Abstract
Purpose
To determine the diagnostic utility of a frozen section biopsy in patients undergoing endoscopic submucosal dissection (ESD) for early gastric neoplasms with obscure margins even with chromoendoscopy using acetic acid and indigo carmine (AI chromoendoscopy).
Materials and Methods
The lateral spread of early gastric neoplasms was unclear even following AI chromoendoscopy in 38 patients who underwent ESD between June 2007 and May 2011. Frozen section biopsies were obtained by agreement of the degree of lateral spread between two endoscopists. Thus, frozen section biopsies were obtained from 23 patients (FBx group) and not in the other 15 patients (AI group).
Results
No significant differences were observed for size, histology, invasive depth, and location of lesions between the AI and FBx groups. No false positive or false negative results were observed in the frozen section diagnoses. Adenocarcinoma was revealed in three patients and tubular adenoma in one, thereby changing the delineation of lesion extent and achieving free lateral margins. The rates of free lateral resection margins and curative resection were significantly higher in the FBx group than those in the AI group.
Conclusions
Frozen section biopsy can help endoscopists perform more safe and accurate ESD in patients with early gastric neoplasm.
Keywords: Frozen section biopsy, Endoscopic submucosal dissection, Chromoendoscopy, Early gastric neoplasm
Introduction
It is important to accurately determine the lateral spreads of early gastric cancer in endoscopic submucosal dissection (ESD). Chromoendoscopy using a dilute indigo carmine dye for this purpose is often difficult because the dye simply contrasts the irregularity of the surface of the carcinoma. Chronic inflammation and atrophy caused by Helicobacter pylori also resulted in errors in the determination of lateral spread of carcinoma. Magnifying endoscopes with/without narrow band image (NBI) can be useful in accurate determination of the area of cancer lesions.(1-4) Acetic acid- enhanced magnifying endoscopy is useful for diagnosis of gastric adenocarcinoma because there are differences of mucosal whitening between normal gastric tissue and cancerous lesion.(5) However, it doesn't seem to be clinically useful method for this purpose because of technical difficulties when manipulating magnifying endoscopes. Recently new chromoendoscopy method using acetic acid and indigo carmine (AI chromoendoscopy) is superior to chromoendoscopy using indigo carmine alone, being useful in accurate determination of free lateral margins of early gastric cancer before ESD.(6-9) However, this method has not been fully evaluated, and diagnostic performance of this method depends on tumor pathology or tumor location.(8,9) If the tumor spreading is unclear in the precise endoscopic assessment, several biopsies are nowadays only one method and still essential to decide the tumor boarder before ESD. Frozen sections are intraoperative consultations used to establish a rapid histopathologic diagnosis or to assess surgical resection margins. Thus we hypothesized that frozen section biopsy during ESD helps the endoscopist to rapidly and accurately decide lateral resection margins in patients with early gastric neoplasms, which showed unclear lateral spread, despite of AI chromoendoscopy. We aimed to test hypotheses that frozen section biopsy provided additional benefit to gastric ESD for these lesions.
Materials and Methods
1. Patients
Four hundred twenty three patients, who underwent ESD for early gastric neoplasms in Institute for Digestive Research of Seoul Hospital of the Soonchunhyang University between June 2007 and May 2011, were enrolled in this study. The exclusion criteria for this study were the followings: 1) early gastric neoplasm with clear lateral margin to the naked eye (i.e. conventional endoscopy alone) 2) early gastric neoplasm with clear lateral margin clarified by AI chromoendoscopy method. The lateral extent of the lesions was easily clarified under observation with the naked eye in 93 patients (21.9%) of them. AI chromoendoscopy helped to clarify these in 292 patients (69.0%) of them. Thus 38 patients in whom the lateral extent of their lesions was unclear even with AI chromoendoscopy were included in this study (Fig. 1).
Fig. 1.
Study design. ESD = endoscopic submucosal dissection.
The patients chosen for our study protocol were divided into two groups according to concordance in the delineation of tumor extent between two endoscopists. An endoscopist, Cho JY had high level of expertise and experience in the field of gastric ESD - He performed ESD for EGC more than 1,300 cases. The other endoscopist was ESD assistant, who had 5-year ESD experience. If the expected lateral extents were not concordant between them after AI chromoendoscopy, frozen section biopsies were additionally performed. Thus 15 patients underwent gastric ESD after AI chromoendoscopy alone (AI group) and 23 patients underwent additional frozen section biopsies during gastric ESD (FBx group). This study was approved by the institutional review board of our hospital, and all patients provide written informed consent before entering the study.
2. Methods
In the AI method, mucus adhering to the mucosa was washed away as thoroughly as possible before examining the entire stomach. Then, commercial acetic acid (4.2%) diluted 4-fold using water was evenly sprinkled over and around the lesion. Indigo carmine (1%) was similarly sprinkled 30~60 s later.
Frozen section biopsies were performed when the lesions showed disconcordance in the assessment of lateral spread between two endoscopists after AI chromoendoscopy. Forceps (MAX2 Reusable Forceps, COOK® RBG-2.4-160, Limerick, Ireland) were placed 1 cm away from the expected lateral extent of the lesion, which was selected by the experienced endoscopist (Cho JY). We marked the outer surface of biopsy specimens with special ink (the Davidson Marking System® Bradely product Inc. Bloomington, USA) to provide a pathologist (Jin SY) orientation of specimens. Specimens were put in saline gauze and then carried to the pathology department. The frozen section report was available after 25 minutes of receiving tissue from the ESD room (Fig. 2).
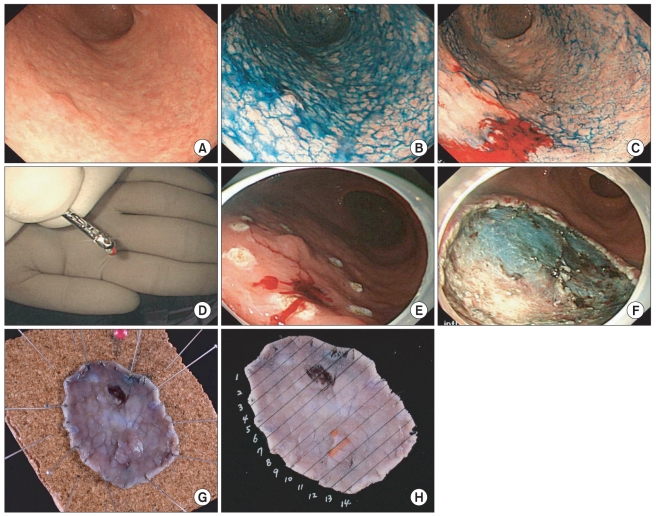
Fig. 2.
A representative case of gastric tubular adenoma in which frozen section biopsy was performed before endoscopic submucosal dissection. (A) A slightly elevated gastric lesion with unclear margin in white light was noticed in the lower third stomach. (B) The lateral spread of the lesion was still obscure despite of chromoendoscopy using acetic acid and indigo carmine. (C) A frozen section biopsy was performed 5mm distal to expected lateral extent of the lesion, which was selected by the experienced endoscopist (Cho JY), because the lesions showed disconcordance in the assessment of lateral spread between two endoscopists. (D) The outer surface of the specimen was marked with special ink (the Davidson Marking System® Bradely product Inc.) to provide a pathologist (Jin SY) orientation of specimens. (E) We marked several spots near the site, where frozen section biopsy was done, because frozen section revealed chronic gastritis with intestinal metaplasia. (F) En bloc resection of the lesion was performed. (G) A site of frozen section biopsy was observed in the resected specimen. (H) Pathologic mapping showed curatively resected tubular adenoma (The orange line indicated the lesion, tubular adenoma).
In markings of several spots outside the lateral margins using argon plasma coagulation, if frozen section revealed no adenocarcinoma or tubular adenoma, we marked several spots near the site (3~5 mm away from the biopsy site), where forceps were placed in purpose of frozen section biopsy. In case of the results of adenocarcinoma or tubular adenoma on the frozen section, we performed additional frozen section biopsy in the site far away from the site of previous frozen section biopsy.
Sodium alginate (1% sodium alginate and normal saline; Taejoon Pharmaceutical, Seoul, Korea) was then injected into the submucosal layer to lift the mucosa. A circumferential mucosal incision 5~10 mm away from the frozen biopsy site was made around the lesion using the IT knife (Olympus Optical Co Ltd, Tokyo, Japan), Flex knife (Olympus), or Fork knife (Kachu Technology Co., Seoul, Korea). Submucosal dissection was performed for the curative removal of the lesions using the IT knife or Fork knife. High frequency generators (ICC200 or VIO 300D; ERBE Elekromedizin, Tubingen, Germany) were used during marking, incision of the gastric mucosa and exfoliation of the gastric submucosa. ESD was performed by an experienced endoscopist (JY Cho).
All patients were sedated by intravenous injection of 5~7.5 mg of midazolam (Roche Korea Co., Ltd, Seoul, Korea). And 1 mg of midazolam was additionally given for conscious sedation as needed throughout the procedure.
3. Histological assessment
These frozen biopsies were reviewed by pathologist Jin SY, who has had lots of experiences of stomach cancer pathology and reviewed ESD for EGC more than 1,300 cases. The excised specimens were sectioned perpendicularly at 2 mm intervals after fixation in formalin. Histologic type, depth of invasion, lateral or vertical margins and lymphovascular invasion were evaluated in each slice according to the Japanese Classification of Gastric Carcinoma.(10) Curative resection was defined as when the removal was achieved with tumor-free lateral and vertical margins, and there was no submucosal invasion deeper than 500 µm from muscularis mucosae and no lymphatic and vascular involvement. Non-curative resection was defined as one that did not meet the curative criteria.
4. End-points
Primary endpoint is free lateral margins rate. And the secondary endpoints are free vertical margins rate and curative resection rate compared between AI group and FBx group.
5. Statistics
Statistical analysis was performed using the statistical software SPSS ver. 12.0 (SPSS, Chicago, IL, USA). Data are presented as median with range. Categorical parameters were compared using chi-square and Fisher's exact test. A P-value<0.05 was considered significant. We computed effect size using G×Power 3.1.3 because of the small sample size; alpha of 5%, Power of 80%, and the Medium Effect size of 0.5.
Results
1. Clinical characteristics of the patients
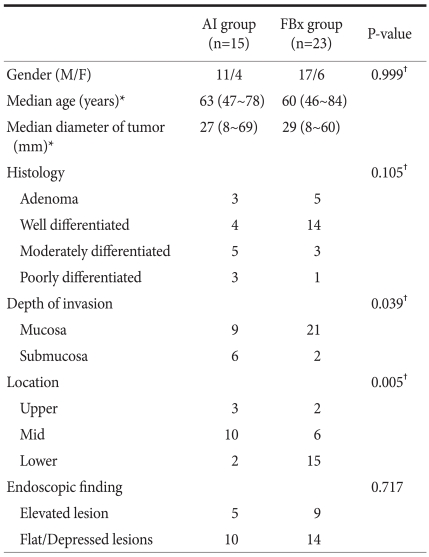
The clinical characteristics of the patients are summarized in Table 1; Thirty eight patients were enrolled in this study; thirty eight are higher than 32 which is the recommend sample size in G×Power. The location of early gastric neoplasm was classified into the upper, middle and lower thirds of the stomach. The endoscopic finding of early gastric neoplasm was divided into elevated type and flat/depressed type.
Table 1.
Clinical characteristics of the patients
*Median with range; †Fisher's exact test.
There were no significant differences in gender ratio, histology, and endoscopic findings between AI group and FBx group. But there were significant difference in depth of invasion, location between these two groups (P-value 0.039, 0.005 respectively).
2. The results of frozen section biopsies
The mean number of frozen section biopsy specimen per each patient was 1.17.
The overall results of frozen section were as follows; chronic gastritis with intestinal metaplasia (n=21), adenocarcinoma (n=3), tubular adenoma (n=1), and chronic gastritis with lymphoid hyperplasia (n=2). There was no false positive diagnosis in the results of frozen section, which means in case of the results of adenocarcinoma or tubular adenoma on the frozen section, there was evidence of adenocarcinoma or tubular adenoma in the biopsy site with ESD specimen. There was also no false negative diagnosis in them, which means if frozen section revealed no adenocarcinoma or tubular adenoma on the frozen section, there was no evidence of malignancy in the biopsy site with ESD specimen. Frozen section biopsy allowed us to make a change in decision of lateral margin in 4 patients, in whom these frozen section results revealed adenocarcinoma or tubular adenoma, thus achieved free lateral margins in all the patients (Fig. 3).
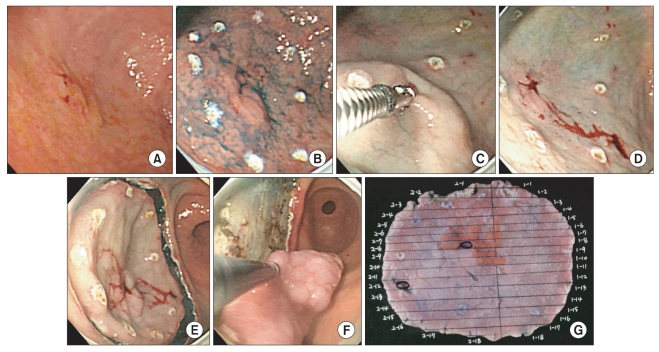
Fig. 3.
A representative case of adenocarcinoma in which frozen section result revealed adenocarcinoma, thus achieved free lateral margin. (A) A slightly elevated gastric lesion with unclear margin in white light was noticed in antrum. (B) The lateral spread of the lesion was still obscure despite of chromoendoscopy using acetic acid and indigo carmine. (C) A frozen section biopsy was performed 3mm away from the expected lateral extent of the lesion, which was selected by the experienced endoscopist (Cho JY), because the lesions showed disconcordance in the assessment of lateral spread between two endoscopists. (D) We marked several spots about 1 cm away from the site, where frozen section biopsy was done, because frozen section revealed adenocarcinoma, well differenciated. The second biopsy revealed chronic gastritis with focal intestinal metaplasia. (E) A circumferential mucosal incision was made around the lesion including frozen section biopsy sites. (F) En bloc resection of the lesion was performed. (G) Pathologic mapping showed curatively resected adenocarcinoma (The orange line indicated the lesion - adenocarcinoma. And blue circles indicated frozen section biopsy sites).
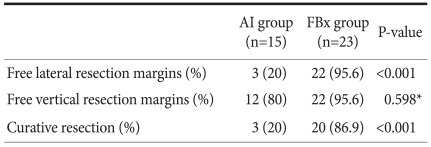
3. Comparisons of ESD outcomes
The comparisons of ESD outcomes between the two groups are summarized in Table 2. The rate of free vertical resection margins was not significantly different between AI group and FBx group, but the rates of free lateral resection margins and curative resection were significantly higher in FBx group compared than AI group.
Table 2.
Comparison of ESD outcomes
*Fisher's exact test. ESD = endoscopic submucosal dissection.
Discussion
The introduction of AI chromoendoscopy and virtual chromoendoscopy, like NBI with magnifying endoscopy, has allowed better endoscopic assessment of early gastric neoplasm.(1-4,6-9) Despite these recent major advances in endoscopic evaluation for early gastric neoplasm, there is no method that is able to accurately determine tumor extent in all patients with early gastric neoplasm. The present study indicates that frozen section biopsy can be a simple and useful method for achieving free lateral resection margins especially in ESD of early gastric neoplasms, which showed obscure margins despite of AI chromoendoscopy.
Magnifying endoscopy with/without NBI has several important limitations; First, these methods require a great amount of experience, skill and time on the part of the endoscopist. Second, these methods have less degree of accuracy in assessment of undifferentiated gastric cancer.(4) Third, these methods are not widely available. Biopsy sampling is ultimately mandatory for reliable assessment of these methods especially in patients with undifferentiated gastric cancer.(4,11)
The lateral extent clarification rate of AI chromoendoscopy depends on the mucus of the lesion area, the concentration of acetic acid, tumor pathology and tumor location.(8,9) Mucus adhesion near the lesion resulted in decreased contrast between the lesion and normal mucosa. Mucus was more secreted from the gastric mucosa by 1.5 % compared with 0.6% acetic acid.(9) Thus 0.6% acetic acid was usually used in the AI chromoendoscopy, but the optimal concentration of acetic acid has not still been validated. Lateral extent clarification rate in diffuse (undifferentiated) type cancer was 76.2% that was significantly lower than in intestinal (differentiated) type cancer. This difference was thought to be attributable to subepithelial spreading, which is often observed in the diffuse type cancer lesions. The diagnostic performance in the antrum or lower body was 89% and 70%, respectively, which was significantly lower than those in the upper body. In the present study, there were lesions with unclear lateral spread even on AI chromoendoscopy in approximately 17%, which was slightly higher than the earlier studies. It is presumed that this resulted from the differences of aforementioned factors such as tumor location or tumor pathology.
Frozen section diagnosis is known for a highly accurate method of diagnosis, with an overall accuracy of 97%.(12,13) In the present study, concordance between the frozen section diagnosis and the final histolopathologic diagnosis was also exceedingly very high. Frozen section biopsy allowed us to make a change in decision of lateral margin in 4 patients, in whom these frozen section results revealed adenocarcinoma or tubular adenoma, thus achieved free lateral margins in all the patients. We could make a rapid therapeutic decision in determination of lateral extent in all patients after short frozen section turnaround time of 25 minutes. However, potentially poorer slide quality and rapid interpretation of intraoperative frozen sections inevitably contribute to the difference in diagnostic accuracy of this method compared with subsequent paraffin sections. The interpretation errors of intraoperative frozen section may mainly result from sampling of nonrepresentative tissue specimens and these also occurred from technical problems in sectioning or staining, misinterpretation, inadequate clinical information, and labeling errors.(13)
However, this study was limited to a single center, small sample size, potentially limiting generalizability of this result. The interpretation problems of frozen section biopsy may result from sampling errors, when this method was performed in the patients with early gastric neoplasm, which showed obscure lateral spread despite of AI chromoendoscopy. Taking greater number of frozen section biopsy can be needed to minimize sampling errors in some cases. Endoscopic expertise, magnifying endoscopy with/without NBI might also be helpful for reduction of sampling errors in frozen section biopsy. In Table 2, there would be a selection bias in AI group and FBx group, which free lateral margin rate was significantly lower in AI group than FBx group (20% vs 95.6%).
In conclusion, frozen section biopsy can help endoscopists to perform more safe and accurate ESD in patients with early gastric neoplasm, which was not easily delineated by AI chromoendoscopy.
References
- 1.Nakayoshi T, Tajiri H, Matsuda K, Kaise M, Ikegami M, Sasaki H. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: correlation of vascular pattern with histopathology (including video) Endoscopy. 2004;36:1080–1084. doi: 10.1055/s-2004-825961. [DOI] [PubMed] [Google Scholar]
- 2.Tamai N, Kaise M, Nakayoshi T, Katoh M, Sumiyama K, Gohda K, et al. Clinical and endoscopic characterization of depressed gastric adenoma. Endoscopy. 2006;38:391–394. doi: 10.1055/s-2005-921207. [DOI] [PubMed] [Google Scholar]
- 3.Otsuka Y, Niwa Y, Ohmiya N, Ando N, Ohashi A, Hirooka Y, et al. Usefulness of magnifying endoscopy in the diagnosis of early gastric cancer. Endoscopy. 2004;36:165–169. doi: 10.1055/s-2004-814184. [DOI] [PubMed] [Google Scholar]
- 4.Yao K, Anagnostopoulos GK, Ragunath K. Magnifying endoscopy for diagnosing and delineating early gastric cancer. Endoscopy. 2009;41:462–467. doi: 10.1055/s-0029-1214594. [DOI] [PubMed] [Google Scholar]
- 5.Yagi K, Aruga Y, Nakamura A, Sekine A, Umezu H. The study of dynamic chemical magnifying endoscopy in gastric neoplasia. Gastrointest Endosc. 2005;62:963–969. doi: 10.1016/j.gie.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 6.Sakai Y, Eto R, Kasanuki J, Kondo F, Kato K, Arai M, et al. Chromoendoscopy with indigo carmine dye added to acetic acid in the diagnosis of gastric neoplasia: a prospective comparative study. Gastrointest Endosc. 2008;68:635–641. doi: 10.1016/j.gie.2008.03.1065. [DOI] [PubMed] [Google Scholar]
- 7.Yamashita H, Kitayama J, Ishigami H, Yamada J, Miyato H, Kaisaki S, et al. Endoscopic instillation of indigo carmine dye with acetic acid enables the visualization of distinct margin of superficial gastric lesion; Usefulness in endoscopic treatment and diagnosis of gastric cancer. Dig Liver Dis. 2007;39:389–391. doi: 10.1016/j.dld.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Iizuka T, Kikuchi D, Hoteya S, Yahagi N. The acetic acid + indigocarmine method in the delineation of gastric cancer. J Gastroenterol Hepatol. 2008;23:1358–1361. doi: 10.1111/j.1440-1746.2008.05528.x. [DOI] [PubMed] [Google Scholar]
- 9.Kawahara Y, Takenaka R, Okada H, Kawano S, Inoue M, Tsuzuki T, et al. Novel chromoendoscopic method using an acetic acid-indigocarmine mixture for diagnostic accuracy in delineating the margin of early gastric cancers. Dig Endosc. 2009;21:14–19. doi: 10.1111/j.1443-1661.2008.00824.x. [DOI] [PubMed] [Google Scholar]
- 10.Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition - Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 11.Kuznetsov K, Lambert R, Rey JF. Narrow-band imaging: potential and limitations. Endoscopy. 2006;38:76–81. doi: 10.1055/s-2005-921114. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman Z, Lew S, Griffel B, Dinbar A. Frozen-section diagnosis in surgical pathology. A prospective analysis of 526 frozen sections. Cancer. 1986;57:377–379. doi: 10.1002/1097-0142(19860115)57:2<377::aid-cncr2820570231>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 13.Howanitz PJ, Hoffman GG, Zarbo RJ. The accuracy of frozen-section diagnoses in 34 hospitals. Arch Pathol Lab Med. 1990;114:355–359. [PubMed] [Google Scholar]