermis [44], it is tempting to speculate that MMP upregulation might be another mechanism by which CXCR4–CXCL12 contributes to LC migration to the dermis; however, whether CXCR4–CXCL12 interaction upregulates MMP in LC remains to be determined.
Is there an analogous multi-step mechanism for DC migration in other major tissues exposed to the external environment? Even though the bronchial and the intestinal lamina propria do not seem to harbor a low-turnover population of DC (analogous to LC in the epidermis), CCR7 is also required for DC migration from these tissues into their corresponding draining lymphoid tissues [32, 33]. Moreover, in the lungs, another chemokine–chemokine receptor pair, CCR5–CCL5, seems to be necessary in order to induce DC maturation and allow subsequent CCR7-dependent migration to bronchial-associated lymphoid tissues [45], suggesting that, at least in the lung, DC migration might also be controlled by a multi-step chemokine-driven mechanism.
Finally, are there any settings in which the upregulation of CXCR4 and CCR7 is uncoupled in LC? The existence of two distinct and mechanistically independent LC migration steps opens the possibility that under some conditions they can be dissociated and LC are arrested in their first stage of migration. In fact, although inflammatory stimuli such as haptens efficiently induce LC maturation and their sequential expression of CXCR4 and CCR7, it has been reported that solar UV radiation induces CXCR4 but not CCR7 on LC [46]. A similar uncoupling can be induced pharmacologically by treating DC with some retinoids [47]. Under these conditions, one may predict that LC would accumulate in the dermis without reaching the LN. This abortive migration may underlie, at least in part, the immunosuppressive effect observed upon skin exposure to UV radiation [48].
As discussed above, the precise role of LC in skin immunity is still a matter of debate. Nonetheless, this newly proposed two-step model suggests that LC migration to LN requires precise regulation. This regulation implies that, in the non-inflamed steady state, CCR7-dependent DC migration to LN mostly consists of dDC, whereas LC would remain confined to the epidermal compartment and would join the dDC pool only during skin inflammation after upregulating CXCR4.
Acknowledgments
We thank Dr. Scott Snapper for critical reading of this paper and Susan Davis for editorial assistance. J.R.M. is indebted to Ingrid Ramos for constant support. J.R.M. is supported by grants from Crohn’s & Colitis Foundation of America (CCFA), Cancer Research Institute (CRI), Center for the Study of IBD (CSIBD, DK 43351), Massachusetts Life Sciences Center (MLSC) and the Howard M. Goodman Fellowship (MGH).
Abbreviations
- dDC
dermal DC
- EpCAM
epithelial cell adhesion molecule
- iDC
immature DC
- LC
Langerhans cells
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Udey MC, Nagao K. Characteristics and functions of murine cutaneous dendritic cells: a synopsis of recent developments. Mucosal Immunol. 2008 doi: 10.1038/mi.2008.37. [DOI] [PubMed] [Google Scholar]
- 2.Shklovskaya E, Roediger B, Fazekas de St Groth B. Epidermal and dermal dendritic cells display differential activation and migratory behavior while sharing the ability to stimulate CD4+ T cell proliferation in vivo. J Immunol. 2008;181:418–430. doi: 10.4049/jimmunol.181.1.418. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan DH, Kissenpfennig A, Clausen BE. Insights into Langerhans cell function from Langerhans cell ablation models. Eur J Immunol. 2008;38:2369–2376. doi: 10.1002/eji.200838397. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham AL, Carbone F, Geijtenbeek TBH. Langerhans cells and viral immunity. Eur J Immunol. 2008;38:2377–2385. doi: 10.1002/eji.200838521. [DOI] [PubMed] [Google Scholar]
- 5.Schuler G, Steinman RM. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med. 1985;161:526–546. doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuo M, Nagata Y, Sato E, Atanackovic D, Valmori D, Chen YT, Ritter G, et al. IFN-gamma enables cross-presentation of exogenous protein antigen in human Langerhans cells by potentiating maturation. Proc Natl Acad Sci USA. 2004;101:14467–14472. doi: 10.1073/pnas.0405947101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ratzinger G, Baggers J, de Cos MA, Yuan J, Dao T, Reagan JL, Munz C, et al. Mature human Langerhans cells derived from CD34+ hematopoietic progenitors stimulate greater cytolytic T lymphocyte activity in the absence of bioactive IL-12p70, by either single peptide presentation or cross-priming, than do dermal-interstitial or monocyte-derived dendritic cells. J Immunol. 2004;173:2780–2791. doi: 10.4049/jimmunol.173.4.2780. [DOI] [PubMed] [Google Scholar]
- 8.Cao T, Ueno H, Glaser C, Fay JW, Palucka AK, Banchereau J. Both Langerhans cells and interstitial DC cross-present melanoma antigens and efficiently activate antigen-specific CTL. Eur J Immunol. 2007;37:2657–2667. doi: 10.1002/eji.200636499. [DOI] [PubMed] [Google Scholar]
- 9.Borkowski TA, Nelson AJ, Farr AG, Udey MC. Expression of gp40, the murine homologue of human epithelial cell adhesion molecule (Ep-CAM), by murine dendritic cells. Eur J Immunol. 1996;26:110–114. doi: 10.1002/eji.1830260117. [DOI] [PubMed] [Google Scholar]
- 10.Bursch LS, Wang L, Igyarto B, Kissenpfennig A, Malissen B, Kaplan DH, Hogquist KA. Identification of a novel population of Langerin+dendritic cells. J Exp Med. 2007;204:3147–3156. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Bennett CL, van Rijn E, Jung S, Inaba K, Steinman RM, Kapsenberg ML, Clausen BE. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol. 2005;169:569–576. doi: 10.1083/jcb.200501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, Tripp CH, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Bennett CL, Noordegraaf M, Martina CA, Clausen BE. Langerhans cells are required for efficient presentation of topically applied hapten to T cells. J Immunol. 2007;179:6830–6835. doi: 10.4049/jimmunol.179.10.6830. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Bursch LS, Kissenpfennig A, Malissen B, Jameson SC, Hogquist KA. Langerin expressing cells promote skin immune responses under defined conditions. J Immunol. 2008;180:4722–4727. doi: 10.4049/jimmunol.180.7.4722. [DOI] [PubMed] [Google Scholar]
- 16.Stoitzner P, Green LK, Jung JY, Price KM, Tripp CH, Malissen B, Kissenpfennig A, et al. Tumor immunotherapy by epicutaneous immunization requires langerhans cells. J Immunol. 2008;180:1991–1998. doi: 10.4049/jimmunol.180.3.1991. [DOI] [PubMed] [Google Scholar]
- 17.Obhrai JS, Oberbarnscheidt M, Zhang N, Mueller DL, Shlomchik WD, Lakkis FG, Shlomchik MJ, Kaplan DH. Langerhans cells are not required for efficient skin graft rejection. J Invest Dermatol. 2008;128:1950–1955. doi: 10.1038/jid.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merad M, Hoffmann P, Ranheim E, Slaymaker S, Manz MG, Lira SA, Charo I, et al. Depletion of host Langerhans cells before transplantation of donor alloreactive T cells prevents skin graft-versus-host disease. Nat Med. 2004;10:510–517. doi: 10.1038/nm1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allan RS, Smith CM, Belz GT, van Lint AL, Wakim LM, Heath WR, Carbone FR. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 20.Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, Knipe DM, Iwasaki A. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J Exp Med. 2003;197:153–162. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villablanca EJ, Russo V, Mora JR. Dendritic cell migration and lymphocyte homing imprinting. Histol Histopathol. 2008;23:897–910. doi: 10.14670/HH-23.897. [DOI] [PubMed] [Google Scholar]
- 22.Sallusto F, Schaerli P, Loetscher P, Schaniel C, Lenig D, Mackay CR, Qin S, Lanzavecchia A. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol. 1998;28:2760–2769. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 23.Sozzani S, Allavena P, D’Amico G, Luini W, Bianchi G, Kataura M, Imai T, et al. Cutting edge: Differential regulation of chemokine receptors during dendritic cell maturation: A model for their trafficking properties. J Immunol. 1998;161:1083–1086. [PubMed] [Google Scholar]
- 24.Huang Q, Liu D, Majewski P, Schulte LC, Korn JM, Young RA, Lander ES, Hacohen N. The plasticity of dendritic cell responses to pathogens and their components. Science. 2001;294:870–875. doi: 10.1126/science.294.5543.870. [DOI] [PubMed] [Google Scholar]
- 25.Ouwehand K, Santegoets S, Bruynzeel D, Scheper R, de Gruijl T, Gibbs S. CXCL12 is essential for migration of activated Langerhans cells from epidermis to dermis. Eur J Immunol. 2008;38 doi: 10.1002/eji.200838384. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan DH, Li MO, Jenison MC, Shlomchik WD, Flavell RA, Shlomchik MJ. Autocrine/paracrine TGFbeta1 is required for the development of epidermal Langerhans cells. J Exp Med. 2007;204:2545–2552. doi: 10.1084/jem.20071401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, Weissman IL, et al. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol. 2002;3:1135–1141. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 29.Ioffreda MD, Whitaker D, Murphy GF. Mast cell degranulation upregulates alpha 6 integrins on epidermal Langerhans cells. J Invest Dermatol. 1993;101:150–154. doi: 10.1111/1523-1747.ep12363632. [DOI] [PubMed] [Google Scholar]
- 30.Price AA, Cumberbatch M, Kimber I, Ager A. Alpha 6 integrins are required for Langerhans cell migration from the epidermis. J Exp Med. 1997;186:1725–1735. doi: 10.1084/jem.186.10.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, Zwirner J, Blankenstein T, et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–288. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 32.Jakubzick C, Tacke F, Llodra J, van Rooijen N, Randolph GJ. Modulation of dendritic cell trafficking to and from the airways. J Immunol. 2006;176:3578–3584. doi: 10.4049/jimmunol.176.6.3578. [DOI] [PubMed] [Google Scholar]
- 33.Jang MH, Sougawa N, Tanaka T, Hirata T, Hiroi T, Tohya K, Guo Z, et al. CCR7 is critically important for migration of dendritic cells in intestinal lamina propria to mesenteric lymph nodes. J Immunol. 2006;176:803–810. doi: 10.4049/jimmunol.176.2.803. [DOI] [PubMed] [Google Scholar]
- 34.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 35.Martin-Fontecha A, Sebastiani S, Hopken UE, Uguccioni M, Lipp M, Lanzavecchia A, Sallusto F. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med. 2003;198:615–621. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gunn MD, Kyuwa S, Tam C, Kakiuchi T, Matsuzawa A, Williams LT, Nakano H. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J Exp Med. 1999;189:451–460. doi: 10.1084/jem.189.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kabashima K, Shiraishi N, Sugita K, Mori T, Onoue A, Kobayashi M, Sakabe J, et al. CXCL12-CXCR4 engagement is required for migration of cutaneous dendritic cells. Am J Pathol. 2007;171:1249–1257. doi: 10.2353/ajpath.2007.070225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scandella E, Men Y, Legler DF, Gillessen S, Prikler L, Ludewig B, Groettrup M. CCL19/CCL21-triggered signal transduction and migration of dendritic cells requires prostaglandin E2. Blood. 2004;103:1595–1601. doi: 10.1182/blood-2003-05-1643. [DOI] [PubMed] [Google Scholar]
- 39.Robbiani DF, Finch RA, Jager D, Muller WA, Sartorelli AC, Randolph GJ. The leukotriene C(4) transporter MRP1 regulates CCL19 (MIP-3beta, ELC)-dependent mobilization of dendritic cells to lymph nodes. Cell. 2000;103:757–768. doi: 10.1016/s0092-8674(00)00179-3. [DOI] [PubMed] [Google Scholar]
- 40.Yen JH, Khayrullina T, Ganea D. PGE2-induced metalloproteinase-9 is essential for dendritic cell migration. Blood. 2008;111:260–270. doi: 10.1182/blood-2007-05-090613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kabashima K, Sugita K, Shiraishi N, Tamamura H, Fujii N, Tokura Y. CXCR4 engagement promotes dendritic cell survival and maturation. Biochem Biophys Res Commun. 2007;361:1012–1016. doi: 10.1016/j.bbrc.2007.07.128. [DOI] [PubMed] [Google Scholar]
- 42.Hanahan D, Lanzavecchia A, Mihich E. Fourteenth Annual Pezcoller Symposium: the novel dichotomy of immune interactions with tumors. Cancer Res. 2003;63:3005–3008. [PubMed] [Google Scholar]
- 43.Bartolome RA, Galvez BG, Longo N, Baleux F, Van Muijen GN, Sanchez-Mateos P, Arroyo AG, Teixido J. Stromal cell-derived factor-1alpha promotes melanoma cell invasion across basement membranes involving stimulation of membrane-type 1 matrix metalloproteinase and Rho GTPase activities. Cancer Res. 2004;64:2534–2543. doi: 10.1158/0008-5472.can-03-3398. [DOI] [PubMed] [Google Scholar]
- 44.Ratzinger G, Stoitzner P, Ebner S, Lutz MB, Layton GT, Rainer C, Senior RM, et al. Matrix metalloproteinases 9 and 2 are necessary for the migration of Langerhans cells and dermal dendritic cells from human and murine skin. J Immunol. 2002;168:4361–4371. doi: 10.4049/jimmunol.168.9.4361. [DOI] [PubMed] [Google Scholar]
- 45.Grayson MH, Ramos MS, Rohlfing MM, Kitchens R, Wang HD, Gould A, Agapov E, Holtzman MJ. Controls for lung dendritic cell maturation and migration during respiratory viral infection. J Immunol. 2007;179:1438–1448. doi: 10.4049/jimmunol.179.3.1438. [DOI] [PubMed] [Google Scholar]
- 46.Mittelbrunn M, Tejedor R, de la Fuente H, Garcia-Lopez MA, Ursa A, Penas PF, Garcia-Diez A, et al. Solar-simulated ultraviolet radiation induces abnormal maturation and defective chemotaxis of dendritic cells. J Invest Dermatol. 2005;125:334–342. doi: 10.1111/j.0022-202X.2005.23824.x. [DOI] [PubMed] [Google Scholar]
- 47.Villablanca EJ, Zhou D, Valentinis B, Negro A, Raccosta L, Mauri L, Prinetti A, et al. Selected natural and synthetic retinoids impair CCR7-and CXCR4-dependent cell migration in vitro and in vivo. J Leukoc Biol. 2008;84:871–879. doi: 10.1189/jlb.0108047. [DOI] [PubMed] [Google Scholar]
- 48.Cooper KD, Oberhelman L, Hamilton TA, Baadsgaard O, Terhune M, LeVee G, Anderson T, Koren H. UV exposure reduces immunization rates and promotes tolerance to epicutaneous antigens in humans: relationship to dose, CD1a-DR+ epidermal macrophage induction, and Langerhans cell depletion. Proc Natl Acad Sci USA. 1992;89:8497–8501. doi: 10.1073/pnas.89.18.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]