Abstract
Purpose
We performed this study to evaluate the clinical presentation as well as the proper surgical intervention for ovarian metastasis from gastric cancers and these tumors were identified during postoperative follow-up. This will help establish the optimal strategy for improving the survival of patients with this entity.
Materials and Methods
22 patients (3.2%) with ovarian metastasis were noted when performing a retrospective chart review of (693) females patients who had undergone a resection for gastric cancer between 1981 and 2008. The covariates used for the survival analysis were the patient age at the time of ovarian relapse, the size of the tumor, the initial TNM stage of the gastric cancer, the interval to metastasis and the presence of gross residual disease after treatment for Krukenberg tumor. The cumulative survival curves for the patient groups were calculated with the Kaplan-Meier method and they were compared by means of the Log-Rank test.
Results
The average age of the patients was 48.6 years (range: 24 to 78 years) and the average survival time of the 22 patients was 18.8 months (the estimated 3-year survival rate was 15.8%) with a range of 2 to 59 months after the diagnosis of Krukenberg tumor. The survival rate for patients without gross residual disease was longer than that of the patients with gross residual disease (P=0.0003). In contrast, patient age, the size of ovarian tumor, the initial stage of gastric adenocarcinoma, the interval to metastasis and adjuvant chemotherapy were not prognostic indicators for survival after the development of ovarian metastasis.
Conclusions
Early diagnosis and complete resection are the only possible hope to improve survival. As the 3-year survival rate after resection of Krukenberg tumor is 15.8%, it seems worthwhile to consider performing tumorectomy as the second cytoreduction.
Keywords: Krukenberg tumor, Stomach neoplasms, Survival
Introduction
The prognosis of ovarian metastases or Krukenberg tumor is known to be poor. Different from the West, it is not rare disease in Korea as it is tumor that has metastasized from gastric cancer and gastric cancer is prevalent in Korea. Krukenberg tumor occur in 0.3~6.7% of the operated gastric cancer patients, and its incidence is much higher in the autopsies of gastric cancer patients (33~41%). (1,2) Numerous studies on Krukenberg tumor have been reported in Korea and other countries, nonetheless, most of them are on synchronous Krukenberg tumors, and there are relatively few studies on the incidence and treatment outcomes of metachronous Krukenberg tumor that developed during the postsurgical follow-up observation period. It has been reported that the incidence of Krukenberg tumor detected during the follow-up observation period after radical gastric resection or the second surgery is 3~4.4%, the median survival time is 12.4~17 months,(3,4) and the inci dence and prognosis are slightly different depending on the reports. Therefore, we analyzed the incidence of Krukenberg tumor that developed during the follow-up observation period after the resection of the primary gastric cancer, and the clinicopathological features of the Krukenberg tumors were confirmed by the second surgery. The treatment methods, the survival rate and the factors mediating effects on them were examined.
Materials and Methods
Among the 41 patients who were pathologically diagnosed with Krukenberg tumor after the second surgery at the Department of Surgery, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine during 27 years from 1981 to 2008, this study was conducted on 22 patients who were considered to have Krukenberg tumor that metastasized from gastric cancer, based on the history of surgery for gastric cancer. First, the incidence of Krukenberg tumor that originated from gastric cancer after gastrectomy was examined by assessing the number of female patients who underwent surgery for gastric cancer during the same period. In addition, the age of onset of the 22 Krukenberg tumor patients, the menopause status, the tumor markers, the tumor node metastasis (TNM) disease stage according to the American Joint Committee on Cancer (AJCC) 6th edition(5) and the presence of factors that could mediate effects on the survival rate were examined by retrospectively assessing the histopathological grade of cell differentiation, the interval from the first surgery to the development of tumor, the surgical methods for Krukenberg tumor, whether or not more than 4 cycles of adjuvant chemotherapy was performed and the survival rate after the second surgery, based on the medical records. The follow-up observation of the patients was performed using the database at the outpatient department or by telephone follow-ups, and the mean follow-up period was 25.2 months. The survival rate of the Krukenberg tumor patients was analyzed by the Kaplan-Meier method and the Log-Rank test, and P-values<0.05 were determined to be significant. All the statistical analyses were performed using the SPSS for Windows 12.0.
Results
The Krukenberg tumor patients were confirmed by the second surgery after the primary surgery for gastric cancer, and 22 patients were thought to have ovarian tumor that metastasized from gastric cancer. In regard to the primary cancer of the 41 Krukenberg tumor patients diagnosed after the second surgery during the same period, 53.7% of them had gastric cancer. In addition, there were 693 female patients who received gastrectomy after the diagnosis of gastric cancer during the same period and so the incidence of Krukenberg tumor that developed during the follow-up period after gastrectomy was 3.2%.
As for the major symptoms of Krukenberg tumor, lower abdominal discomfort and intermittent abdominal pain were noted in 10 cases (45.5%), no symptoms other than the detection of an ovarian mass by abdominal ultrasonography during regular physical check-ups was noted in 6 cases (27.3%), palpation of a mass in the lower abdomen was noted in 4 cases (18.2%) and abdominal distention associated with ascites, abscess within the pelvis and fever was noted in one case each (4.5%). As for the diagnostic method, except for 1 patient with an intrapelvic abscess, 21 cases (95.5%) were diagnosed with an ovarian mass that was suspicious to be malignant by the abdominal sonography and abdominal CT performed prior to the second surgery. These patients and their masses were subsequently definitely diagnosed by examining the frozen sections during surgery.
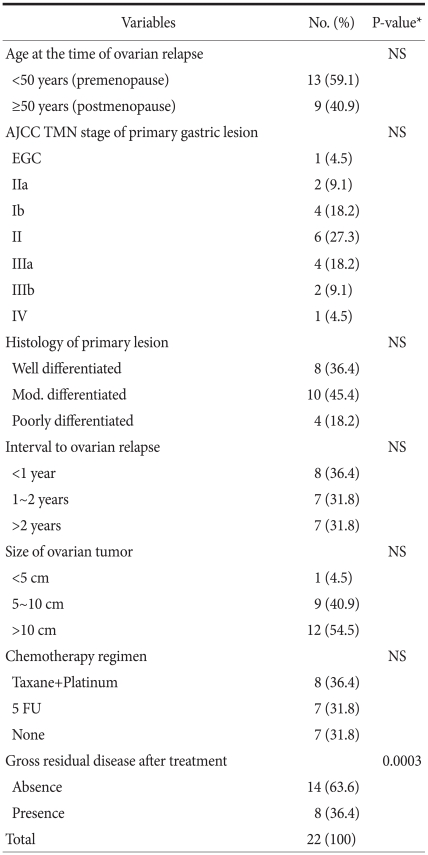
The interval from the first surgery to the resurgery for Krukenberg tumor was on average 16.5 months (range: 3~34 months). The mean age of the patients was 48.6 years (range: 24~78 years), 13 patients were premenopausal women (59.1%) and there were 9 postmenopausal women (40.9%). During the first surgery, concerning the disease stage of the primary tumor according to the AJCC TNM disease stage classification, 6th edition, there was 1 case of stage 0 (4.5%), there were 2 cases of stage IA (9.1%), there were 4 cases of stage IB (18.2%), there were 6 cases of stage II (27.3%), there were 4 cases of stage IIIA (18.2%) and 1 case of stage IV (4.5%). The disease stage II was the most prevalent. In addition, with regard to the depth of lesions, there was 1 case of Tis (4.5%), there were 7 cases of T1 (31.8%), there were 6 case of T2a/2b (27.3%), there were 7 cases of T3 (73.8%) and 1 case of T4 (4.5%). Regarding lymphovascular involvement, there were 5 cases of N0 (22.7%), 9 cases of N1 (40.9%) and 1 case of (4.5%). Regarding the histologic grade, 10 patients had moderately differentiated tumor (45.5%) and 8 patients had well differentiated tumor (36.4%).
As for the clinical characteristics of Krukenberg tumor, the average long axis was 12.9 cm (range: 4.6~18.0 cm), 18 patients had bilateral tumors (81.8%) and 4 patients had unilateral tumor (18.2%). As the recurrence patterns, 11 patients had tumor limited to the ovary (50.0%), 5 patients recurred within the pelvic cavity (22.7%) and 6 patients' tumor had metastasized to organs other than the abdomen and the pelvis (27.3%). Concerning surgical treatments, laparotomy was performed on all 22 patients, and bilateral oophorectomy was performed on all 22 patients. Hysterectomy was performed simultaneously on 10 patients, including 5 patients for whom invasion to the uterus was suspected. There were 14 patients without macroscopic residual tumors after tumor resection (63.6%). Including the 3 patients (13.6%) whose tumor had metastasized to the great omentum at the time of the second surgery, 8 (36.4%) patients' tumor metastasized to the intraperitoneal peritoneum and thus the complete resection of the metastasized ovarian lesions was difficult and only palliative resection was performed, and this resulted in the presence of macroscopic residual cancer cells. After the second surgery, more than 4 cycles chemotherapy was performed for 15 patents (68.2%). Eight patients (36.4%) were treated with the combination of Taxane and platinum and 7 patients (31.8%) were treated with 5-FU monotherapy.
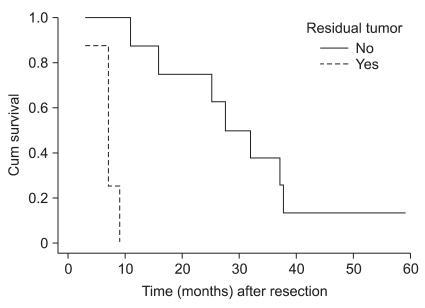
The average postsurgical survival time of all the patients was 18.8 months (range: 2.0~59.0 months, including the 2 surviving patients), the 3-year survival rate was 15.8%, the survival period of the 16 patients (R0) for whom tumor resection could be performed without leaving residual tumors was on average 23.7 months, and it was significantly higher than the 6.0 months average survival time of the 8 patients (R2) for whom only palliative resection was performed because of the invasion to the adjacent pelvis, etc. (P=0.0003) (Fig. 1). However, the age of the patient at the time of the diagnosis of tumors, the menopausal status, the AJCC TMN disease stage of the primary tumor, the grade of differentiation of the tumor cells, the interval from the first surgery to tumor development, the tumor size and the status of chemotherapy were not associated with the survival rate (Table 1).
Fig. 1.
Overall survival curves for the patients with Krukenberg tumor from gastric cancer. The patients without gross residual disease survived longer than the patients with gross residual disease (P=0.0003).
Table 1.
Factors associated with survival aft er the development of Krukenberg Tumor from gastric cancers treated by resection
NS = not significant; AJCC = American Joint Commitee on Cancer; TMN = tumor node metastasis; EGC = early gastric cancer; FU = fluorouracil. *P>0.05.
Among all 22 patients, 9 patients were tested for two tumor markers, serum CEA and CA-125, which may be of help to make an early diagnosis of Krukenberg tumor. Of the 9 patients, the CA-125 level was shown to be higher than normal (normal: lower than 35 U/ml) in 8 patients out of the 9 patients (88.9%), and the average value was 45.5±25.3 U/ml. The CEA was elevated in 1 patient (11.1%) to 16.2 ng/ml, which was slightly high, and the rest of the patients were within the normal range (normal: lower than 10 ng/ml).
Discussion
The metastasis mechanism of Krukenberg tumor is particular, for example, it has been reported to develop even after the radical resection of early gastric cancer,(6) and so we studied the clinical characteristics, including the metastasis patterns. It has been reported that Krukenberg tumor is rare and its prognosis is poor, yet the studies performed in the West on Krukenberg tumor were on patients with primary colon cancer in most cases. Thus, it was considered that the incidence and prognosis in Korea, where gastric cancer is more prevalent, would be different. In our study, statistical analysis was not performed on all the 693 gastric cancer patients treated during the same period and so comparison with all the patients who had undergone a curative gastric resection for stomach cancer was difficult. It was particular that 6 patients (27.3%) belonged to the AJCC disease stage 2, which was the most prevalent stage. Although this may be due to several reasons, we can speculated that this due to the effect of mass screening programs that have been widely performed recently, and so the number of patients lower than stage 2 became abundant. Indeed, according to the result of studies recently performed by Nam et al.(7) at the National Cancer Center, of the 18,414 patients who received physical check-ups, gastric cancer was detected in 81 patients (0.44%). Among these 81 patients, 80% had early gastric cancer, which shows that the disease stage of the diagnosed gastric cancer has become substantially lower.
Krukenberg tumor was reported for the first time in 1896 by Freidrich Ernst Krukenberg. Afterward, the definition of the disease and the diagnostic criteria were very confusing. Initially, all metastatic ovarian cancers were termed as Krukenberg tumor. Since the confusion was induced by the term, Novak and Gray(8) in 1938 proposed the diagnostic criteria that classical Krukenberg tumor should composed of signet-ring cell carcinoma within a dense fibroblastic stroma in the ovary. The WHO(9) in 1973 established the diagnostic standard, which is the diagnostic standard that has been applied until now. Almost all Krukenberg tumors are the diffused type according to the Luaren's classification of primary gastric cancer. Nevertheless, it has been reported that although it is a very rare tumor, this tumor is almost always associated with disseminated disease like the intestinal type adenocarcinoma of gastric cancer, which has metastasizes to the ovary.(10) Involvement of ovary by intestinal type gastric carcinoma compared with that on signet-ring cell carcinoma that result in the Krukenberg tumor may be confused with primary ovarian mucinous neoplasm, but such cases were not detected at our series.
Several mechanism have been suggested to explain the progression and recurrence pathway of gastric cancer such as lymphatic spread, hematogenous spread, direct invasion, peritoneal seeding, etc. Among them, the incidence of hematogenous recurrence is highest. Although Krukenberg tumor also may be induced by complex mechanisms, as a factor for metastasis, lymph node metastasis is considered to be the most potent risk factor for recurrence.(11,12) Therefore, if the national cancer screening program, including esophago-gastroscopic screening, becomes active and so the diagnosis of early gastric cancer is increased and appropriate treatments could be administered early, the incidence of Krukenberg tumor also may be changed, and the distribution of the primary disease stage may become different.
It has been reported that the incidence of Krukenberg tumor is approximately 30~40% of all metastatic ovarian cancers, it is a rare tumor (approximately 2% of all ovarian cancers), and the prognosis is awful to the level that all the patients die within 1 year after diagnosis.(13) In the east Asia where the incidence of gastric cancer is high, Krukenberg tumor has been shown to occur relatively frequently as approximately 4.4~6.7% within 3 years after surgery in the female patients who have received gastrectomy.(1,3,11) In this study, similarly, although it was difficult to infer the incidence of metachronous tumor during the same period, during the relatively long follow-up observation (27 years), approximately 3.2% of the patients who received gastrectomy after the diagnosis of gastric cancer developed Krukenberg tumor. The primary lesion of Krukenberg tumor is in the order of the colon, pancreas and breast in the West, and the prognosis varies depending on the primary lesion. On the other hand, in the east Asia, the cause is gastric cancer in many cases, followed by cancer of the colon, breast, small intestine, gallbladder and bladder.(13-15)
Most patients with Krukenberg tumors present with symtoms related to ovarian tumors including abdominal pain and the tumor can be palpated in more than 50% of cases. Rarely, patients may present with abnormal uterine bleeding.(16) In such situation, early clinical symptoms of Krukenberg tumors are vague and the primary gastric cancer may not manifest itself until later in most cases. So making an early diagnosis is difficult and this results in often detecting inoperable cases. In our study, unexpectedly, there was only one patients case in whom typical ascites was detected by a physical examination, and other than that, most early symptoms were lower abdominal pain, discomfort, etc. and they were considered to be nonspecific symptoms, so making the early diagnosis was difficult, and this may lower the postsurgical survival rate. Therefore, the most important factor for allowing the complete resection of tumors may be physicians being aware of the possibility of Krukenberg tumor. In several studies, it has been reported that premenopausal Krukenberg tumor patients were more prevalent and the tumor was multicentric in most cases.(17) In our study, similarly, there were 13 premenopausal patients (59.1%) and 18 patients (81.8%) with bilateral tumor. Thus, additional tests must be performed on the bilateral ovarian tumors detected in premenopausal women after they have undergone surgery for gastric cancer. Koyama et al.(18) have reported that even if ascites, abdominal pain or other symptoms are not detected by physical examination, for patients with the past history of surgery for gastric cancer or other digestive tract cancer, the presence or absence of ascites should be assessed by abdominal ultrasonography or CT, and efforts should be made to diagnose ovarian cancer early. In addition, Krukenberg tumor should be always be suspected if on CT the tumor is bilateral and it is characterized by lobulated solid tumors, and enhancement with contrast medium is noticeable in the ovary. So, only an early diagnosis that allows the complete resection of tumor can improve the survival rate of Krukenberg tumor, and McGill et al.(19) stated that gynecologists should comprehensively examine the upper abdominal area during surgery, and surgeons should comprehensively examine the lower abdominal area during surgery. This should be encouraged now that endoscopic submucosal dissection, laparoscopic gastrectomy and robotic surgery are becoming more common.
Concerning the prognosis of Krukenberg tumor, the radical resection of Krukenberg tumor without leaving macroscopic residual lesions is difficult in many cases, and it has been reported that the median duration of survival ranges from 7.7 to 14.0 months.(1,3,13) In our study, the average survival rate was 18.8 months and this includes 2 patients who are still alive 22 and 59 months after surgery, the overall 3-year survival rate was approximately 15.7% and the prognosis was not good, but it was better than that of other reports. The reasons that the survival rate in our study were slightly high may be diverse, yet it may be due to the proportion of patients incidentally detected during physical examination. So an early diagnosis was possible for 6 patients, and the patients for whom complete resection of Krukenberg tumor was difficult were transferred to other hospitals and so there were relatively fewer patients with the incomplete resection of tumors. Yada-Hashimoto et al.(2) have reported that even for patients with metastatic ovarian cancer originated from tumors in non-intrapelvic organs, including Krukenberg tumor, a postsurgical 5-year survival rate higher than 19% could be anticipated. They also mentioned that if the metastatic ovarian cancer is completely resected, then better results than anticipated could be obtained, so they emphasized aggressive resection. Of course, for all metachronous Krukenberg tumors, the feasibility of resection may be determined according to the extraovarian metastasis at the time of ovarian metasectomy. The survival rate for our patients who underwent aggressive tumor resection without residual cancer was on average 23.7 months, and this was significantly higher than the 6.0 months for the average survival period of the patients who underwent palliative resection because of tumor invasion to other organs and the adjacent pelvis. This suggests that only an early diagnosis and efforts to remove tumor-burdened intrapelvic organs could increase the survival period. In regard to the factors exerting effects on the postsurgical survival rate, most studies have reported that the age of patients at the time of the diagnosis of Krukenberg tumor, the menopause status, the primary tumor site, the grade of differentiation of the tumor cells and the disease stage were not associated with the postsurgical survival rate, and similar to other malignant tumors, only aggressive resection could increase the survival rate.(1,3,15) It has recently been reported that postsurgical adjuvant chemoradiation therapy had a major impact on the local recurrence in patients with resectable gastric cancer.(20) However, the effect of adjuvant chemoradiation therapy after the resection of Krukenberg tumor is still controversial. Numerous studies have reported that it did not mediate effects on the survival rate.(1,21) In our study, similarly, the survival rate of the 15 patients who underwent more than 4 cycles of the therapy was not different from the 15 patients who did not receive adjuvant therapies, and the survival rate was also not associated with the types of chemotherapeutic agents.
When performing colectomy for colorectal cancer, for women older than 40 years, attempts to perform prophylactic bilateral ovariectomy have been made in some cases,(22) but this is still controversial for the patients who have undergone gastrectomy. Yamamoto et al.(23) stated that prophylactic ovariectomy could be considered if it is not possible to resect the tumor from the ovary without macroscopic abnormalities and if the CEA value is high after washing the peritoneal cavity with approximately 100 ml saline. The feasibility of the early detection of Krukenberg tumor by tumor markers has been reported in several studies, yet most of the markers have been shown to be non-specific. It has been reported that the rapid elevation of the CA-125 level has been widely applied as a marker of ovarian cancer and this is of help to make the early diagnosis of Krukenberg tumor.(2) In our study, there were only 2 patients (9.1%) with the rapid increase of the CA-125 level to higher than 100 U/ml and the percentage of patients with a higher than average value were only 45.5%, and so this marker was not of great help. As compared with the observation that the CEA and CA 19-9 levels were not changed in most patients, among 9 patients for whom the measurement of CA-125 was performed, it was shown to be elevated in 8 patients. Since CA-125 was helpful to predict the development of Krukenberg tumor after surgery for cancer in the digestive tract, it may be better to test the CA-125 tumor marker during the follow-up observation period.
In summary, the incidence of the metastatic ovarian cancer that developed during the follow-up observation period after gastrectomy (Krukenberg tumor) was 3.2%. Even if surgery was performed, the mean survival period was 18.8 months, and the prognosis was poor. Nonetheless, the 3-year survival rate was 15.8%, and so attempts to resect tumors as the second tumor reduction seem worthwhile. Although several factors may mediate effects on the survival rate, the most important factor that affects the survival rate is the complete resection of tumors. Yet the recurrence patterns are important, and aggressive efforts not to leave residual cancer cells at the second surgery are required. In addition, for all female gastric cancer patients who have undergone a gastrectomy and especially young premenopausal women, a suspicion of Krukenberg tumor can help make an early diagnosis by comprehensively assessing the CA-125 level and other tumor markers, as well as by performing lower abdominal ultrasonography.
Footnotes
This study was sponsored by Hyoseok Research Fund.
References
- 1.Wang J, Shi YK, Wu LY, Wang JW, Yang S, Yang JL, et al. Prognostic factors for ovarian metastases from primary gastric cancer. Int J Gynecol Cancer. 2008;18:825–832. doi: 10.1111/j.1525-1438.2007.01078.x. [DOI] [PubMed] [Google Scholar]
- 2.Yada-Hashimoto N, Yamamoto T, Kamiura S, Seino H, Ohira H, Sawai K, et al. Metastatic ovarian tumors: a review of 64 cases. Gynecol Oncol. 2003;89:314–317. doi: 10.1016/s0090-8258(03)00075-1. [DOI] [PubMed] [Google Scholar]
- 3.Cheong JH, Hyung WJ, Chen J, Kim J, Choi SH, Noh SH. Survival benefit of metastasectomy for Krukenberg tumors from gastric cancer. Gynecol Oncol. 2004;94:477–482. doi: 10.1016/j.ygyno.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Kiyokawa T, Young RH, Scully RE. Krukenberg tumors of the ovary: a clinicopathologic analysis of 120 cases with emphasis on their variable pathologic manifestations. Am J Surg Pathol. 2006;30:277–299. doi: 10.1097/01.pas.0000190787.85024.cb. [DOI] [PubMed] [Google Scholar]
- 5.Sobin LH, Wittekind C, editors. TNM Classification of Malignant Tumours (UICC) 6th ed. New York: Wiley-Liss; 2002. pp. 65–68. [Google Scholar]
- 6.Kakushima N, Kamoshida T, Hirai S, Hotta S, Hirayama T, Yamada J, et al. Early gastric cancer with Krukenberg tumor and review of cases of intramucosal gastric cancers with Krukenberg tumor. J Gastroenterol. 2003;38:1176–1180. doi: 10.1007/s00535-003-1227-3. [DOI] [PubMed] [Google Scholar]
- 7.Nam SY, Choi IJ, Park KW, Kim CG, Lee JY, Kook MC, et al. Effect of repeated endoscopic screening on the incidence and treatment of gastric cancer in health screenees. Eur J Gastroenterol Hepatol. 2009;21:855–860. doi: 10.1097/MEG.0b013e328318ed42. [DOI] [PubMed] [Google Scholar]
- 8.Novak C, Gray LA. Krukenberg tumor of the ovary: clinical and pathological study of four cases. Surg Gynecol Obstet. 1938;66:157–165. [Google Scholar]
- 9.Serov SF, Scully RE, Sobin LH. Histological Typing of Ovarian Tumours. International Histological Classification of Tumours. No 9. Geneva: WHO; 1973. pp. 17–54. [Google Scholar]
- 10.Lerwill MF, Young RH. Ovarian metastases of intestinal-type gastric carcinoma: a clinicopathologic study of 4 cases with contrasting features to those of the Krukenberg tumor. Am J Surg Pathol. 2006;30:1382–1388. doi: 10.1097/01.pas.0000213256.75316.4a. [DOI] [PubMed] [Google Scholar]
- 11.Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87:236–242. doi: 10.1046/j.1365-2168.2000.01360.x. [DOI] [PubMed] [Google Scholar]
- 12.Shin DW, Hyung WJ, Noh SH, Min JS. Risk factors for recurrence aft er curative surgery for early gastric cancer. J Korean Gastric Cancer Assoc. 2001;1:106–112. [Google Scholar]
- 13.Berek JS, Natarajan S. Ovarian and fallopian tube cancer. In: Berek JS, editor. Berek and Novak's Gynecology. 14th ed. Philadelphia: Lippincott Williams and Wilkins; 2007. pp. 1457–1547. [Google Scholar]
- 14.McGill F, Ritter DB, Rickard C, Kaleya RN, Wadler S, Greston WM. Management of Krukenberg tumors: an 11-year experience and review of literature. Prim Care Update Ob Gyns. 1998;5:157–158. doi: 10.1016/s1068-607x(98)00047-x. [DOI] [PubMed] [Google Scholar]
- 15.Kim HK, Heo DS, Bang YJ, Kim NK. Prognostic factors of Krukenberg's tumor. Gynecol Oncol. 2001;82:105–109. doi: 10.1006/gyno.2001.6210. [DOI] [PubMed] [Google Scholar]
- 16.Shen-Gunther J, Mannel RS. Ascites as a predictor of ovarian malignancy. Gynecol Oncol. 2002;87:77–83. doi: 10.1006/gyno.2002.6800. [DOI] [PubMed] [Google Scholar]
- 17.Mateş IN, Iosif C, Bănceanu G, Ionescu M, Peltecu G, Dinu D, et al. Features of Krukenberg-type tumors--clinical study and review. Chirurgia (Bucur) 2008;103:23–38. [PubMed] [Google Scholar]
- 18.Koyama T, Mikami Y, Saga T, Tamai K, Togashi K. Secondary ovarian tumors: spectrum of CT and MR features with pathologic correlation. Abdom Imaging. 2007;32:784–795. doi: 10.1007/s00261-007-9186-4. [DOI] [PubMed] [Google Scholar]
- 19.McGill FM, Ritter DB, Rickard CS, Kaleya RN, Wadler S, Greston WM, et al. Krukenberg tumors: can management be improved? Gynecol Obstet Invest. 1999;48:61–65. doi: 10.1159/000010136. [DOI] [PubMed] [Google Scholar]
- 20.Dikken JL, Jansen EP, Cats A, Bakker B, Hartgrink HH, Kranenbarg EM, et al. Impact of extent of surgery and postoperative chemoradiotherapy on recurrence patterns in gastric cancer. J Clin Oncol. 2010;28:2430–2436. doi: 10.1200/JCO.2009.26.9654. [DOI] [PubMed] [Google Scholar]
- 21.Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903–2909. doi: 10.1200/JCO.2005.05.0245. [DOI] [PubMed] [Google Scholar]
- 22.Schütt U, Wedell J, Köppen P, Reichmann J. Preventive ovariectomy in colorectal cancer. Chirurg. 1993;64:1040–1043. [PubMed] [Google Scholar]
- 23.Yamamoto M, Baba H, Kakeji Y, Endo K, Ikeda Y, Toh Y, et al. Prognostic significance of tumor markers in peritoneal lavage in advanced gastric cancer. Oncology. 2004;67:19–26. doi: 10.1159/000080281. [DOI] [PubMed] [Google Scholar]