Abstract
Purpose
α-fetoprotein (AFP)-producing gastric cancer is a rare tumor with high rates of liver metastasis and a poor prognosis. Many studies have been performed but there have been no comprehensive investigations of the clinicopathological and prognosis.
Materials and Methods
Six hundred ninety four patients with gastric cancer who underwent a curative gastric resection in Hanyang University Hospital from February 2001 to December 2008 were evaluated retrospectively after excluding active or chronic hepatits, liver cirrhosis and preoperative distant metastasis. Among them, thirty five patients had an elevated serum level of AFP (>7 ng/ml) preoperatively. The clinicopathological features of AFP-producing gastric cancer were analyzed.
Results
There was poorer differentiation, a higher incidence of lymph node metastasis, more marked lymphatic and vascular invasion in the AFP-positive group than in the AFP-negative group. The 5-year survival rate of the AFP-positive group was significantly poorer than that in the AFP-negative group (66% vs. 80%, P=0.002). A significantly higher incidence of liver metastasis was observed in the AFP-positive group than in the AFP-negative group (14.3% vs. 3.6%, P=0.002) with a shorter median time period from the operation to the metachronous liver metastasis (3.7 months vs. 14.1 months, P=0.043). Multivariate survival analysis revealed the depth of invasion, degree of lymph node metastasis and AFP-positivity to be the independent prognostic factors.
Conclusions
AFP-producing gastric cancers have an aggressive behavior with a high metastatic potential to the liver. In addition, their clinicopathological features are quite different from the more common AFP-negative gastric cancer.
Keywords: Stomach neoplasms, Alpha-fetoproteins, Liver metastasis, Prognosis
Introduction
Alpha-fetoprotein (AFP) was initially found in the human fetus and is normally produced in the fetal liver and yolk sac.(1) The elevation of serum AFP level is often considered as abnormal in adults, and in clinical practice, AFP is a well-known tumor marker for screening or monitoring hepatocellular carcinoma and yolk sac tumor. Some studies showed that AFP could be produced in other cancers including primary gastric carcinoma.(2)
A case of AFP-producing gastric cancer with liver metastasis was first reported in 1970. Since then, scattered cases of early and advanced AFP-producing gastric cancer have been reported, some of them showing poor prognosis with lymphatic and venous microinvasion along with high rates of liver metastasis, of both synchronous and metachronous types.(3-5) Furthermore, AFP-producing gastric cancer showed significantly poorer survival than the AFP-negative group.(6) It is reported that AFP-producing gastric cancer often has high proliferative activity, weak apoptosis and rich neovascularization, as compared with AFP-negative gastric cancer.(7) Recently, others have also reported the aggressiveness of AFP-producing gastric cancer after observing frequent c-Met overexpression in AFP-producing gastric cancer, as compared with stage-matched gastric cancer not producing AFP.(8)
All these studies reflect the aggressive clinical behavior of AFP-producing gastric cancer, which isconsidered as a special subtype of gastric cancer. However, most of these studies were case reports, and there were few reports concerning the clinicopathological or prognosis of AFP-producing gastric cancer. These issues are clarified here, especially with respect to the characteristics of liver metastasis.
Materials and Methods
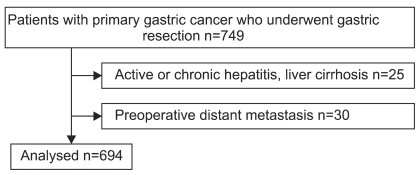
In this study, 694 patients with histologically confirmed primary gastric cancer who underwent curative gastric resection with D2 or more extended lymph node dissection at Hanyang University Hospital from February 2001 to December 2008 were selected and evaluated retrospectively. A total of 25 patients with active or chronic hepatitis and liver cirrhosis, as well as 30 patients with preoperative distant metastasis, were excluded from this study (Fig. 1). Preoperative serum AFP levels were measured in all patients during the week before surgery, using the electrochemiluminance immunoassay (ECLIA) method with Cobas™ immunoassay analyzers (Roche Diagnostics GmbH, D-68298 Mannheim). Serum AFP level above 7 ng/ml was defined as AFP-positive according to the manufacturer's instructions. There were 35 patients with elevated serum level of AFP preoperatively, with a median follow up period of 37.7 months.
Fig. 1.
Patients selection.
Before the operation, all patients routinely underwent esophagogastroduodenoscopy and abdominal computed tomography in order to evaluate tumor location, size and depth, as well as the status of lymph node and distant metastasis. Postoperative follow up was done with routine blood tests, tumor marker tests and the diagnostic tools mentioned above, every 3 months for the first 2 years and every 6 months thereafter until 5 years postoperatively. The diagnosis of postoperative recurrence was performed using abdominal ultrasonography or abdominal computed tomography. If these examinations did not confirm recurrence, histological biopsy or Positron Emission Tomography-Computed Tomography (PET-CT) were also performed.
Node status and disease stage were reassessed according to the UICC TNM classification (6th edition),(9) and surgicopathological findings were recorded according to the Borrmann, Lauren and WHO International Histological Classification (1997).
Median values were used as the measured values of continuous variables, according to the standard distribution. The survival rates were analyzed using the Life table or Kaplan-Meier method, depending on the size of the patient group. Differences were examined with Gehan's Wilcoxon or the log-rank test, respectively. A multivariate analysis of the prognostic factors was evaluated using the Cox proportional hazards model (forward stepwise method). Analysis was performed with the use of SPSS software version 18.0 (SPSS, Inc., Chicago, IL). P-values less than 0.05 were considered statistically significant.
Results
1. Preoperative serum AFP level
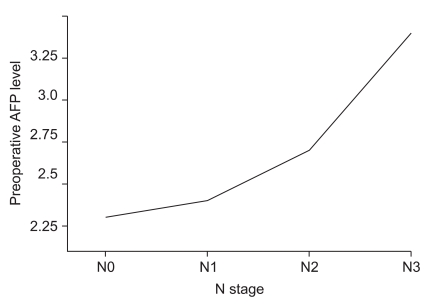
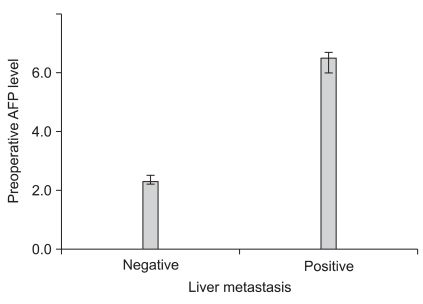
The median value of preoperative serum AFP level was 2.3 ng/ml (range, 0.1~1210.0). An increase of AFP level according to each pathological N stage was observed along with higher AFP levels in hepatic metastasis patients as compared to those in patients withnon-hepatic metastasis (Fig. 2, 3).
Fig. 2.
Relationship between preoperative serum AFP level and N stage. There was a signifi cant increase of median serum AFP level according to each N stage (2.3 vs. 2.4 vs. 2.7 vs. 3.4, P=0.001). AFP = alpha-fetoprotein.
Fig. 3.
Relationship between preoperative serum AFP level and liver metastasis. There was a signifi cant increase of median AFP level in liver metastasis positive group compared to liver metastasis negative group (6.5 vs. 2.3, P=0.000). AFP = alpha-fetoprotein.
2. Clinicopathological features according to AFP positivity
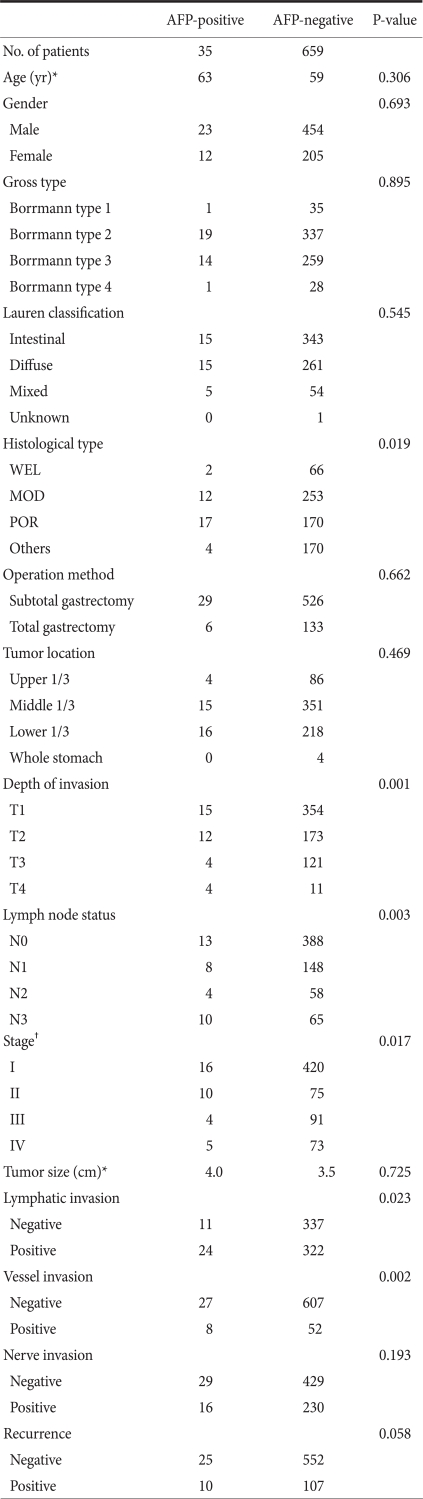
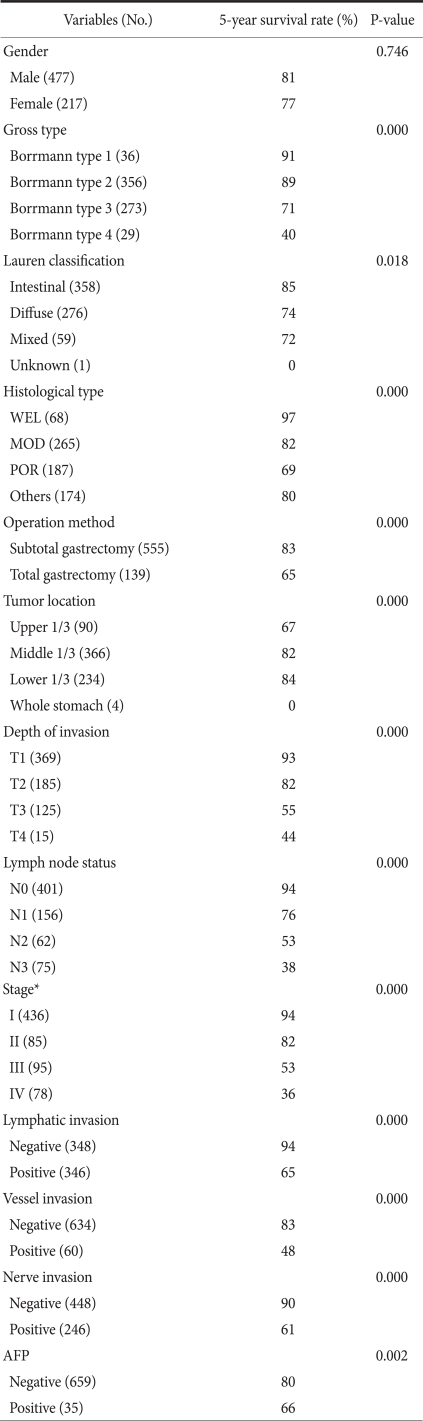
Clinicopathological characteristics were comparatively analyzed by dividing samples into AFP-positive and AFP-negative groups (Table 1). In histological classification, a poorer differentiation was observed more frequently in the AFP-positive group as compared with the AFP-negative group. There was a higher incidence of lymph node metastasis and a more marked lymphatic and vascular invasion in the AFP-positive group compared with AFP-negative group. With regard to the depth of invasion, high incidence of AFP positivity was observed in T4 patients (26.7%) without a gradual increase according to the depth of invasion. A significant difference was observed only with the TNM stage. No significant correlation was found according to age, gender, Borrmann classification, Lauren classification, extent of resection, tumor location, tumor size, neural invasion or recurrence.
Table 1.
Clinicopathological findings of AFP-positive gastric cancer in comparison to AFP-negative gastric cancer
AFP = alpha-fetoprotein; WEL = well differentiated tubular adenocarcinoma; MOD = moderately differentiated tubular adenocarcinoma; POR = poorly differentiated tubular adenocarcinoma. *Median value; †UICC TNM staging system (6th ed).
3. Univariate survival analysis according to clinicopathological factors
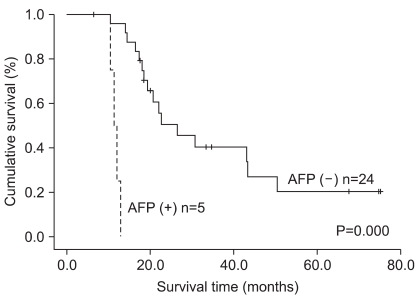
The 5-year survival rate differed in association with the Borrmann classification, Lauren classification, histological classification, extent of resection, tumor location, depth of invasion, lymph node metastasis, TNM stage, lymphatic invasion, vessel invasion, neural invasion and AFP positivity, but not with gender (Table 2). The 5-year survival rate of the AFP-positive group was significantly poorer than that of the AFP-negative group. In gastric cancer with liver metastasis, the AFP-positive group also had a significantly poorer 5-year survival rate compared to the AFP-negative group (Fig. 4).
Table 2.
Univariate survival analysis according to clinicopathological factors
No. = number; WEL = well differentiated tubular adenocarcinoma; MOD = moderately differentiated tubular adenocarcinoma; POR = poorly differentiated tubular adenocar cinoma; AFP = alpha-fetoprotein. *UICC TNM staging system (6th ed).
Fig. 4.
Overall survival curves of gastric cancer patients with liver metastasis according to AFP-positivity (5-year survival rate 20% vs. 0%, median survival time 26.4 months vs. 11.4 months). AFP = alpha-fetoprotein.
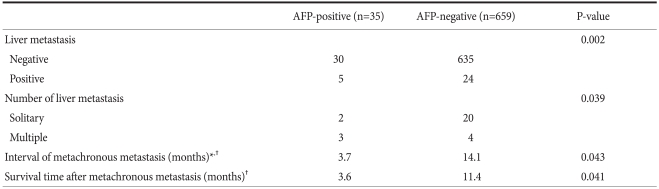
4. Liver metastasis
We observed a higher incidence of liver metastasis in the AFP-positive group compared with the AFP-negative group (14.3% vs. 3.6%), along with a higher incidence of multiple liver metastases rather than solitary liver metastasis (60% vs. 16.7%) (Table 3).
Table 3.
Characteristics of liver metastasis according to the positivity of AFP in gastric cancers
AFP = alpha-fetoprotein. *Time period from the operation to metachronous liver metastasis; †Median value.
The time period from the operation to metachronous liver metastasis was significantly shorter in the AFP-positive group than in the negative group. Furthermore, thesurvival time from the point of liver metastasis diagnosis revealed significantly shorter survival time in the AFP-positive group compared with the AFP-negative group.
All 5 AFP-positive liver metastasis patients died within 14 months, while AFP-negative liver metastasis patients survived as long as 75 months. There were no liver metastatic patients who had curative liver resection in our facility, but 3 patients underwent transarterial chemoembolization, 4 patients received radiofrequency thermoablation therapy, and the rest of the patients underwent systemic chemotherapy. No patients other than those in the systemic chemotherapy group survived more than 1 year.
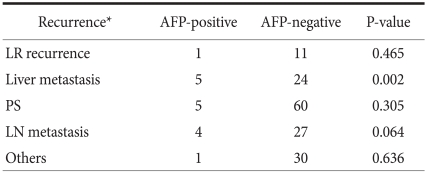
There was no significant correlation between AFP positivity and the location of recurrence except for liver metastasis (which included overlapped metastasis), as shown in Table 4.
Table 4.
Comparison of the recurrence site according to the positivity of AFP in gastric cancers
AFP = alpha-fetoprotein; LR = loco-regional; PS = peritoneal seeding; LN = lymph node. *Recurrence site includes overlapped metastasis.
5. Multivariate survival analysis according to clinicopathological factors
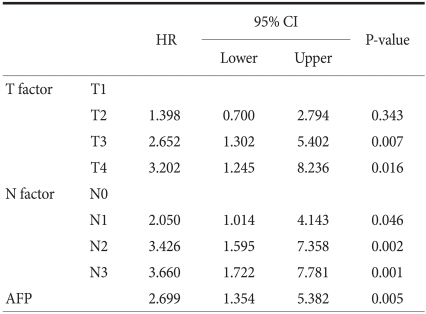
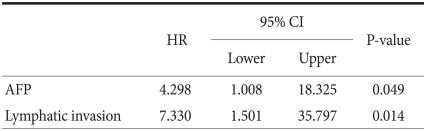
As there is a possibility of mutual correlation between significant variables observed in univariate survival analysis, the TNM stage was excluded initially and when the rest of the significant variables were added to the Cox proportional hazard models. Significant prognostic factors included the following: depth of invasion, lymph node metastasis and AFP, as shown in Table 5. Multivariate analysis limited to gastric cancer patients with liver metastasis revealed that AFP and lymphatic invasion were independent prognostic factors, as shown in Table 6.
Table 5.
Independent prognostic factors in multivariate analysis by the Cox proportional hazards model
HR= hazard ratio; 95% CI = 95 percent confidence interval; AFP = alpha-fetoprotein.
Table 6.
Independent prognostic factors of liver metastasis among recurred gastric cancer patients in multivariate analysis by the Cox proportional hazards model
HR = hazard ratio; 95% CI = 95 percent confi dence interval; AFP = alpha-fetoprotein.
Discussion
AFP was first identified in the human fetus in 1956.(1) It is an albumin-like glycoprotein with a molecular weight of 70,000 daltons. AFP is formed in the yolk sac, non-differentiated liver cells, and the fetal gastrointestinal tract and is synthesized as early as 6 months of gestation to birth.(10) Elevation of the serum AFP level at more than 1 year of age implies hepatocellular carcinoma or yolk sac tumor. Notably, 70~95% of primary hepatocellular carcinoma patients have elevated AFP levels.(11) Although AFP is a useful marker for predicting survival and for screening or monitoring in hepatocellular carcinoma, its correlation with other tumors remains to be clarified. However, some studies showed production of AFP in gastrointestinal tracts such as gastric cancers along with rectal cancers, gallbladder cancers, lung cancers and bladder cancers.(12-16) An elevation of AFP level can also be seen when damaged liver cells regenerate, as in alcoholics, individuals with chronic liver cirrhosis and HBsAg-carrier patients. It is important to exclude these patients from the study, in order to eliminate selection bias.(17)
Pre- and postoperative chemotherapy can affect survival analysis. In order to eliminate the bias from survival analysis, pre- and postoperative chemotherapy must be fully considered. Unfortunately, diverse chemotherapy strategies and differing treatment times were applied to each one of our patients. Considering chemotherapy as an analysis factor created a barrier to precise survival analysis. Therefore, chemotherapy was excluded as an analysis factor in our study.
The prevalence of AFP-producing gastric cancer is reported to be 6.2~6.3%(18,19) in Korea, 1.3~5% in Japan,(20,21) 2.5% in China(22) and 15% in the United States.(23) The discrepancies in prevalence might be due to the different methods used to measure serum AFP level or may represent regional or racial differences. Korean studies usually measured AFP level in patient serum, while studies from other countries used immunohistochemical evaluation. One study reported that 104 of 111 (93.7%) of patients with preoperatively elevated serum AFP level were proven to be carriers of AFP-producing gastric cancer by immunohistochemistry, a proportion identical to that of other studies. This implies that the serum AFP level is quite precise and more useful than immunohistochemistry, considering its cost-effectiveness and conveniencex. (20,21) We observed an incidence of AFP-positive gastric cancer of 5.0%, which is similar to previously reported values.
The prognosis of AFP-producing gastric cancer is reported to be poor. One study reported that the 1-, 3- and 5-year survival rates of AFP-producing gastric cancer were 53%, 35% and 28%, respectively.(22) Another reported the 5-year survival rate of AFP-positive gastric cancer to be 28.4%.(21) In Korea, a study of 812 cases of gastric cancer revealed a 5-year survival rate of 46.6% in the AFP-positive gastric cancer group.(18) Our study reported a 5-year survival rate of 66%, with a median survival time of 72 months, which is relatively high compared to other reports.
Liver metastasis, in particular, is a very important prognostic factor in controlling AFP-positive gastric cancer because 14.3% of the AFP-positive group in our study consequently developed liver metastasis. The tendency to exhibit multiple liver metastases was high in the AFP-positive group with a significantly shorter time period from operation to metachronous liver metastasis as previously mentioned. These observations indicate that AFP-producing gastric cancer has clinical biological behavior that differs drastically from that of the AFP-negative group. For some reason, the liver may be an environment conduciveto the proliferation of this type of cancer. Metachronous second primary tumor was defined as when the new primary cancer was diagnosed after 2 months of the original primary cancer, based on criteria used by the SEER Programme of the National Cancer Institute in the USA.
How can we explain this aggressive clinical behavior of AFP-producing gastric cancer? Unfortunately, the exact molecular mechanism that could explain this is still unclear and far limited. Generally, AFP-producing gastric cancer is associated with higher proliferative activity, weaker apoptosis and richer neovascularization. Some authors suggested that high levels of CD10 and low levels of CDX expression might be associated with aggressive behavior, particularly in hepatoid carcinoma.(24) Others, as previously mentioned, proposed that overexpression of c-Met was frequently observed in AFP-positive gastric cancer and that this receptor, encoded by the c-Met proto-oncogene, regulates cell proliferation with its ligand, hepatocyte growth factor (HGF), forming an HGF/c-Met complex, which was recently found in gastric cancer cells.(25) Thus, this HGF/c-Met complex could be a clue to the exact mechanism of AFP-positive gastric cancer, which might provide a solution for the treatment of AFP-positive gastric cancer. With regard to treatment, there was a recent report on the inhibition of growth and migration of a gastric cancer cell line when treated with antisense c-Met oligonucleotides.(25) Far more studies are necessary in order to elucidate the exact mechanism of AFP-producing gastric cancer and to optimize treatment.
Because the exact mechanism of liver metastasis in AFP-positive gastric cancer has not been established, the optimal approach to treatment remains controversial. Our study showed that all AFP-positive gastric cancer patients with liver metastasis died within 14 months despite adjuvant treatment. Some authors suggested that percutaneous ethanol injection might be an effective treatment for liver metastasis, showing a survival of 18 months, which was the longest survival time compared to other treatments, including partial resection of the liver and systemic chemotherapy (after which all patientsdied within 7 months).(22) Another study initially proposed that in cases with liver metastasis, the solitary liver metastasis should be resected, and tumor reduction of multiple liver metastases should be performed, with partial resection of liver followed by transarterial continuous-infusion chemotherapy for the remnant tumor. However, all these patients died within 8 months, demonstrating that hepatic resection must be considered carefully.(21) Although there are some case reports of successful liver resection in AFP-positive gastric cancer with liver metastasis, most of these cases are limited to small patient pools. Therefore, many more studies with larger patient pools are required regarding the treatment of AFP-positive gastric cancer with liver metastasis.(26,27)
Depth of invasion, lymph node metastasis and AFP were found to be the independent prognostic factors in multivariate analysis, with AFP having a hazard ratio of 2.699. This value is a slightly low hazard ratio compared to other significant values, thus implying that AFP might not be a major risk factor in gastric cancer. However, when multivariate analysis was limited to gastric cancer with liver metastasis, the hazard ratio increased to 4.298, showing that AFP could be an important risk factor when liver metastasis has occurred.
AFP-producing gastric cancer is a small subgroup of gastric cancer with a high likelihood of rapid metastasis to the liver. Its aggressive biological behavior in addition to its unique clinicopathological features should be studied further at the cellular and molecular levels in order to develop an effective multimodal therapy against AFP-producing gastric cancer.
References
- 1.Bergstrand CG, Czar B. Demonstration of a new protein fraction in serum from the human fetus. Scand J Clin Lab Invest. 1956;8:174. doi: 10.3109/00365515609049266. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura H, Okamoto Y, Takahashi M, Fujita T. Occurrence of alpha-fetoprotein, Regan isoenzyme, and variant alkaline phosphatase in the serum of a patient with gastric cancer. Gastroenterology. 1976;71:497–499. [PubMed] [Google Scholar]
- 3.Bourreille J, Metayer P, Sauger F, Matray F, Fondimare A. Existence of alpha feto protein during gastric-origin secondary cancer of the liver. Presse Med. 1970;78:1277–1278. [PubMed] [Google Scholar]
- 4.Chang YC, Nagasue N, Abe S, Kohno H, Yamanoi A, Uchida M, et al. The characters of AFP-producing early gastric cancer. Nippon Geka Gakkai Zasshi. 1990;91:1574–1580. [PubMed] [Google Scholar]
- 5.Motoyama T, Aizawa K, Watanabe H, Fukase M, Saito K. Alpha-Fetoprotein producing gastric carcinomas: a comparative study of three different subtypes. Acta Pathol Jpn. 1993;43:654–661. doi: 10.1111/j.1440-1827.1993.tb02549.x. [DOI] [PubMed] [Google Scholar]
- 6.Chang YC, Nagasue N, Abe S, Taniura H, Kumar DD, Nakamura T. Comparison between the clinicopathologic features of AFP-positive and AFP-negative gastric cancers. Am J Gastroenterol. 1992;87:321–325. [PubMed] [Google Scholar]
- 7.Koide N, Nishio A, Igarashi J, Kajikawa S, Adachi W, Amano J. Alpha-fetoprotein-producing gastric cancer: histochemical analysis of cell proliferation, apoptosis and angiogenesis. Am J Gastroenterol. 1999;94:1658–1663. doi: 10.1111/j.1572-0241.1999.01158.x. [DOI] [PubMed] [Google Scholar]
- 8.Amemiya H, Kono K, Mori Y, Takahashi A, Ichihara F, Iizuka H, et al. High frequency of c-Met expression in gastric cancers producing alpha-fetoprotein. Oncology. 2000;59:145–151. doi: 10.1159/000012152. [DOI] [PubMed] [Google Scholar]
- 9.Sobin LH, Wittekind CH, editors. TNM Classifi cation of Malignant Tumors. 6th ed. New York, NY: Wiley-Liss; 2002. [Google Scholar]
- 10.Gitlin D, Boesman M. Serum alpha-fetoprotein, albumin, and gamma-G-globulin in the human conceptus. J Clin Invest. 1966;45:1826–1838. doi: 10.1172/JCI105486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramsey WH, Wu GY. Hepatocellular carcinoma: update on diagnosis and treatment. Dig Dis. 1995;13:81–91. doi: 10.1159/000171490. [DOI] [PubMed] [Google Scholar]
- 12.Gitlin D, Perricelli A, Gitlin GM. Synthesis of alpha-fetoprotein by liver, yolk sac, and gastrointestinal tract of the human conceptus. Cancer Res. 1972;32:979–982. [PubMed] [Google Scholar]
- 13.Taguchi J, Yano H, Sueda J, Yamaguchi R, Kojiro M, Shirouzu G, et al. Alpha-Fetoprotein-producing rectal carcinoma--a case report. Kurume Med J. 1997;44:339–348. doi: 10.2739/kurumemedj.44.339. [DOI] [PubMed] [Google Scholar]
- 14.Sugaya Y, Sugaya H, Kuronuma Y, Hisauchi T, Harada T. A case of gallbladder carcinoma producing both alpha-fetoprotein (AFP) and carcinoembryonic antigen (CEA) Gastroenterol Jpn. 1989;24:325–331. doi: 10.1007/BF02774332. [DOI] [PubMed] [Google Scholar]
- 15.Hiroshima K, Iyoda A, Toyozaki T, Haga Y, Baba M, Fujisawa T, et al. Alpha-fetoprotein-producing lung carcinoma: report of three cases. Pathol Int. 2002;52:46–53. doi: 10.1046/j.1440-1827.2002.01311.x. [DOI] [PubMed] [Google Scholar]
- 16.Takayama H. A case of bladder cancer producing alpha-fetoprotein (AFP) Hinyokika Kiyo. 1995;41:387–389. [PubMed] [Google Scholar]
- 17.Stuart KE, Anand AJ, Jenkins RL. Hepatocellular carcinoma in the United States. Prognostic features, treatment outcome, and survival. Cancer. 1996;77:2217–2222. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2217::AID-CNCR6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 18.Kim T, Yu WS. Prognostic value of preoperative serum alpha-fetoprotein level in resectable gastric cancer. J Korean Gastric Cancer Assoc. 2003;3:33–37. [Google Scholar]
- 19.Joo YH, Jung HY, Kang GH, Youn HJ, Kim SY, Yang SK, et al. Clinico-pathological characteristics of the alpha-fetoprotein producing gastric carcinoma. Korean J Gastroenterol. 2000;36:54–60. [Google Scholar]
- 20.Chang YC, Nagasue N, Kohno H, Taniura H, Uchida M, Yamanoi A, et al. Clinicopathologic features and long-term results of alpha-fetoprotein-producing gastric cancer. Am J Gastroenterol. 1990;85:1480–1485. [PubMed] [Google Scholar]
- 21.Kono K, Amemiya H, Sekikawa T, Iizuka H, Takahashi A, Fujii H, et al. Clinicopathologic features of gastric cancers producing alpha-fetoprotein. Dig Surg. 2002;19:359–365. doi: 10.1159/000065838. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Cheng Y, Sheng W, Lu H, Xu Y, Long Z, et al. Clinicopathologic features and prognostic factors in alpha-fetoprotein-producing gastric cancers: analysis of 104 cases. J Surg Oncol. 2010;102:249–255. doi: 10.1002/jso.21624. [DOI] [PubMed] [Google Scholar]
- 23.McIntire KR, Waldmann TA, Moertel CG, Go VL. Serum alpha-fetoprotein in patients with neoplasms of the gastrointestinal tract. Cancer Res. 1975;35:991–996. [PubMed] [Google Scholar]
- 24.Kumashiro Y, Yao T, Aishima S, Hirahashi M, Nishiyama K, Yamada T, et al. Hepatoid adenocarcinoma of the stomach: histogenesis and progression in association with intestinal phenotype. Hum Pathol. 2007;38:857–863. doi: 10.1016/j.humpath.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 25.Kaji M, Yonemura Y, Harada S, Liu X, Terada I, Yamamoto H. Participation of c-met in the progression of human gastric cancers: anti-c-met oligonucleotides inhibit proliferation or invasiveness of gastric cancer cells. Cancer Gene Ther. 1996;3:393–404. [PubMed] [Google Scholar]
- 26.Sato Y, Nishimaki T, Date K, Shirai Y, Kurosaki I, Saito Y, et al. Successful resection of metachronous liver metastasis from alpha-fetoprotein-producing gastric cancer: report of a case. Surg Today. 1999;29:1075–1078. doi: 10.1007/s005950050647. [DOI] [PubMed] [Google Scholar]
- 27.Tsurumachi T, Yamamoto H, Watanabe K, Honda I, Watanabe S, Yamada S, et al. Resection of liver metastasis from alpha-fetoprotein-producing early gastric cancer: report of a case. Surg Today. 1997;27:563–566. doi: 10.1007/BF02385813. [DOI] [PubMed] [Google Scholar]