Abstract
The use of TRAIL/APO2L and monoclonal antibodies targeting TRAIL receptors for cancer therapy holds great promise, due to their ability to restore cancer cell sensitivity to apoptosis in association with conventional chemotherapeutic drugs in a large variety of tumors. TRAIL-induced cell death is tightly regulated right from the membrane and at the DISC (Death-Inducing Signaling Complex) level. The following patent and literature review aims to present and highlight recent findings of the deadly discussion that determines tumor cell fate upon TRAIL engagement.
Keywords: Chemotherapy, death domain, death effector domain, DISC, FADD, c-FLIP, scaffold, TRAIL, TRAIL-R4.
INTRODUCTION
TRAIL, also known as APO2-L or TNF-Related Apoptosis-Inducing Ligand and its derivatives, including agonistic antibodies targeting TRAIL receptors or PARAs (ProApoptotic Receptor Agonists), are attractive compounds for cancer therapy due to their ability to induce tumor regression without significant side effects [1]. Extensive efforts are being made to evaluate the efficacy and the safety of these combinations in clinical trials [2], and there are many instances in the patent literature of efforts to use polypeptides derived from the TRAIL ligand, [3-10] as therapy against cancerous cells. Other patent applications seek to use agonistic antibodies directed against the TRAIL receptors in order to induce the TRAIL apoptotic pathway [11-19], or TRAIL ligand gene transfer [20]. Amgen has recently published interesting results of a phase Ib study on twenty five patients with advanced nonsquamous non-small-cell lung cancer, treated with recombinant TRAIL (Dulanermin / AMG 951) combined with paclitaxel, carboplatine and Bevacizumab (PCB). Combining Dulanermin with PCB was well tolerated in patients, but importantly was more efficient than PCB alone for first line treatments, with an overall response rate of 58% as compared to 35% for PCB [21]. For a review on current ongoing clinical trials using PARAs see [22].
TRAIL belongs to the TNF (Tumor Necrosis Factor) superfamily of ligands and receptors. Ligands of this family generally recognize and bind to a limited subset of cognate receptors on the cell surface, leading to signal transduction cascades downstream of the receptor, allowing the activation of a large panel of signaling pathways including NF-kB- or caspase-activation. These type I transmembrane proteins contain two to four cysteine-rich domains (CRDs) in their extracellular region, and an intracellular domain that enables the recruitment of adaptor proteins, driving the activation of a particular signaling pathway.
The receptors of this family, which includes TNFR1, CD95/Fas, TRAIL-R1/DR4, TRAILR2/DR5, DR3, and DR6, contain an intracellular stretch of approximately 80 amino acids, called the Death Domain (DD), which is necessary and sufficient for the triggering of the apoptotic programme [23, 24]. With the exception of DR6, whose ligand has only recently been proposed to be a beta-amyloid precursor protein [25], death domain containing receptors are recognized by ligands of the TNF superfamily. These cognate ligands share a common structural motif, the TNF homology domain, which allows their binding to the CRD of TNF receptors [26]. They can be cleaved by metalloproteinases to form soluble cytokines, however, the capacity of the soluble forms of the death ligands to induce apoptosis is significantly lower than the membrane-bound forms [27, 28]. Ligands such as TRAIL, FasL and TNF can, however, be produced as recombinant proteins and used for anticancer therapy [29]. Unlike DR3, whose expression is mainly restricted to T lymphocytes [30], TNFR1, Fas, TRAIL-R1 and TRAIL-R2 were demonstrated to be widely expressed by tumor cells, which prompted the evaluation of their cognate ligands for cancer therapy. TNF and Fas ligand, however, were rapidly shown to be toxic in vivo. Their administration triggers fulminant hepatic failure in mice [31], hampering their application for cancer therapy. TRAIL, unlike Fas and TNF, was shown to be safe in experimental animal models [32], as well as in patients, as demonstrated by ongoing clinical trials [33]. Similarly, antibodies targeting agonistic TRAIL receptors, including mapatumumab or lexatumumab, are also well tolerated in patients [33-35].
Besides its lack of evident toxicity in vivo, TRAIL has gained increasing interest for cancer therapy due to at least four major properties. First of all, TRAIL is naturally involved in tumor metastasis immune surveillance by NK cells [36]. Accordingly, TRAIL-null mice are tumor prone [37] and TRAIL-R-deficient mice exhibit enhanced lymph node metastasis in a model of drug-induced skin carcinogenesis [38]. Second, amongst the ligands of the TNF superfamily, TRAIL is the only member that exhibits a relative selectivity for tumor cells [39, 40]. Hence, it has been demonstrated that while both normal and immortalized cells are resistant to TRAIL-induced apoptosis, Ras- or myc-transformed cells become sensitive [39, 41]. Third, TRAIL-induced cell death is largely independent of p53 [42]. It should be noted however that TRAIL and its receptors are p53 targets [43-46] and that sensitization to TRAIL-induced cell death by chemotherapeutic drugs has sometimes been associated with p53-induced mitochondrial activation either through the activation of Bax [47] or puma [48], as well as through the upregulation of TRAIL-R2 [43, 49] or TRAIL [50]. On the other hand, activation of p53 by some chemotherapeutic drugs may be detrimental to TRAIL-induced apoptosis. Likewise, the combination of TRAIL and oxaliplatin in p53 wt colon carcinoma cell lines was shown to be inefficient due to the p53-dependent up-regulation of TRAIL-R3 [51]. Finally, combinations that associate TRAIL with chemotherapy generally restore tumour cell sensitivity to apoptosis [6, 7, 12], irrespective of TRAIL-R4 expression, or mitochondrial inhibition [52], while having little effect on normal cells [53]. The molecular mechanisms that underlie sensitization to cell death induced by death domain containing receptors encompass a wide panel of events, and depend on both the drug and the cell type [54].
At the proximal level, sensitization to TRAIL or Fas ligand was shown to involve receptor up-regulation [55-60], c-FLIP downregulation [42, 61-64], restoration of caspase-8 expression [65, 66] or enhanced DISC formation [67-71]. Downstream of the DISC, sensitization to TRAIL-induced apoptosis was associated with the deregulation of cell survival proteins including, Bcl-2, Bcl-XL, Mcl-1, HSP27, survivin, IAPs [60, 72-75] or pathways such as AKT and NF-kB [76-79].
TRAIL-induced cell death engagement is subject to an exceptional level of control, with many different proteins interacting throughout the apoptotic cascade. The following chapters will focus on the regulation of TRAIL signaling at the membrane and DISC level.
TRAIL SIGNALING
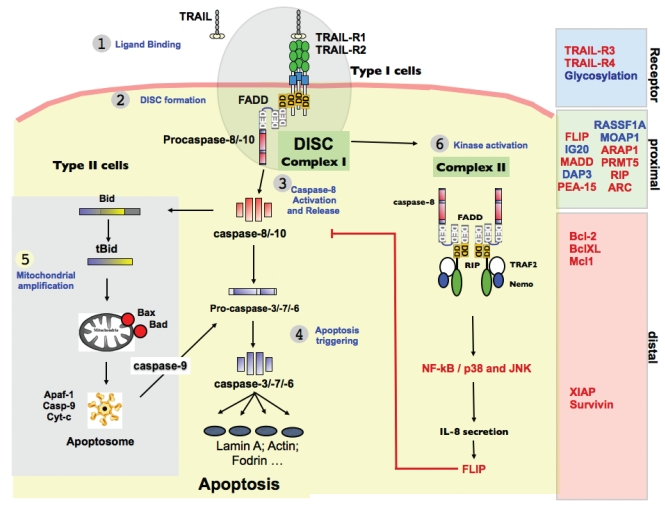
TRAIL-induced apoptosis involves several major events. The main constituents of the TRAIL receptor DISC and experimental evidence for their presence and function as compared to TNFR1 or Fas are summarized in Table I [80-11]. Initiation by ligand binding to the receptors is followed by recruitment of adaptor proteins to the intracellular region of the receptors. The adaptor proteins in turn recruit initiator caspases, forming the Death-Inducing Signaling Complex (DISC), a large macromolecular complex in which caspase-8 and -10 are activated and released for the triggering of apoptosis either directly or indirectly through the mitochondria via the protein Bid Fig. (1).
Table I.
Components of the Death Inducing Signaling Complex for Fas, TRAIL and TNF. *Membrane Bound Complex. Main Evidences from native Immunoprecipitation Experiments or from Yeast Two-hybrid and Co-immunoprecipitation Assays.
| TNF-R1 | TRAIL-R1, TRAIL-R2 | Fas | |
|---|---|---|---|
| Apoptosis | Complex II | Complex I* | Complex I* |
| (in absence of TNF-R1) | FADD [80,81] | FADD [82,87,111] | |
| RIP [83,85,89] | Caspase-8 [80,92] | Caspase-8 [86] | |
| TRADD [83,85,89] | Caspase-10 [88,93,96] | Caspase-10 [88,90,93,95] | |
| FADD [83,85,89] | c-FLIP [84,94,97] | c-FLIP [84,91,94] | |
| Caspase-8 [83,85,89] | |||
| Caspase-10 [89] | |||
| c-FLIP [89] | |||
| Non-apoptotic signalling | Complex I * | Complex II | Complex II |
| TRADD [102-104] | RIP-1 [85,110] | (in absence of CD95) | |
| TRAF-2 [102,104] | TRAF2 [110] | FADD [105] | |
| RIP1 [102] | Caspase-8 [110] | Caspase-8 [105] | |
| IKKγ [98] | FADD [110] | cFLIP [105] | |
| IKKα, IKKβ [98,99] | IKKγ [85,110] | RIP1 [100] | |
| cIAP1 [106-108] | TRADD [85] | ||
| cIAP2 [106,107] | |||
| LUBAC ligase complex [101,109] | |||
Fig. (1).
TRAIL signaling pathway and regulatory proteins. Binding of the TRAIL ligand to TRAIL-R1 or TRAIL-R2 (1) induces the recruitment of FADD and caspase-8 to these receptors, forming the membrane DISC or complex I (2), in which pro-caspase-8 is activated, leading to the release of the active caspase-8 in the cytosol (3) and allowing the engagement of the apoptotic cascade (4). The mitochondrial amplification loop (5) can be required in some cells to induce the activation of the effector caspase-3. A second complex has recently been described (6), which induces activation of survival signaling pathways leading to transcription factors, which can result in cytokine secretion and increase levels of the inhibitory protein c-FLIP. Regulatory processes of the TRAIL pathway can be divided into three groups: receptor level, proximal, and distal regulation. Inhibitory proteins are shown in red.
TRAIL triggers apoptosis following binding to one of its cognate death receptors, TRAIL-R1 (DR4) and TRAIL-R2 (DR5). Like Fas, but unlike TNFR1[89], TRAIL binding to TRAIL-R1 and TRAIL-R2 induces the formation of the DISC at the membrane level, through homotypic interactions Fig. (1). The DD of the agonistic receptors and that of the adaptor protein FADD allow the recruitment of caspase-8, caspase-10 or c-FLIP through their respective DED (Death effector Domain) [80, 112]. TRAIL-induced cell death can efficiently be regulated at the receptor level by antagonistic receptors [53], at the proximal level by c-FLIP [113] or further downstream by Bcl-2 family members [114] or inhibitors such as XIAP [115, 116] or Survivin [117] Fig. (1).
TRAIL non-apoptotic signaling activities include NF-kB, ERK or p38 activation. A secondary complex, which is not membrane bound (Table I), has been proposed to arise sequentially from complex I to trigger MAPK activation [110]. Sequential generation of two distinct functional complexes provide clues to TRAIL's pleiotropic signaling activities, that depending on the cell type, lead to apoptosis, survival or cell differentiation. This secondary complex may explain why, for instance, terminal keratinocyte differentiation induced by TRAIL proceeds both through MAPK and caspase activation [118]. Albeit less characterized (Table I), a similar secondary complex may also arise upon Fas stimulation [105]. Keeping in mind that keratinocytes express large amounts of intracellular Fas ligand [119], it could be of interest to define whether this death ligand/receptor set may substitute for TRAIL deficiency to induce cell differentiation, or whether the Fas pathway only affords apoptotic triggering in this cell type. Other differentiation functionalities, associated with MAPK activation have been attributed to TRAIL, including in intestinal cells [120], skeletal myoblasts [121, 122], osteoclasts [123], T helper cells [124] and in dendritic cells [125].
Although of great interest, these non-apoptotic features of TRAIL will not be developed any further here. Rather, the following review will mainly focus on TRAIL-induced cell death regulation from complex I.
CONTROLLING TRAIL-INDUCED CELL DEATH AT THE MEMBRANE LEVEL
Antagonistic Receptors
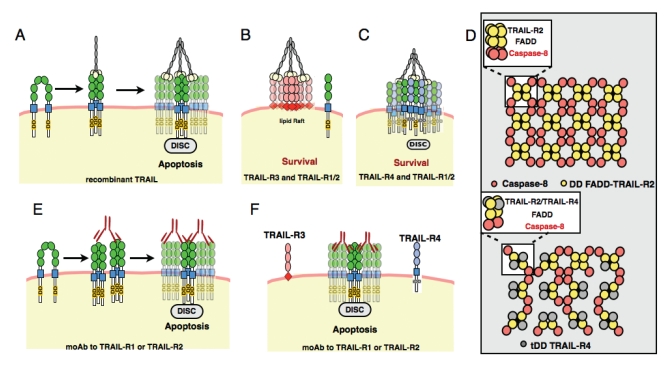
TRAIL-R3 (DcR1) and TRAIL-R4 (DcR2) can specifically inhibit TRAIL signaling at the membrane level [45, 126]. These two antagonistic receptors lack a functional DD [53] and impair TRAIL-induced cell death through distinct mechanisms Fig. (2A). TRAIL-R3 is devoid of an intracellular domain, but harbours a GPI anchor which drives its expression to lipid rafts [127]. TRAIL-R3 prevents DISC assembly through its ability to compete for TRAIL binding, resulting in the titration of TRAIL within the lipid rafts Fig. (2B). TRAIL-R4 on the other hand, is much more similar to TRAIL-R2, and contains an intracellular domain that includes a truncated death domain. Unlike TRAIL-R3, TRAIL-R4 is recruited with TRAIL-R2 within the DISC upon TRAIL engagement, and inhibits initiator caspase activation Fig. (2C), probably through steric hindrance [23]. TRAIL-R4 has been shown to form a specific heteromeric complex with TRAIL-R2 through the preligand assembly domain (PLAD), a domain encompassing the first CRD of both receptors but, contrary to our findings, PLAD-mediated TRAIL-R4 and TRAIL-R2 association was suggested to be ligand-independent [128]. The interaction of death-domain containing receptors via the PLAD is proposed to induce a parallel dimeric conformation of the receptors that can account for homotypic as well as heterotypic associations in the absence of ligand Fig. (2A). Ligand binding causes a conformational change in the pre-assembled receptor complex that facilitates receptor clustering and DISC formation Fig. (2A).
Fig. (2).
TRAIL signaling at the receptor level. A, TRAIL ligand induces aggregation of the TRAIL receptor at the membrane and activates the apoptotic cascade. B, TRAIL-R3 competes for TRAIL binding, sequestering TRAIL in lipid rafts. C, TRAIL-R4 forms heteromeric complexes with TRAIL-R2, inhibiting caspase-8 activation. D, Tentative model of inhibition of caspase-8 activation by TRAIL-R4. Upon engagement of TRAIL, the receptors aggregate into a highly regular array, whose minimal arrangement is represented as a side view in the white square. This tetrameric interaction module is composed of FADD-TRAIL-R2/Caspase-8 (Yellow and Red circles). Modular arrangement of this module into a platform enhances the proximity-induced dimerization and activation of caspase-8. Recruitment of TRAILR4 (lower panel, grey circles) disrupts caspase-8 arrangement, and thus limits caspase-8 activation. E-F, receptor specific agonistic antibodies engage TRAIL DISC, irrespective of TRAIL-R3 or TRAIL-R4 expression.
Similar to TRAIL, some agonistic antibodies are able to engage TRAIL signaling through DISC formation Fig. (2E). These antibodies, which selectively target either TRAIL-R1 or TRAIL-R2, efficiently induce cell death in cells that express TRAIL-R3 or TRAIL-R4, unlike TRAIL ligand itself Fig. (2E-F). Regardless of the stoichiometry of the DISC components, the key common event for the triggering of signaling activity is oligomerization, which allows neighbouring initiator caspases to form specific activating dimers Fig. (2D). A proximity-induced dimerization model was proposed to explain the activation of caspase-8 [129]. Recently, a very elegant approach of reconstitution of the Fas DISC using recombinant proteins, revealed a two-step activation mechanism involving both dimerization and proteolytic cleavage of procaspase-8 as obligatory steps for death-receptor-induced apoptosis [130]. Little is known about the stoichiometry of this scaffold. The DD and the DED, like the caspase recruitment domain (CARD) family or the pyrin domain (PYD), share a six-helical bundle structural fold feature that accounts for protein-protein interaction, the arrangement of which defines the stoichiometry of the multimolecular scaffold to which they are recruited. A crystal structure of RAIDD and PIDD, two DD-containing proteins, which are not required for TRAIL signaling but are closely related to the adaptor protein FADD, revealed an asymmetric core complex comprised of seven RAIDD DDs and five PIDD DDs assembled through 3 major interfaces [131]. More recently, the crystal structure of FADD and Fas was obtained, unveiling a tetrameric arrangement [132]. The crystal structure of FADD and TRAIL-R2 or TRAIL-R1 is not known for the moment but, assuming that the assembly of the TRAIL DISC mimics that of Fas, the formation of heteromers of TRAIL-R2 and TRAIL-R4 is likely to disturb the highly ordered arrangement that accounts for caspase-8 activation within the TRAIL DISC since the truncated death domain of TRAIL-R4 is unable to interact with FADD and thus cannot recruit a caspase-8 monomer. According to this hypothesis, we propose a model of DISC arrangement disruption by TRAIL-R4 as compared to the arrangement of a DISC composed of TRAIL-R2 and TRAIL-R1 Fig. (2D). In the latter complex, each receptor recruits an initiator caspase, the proximity of which is favourable for a full activation of caspase-8. Recruitment of TRAIL-R4 within the TRAIL DISC, however, alters caspase-8 dimer formation and therefore inhibits caspase-8 activation within the DISC Fig. (2D). Arrangement of the DISC and, in particular, caspase-8 proximity is a limiting step for the initiation of the apoptotic signal. Accordingly, it has been demonstrated that enforced ligand covalent trimerization accelerates TRAIL-induced caspase-8 activation and cell death [133]. In line with the requirement of these adaptor proteins to build a proper scaffold for caspase-8 activation, US6015712 raises the possibility of inhibiting FADD expression for therapeutic intervention related to diseases in which the death signaling pathway is activated inappropriately [134].
LIPID RAFTS AND TRAIL
Death domain-containing receptors of the TNF superfamily have a tendency to self-aggregate, owing to their DD. Their overexpression triggers apoptosis [135]. Therefore, it may be assumed that initiation of DISC formation is tightly controlled at the membrane level. The Silencer Of Death Domain (SODD), a DD containing protein identified by yeast two hybrid assay to interact with TNFR1, was proposed to prevent constitutive signaling of tumor necrosis factor receptor 1 (TNFR1) in the absence of TNFα [136]. Generation of mice deficient for SODD, however, failed to support a function regarding the control of TNFR1 aggregation [137]. At the moment it is not clear how these receptors are maintained in an inactive state at the membrane. Membrane lipid composition and fluidity could take part in avoiding receptor self-association. Supporting this hypothesis, ionizing radiation and UV rays [138, 139], which are known to change membrane fluidity, as does cholesterol depletion, induce Fas receptor clustering on the cell surface independently of FasL [140-142]. Alternatively, DISC formation in lipid rafts may account for efficient Fas-induced apoptosis triggering [143]. Cholesterol enriched membranes, however, have not been associated with TRAIL-DISC formation [144-148] with the exception of one study in which TRAIL-DISC formation in lipid rafts was clearly demonstrated [149]. Another indication suggesting that TRAIL signaling might occur within the cholesterol rich membrane domains was the discovery that palmitoylation is required to target TRAIL-R1 to lipid rafts [146]. In a mechanism similar to that of Fas, palmitoylation of TRAIL-R1 was shown to be required for redistribution of actin cytoskeleton-linked rafts, receptor oligomerization and cell death. However it was also found that TRAIL-R2 was not palmitoylated. Although some TRAIL DISC components can be found within lipid rafts [149], thus far it has not been clearly demonstrated that caspase-8 activation occurs within these structures. In addition, with the exception of TRAIL-R3, which localizes readily within lipid rafts, TRAIL-R1, TRAIL-R2 and TRAIL-R4 are mainly expressed in non-lipid raft-containing membranes at the steady state, where most DISC analysis assays demonstrate capase-8 activation upon TRAIL stimulation [23]. In line with these findings, it should be noted that edelfosine-induced cell death requires Fas translocation and aggregation within lipid rafts, but not TRAIL receptors [150].
O-GLYCOSYLATION
An additional level of complexity regarding the regulation of TRAIL signaling was recently found, following the discovery that O-glycosylation of TRAIL-R1 and TRAIL-R2 is a prerequisite for DISC formation and apoptotic triggering [151]. The finding that O-glycosylation controls cell sensitivity to TRAIL-induced cell death could be an important finding, as alterations in glycosylation profiles are often found in cancer patients [152] and during cancer progression [153]. Of particular interest are the findings that in normal human mammary epithelial cells, RAS-induced transformation triggers drastic changes in the glycosylation profile of cell surface proteins [154], and enhances TRAIL DISC formation and caspase-8 activation upon TRAIL stimulation [39]. It remains, however, to be determined whether these changes are sufficient to account for TRAIL tumor cell selectivity.
RECEPTOR TURN-OVER/TRAFFICKING
Little is known about TRAIL receptor trafficking, yet the first requirement to engage TRAIL-induced cell death is the availability of TRAIL agonistic receptors at the cell surface. Epigenetic dysregulation of TRAIL antagonistic receptors, TRAIL-R3 and TRAIL-R4, or of TRAIL agonistic receptors TRAIL-R1 and TRAIL-R2, has been documented to varying extents [155-157], leading to the loss of expression of the receptors in tumor cells and giving rise to resistance to TRAIL-induced cell death [158]. Recently, a yeast two-hybrid screen uncovered ARAP1, an ArfGAP and RhoGAP adapter protein, as a TRAIL-R1-binding partner. ARAP1 was shown to bind to TRAIL-R1 and TRAIL-R2 in co-expression experiments, but was unable to interact with DR6, another death-domain containing receptor. At the endogenous level, ARAP1 interacted with TRAIL-R1 in a TRAIL- and time-dependent manner. Downregulation of ARAP1 induced a loss of membrane expression of TRAIL-R1 and partly impaired TRAIL-induced cell death [159]. Since ARAP-1 was shown to regulate EGFR endocytosis [160], it was proposed that ARAP1 could play a role in regulating TRAIL-R1 trafficking and thus TRAIL-induced signaling.
CONTROLLING TRAIL-INDUCED CELL DEATH AT THE DISC LEVEL
The stoichiometry and composition of the TRAIL DISC is not clearly defined, but important regulatory proteins are proposed to be involved in the regulation of TRAIL signaling, owing to their ability to be recruited to the DISC or to interfere with proteins participating in TRAIL DISC formation.
c-FLIP
Cellular FLIP is probably the most important inhibitor of receptors containing death domains. Three main isoforms of c-FLIP are expressed in human cells: c-FLIPL, c-FLIPS, and c-FLIPR. Depending on the cell line and on the levels of FLIP expression, all three proteins can be found within the Fas DISC due to their N-terminal domain which contains two DED repeats similar to caspase-8 or caspase-10 [54]. The contribution of c-FLIPR regarding the control of TRAIL-induced cell death has not yet been characterised. The short isoforms of c-FLIP, c-FLIPS and c-FLIPR both possess a truncated C-terminus. In addition to the tandem DED repeat, c-FLIPL harbours an extended C-terminal domain that is structurally similar to procaspase-8, but is devoid of an active catalytic domain. The cysteine residue that is normally required for caspase-8 function is replaced by a tyrosine that renders c-FLIPL inactive [113]. These isoforms of c-FLIP, although generally expressed at a lower level compared to caspase-8 in most tumor cell lines, are recruited within the DISC together with caspase-8 where they inhibit the activation of the initiator caspases, impairing apoptotic triggering. The molecular mechanisms by which the long and the short FLIP isoforms inhibit TRAIL-induced cell death differ substantially. In the absence of c-FLIP, caspase-8 is activated in two-steps: dimerization, followed by cleavage [130]. One caspase-8 molecule brought in close proximity within the DISC to another caspase-8 can cleave itself and the other caspase-8 to induce the release of the catalytic subunits, p10 and p20, which form the mature caspase-8 that initiates the triggering of apoptosis. In the presence of c-FLIPS, procaspase-8 remains inactive within the DISC and the cells survive. Heterodimerization of c-FLIPL with procaspase-8 within the DISC, however, mimics procaspase-8 dimerization and leads to caspase-8 activation in the absence of procaspase-8 cleavage [161]. Caspase-8 is maintained within the DISC and cannot be released to the cytosol because the generation of the p20 subunit of caspase-8 cannot occur in the presence of c-FLIPL. Active caspase-8 therefore remains sequestered within the DISC, where it can still induce the cleavage of a number of substrates including c-FLIP, RIP and as yet to be discovered unidentified proteins, recruited within the DISC or in close proximity [130, 161]. While all isoforms of c-FLIP efficiently inhibit Fas ligand- and TRAIL-induced cell death, subcellular confinement of active caspase-8 is only asscociated with c- FLIPL so far. The finding that c-FLIPL induces caspase-8 activation within the DISC represents another degree of control regarding the regulation of TRAIL signaling. RIP cleavage at the DISC level in these circumstances could play a role in controlling TRAIL-induced necrosis [162], NF-kB activation [163-165] or other non-apoptotic functions. The possibility of using of RNA interference to inhibit cFLIP to circumvent TRAIL resistance has been proposed in the patent application US20040126791 [166].
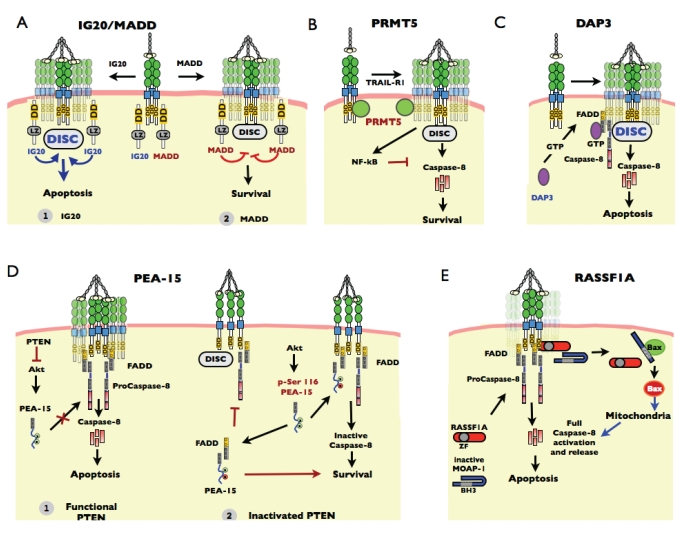
MADD-IG20
The MAPK-Activating Death Domain (MADD) variant, also coined Rab3-GAP, which is constitutively expressed in many cancer cells [167], was the first member of the family found to harbour a low homology DD and to interact with TNFR1 [168]. All of these splice variants contain a DD, but their contribution to the regulation of death receptor differs. IG20 was found to interact with both TRAIL-R1 and TRAIL-R2 and to enhance TRAIL DISC formation, thus increasing TRAIL-induced cell death Fig. (3A) [169]. MADD on the other hand, albeit structurally close to IG20 since both isoforms contain a DD and a leucine zipper domain, was demonstrated to behave as a negative regulator of TRAIL [169]. Similar to IG20, MADD was shown to interact with TRAIL-R1, but its expression was suggested to impair TRAIL DISC formation through the inhibition of caspase-8 recruitment Fig. (3A), leading to survival of the cells expressing MADD. Modulation of MADD to overcome resistance to TRAIL has been suggested as a possible therapy [170].
Fig. (3).
Mechanisms of TRAIL signaling regulation by different proteins. See text for details.
PRMT5
The protein arginine methyltransferase 5 (PRMT5) was found to interact with TRAIL-R1 by a proteomic screen [171]. Coexpression experiments revealed that PRMT5 could also interact with TRAIL-R2 but not other receptors of the TNF family, including Fas or TNFR1 Fig. (3B). Knockdown of PRMT5 was shown to sensitize tumor cells to TRAIL-induced cell death, while PRMT5 overexpression conferred TRAIL resistance [171]. PRMT5-mediated TRAIL resistance required NF-kB activation but was demonstrated to be methyl transferase-independent [171]. It is unclear for the moment how PRMT5 binds to TRAIL-R1. PRMT5, besides its methyl transferase activity, has no DD and no DED. Like IG20 and MADD, recruitment of PRMT5 to TRAIL-R1 appeared to be ligand independent. Finally, PRMT5 inhibitory function was suggested to occur regardless of TRAIL DISC formation.
DAP3
DAP3 (Death Associated Protein 3) is a GTP-binding adapter protein that interacts directly with the FADD DED Fig. (3C). Though the predominant function of DAP3 concerns maintenance of mitochondrial function, it is also able to influence apoptotic signaling [172]. Its recruitment to the TRAIL DISC was shown to regulate caspase-8 activation in a GTP-dependent fashion [173], so it was therefore proposed that DAP3 could be a direct regulator of TRAIL-induced caspase-8 activation and cell death Fig. (3C). It was later found that DAP3 is a ribosomal protein that is mainly localized to the mitochondrial matrix, and which cannot interact with FADD unless subcellular compartments are compromised [174, 175]. While the relative expression level of DAP3 in the cytosolic fraction remains unclear, targeted gene inactivation of dap3 confirmed the regulatory function of this GTP-binding adapter protein regarding TRAIL-induced cell death in particular, but also apoptosis induced by death receptors including TNFR1 or Fas [172]. Inactivation of dap3, however, had little or no impact on apoptosis induced by staurosporine or etoposide, two chemotherapeutic compounds known to target the intrinsic mitochondrial pathway. The question as to how DAP3 controls caspase-8 activation within the TRAIL DISC remains open. The master kinase STK11 was suggested to play a significant regulatory function upstream of DAP3 in osteosarcomas. In this study, STK11 was found to interact with DAP3 and enhance TRAIL-induced cell death through its serine/threonine kinase activity [176].
PEA15/PED
Phosphoprotein enriched in astrocytes (PEA15, also known as PED or HTMA), is a small protein (15kDa) composed of a N-terminal DED and a C-terminal tail of irregular structure. PEA15 was first reported to inhibit apoptosis induced by Fas and TNFR1 [177] and later found to be recruited to the TRAIL DISC, and to inhibit TRAIL-induced cell death, thus accounting for cell resistance in gliomas [178]. PEA15 is an endogenous substrate of kinases including PKC, Akt and CAMKII, and phosphorylation of PEA-15 on the serine 116 promotes FADD-binding [179]. It has been demonstrated recently that PEA-15 could promote mitochondrial-dependent type II Fas-induced cell death in cells inactivated for PTEN [180]. It is reported, however, that Akt-mediated phosphorylation of PEA-15 on the serine 116 residue impairs Fas DISC formation through the sequestration of FADD and not through the recruitment of PEA-15 within the Fas DISC. However when PTEN is functional, Akt is inactivated, serine 116 of PEA-15 is not phosphorylated and PEA-15 is unable to interact with FADD, allowing FADD recruitment, TRAIL DISC formation and apoptosis after TRAIL stimulation Fig. (3D). Interestingly, TPA, a phorbol ester also coined PMA, which has been known for a long time to inhibit Fas and TRAIL-induced apoptosis [181], and to impair DISC formation [182, 183], upregulates PEA-15 expression and enhances PEA-15 phosphorylation at serine 116 [184].
RASSF1A/MOAP1
The RAS association domain family 1A (RASSF1A) protein, although devoid of any characterized DD or DED, is a tumor suppressor that is shown to interact with the DD of TNFR1 and TRAIL-R1 [185]. RASSF1A links death receptors to the mitochondrial pathway through the protein modulator of apoptosis 1 (MOAP-1). Ectopic expression of RASSF1A enhances death receptor induced cell death while downregulation of RASSF1A or MOAP-1 inhibits bax activation and cytochrome c release [186]. This adaptor protein is found in an inactive state in the cytoplasm, and is activated upon recruitment with RASSF1A within the TRAIL DISC upon stimulation Fig. (3E). Release of MOAP-1 from the DISC is proposed to induce a conformational change that allows Bax recruitment and activation, leading to the activation of the mitochondrial amplification loop that sustains caspase activation and apoptosis [185]. Given that epigenetic-driven loss of RASSF1A protein expression is often observed in tumors of higher grade [187] and that RASSF1A is a potential regulator of TRAIL, demethylation agents could prove useful to restore TRAIL sensitivity in high grade tumors.
OTHER DD- OR DED-CONTAINING ADAPTOR PROTEINS INVOLVED IN THE REGULATION OF TRAIL AT THE DISC LEVEL (DEDD, RIP, TRADD, ARC)
All DD-containing proteins may potentially regulate TRAIL-induced apoptosis owing to their ability to induce homotypic interactions with TRAIL-R1, TRAIL-R2 or FADD. Likewise, RIP and TRADD contain a DD and are likely to participate in the TRAIL DISC. However, so far their recruitment has been found to be cell dependent, as neither TRADD nor RIP are recruited to the DISC in BJAB cells, a B lymphoma [188] while RIP is shown to be recruited in colon cancer cell lines [189]. Similar to TNFR1 [89], TRAIL induces the sequential formation of two distinct complexes Fig. (1).
TRAIL complex I, corresponds to the DISC. Complex I is localized at the cell membrane and is essential for caspase-8 activation while Complex II, a complex arising from the membrane TRAIL DISC, has been proposed to activate survival pathways due to the integration of several adaptor proteins including TRADD and RIP [110]. RIP recruitment at the cell membrane can occur in the absence of FADD or TRADD in the TNFRI complex I, as well as independently of FADD in the Fas DISC [85, 162]. Therefore, it is likely that the recruitment of RIP to the TRAIL DISC or to complex II is independent of these adaptor proteins. However, indirect interactions cannot be excluded despite the finding that RIP and TRADD are able to interact directly with FADD and some death receptors in overexpression experiments [164]. In addition to its DD, RIP contains a kinase domain whose activity is required for TRAIL-induced necrosis, but is compulsory for NF-kB activation upon TRAIL stimulation [162]. Though RIP itself is thought to be essential to trigger NF-kB activation upon TNF stimulation [163], it has recently been demonstrated that RIP is in fact not essential for TNFR1-induced NF-kB activation [190]. Whether this holds true for TRAIL remains to be determined. Inhibition of RIP expression, nonetheless, promotes TRAIL-induced cell death [191, 192].
Similar to DD-containing proteins, proteins that harbour a DED such as DEDD or DEDD2, are capable of interfering with known DISC components, including c-FLIP or caspase-8. Accordingly, DEDD2, a DED-containing protein that exhibits a close sequence homology with DEDD, was shown to interact with c-FLIP and to enhance apoptosis induced by Fas and TRAIL [193]. However, DEDD2 was unable to interact with FADD or caspase-8, even though DEDD2-mediated sensitization to apoptosis was restricted to Fas and TRAIL. Its overexpression failed to enhance staurosporine- or bax-induced cell death.
DD- and DED-containing proteins feature a 6-alpha-helical bundle structure fold that mediates dimerization by electrostatic interactions. It is generally accepted that homotypic interactions occur between similar domains, but unconventional heterotypic interactions may also account for the regulation of TRAIL signaling. Accordingly, Arc a protein containing a CARD domain, which is a protein interaction module similar to the DD or the DED, was found to interfere with death receptor induced cell death, owing to its ability to bind to FADD and to inhibit DISC formation [194].
CURRENT & FUTURE DEVELOPMENTS
One important bottle neck for TRAIL signaling is probably the engagement of the apoptotic signaling complex from the membrane. Since this signaling pathway seems primarily dedicated to cell killing in vivo, TRAIL signaling has to be tightly controlled at the cell surface. This control can be specifically acheived by TRAIL antagonistic receptors but also less selectively by c-FLIP, both of which are found to be conserved throughout evolution. Plasma membrane lipid composition, although not specific to TRAIL, is also likely to play an important role in controlling DISC formation and apopotosis triggering (see Segui and Dimanche-Boitrel, this issue). Moreover, since the discovery of the membrane-bound DISC, composed of the receptor, the adaptor protein FADD, the initiator caspases-10 and -8 and their inhibitor c-FLIP, a plethora of binding partners have been shown to contribute to the regulation of the deadly signal. These proteins act both at the membrane level or at the proximal level due to their ability to interact with DISC or secondary complex components. Regulation of caspase-8 activation from the DISC and initiation of apoptosis is associated with either the disruption or the enhancement of TRAIL-induced scaffold complexes, as well as with the regulation of the mitochondrial pathway. There is an incredible diversity of interacting molecules that have been described so far to take part in the regulation of TRAIL-signaling which add another level of complexity to our understanding of the TRAIL Discussion. Current developments for targeting the TRAIL pathway show promise for cancer therapies, but a deeper understanding of the TRAIL DISC composition and stoichiometry will be crucial for the development of effective TRAIL-based therapeutic approaches.
ACKNOWLEDGMENTS
This work is supported by grants of the Conseil Regional de Bourgogne, the INCa (Institut National du Cancer), Cancéropôle Grand-Est, ANR (Agence Nationale de la Recherche, ANR-06-JCJC-0103 and 07-PCV-0031), and the European Community (ApopTrain Marie Curie RTN) (O.M.). A.M., is supported by fellowships from the Ministry of Research and Education and the ARC (Association pour la Recherche sur le Cancer). S.S. is supported by the INCa.
CONFLICT OF INTEREST
We have no conflict of interest to present.
REFERENCES
- 1.Ashkenazi A, Holland P, Eckhardt SG. Ligand-based targeting of apoptosis in cancer: The potential of recombinant human apoptosis ligand 2/Tumor necrosis factor-related apoptosis-inducing ligand (rhApo2L/TRAIL) J Clin Oncol. 2008;26(21):3621–30. doi: 10.1200/JCO.2007.15.7198. [DOI] [PubMed] [Google Scholar]
- 2.Newsom-Davis T, Prieske S, Walczak H. Is TRAIL the holy grail of cancer therapy? Apoptosis. 2009;14(4):607–23. doi: 10.1007/s10495-009-0321-2. [DOI] [PubMed] [Google Scholar]
- 3.Angell YM, Bhandari A, Green J, Schatz. PJ, Holmes CP. Compounds and peptides that bind the trail receptor. US20090131317. 2009
- 4.Ashkenazi A. Apo-2LI and APO-3 polypeptides. US6469144. 2002
- 5.Ashkenazi A, Kelley RF, O'connell MP, Pitti RM, Schwall RA. Substitutional variants of APO-2 ligand. US6740739. 2004
- 6.Ashkenazi A, Benyunes M, Schwall R. APO-2L receptor agonist and CPT-11 synergism. US20070026000. 2007
- 7.Nagane M, Cavenee W, Huang S. Compositions of trail and DNA damaging drugs and uses thereof. US6444640. 2002
- 8.Ni J, Rosen CA, Pan JG, Gentz RL, Dixit VM. Death domain containing receptor 4. US20050244857. 2005
- 9.Ni J, Gentz RL, Yu G-l, Rosen CA. Death domain containing receptor 5. US20050233958. 2005
- 10.Wiley SR, Goodwin RG. Use of TRAIL polypeptides to induce apoptosis. US7736637. 2010
- 11.Chuntharapai A, Kim KJ. Human dr4 antibodies and uses thereof. US20040147725. 2004
- 12.Kimberly RP, Koopman WJ, Lobuglio AF, Zhou T, Buchsbaum DJ, Ichikawa K, Oshumi J. Combinations of antibodies selective for a tumor necrosis factor-related apoptosis-inducing ligand receptor and other therapeutic agents. US20090022707. 2009
- 13.Li B, Sidhu SS. Dr5 antibodies and uses thereof. US20080248037. 2008
- 14.Lynch DH. Bispecific antibodies that bind TRAIL-R1 and TRAIL-R2. US20020155109. 2002
- 15.Ni J, Rosen CA, Pan JG, Gentz RL, Dixit VM. Death domain containing receptor-4 antibodies. US6461823. 2002
- 16.Ni J, Gentz RL, Yu G-l, Rosen CA. Death domain containing receptor 5 antibodies. US6872568. 2005
- 17.Salcedo T, Ruben. SM, Rosen CA, Albert VR, Dobson C, Vaughan T. Antibodies that immunospecifically bind to trail receptors. US7064189. 2006
- 18.Wiley SR, Goodwin RG. Antibodies directed against trail. US6521228. 2003
- 19.Zhou T, Ichikawa K, Kimberly RP, Koopman WJ, Ohsumi J, Lobuglio AF, Buchsbaum DJ. Combinations of antibodies selective for DR5 and other theurapeutic agents. US7704502. 2010
- 20.Griffith TS, Ratliff T. Method of inducing tumor cell apoptosis using trail/APO-2 ligand gene transfer. US6900185. 2005
- 21.Soria JC, Smit E, Khayat D, Besse B, Yang X, Hsu CP, et al. Phase 1b study of dulanermin (recombinant human APO2L/TRAIL) in combination with paclitaxel, carboplatin, and bevacizumab in patients with advanced non-squamous non-small-cell lung cancer. J Clin Oncol. 2010;28(9):1527–33. doi: 10.1200/JCO.2009.25.4847. [DOI] [PubMed] [Google Scholar]
- 22.Fox NL, Humphreys R, Luster TA, Klein J, Gallant G. Tumor Necrosis Factor-related apoptosis-inducing ligand (TRAIL) Receptor-1 and Receptor-2 agonists for cancer therapy. Expert Opin Biol Ther. 2010;10(1):1–18. doi: 10.1517/14712590903319656. [DOI] [PubMed] [Google Scholar]
- 23.Merino D, Lalaoui N, Morizot A, Schneider P, Solary E, Micheau O. Differential inhibition of TRAIL-mediated DR5-DISC formation by decoy receptors 1 and 2. Mol Cell Biol. 2006;26(19):7046–55. doi: 10.1128/MCB.00520-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tartaglia LA, Ayres TM, Wong GH, Goeddel DV. A novel domain within the 55 kd TNF receptor signals cell death. Cell. 1993;74(5):845–53. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- 25.Nikolaev A, McLaughlin T, O'Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457(7232):81–9. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Bodmer JL, Schneider P, Tschopp J. The molecular architecture of the TNF superfamily. Trends Biochem Sci. 2002;27(1):19–26. doi: 10.1016/s0968-0004(01)01995-8. [DOI] [PubMed] [Google Scholar]
- 27.Schneider P, Holler N, Bodmer JL, Hahne M, Frei K, Fontana A, et al. Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J Exp Med. 1998;187(8):1205–13. doi: 10.1084/jem.187.8.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wajant H, Moosmayer D, Wuest T, Bartke T, Gerlach E, Schonherr U, et al. Differential activation of TRAIL-R1 and -2 by soluble and membrane TRAIL allows selective surface antigen-directed activation of TRAIL-R2 by a soluble TRAIL derivative. Oncogene. 2001;20(30):4101–6. doi: 10.1038/sj.onc.1204558. [DOI] [PubMed] [Google Scholar]
- 29.Schneider P. Production of recombinant TRAIL and TRAIL receptor: Fc chimeric proteins. Methods Enzymol. 2000;322:325–45. doi: 10.1016/s0076-6879(00)22031-4. [DOI] [PubMed] [Google Scholar]
- 30.Screaton GR, Xu XN, Olsen AL, Cowper AE, Tan R, McMichael AJ, et al. LARD: a new lymphoid-specific death domain containing receptor regulated by alternative pre-mRNA splicing. Proc Natl Acad Sci USA. 1997;94(9):4615–9. doi: 10.1073/pnas.94.9.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leist M, Gantner F, Kunstle G, Bohlinger I, Tiegs G, Bluethmann H, et al. The 55-kD tumor necrosis factor receptor and CD95 independently signal murine hepatocyte apoptosis and subsequent liver failure. Mol Med. 1996;2(1):109–24. [PMC free article] [PubMed] [Google Scholar]
- 32.Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104(2):155–62. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herbst RS, Mendolson DS, Ebbinghaus S, Gordon MS, O'Dwyer M, Lieberman G, et al. A phase I safety and pharmacokinetic (PK) study of recombinant Apo2L/TRAIL, an apoptosis-inducing protein in patients with advanced cancer. J Clinl Oncol. 2006;24(18S):3013. [Google Scholar]
- 34.Hotte SJ, Hirte HW, Chen EX, Siu LL, Le LH, Corey A, et al. A phase 1 study of mapatumumab (fully human monoclonal antibody to TRAIL-R1) in patients with advanced solid malignancies. Clin Cancer Res. 2008;14(11):3450–5. doi: 10.1158/1078-0432.CCR-07-1416. [DOI] [PubMed] [Google Scholar]
- 35.Wakelee HA, Patnaik A, Sikic BI, Mita M, Fox NL, Miceli R, et al. Phase I and pharmacokinetic study of lexatumumab (HGS-ETR2) given every 2 weeks in patients with advanced solid tumors. Ann Oncol. 2010;21(2):376–81. doi: 10.1093/annonc/mdp292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, et al. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med. 2001;7(1):94–100. doi: 10.1038/83416. [DOI] [PubMed] [Google Scholar]
- 37.Cretney E, Takeda K, Yagita H, Glaccum M, Peschon JJ, Smyth MJ. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J Immunol. 2002;168(3):1356–61. doi: 10.4049/jimmunol.168.3.1356. [DOI] [PubMed] [Google Scholar]
- 38.Grosse-Wilde A, Voloshanenko O, Bailey SL, Longton GM, Schaefer U, Csernok AI, et al. TRAIL-R deficiency in mice enhances lymph node metastasis without affecting primary tumor development. J Clin Invest. 2008;118(1):100–10. doi: 10.1172/JCI33061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nesterov A, Nikrad M, Johnson T, Kraft AS. Oncogenic Ras sensitizes normal human cells to tumor necrosis factor-alpha-related apoptosis-inducing ligand-induced apoptosis. Cancer Res. 2004;64(11):3922–7. doi: 10.1158/0008-5472.CAN-03-2219. [DOI] [PubMed] [Google Scholar]
- 40.Klefstrom J, Verschuren EW, Evan G. c-Myc augments the apoptotic activity of cytosolic death receptor signaling proteins by engaging the mitochondrial apoptotic pathway. J Biol Chem. 2002;277(45):43224–32. doi: 10.1074/jbc.M206967200. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Quon KC, Knee DA, Nesterov A, Kraft AS. RAS, MYC, and sensitivity to tumor necrosis factor-alpha-related apoptosis-inducing ligand-induced apoptosis. Cancer Res. 2005;65(4):1615–6. doi: 10.1158/0008-5472.CAN-04-2757. [DOI] [PubMed] [Google Scholar]
- 42.Galligan L, Longley DB, McEwan M, Wilson TR, McLaughlin K, Johnston PG. Chemotherapy and TRAIL-mediated colon cancer cell death: The roles of p53, TRAIL receptors, and c-FLIP. Mol Cancer Ther. 2005;4(12):2026–36. doi: 10.1158/1535-7163.MCT-05-0262. [DOI] [PubMed] [Google Scholar]
- 43.Wu GS, Burns TF, McDonald ER, 3rd, Meng RD, Kao G, Muschel R, et al. Induction of the TRAIL receptor KILLER/DR5 in p53-dependent apoptosis but not growth arrest. Oncogene. 1999;18(47):6411–8. doi: 10.1038/sj.onc.1203025. [DOI] [PubMed] [Google Scholar]
- 44.Sheikh MS, Huang Y, Fernandez-Salas EA, El-Deiry WS, Friess H, Amundson S, et al. The antiapoptotic decoy receptor TRID/TRAIL-R3 is a p53-regulated DNA damage-inducible gene that is overexpressed in primary tumors of the gastrointestinal tract. Oncogene. 1999;18(28):4153–9. doi: 10.1038/sj.onc.1202763. [DOI] [PubMed] [Google Scholar]
- 45.Meng RD, McDonald ER, 3rd, Sheikh MS, Fornace AJ, Jr, El-Deiry WS. The TRAIL decoy receptor TRUNDD (DcR2, TRAIL-R4) is induced by adenovirus-p53 overexpression and can delay TRAIL-, p53-, and KILLER/DR5-dependent colon cancer apoptosis. Mol Ther. 2000;1(2):130–44. doi: 10.1006/mthe.2000.0025. [DOI] [PubMed] [Google Scholar]
- 46.Tomasetti M, Andera L, Alleva R, Borghi B, Neuzil J, Procopio A. Alpha-tocopheryl succinate induces DR4 and DR5 expression by a p53-dependent route: Implication for sensitisation of resistant cancer cells to TRAIL apoptosis. FEBS Lett. 2006;580(8):1925–31. doi: 10.1016/j.febslet.2006.02.054. [DOI] [PubMed] [Google Scholar]
- 47.Hu H, Jiang C, Schuster T, Li GX, Daniel PT, Lu J. Inorganic selenium sensitizes prostate cancer cells to TRAIL-induced apoptosis through superoxide/p53/Bax-mediated activation of mitochondrial pathway. Mol Cancer Ther. 2006;5(7):1873–82. doi: 10.1158/1535-7163.MCT-06-0063. [DOI] [PubMed] [Google Scholar]
- 48.Lee DH, Rhee JG, Lee YJ. Reactive oxygen species up-regulate p53 and Puma; a possible mechanism for apoptosis during combined treatment with TRAIL and wogonin. Br J Pharmacol. 2009;157(7):1189–202. doi: 10.1111/j.1476-5381.2009.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carter BZ, Mak DH, Schober WD, Dietrich MF, Pinilla C, Vassilev LT, et al. Triptolide sensitizes AML cells to TRAIL-induced apoptosis via decrease of XIAP and p53-mediated increase of DR5. Blood. 2008;111(7):3742–50. doi: 10.1182/blood-2007-05-091504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuribayashi K, Krigsfeld G, Wang W, Xu J, Mayes PA, Dicker DT, et al. TNFSF10 (TRAIL), a p53 target gene that mediates p53-dependent cell death. Cancer Biol Ther. 2008;7(12):2034–8. doi: 10.4161/cbt.7.12.7460. [DOI] [PubMed] [Google Scholar]
- 51.Toscano F, Fajoui ZE, Gay F, Lalaoui N, Parmentier B, Chayvialle JA, et al. P53-mediated upregulation of DcR1 impairs oxaliplatin/TRAIL-induced synergistic anti-tumour potential in colon cancer cells. Oncogene. 2008;27(30):4161–71. doi: 10.1038/onc.2008.52. [DOI] [PubMed] [Google Scholar]
- 52.Morizot A, Merino D, Lalaoui N, Jacquemin G, Granci V, Iessi E, et al. Chemotherapy overcomes TRAIL-R4-mediated TRAIL resistance at the DISC level. Cell Death Differ. 2011;18(4):700–11. doi: 10.1038/cdd.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Merino D, Lalaoui N, Morizot A, Solary E, Micheau O. TRAIL in cancer therapy: Present and future challenges. Expert Opin Ther Targets. 2007;11(10):1299–314. doi: 10.1517/14728222.11.10.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Micheau O. Cellular FLICE-inhibitory protein: An attractive therapeutic target? Expert Opin Ther Targets. 2003;7(4):559–73. doi: 10.1517/14728222.7.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Micheau O, Solary E, Hammann A, Martin F, Dimanche-Boitrel MT. Sensitization of cancer cells treated with cytotoxic drugs to fas-mediated cytotoxicity. J Natl Cancer Inst. 1997;89(11):783–9. doi: 10.1093/jnci/89.11.783. [DOI] [PubMed] [Google Scholar]
- 56.Ivanov VN, Hei TK. Sodium arsenite accelerates TRAIL-mediated apoptosis in melanoma cells through upregulation of TRAIL-R1/R2 surface levels and downregulation of cFLIP expression. Exp Cell Res. 2006;312(20):4120–38. doi: 10.1016/j.yexcr.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frese S, Frese-Schaper M, Andres AC, Miescher D, Zumkehr B, Schmid RA. Cardiac glycosides initiate Apo2L/TRAIL-induced apoptosis in non-small cell lung cancer cells by up-regulation of death receptors 4 and 5. Cancer Res. 2006;66(11):5867–74. doi: 10.1158/0008-5472.CAN-05-3544. [DOI] [PubMed] [Google Scholar]
- 58.Hetschko H, Voss V, Seifert V, Prehn JH, Kogel D. Upregulation of DR5 by proteasome inhibitors potently sensitizes glioma cells to TRAIL-induced apoptosis. FEBS. J. 2008;275(8):1925–36. doi: 10.1111/j.1742-4658.2008.06351.x. [DOI] [PubMed] [Google Scholar]
- 59.Nagane M, Pan G, Weddle JJ, Dixit VM, Cavenee WK, Huang HJ. Increased death receptor 5 expression by chemotherapeutic agents in human gliomas causes synergistic cytotoxicity with tumor necrosis factor-related apoptosis-inducing ligand in vitro and in vivo. Cancer Res. 2000;60(4):847–53. [PubMed] [Google Scholar]
- 60.Sung B, Park B, Yadav VR, Aggarwal BB. Celastrol, a triterpene, enhances TRAIL-induced apoptosis through the down-regulation of cell survival proteins and up-regulation of death receptors. J Biol Chem. 2010;285(15):11498–507. doi: 10.1074/jbc.M109.090209. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Zeise E, Weichenthal M, Schwarz T, Kulms D. Resistance of human melanoma cells against the death ligand TRAIL is reversed by ultraviolet-B radiation via downregulation of FLIP. J Invest Dermatol. 2004;123(4):746–54. doi: 10.1111/j.0022-202X.2004.23420.x. [DOI] [PubMed] [Google Scholar]
- 62.Kinoshita H, Yoshikawa H, Shiiki K, Hamada Y, Nakajima Y, Tasaka K. Cisplatin (CDDP) sensitizes human osteosarcoma cell to Fas/CD95-mediated apoptosis by down-regulating FLIP-L expression. Int J Cancer. 2000;88(6):986–91. doi: 10.1002/1097-0215(20001215)88:6<986::aid-ijc23>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 63.Palacios C, Yerbes R, Lopez-Rivas A. Flavopiridol induces cellular FLICE-inhibitory protein degradation by the proteasome and promotes TRAIL-induced early signaling and apoptosis in breast tumor cells. Cancer Res. 2006;66(17):8858–69. doi: 10.1158/0008-5472.CAN-06-0808. [DOI] [PubMed] [Google Scholar]
- 64.Kim JY, Kim EH, Park SS, Lim JH, Kwon TK, Choi KS. Quercetin sensitizes human hepatoma cells to TRAIL-induced apoptosis via Sp1-mediated DR5 up-regulation and proteasome-mediated c-FLIPS down-regulation. J Cell Biochem. 2008;105(6):1386–98. doi: 10.1002/jcb.21958. [DOI] [PubMed] [Google Scholar]
- 65.Fulda S, Debatin KM. 5-Aza-2?-deoxycytidine and IFN-gamma cooperate to sensitize for TRAIL-induced apoptosis by upregulating caspase-8. Oncogene. 2006;25(37):5125–33. doi: 10.1038/sj.onc.1209518. [DOI] [PubMed] [Google Scholar]
- 66.Fulda S, Kufer MU, Meyer E, van Valen F, Dockhorn-Dworniczak B, Debatin KM. Sensitization for death receptor- or drug-induced apoptosis by re-expression of caspase-8 through demethylation or gene transfer. Oncogene. 2001;20(41):5865–77. doi: 10.1038/sj.onc.1204750. [DOI] [PubMed] [Google Scholar]
- 67.Ducoroy P, Micheau O, Perruche S, Dubrez-Daloz L, de Fornel D, Dutartre P, et al. LF 15-0195 immunosuppressive agent enhances activation-induced T-cell death by facilitating caspase-8 and caspase-10 activation at the DISC level. Blood. 2003;101(1):194–201. doi: 10.1182/blood-2002-02-0603. [DOI] [PubMed] [Google Scholar]
- 68.Lacour S, Micheau O, Hammann A, Drouineaud V, Tschopp J, Solary E, et al. Chemotherapy enhances TNF-related apoptosis-inducing Ligand DISC assembly in HT29 human colon cancer cells. Oncogene. 2003;22(12):1807–16. doi: 10.1038/sj.onc.1206127. [DOI] [PubMed] [Google Scholar]
- 69.Ganten TM, Haas TL, Sykora J, Stahl H, Sprick MR, Fas SC, et al. Enhanced caspase-8 recruitment to and activation at the DISC is critical for sensitisation of human hepatocellular carcinoma cells to TRAIL-induced apoptosis by chemotherapeutic drugs. Cell Death Differ. 2004;11 Suppl 1:S86–96. doi: 10.1038/sj.cdd.4401437. [DOI] [PubMed] [Google Scholar]
- 70.Micheau O, Solary E, Hammann A, Dimanche-Boitrel MT. Fas ligand-independent, FADD-mediated activation of the Fas death pathway by anticancer drugs. J Biol Chem. 1999;274(12):7987–92. doi: 10.1074/jbc.274.12.7987. [DOI] [PubMed] [Google Scholar]
- 71.Inoue S, Harper N, Walewska R, Dyer MJ, Cohen GM. Enhanced Fas-associated death domain recruitment by histone deacetylase inhibitors is critical for the sensitization of chronic lymphocytic leukemia cells to TRAIL-induced apoptosis. Mol Cancer Ther. 2009;8(11):3088–97. doi: 10.1158/1535-7163.MCT-09-0451. [DOI] [PubMed] [Google Scholar]
- 72.Zhuang H, Jiang W, Cheng W, Qian K, Dong W, Cao L, et al. Down-regulation of HSP27 sensitizes TRAIL-resistant tumor cell to TRAIL-induced apoptosis. Lung Cancer. 2010;68(1):27–38. doi: 10.1016/j.lungcan.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 73.Huerta-Yepez S, Vega M, Jazirehi A, Garban H, Hongo F, Cheng G. Nitric oxide sensitizes prostate carcinoma cell lines to TRAIL-mediated apoptosis via inactivation of NF-kappa B and inhibition of Bcl-xl expression. Oncogene. 2004;23(29):4993–5003. doi: 10.1038/sj.onc.1207655. [DOI] [PubMed] [Google Scholar]
- 74.Fandy TE, Srivastava RK. Trichostatin A sensitizes TRAIL-resistant myeloma cells by downregulation of the antiapoptotic Bcl-2 proteins. Cancer Chemother Pharmacol. 2006;58(4):471–7. doi: 10.1007/s00280-005-0184-3. [DOI] [PubMed] [Google Scholar]
- 75.Siegelin MD, Gaiser T, Habel A, Siegelin Y. Myricetin sensitizes malignant glioma cells to TRAIL-mediated apoptosis by down-regulation of the short isoform of FLIP and bcl-2. Cancer Lett. 2009;283(2):230–8. doi: 10.1016/j.canlet.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 76.Peuhu E, Rivero-Muller A, Stykki H, Torvaldson E, Holmbom T, Eklund P, et al. Inhibition of Akt signaling by the lignan matairesinol sensitizes prostate cancer cells to TRAIL-induced apoptosis. Oncogene. 2010;29(6):898–908. doi: 10.1038/onc.2009.386. [DOI] [PubMed] [Google Scholar]
- 77.Festa M, Petrella A, Alfano S, Parente L. R-roscovitine sensitizes anaplastic thyroid carcinoma cells to TRAIL-induced apoptosis via regulation of IKK/NF-kappaB pathway. Int J Cancer. 2009;124(11):2728–36. doi: 10.1002/ijc.24260. [DOI] [PubMed] [Google Scholar]
- 78.Ammann JU, Haag C, Kasperczyk H, Debatin KM, Fulda S. Sensitization of neuroblastoma cells for TRAIL-induced apoptosis by NF-kappaB inhibition. Int J Cancer. 2009;124(6):1301–11. doi: 10.1002/ijc.24068. [DOI] [PubMed] [Google Scholar]
- 79.Lee TJ, Jang JH, Noh HJ, Park EJ, Choi KS, Kwon TK. Overexpression of Par-4 sensitizes TRAIL-induced apoptosis via inactivation of NF-kappaB and Akt signaling pathways in renal cancer cells. J Cell Biochem. 2010;109(5):885–95. doi: 10.1002/jcb.22504. [DOI] [PubMed] [Google Scholar]
- 80.Bodmer JL, Holler N, Reynard S, Vinciguerra P, Schneider P, Juo P, et al. TRAIL receptor-2 signals apoptosis through FADD and caspase-8. Nat Cell Biol. 2000;2(4):241–3. doi: 10.1038/35008667. [DOI] [PubMed] [Google Scholar]
- 81.Chaudhary PM, Eby M, Jasmin A, Bookwalter A, Murray J, Hood L. Death receptor 5, a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NF-kappaB pathway. Immunity. 1997;7(6):821–30. doi: 10.1016/s1074-7613(00)80400-8. [DOI] [PubMed] [Google Scholar]
- 82.Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81(4):505–12. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 83.Huang Y, Chen L, Zhou Y, Liu H, Yang J, Liu Z, et al. UXT-V1 protects cells against TNF-induced apoptosis through modulating complex II formation. Mol Biol Cell. 2011 doi: 10.1091/mbc.E10-10-0827. mbc.E10-10-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388(6638):190–5. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 85.Jin Z, El-Deiry WS. Distinct signaling pathways in TRAIL- versus tumor necrosis factor-induced apoptosis. Mol Cell Biol. 2006;26(21):8136–48. doi: 10.1128/MCB.00257-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Juo P, Kuo CJ, Yuan J, Blenis J. Essential requirement for caspase-8/FLICE in the initiation of the Fas-induced apoptotic cascade. Curr Biol. 1998;8(18):1001–8. doi: 10.1016/s0960-9822(07)00420-4. [DOI] [PubMed] [Google Scholar]
- 87.Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. Embo J. 1995;14(22):5579–88. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kischkel FC, Lawrence DA, Tinel A, LeBlanc H, Virmani A, Schow P, et al. Death receptor recruitment of endogenous caspase-10 and apoptosis initiation in the absence of caspase-8. J Biol Chem. 2001;276(49):46639–46. doi: 10.1074/jbc.M105102200. [DOI] [PubMed] [Google Scholar]
- 89.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114(2):181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 90.Milhas D, Cuvillier O, Therville N, Clave P, Thomsen M, Levade T, et al. Caspase-10 triggers Bid cleavage and caspase cascade activation in FasL-induced apoptosis. J Biol Chem. 2005;280(20):19836–42. doi: 10.1074/jbc.M414358200. [DOI] [PubMed] [Google Scholar]
- 91.Scaffidi C, Schmitz I, Krammer PH, Peter ME. The role of c-FLIP in modulation of CD95-induced apoptosis. J Biol Chem. 1999;274(3):1541–8. doi: 10.1074/jbc.274.3.1541. [DOI] [PubMed] [Google Scholar]
- 92.Soderstrom TS, Poukkula M, Holmstrom TH, Heiskanen KM, Eriksson JE. Mitogen-activated protein kinase/extracellular signal-regulated kinase signaling in activated T cells abrogates TRAIL-induced apoptosis upstream of the mitochondrial amplification loop and caspase-8. J Immunol. 2002;169(6):2851–60. doi: 10.4049/jimmunol.169.6.2851. [DOI] [PubMed] [Google Scholar]
- 93.Sprick MR, Rieser E, Stahl H, Grosse-Wilde A, Weigand MA, Walczak H. Caspase-10 is recruited to and activated at the native TRAIL and CD95 death-inducing signalling complexes in a FADD-dependent manner but can not functionally substitute caspase-8. Embo J. 2002;21(17):4520–30. doi: 10.1093/emboj/cdf441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, et al. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386(6624):517–21. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 95.Vincenz C, Dixit VM. Fas-associated death domain protein interleukin-1beta-converting enzyme 2 (FLICE2), an ICE/Ced-3 homologue, is proximally involved in CD95- and p55-mediated death signaling. J Biol Chem. 1997;272(10):6578–83. doi: 10.1074/jbc.272.10.6578. [DOI] [PubMed] [Google Scholar]
- 96.Wang J, Chun HJ, Wong W, Spencer DM, Lenardo MJ. Caspase-10 is an initiator caspase in death receptor signaling. Proc Natl Acad Sci USA. 2001;98(24):13884–8. doi: 10.1073/pnas.241358198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang J, Lobito AA, Shen F, Hornung F, Winoto A, Lenardo MJ. Inhibition of Fas-mediated apoptosis by the B cell antigen receptor through c-FLIP. Eur J Immunol. 2000;30(1):155–63. doi: 10.1002/1521-4141(200001)30:1<155::AID-IMMU155>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 98.Devin A, Cook A, Lin Y, Rodriguez Y, Kelliher M, Liu Z. The distinct roles of TRAF2 and RIP in IKK activation by TNF-R1: TRAF2 recruits IKK to TNF-R1 while RIP mediates IKK activation. Immunity. 2000;12(4):419–29. doi: 10.1016/s1074-7613(00)80194-6. [DOI] [PubMed] [Google Scholar]
- 99.Devin A, Lin Y, Yamaoka S, Li Z, Karin M, Liu Z. The alpha and beta subunits of IkappaB kinase (IKK) mediate TRAF2-dependent IKK recruitment to tumor necrosis factor (TNF) receptor 1 in response to TNF. Mol Cell Biol. 2001;21(12):3986–94. doi: 10.1128/MCB.21.12.3986-3994.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Geserick P, Hupe M, Moulin M, Wong WW, Feoktistova M, Kellert B. Cellular IAPs inhibit a cryptic CD95-induced cell death by limiting RIP1 kinase recruitment. J Cell Biol. 2009;187(7):1037–54. doi: 10.1083/jcb.200904158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Haas TL, Emmerich CH, Gerlach B, Schmukle AC, Cordier SM, Rieser E, et al. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol Cell. 2009;36(5):831–44. doi: 10.1016/j.molcel.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 102.Hsu H, Huang J, Shu HB, Baichwal V, Goeddel DV. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 1996;4(4):387–96. doi: 10.1016/s1074-7613(00)80252-6. [DOI] [PubMed] [Google Scholar]
- 103.Hsu H, Shu HB, Pan MG, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84(2):299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 104.Hsu H, Xiong J, Goeddel DV. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell. 1995;81(4):495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 105.Lavrik IN, Mock T, Golks A, Hoffmann JC, Baumann S, Krammer PH. CD95 stimulation results in the formation of a novel death effector domain protein-containing complex. J Biol Chem. 2008;283(39):26401–8. doi: 10.1074/jbc.M800823200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mahoney DJ, Cheung HH, Mrad RL, Plenchette S, Simard C, Enwere E. Both, cIAP1 and cIAP2 regulate TNFalpha-mediated NF-kappaB activation. Proc Natl Acad Sci USA. 2008;105(33):11778–83. doi: 10.1073/pnas.0711122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Petersen SL, Peyton M, Minna JD, Wang X. Overcoming cancer cell resistance to Smac mimetic induced apoptosis by modulating cIAP-2 expression. Proc Natl Acad Sci USA. 2010;107(26):11936–41. doi: 10.1073/pnas.1005667107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shu HB, Takeuchi M, Goeddel DV. The tumor necrosis factor receptor 2 signal transducers TRAF2 and c-IAP1 are components of the tumor necrosis factor receptor 1 signaling complex. Proc Natl Acad Sci USA. 1996;93(24):13973–8. doi: 10.1073/pnas.93.24.13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tokunaga F, Sakata S, Saeki Y, Satomi Y, Kirisako T, Kamei K, et al. Involvement of linear polyubiquitylation of NEMO in NF-kappaB activation. Nat Cell Biol. 2009;11(2):123–32. doi: 10.1038/ncb1821. [DOI] [PubMed] [Google Scholar]
- 110.Varfolomeev E, Maecker H, Sharp D, Lawrence D, Renz M, Vucic D, et al. Molecular determinants of kinase pathway activation by Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand. J Biol Chem. 2005;280(49):40599–608. doi: 10.1074/jbc.M509560200. [DOI] [PubMed] [Google Scholar]
- 111.Juo P, Woo MS, Kuo CJ, Signorelli P, Biemann HP, Hannun YA, et al. FADD is required for multiple signaling events downstream of the receptor Fas. Cell Growth Differ. 1999;10(12):797–804. [PubMed] [Google Scholar]
- 112.LeBlanc H, Lawrence D, Varfolomeev E, Totpal K, Morlan J, Schow P, et al. Tumor-cell resistance to death receptor--induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat Med. 2002;8(3):274–81. doi: 10.1038/nm0302-274. [DOI] [PubMed] [Google Scholar]
- 113.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388(6638):190–5. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 114.Fulda S, Meyer E, Debatin KM. Inhibition of TRAIL-induced apoptosis by Bcl-2 overexpression. Oncogene. 2002;21(15):2283–94. doi: 10.1038/sj.onc.1205258. [DOI] [PubMed] [Google Scholar]
- 115.Cummins JM, Kohli M, Rago C, Kinzler KW, Vogelstein B, Bunz F. X-linked inhibitor of apoptosis protein (XIAP) is a nonredundant modulator of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis in human cancer cells. Cancer Res. 2004;64(9):3006–8. doi: 10.1158/0008-5472.can-04-0046. [DOI] [PubMed] [Google Scholar]
- 116.Ndozangue-Touriguine O, Sebbagh M, Merino D, Micheau O, Bertoglio J, Breard J. A mitochondrial block and expression of XIAP lead to resistance to TRAIL-induced apoptosis during progression to metastasis of a colon carcinoma. Oncogene. 2008;27(46):6012–22. doi: 10.1038/onc.2008.197. [DOI] [PubMed] [Google Scholar]
- 117.Siegelin MD, Reuss DE, Habel A, Rami A, von Deimling A. Quercetin promotes degradation of survivin and thereby enhances death-receptor-mediated apoptosis in glioma cells. Neuro Oncol. 2009;11(2):122–31. doi: 10.1215/15228517-2008-085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wu NL, Lee TA, Tsai TL, Lin WW. TRAIL-Induced Keratinocyte Differentiation Requires Caspase Activation and p63 Expression. J Invest Dermatol. 2011;jid:2010–402. doi: 10.1038/jid.2010.402. [DOI] [PubMed] [Google Scholar]
- 119.Viard-Leveugle I, Bullani RR, Meda P, Micheau O, Limat A, Saurat JH, et al. Intracellular localization of keratinocyte Fas ligand explains lack of cytolytic activity under physiological conditions. J Biol Chem. 2003;278(18):16183–8. doi: 10.1074/jbc.M212188200. [DOI] [PubMed] [Google Scholar]
- 120.Rimondi E, Secchiero P, Quaroni A, Zerbinati C, Capitani S, Zauli G. Involvement of TRAIL/TRAIL-receptors in human intestinal cell differentiation. J Cell Physiol. 2006;206(3):647–54. doi: 10.1002/jcp.20512. [DOI] [PubMed] [Google Scholar]
- 121.Freer-Prokop M, O'Flaherty J, Ross JA, Weyman CM. Non-canonical role for the TRAIL receptor DR5/FADD/caspase pathway in the regulation of MyoD expression and skeletal myoblast differentiation. Differentiation. 2009;78(4):205–12. doi: 10.1016/j.diff.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.O'Flaherty J, Mei Y, Freer M, Weyman CM. Signaling through the TRAIL receptor DR5/FADD pathway plays a role in the apoptosis associated with skeletal myoblast differentiation. Apoptosis. 2006;11(12):2103–13. doi: 10.1007/s10495-006-0196-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yen ML, Tsai HF, Wu YY, Hwa HL, Lee BH, Hsu PN. TNF-related apoptosis-inducing ligand (TRAIL) induces osteoclast differentiation from monocyte/macrophage lineage precursor cells. Mol Immunol. 2008;45(8):2205–13. doi: 10.1016/j.molimm.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 124.Zhang XR, Zhang LY, Devadas S, Li L, Keegan AD, Shi YF. Reciprocal expression of TRAIL and CD95L in Th1 and Th2 cells: role of apoptosis in T helper subset differentiation. Cell Death Differ. 2003;10(2):203–10. doi: 10.1038/sj.cdd.4401138. [DOI] [PubMed] [Google Scholar]
- 125.Cho YS, Challa S, Clancy L, Chan FK. Lipopolysaccharide-induced expression of TRAIL promotes dendritic cell differentiation. Immunology. 2010;130(4):504–15. doi: 10.1111/j.1365-2567.2010.03266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997;277(5327):815–8. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- 127.Schneider P, Bodmer JL, Thome M, Hofmann K, Holler N, Tschopp J. Characterization of two receptors for TRAIL. FEBS Lett. 1997;416(3):329–34. doi: 10.1016/s0014-5793(97)01231-3. [DOI] [PubMed] [Google Scholar]
- 128.Clancy L, Mruk K, Archer K, Woelfel M, Mongkolsapaya J, Screaton G, et al. Preligand assembly domain-mediated ligand-independent association between TRAIL receptor 4 (TR4) and TR2 regulates TRAIL-induced apoptosis. Proc Natl Acad Sci USA. 2005;102(50):18099–104. doi: 10.1073/pnas.0507329102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Muzio M, Stockwell BR, Stennicke HR, Salvesen GS, Dixit VM. An induced proximity model for caspase-8 activation. J Biol Chem. 1998;273(5):2926–30. doi: 10.1074/jbc.273.5.2926. [DOI] [PubMed] [Google Scholar]
- 130.Hughes MA, Harper N, Butterworth M, Cain K, Cohen GM, MacFarlane M. Reconstitution of the death-inducing signaling complex reveals a substrate switch that determines CD95-mediated death or survival. Mol Cell. 2009;35(3):265–79. doi: 10.1016/j.molcel.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 131.Park HH, Logette E, Raunser S, Cuenin S, Walz T, Tschopp J, et al. Death domain assembly mechanism revealed by crystal structure of the oligomeric PIDDosome core complex. Cell. 2007;128(3):533–46. doi: 10.1016/j.cell.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Scott FL, Stec B, Pop C, Dobaczewska MK, Lee JJ, Monosov E, et al. The Fas-FADD death domain complex structure unravels signalling by receptor clustering. Nature. 2009;457(7232):1019–22. doi: 10.1038/nature07606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Berg D, Lehne M, Muller N, Siegmund D, Munkel S, Sebald W, et al. Enforced covalent trimerization increases the activity of the TNF ligand family members TRAIL and CD95L. Cell Death Differ. 2007;14(12):2021–34. doi: 10.1038/sj.cdd.4402213. [DOI] [PubMed] [Google Scholar]
- 134.Monia BP, Baker BF, Zhang H, Cowsert LM. Antisense modulation of FADD expression. US6015712. 2000
- 135.Yeh WC, Pompa JL, McCurrach ME, Shu HB, Elia AJ, Shahinian A, et al. FADD: Essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279(5358):1954–8. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- 136.Jiang Y, Woronicz JD, Liu W, Goeddel DV. Prevention of constitutive TNF receptor 1 signaling by silencer of death domains. Science. 1999;283(5401):543–6. doi: 10.1126/science.283.5401.543. [DOI] [PubMed] [Google Scholar]
- 137.Takada H, Chen NJ, Mirtsos C, Suzuki S, Suzuki N, Wakeham A, et al. Role of SODD in regulation of tumor necrosis factor responses. Mol Cell Biol. 2003;23(11):4026–33. doi: 10.1128/MCB.23.11.4026-4033.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Berroud A, Le Roy A, Voisin P. Membrane oxidative damage induced by ionizing radiation detected by fluorescence polarization. Radiat Environ Biophys. 1996;35(4):289–95. doi: 10.1007/s004110050042. [DOI] [PubMed] [Google Scholar]
- 139.Gaboriau F, Morliere P, Marquis I, Moysan A, Geze M, Dubertret L. Membrane damage induced in cultured human skin fibroblasts by UVA irradiation. Photochem Photobiol. 1993;58(4):515–20. doi: 10.1111/j.1751-1097.1993.tb04924.x. [DOI] [PubMed] [Google Scholar]
- 140.Aragane Y, Kulms D, Metze D, Wilkes G, Poppelmann B, Luger TA, et al. Ultraviolet light induces apoptosis via direct activation of CD95 (Fas/APO-1) independently of its ligand CD95L. J Cell Biol. 1998;140(1):171–82. doi: 10.1083/jcb.140.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Huang HL, Fang LW, Lu SP, Chou CK, Luh TY, Lai MZ. DNA-damaging reagents induce apoptosis through reactive oxygen species-dependent Fas aggregation. Oncogene. 2003;22(50):8168–77. doi: 10.1038/sj.onc.1206979. [DOI] [PubMed] [Google Scholar]
- 142.Gniadecki R. Depletion of membrane cholesterol causes ligand-independent activation of Fas and apoptosis. Biochem Biophys Res Commun. 2004;320(1):165–9. doi: 10.1016/j.bbrc.2004.05.145. [DOI] [PubMed] [Google Scholar]
- 143.Segui B, Legembre P. Redistribution of CD95 into the lipid rafts to treat cancer cells? Recent Pat Anticancer Drug Discov. 2010;5(1):22–8. doi: 10.2174/157489210789702190. [DOI] [PubMed] [Google Scholar]
- 144.Gajate C, Mollinedo F. Cytoskeleton-mediated death receptor and ligand concentration in lipid rafts forms apoptosis-promoting clusters in cancer chemotherapy. J Biol Chem. 2005;280(12):11641–7. doi: 10.1074/jbc.M411781200. [DOI] [PubMed] [Google Scholar]
- 145.Maldonado-Celis ME, Bousserouel S, Gosse F, Lobstein A, Raul F. Apple procyanidins activate apoptotic signaling pathway in human colon adenocarcinoma cells by a lipid-raft independent mechanism. Biochem Biophys Res Commun. 2009;388(2):372–6. doi: 10.1016/j.bbrc.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 146.Rossin A, Derouet M, Abdel-Sater F, Hueber AO. Palmitoylation of the TRAIL receptor DR4 confers an efficient TRAIL-induced cell death signalling. Biochem J. 2009;419(1):185–92. doi: 10.1042/BJ20081212. [DOI] [PubMed] [Google Scholar]
- 147.Min Y, Shi J, Zhang Y, Liu S, Liu Y, Zheng D. Death receptor 5-recruited raft components contributes to the sensitivity of Jurkat leukemia cell lines to TRAIL-induced cell death. IUBMB Life. 2009;61(3):261–7. doi: 10.1002/iub.166. [DOI] [PubMed] [Google Scholar]
- 148.Xu L, Qu X, Zhang Y, Hu X, Yang X, Hou K, et al. Oxaliplatin enhances TRAIL-induced apoptosis in gastric cancer cells by CBL-regulated death receptor redistribution in lipid rafts. FEBS Lett. 2009;583(5):943–8. doi: 10.1016/j.febslet.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 149.Song JH, Tse MC, Bellail A, Phuphanich S, Khuri F, Kneteman NM, et al. Lipid rafts and nonrafts mediate tumor necrosis factor related apoptosis-inducing ligand induced apoptotic and nonapoptotic signals in non small cell lung carcinoma cells. Cancer Res. 2007;67(14):6946–55. doi: 10.1158/0008-5472.CAN-06-3896. [DOI] [PubMed] [Google Scholar]
- 150.Gajate C, Mollinedo F. Edelfosine and perifosine induce selective apoptosis in multiple myeloma by recruitment of death receptors and downstream signaling molecules into lipid rafts. Blood. 2007;109(2):711–9. doi: 10.1182/blood-2006-04-016824. [DOI] [PubMed] [Google Scholar]
- 151.Wagner KW, Punnoose EA, Januario T, Lawrence DA, Pitti RM, Lancaster K, et al. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med. 2007;13(9):1070–7. doi: 10.1038/nm1627. [DOI] [PubMed] [Google Scholar]
- 152.Rostenberg I, Guizar-Vazquez J, Suarez P, Rico R, Nungaray L, Dominguez C. Distinct glycosylation of serum proteins in patients with cancer: Brief communication. J Natl Cancer Inst. 1978;60(1):83–7. doi: 10.1093/jnci/60.1.83. [DOI] [PubMed] [Google Scholar]
- 153.Dennis JW, Granovsky M, Warren CE. Glycoprotein glycosylation and cancer progression. Biochim Biophys Acta. 1999;1473(1):21–34. doi: 10.1016/s0304-4165(99)00167-1. [DOI] [PubMed] [Google Scholar]
- 154.Rak JW, Basolo F, Elliott JW, Russo J, Miller FR. Cell surface glycosylation changes accompanying immortalization and transformation of normal human mammary epithelial cells. Cancer Lett. 1991;57(1):27–36. doi: 10.1016/0304-3835(91)90059-q. [DOI] [PubMed] [Google Scholar]
- 155.van Noesel MM, van Bezouw S, Salomons GS, Voute PA, Pieters R, Baylin SB, et al. Tumor-specific down-regulation of the tumor necrosis factor-related apoptosis-inducing ligand decoy receptors DcR1 and DcR2 is associated with dense promoter hypermethylation. Cancer Res. 2002;62(7):2157–61. [PubMed] [Google Scholar]
- 156.Horak P, Pils D, Haller G, Pribill I, Roessler M, Tomek S, et al. Contribution of epigenetic silencing of tumor necrosis factor-related apoptosis inducing ligand receptor 1 (DR4) to TRAIL resistance and ovarian cancer. Mol Cancer Res. 2005;3(6):335–43. doi: 10.1158/1541-7786.MCR-04-0136. [DOI] [PubMed] [Google Scholar]
- 157.Margetts CD, Astuti D, Gentle DC, Cooper WN, Cascon A, Catchpoole D, et al. Epigenetic analysis of HIC1, CASP8, FLIP, TSP1, DCR1, DCR2, DR4, DR5, KvDMR1, H19 and preferential 11p15.5 maternal-allele loss in von Hippel-Lindau and sporadic phaeochromocytomas. Endocr Relat Cancer. 2005;12(1):161–72. doi: 10.1677/erc.1.00865. [DOI] [PubMed] [Google Scholar]
- 158.Elias A, Siegelin MD, Steinmuller A, von Deimling A, Lass U, Korn B, et al. Epigenetic silencing of death receptor 4 mediates tumor necrosis factor-related apoptosis-inducing ligand resistance in gliomas. Clin Cancer Res. 2009;15(17):5457–65. doi: 10.1158/1078-0432.CCR-09-1125. [DOI] [PubMed] [Google Scholar]
- 159.Simova S, Klima M, Cermak L, Sourkova V, Andera L. Arf and Rho GAP adapter protein ARAP1 participates in the mobilization of TRAIL-R1/DR4 to the plasma membrane. Apoptosis. 2008;13(3):423–36. doi: 10.1007/s10495-007-0171-8. [DOI] [PubMed] [Google Scholar]
- 160.Yoon HY, Lee JS, Randazzo PA. ARAP1 regulates endocytosis of EGFR. Traffic. 2008;9(12):2236–52. doi: 10.1111/j.1600-0854.2008.00839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Micheau O, Thome M, Schneider P, Holler N, Tschopp J, Nicholson DW, et al. The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. J Biol Chem. 2002;277(47):45162–71. doi: 10.1074/jbc.M206882200. [DOI] [PubMed] [Google Scholar]
- 162.Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, et al. et alFas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1(6):489–95. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 163.Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998;8(3):297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- 164.Schneider P, Thome M, Burns K, Bodmer JL, Hofmann K, Kataoka T, et al. TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate NF-kappaB. Immunity. 1997;7(6):831–6. doi: 10.1016/s1074-7613(00)80401-x. [DOI] [PubMed] [Google Scholar]
- 165.Golks A, Brenner D, Fritsch C, Krammer PH, Lavrik IN. l c-FLIPR a new regulator of death receptor-induced apoptosis. J Biol Chem. 2005;280(15):14507–13. doi: 10.1074/jbc.M414425200. [DOI] [PubMed] [Google Scholar]
- 166.Wajant H, Pfizenmaier K, Limmer S, Kreutzer R, Vornlocher H. Compositions and methods for treating trail-resistant cancer cells. US20040126791. 2004
- 167.Al-Zoubi AM, Efimova EV, Kaithamana S, Martinez O, El-Idrissi Mel A, Dogan RE, et al. Contrasting effects of IG20 and its splice isoforms, MADD and DENN-SV, on tumor necrosis factor alpha-induced apoptosis and activation of caspase-8 and -3. J Biol Chem. 2001;276(50):47202–11. doi: 10.1074/jbc.M104835200. [DOI] [PubMed] [Google Scholar]
- 168.Schievella AR, Chen JH, Graham JR, Lin LL. MADD, a novel death domain protein that interacts with the type 1 tumor necrosis factor receptor and activates mitogen-activated protein kinase. J Biol Chem. 1997;272(18):12069–75. doi: 10.1074/jbc.272.18.12069. [DOI] [PubMed] [Google Scholar]
- 169.Ramaswamy M, Efimova EV, Martinez O, Mulherkar NU, Singh SP, Prabhakar BS. IG20 (MADD splice variant-5), a proapoptotic protein, interacts with DR4/DR5 and enhances TRAIL-induced apoptosis by increasing recruitment of FADD and caspase-8 to the DISC. Oncogene. 2004;23(36):6083–94. doi: 10.1038/sj.onc.1207804. [DOI] [PubMed] [Google Scholar]
- 170.Prabhakar BS, Mulherkar N. IG20 splice variants therapeutics for cancer. US20090075929. 2009
- 171.Tanaka H, Hoshikawa Y, Oh-hara T, Koike S, Naito M, Noda T, et al. PRMT5, a novel TRAIL receptor-binding protein, inhibits TRAIL-induced apoptosis via nuclear factor-kappaB activation. Mol Cancer Res. 2009;7(4):557–69. doi: 10.1158/1541-7786.MCR-08-0197. [DOI] [PubMed] [Google Scholar]
- 172.Kim HR, Chae HJ, Thomas M, Miyazaki T, Monosov A, Monosov E, et al. Mammalian dap3 is an essential gene required for mitochondrial homeostasis in vivo and contributing to the extrinsic pathway for apoptosis. FASEB J. 2007;21(1):188–96. doi: 10.1096/fj.06-6283com. [DOI] [PubMed] [Google Scholar]
- 173.Miyazaki T, Reed JC. A GTP-binding adapter protein couples TRAIL receptors to apoptosis-inducing proteins. Nat Immunol. 2001;2(6):493–500. doi: 10.1038/88684. [DOI] [PubMed] [Google Scholar]
- 174.Berger T, Kretzler M. TRAIL-induced apoptosis is independent of the mitochondrial apoptosis mediator DAP3. Biochem Biophys Res Commun. 2002;297(4):880–4. doi: 10.1016/s0006-291x(02)02310-0. [DOI] [PubMed] [Google Scholar]
- 175.Berger T, Kretzler M. Interaction of DAP3 and FADD only after cellular disruption. Nat Immunol. 2002;3(1): 3–5. doi: 10.1038/ni0102-3b. [DOI] [PubMed] [Google Scholar]
- 176.Takeda S, Iwai A, Nakashima M, Fujikura D, Chiba S, Li HM, et al. LKB1 is crucial for TRAIL-mediated apoptosis induction in osteosarcoma. Anticancer Res. 2007;27(2):761–8. [PubMed] [Google Scholar]
- 177.Condorelli G, Vigliotta G, Cafieri A, Trencia A, Andalo P, Oriente F, et al. PED/PEA-15: An anti-apoptotic molecule that regulates FAS/TNFR1-induced apoptosis. Oncogene. 1999;18(31):4409–15. doi: 10.1038/sj.onc.1202831. [DOI] [PubMed] [Google Scholar]
- 178.Xiao C, Yang BF, Asadi N, Beguinot F, Hao C. Tumor necrosis factor-related apoptosis-inducing ligand-induced death- inducing signaling complex and its modulation by c-FLIP and PED/PEA-15 in glioma cells. J Biol Chem. 2002;277(28):25020–5. doi: 10.1074/jbc.M202946200. [DOI] [PubMed] [Google Scholar]
- 179.Renganathan H, Vaidyanathan H, Knapinska A, Ramos JW. Phosphorylation of PEA-15 switches its binding specificity from ERK/MAPK to FADD. Biochem J. 2005;390(Pt 3):729–35. doi: 10.1042/BJ20050378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Peacock JW, Palmer J, Fink D, Ip S, Pietras EM, Mui AL, et al. PTEN loss promotes mitochondrially dependent type II Fas-induced apoptosis via PEA-15. Mol Cell Biol. 2009;29(5):1222–34. doi: 10.1128/MCB.01660-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Trauzold A, Wermann H, Arlt A, Schutze S, Schafer H, Oestern S, et al. CD95 and TRAIL receptor-mediated activation of protein kinase C and NF-kappaB contributes to apoptosis resistance in ductal pancreatic adenocarcinoma cells. Oncogene. 2001;20(31):4258–69. doi: 10.1038/sj.onc.1204559. [DOI] [PubMed] [Google Scholar]
- 182.Harper N, Hughes MA, Farrow SN, Cohen GM, MacFarlane M. Protein kinase C modulates tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by targeting the apical events of death receptor signaling. J Biol Chem. 2003;278(45):44338–47. doi: 10.1074/jbc.M307376200. [DOI] [PubMed] [Google Scholar]
- 183.Meng XW, Heldebrant MP, Kaufmann SH. Phorbol 12-myristate 13-acetate inhibits death receptor-mediated apoptosis in Jurkat cells by disrupting recruitment of Fas-associated polypeptide with death domain. J Biol Chem. 2002;277(5):3776–83. doi: 10.1074/jbc.M107218200. [DOI] [PubMed] [Google Scholar]
- 184.Perfetti A, Oriente F, Iovino S, Alberobello AT, Barbagallo AP, Esposito I, et al. Phorbol esters induce intracellular accumulation of the anti-apoptotic protein PED/PEA-15 by preventing ubiquitinylation and proteasomal degradation. J Biol Chem. 2007;282(12):8648–57. doi: 10.1074/jbc.M608359200. [DOI] [PubMed] [Google Scholar]
- 185.Foley CJ, Freedman H, Choo SL, Onyskiw C, Fu NY, Yu VC. Dynamics of RASSF1A/MOAP-1 association with death receptors. Mol Cell Biol. 2008;28(14):4520–35. doi: 10.1128/MCB.02011-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Baksh S, Tommasi S, Fenton S, Yu VC, Martins LM, Pfeifer GP. The tumor suppressor RASSF1A and MAP-1 link death receptor signaling to Bax conformational change and cell death. Mol Cell. 2005;18(6):637–50. doi: 10.1016/j.molcel.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 187.Hesson LB, Cooper WN, Latif F. The role of RASSF1A methylation in cancer. Dis Markers. 2007;23(1-2):73–87. doi: 10.1155/2007/291538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kischkel FC, Lawrence DA, Chuntharapai A, Schow P, Kim KJ, Ashkenazi A. Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity. 2000;12(6):611–20. doi: 10.1016/s1074-7613(00)80212-5. [DOI] [PubMed] [Google Scholar]
- 189.Siegmund D, Klose S, Zhou D, Baumann B, Roder C, Kalthoff H, et al. Role of caspases in CD95L- and TRAIL-induced non-apoptotic signalling in pancreatic tumour cells. Cell Signal. 2007;19(6):1172–84. doi: 10.1016/j.cellsig.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 190.Wong WW, Gentle IE, Nachbur U, Anderton H, Vaux DL, Silke J. RIPK1 is not essential for TNFR1-induced activation of NF-kappaB. Cell Death Differ. 2010;17(3):482–7. doi: 10.1038/cdd.2009.178. [DOI] [PubMed] [Google Scholar]
- 191.Palacios C, Lopez-Perez AI, Lopez-Rivas A. Down-regulation of RIP expression by 17-dimethylaminoethylamino-17-demethoxygeldanamycin promotes TRAIL-induced apoptosis in breast tumor cells. Cancer Lett. 2010;287(2):207–15. doi: 10.1016/j.canlet.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 192.Wang P, Zhang J, Bellail A, Jiang W, Hugh J, Kneteman NM, et al. Inhibition of RIP and c-FLIP enhances TRAIL-induced apoptosis in pancreatic cancer cells. Cell Signal. 2007;19(11):2237–46. doi: 10.1016/j.cellsig.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 193.Roth W, Stenner-Liewen F, Pawlowski K, Godzik A, Reed JC. Identification and characterization of DEDD2, a death effector domain-containing protein. J Biol Chem. 2002;277(9):7501–8. doi: 10.1074/jbc.M110749200. [DOI] [PubMed] [Google Scholar]
- 194.Nam YJ, Mani K, Ashton AW, Peng CF, Krishnamurthy B, Hayakawa Y, et al. Inhibition of both the extrinsic and intrinsic death pathways through nonhomotypic death-fold interactions. Mol Cell. 2004;15(6):901–12. doi: 10.1016/j.molcel.2004.08.020. [DOI] [PubMed] [Google Scholar]