Abstract
Purpose
Bone metastasis from stomach cancer occurs only rarely and it is known to have a very poor prognosis. This study examined the clinical characteristics and prognosis of patients who were diagnosed with stomach cancer and bone metastasis.
Materials and Methods
The subjects were 19 patients who were diagnosed with stomach cancer at Hanyang University Medical Center from June 1992 to August 2010 and they also had bone metastasis. The survival rate according to many clinicopathologic factors was retrospectively analyzed.
Results
11 patients out of 18 patients (61%) who received an operation were in stage IV and the most common bone metastasis location was the spine. Bone scintigraphy was mostly used for diagnosing bone metastasis and PET-CT and magnetic resonance imaging were used singly or together. The serum alkaline phosphatase at the time of diagnosis had increased in 12 cases and there were clinical symptoms (bone pain) in 16 cases. Treatment was given to 14 cases and it was mostly radiotherapy. There were 2 cases of discovering bone metastasis at the time of diagnosing stomach cancer. The interval after operation to the time of diagnosing bone metastasis for the 18 cases that received a stomach cancer operation was on average 14.9±17.3 months and the period until death after the diagnosis of bone metastasis was on average 3.8±2.6 months. As a result of univariate survival rate analysis, the group that was treated for bone metastasis had a significantly better survival period when the bone metastasis was singular rather than multiple, as compared to the non-treatment group, yet both factors were not independent prognosis factors on multivariate survival analysis.
Conclusions
An examination to confirm the status of bone metastasis when conducting a radio-tracer test after the initial diagnosis and also after an operation is needed for stomach cancer patients, and bone scintigraphy is the most helpfully modality. Making the diagnosis at the early stage and suitable treatments are expected to enhance the survival rate and improve the quality of life even for the patients with bone metastasis.
Keywords: Stomach neoplasms, Bone metastasis, Diagnosis, Prognosis
Introduction
The proportion of gastric cancer patients among the annually registered cancer patients in Korea is as high as approximately 20%.(1) It is very important to assess the presence or absence of metastasis to establish the treatment plans for gastric cancer, which occurs at such a high rate, and to predict the prognosis. Gastric cancer generally metastasizes to the peritoneal membrane, liver, lymph nodes, etc., and it may metastasize to the spleen, adrenalin, ovary, lung, brain and skin.
Bone metastasis generally occurs in patients with prostate cancer, breast cancer and lung cancer, and bone metastasis in gastric cancer patients has been shown to be very rare.(2-4) Bone metastasis is usually associated with disseminated vascular coagulation, hemolytic anemia and other hematological complications, and the prognosis is very poor.(5) The diverse incidence and prognosis of bone metastasis from gastric cancer have been reported in numerous studies, but this has not been sufficiently established in Korea. Therefore, we conducted this study to examine the clinical characteristics of the patients diagnosed with gastric cancer by gastroscopy as well as histological tests, and these patients had bone metastasis detected simultaneously or later, and we also wanted to assess their prognosis.
Materials and Methods
1. The subjects
The study was conducted on 19 patients who were diagnosed as having gastric caner with bone metastasis by histological tests from June 1992 to August 2010 in the Department of Surgery, Hanyang University Hospital.
2. Methods
Bone scintigraphy (Fig. 1), PET-CT (Fig. 2), and magnetic resonance imaging (MRI) were used as the methods of diagnosing bone metastasis. All the patients with bone metastasis were classified according to Borrmann's morphology as assessed by the gastroscopic findings, the location of the gastric cancer and the histological types, and their correlations with bone metastasis were analyzed. In addition, the preferential site of bone metastasis, and the association with lung metastasis and brain metastasis were examined. In addition, by measuring the serum alkaline phosphatase (ALP) value, the changes of the ALP value in patients with bone metastasis was examined (Table 1), and the difference of the mortality rate according to with or without treatments for bone metastasis was analyzed, as well as the treatment methods SPSS 13.0 (statistical Package for Social Science version 13.0, SPSS Inc., Chicago, IL, USA) was used for the data analysis, the survival rate was obtained by the Kaplan-Meier method and the significance of the difference of the survival rate was validated by the log-rank test. Cox's proportional hazard model was used for multivariate analysis of the survival rate and P-values less than 0.05 were considered to be statistically significant.
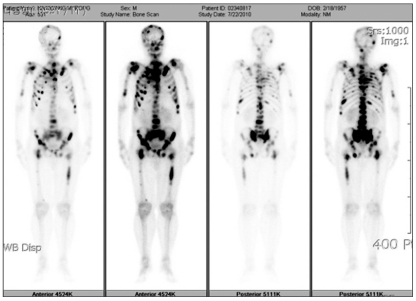
Fig. 1.
Bone scintigraphy shows increased uptake in multifocal area.
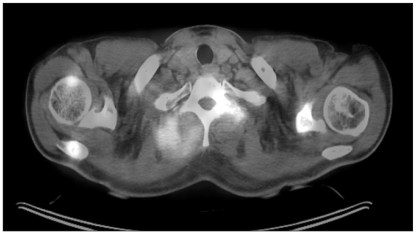
Fig. 2.
PET-CT shows increased uptake in the vertebra, paravertebral muscle.
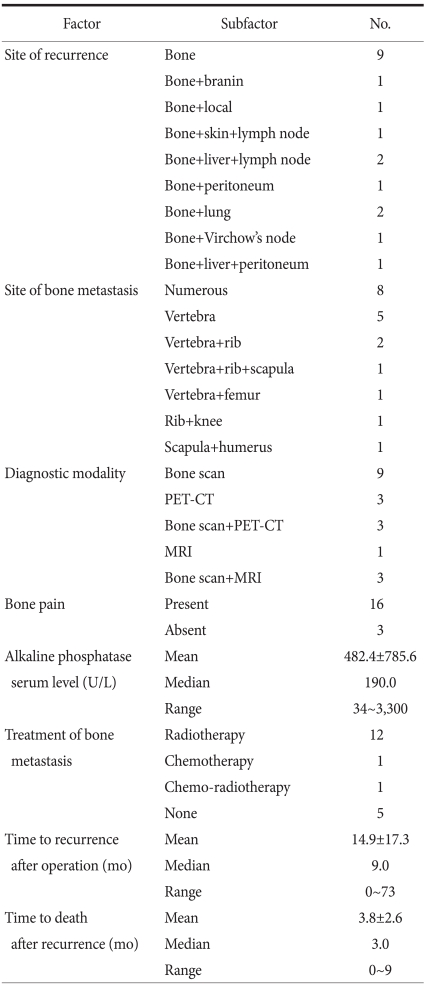
Table 1.
Clinicopathological characteristics
No. = number; yr = year; TNM = tumor node metastasis; WEL = well differentiated adeno carcinoma; MOD = moderated differentiated adenocarcinoma; POR = poorly differentiated adenocarcinoma; SIG = signet ring cell carcinoma; MUC = mucinous carcinoma.
Results
1. Clinicopathological characteristics of the gastric cancer
Eighteen among the 19 subjects received surgery. Total gastrectomy was performed on 10 patients, subtotal gastrectomy was performed on 7 patients and gastrojejunostomy was performed on 1 patient. In regard to the disease stage of gastric cancer according to the AJCC 6th edition, Ib was 1 case (5%), II was 3 cases (15%), IIIa was 2 cases (10%), IIIb was 1 case (5%), and IV was 12 cases (63%). Stage IV was the most prevalent. The patients were divided into early gastric cancer and advanced gastric cancer according to the macroscopic morphology observed on gastroscopy, and advanced gastric cancer was defined as cases from Borrmann's type 1 to type 4. In the 19 patients, there were 4 cases of Borrmann's type 2 (21%), 12 case of Borrmann's type 3 (63%) and 3 cases of Borrmann's type 4 (16%). Concerning the histological types, there was 1 case of highly differentiated cancer, 4 cases of moderately differentiated cancer, 8 cases of poorly differentiated cancer, 5 cases of signet ring cell carcinoma and 1 case of mucinous adenocarcinoma. The undifferentiated type (14/19, 73%) was more abundant than the differentiated types (5/19, 27%) (Table 1).
2. Clinicopathological characteristics of bone metastasis
The area of bone metastasis was in the order of the vertebrae (17 cases, 89%), the costa (12 cases, 63%), the lower extremities (2 cases, 10 %), the scapula (2 cases, 10%) and the upper extremities (1 case, 5%). In regard to the diagnosis of bone metastasis lesions, there were 9 patients diagnosed by bone scintigraphy alone, 3 patients were diagnosed by using only positron emission tomographycomputed tomography and 1 patient was diagnosed by magnetic resonance imaging alone. In the remaining 6 cases, together with bone scintigraphy, positron emission tomography-computed tomography and magnetic resonance imaging were used simultaneously. In other words, bone scintigraphy was used most frequently for the diagnosis. There were 5 patients (26%) with a solitary lesion at the time of diagnosis and there were 14 patients (74%) with multiple lesions at the time of diagnosis. Concerning the serum ALP level at the time of the diagnosis of bone metastasis, it was higher than the normal values (30~110 U/L) in 12 cases (66%), the median value was 190 U/L and the average value was 484.4±785.6 U/L. Backache, bone pain and other symptoms pertinent to bone metastasis were noted in 16 cases (88%). Nine patients (47%), were diagnosed bone metastasis only without distant metastasis, there was 1 case of bone metastasis together with brain metastasis, one case was associated with metastasis in the skin and periaortic lymph node metastasis, there were 2 cases associated with liver metastasis and periaortic lymph node metastasis, there was 1 case associated with liver metastasis and peritoneal dissemination, there was 1 case associated with peritoneal dissemination only, there were 2 cases associated with lung metastasis and one case was associated with Virchow's node metastasis. Excluding the 5 cases whose general condition deteriorated due to the progression of gastric cancer and so they could not be treated, 14 cases (73%) were treated. Twelve cases were treated with radiation therapy alone, 1 case was treated with systemic injection of chemotherapeutics and 1 case was treated with the combination treatment of systemic injection of chemotherapeutic agents and radiation therapy. All 13 patients treated with radiation therapy presented with bone pain caused by bone metastasis. After irradiation with 3,000~3,500 rad, 9 patients (69.2%) showed the amelioration of symptoms. One patient who was administered systemic chemotherapeutic agents did not have symptoms associated with bone metastasis. In 2 cases, bone metastasis was detected at the time of the diagnosis of gastric cancer. In 18 cases, the interval from surgery to the diagnosis of bone metastasis was on average 14.9±17.3 months (median value: 9 months, range: 0 ~73 months). The period from the diagnosis of bone metastasis to death was on average 3.8±2.6 months (median value: 3 months, range: 1~9 months (Table 2).
Table 2.
Clinicopathological characteristics of bone metastasis
No. = number; MRI = magnetic resonance imaging; mo = month.
3. The survival period of the gastric cancer patients with bone metastasis
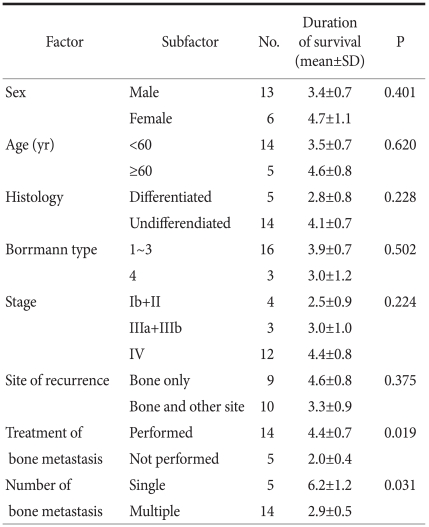
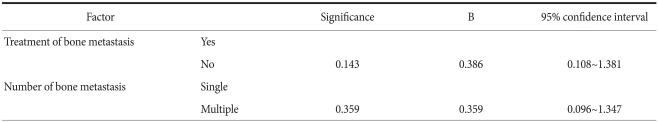
In regard to the average survival period according to gender, that for the males was 3.4±0.7 months, that for the females was 4.7±1.1 months and there was no significant difference (P=0.401). Based on the age of 60 years, the survival rate of the 5 patients older than 60 years and the 14 patients older than 60 years was 4.6±0.8 months and 3.5±0.7 months, respectively, and a significant difference was not shown (P=0.620) (Table 1). For the univariate analysis of the survival rate according to the number of bone metastasis lesions, the average survival period was 6.2±1.2 months and 2.9±0.5 months for patients with solitary and multiple lesions, respectively, and the survival period of the patients with a solitary lesion was significantly longer than that of the patients with multicentric lesions (P=0.031). Nonetheless, the results of multivariate analysis showed that the number of metastatic lesions was not an independent prognostic factor. The survival period of the 16 cases of Borrmann's types 2 and 3 was compared with that of the 3 cases of Borrmann's type 4. The survival period was 3.9±0.7 months and 3.0±1.2 months, respectively, and a statistically significant difference was not shown (P=0.502). On the analysis of the survival period according to the disease stage, the survival period of the three groups (4 cases of stages 1 and 2, 3 cases of stage 3 and 12 cases of stage 4) after the diagnosis of bone metastasis was 2.5±0.9 months, 3.0±1.0 months and 4.4±0.8 months, respectively, and a statistically significant difference was not shown (P=0.224). When the survival period according to the histological types was compared, that for the differentiated type was 2.8±0.8 months, that for the undifferentiated type was 4.1±0.7 months and a statistically significant difference was not shown (P=0.228). The survival period for the 9 cases with metastatic lesions in the bone only was compared with that of the 10 cases with metastasis in other organs in addition to the bone was compared, and it was 4.6±0.8 months and 3.3±0.9 months, respectively, and the difference between the two groups was not significant (P=0.375). The survival period of the 14 patients treated for bone metastasis was compared with the 5 patients who were not treated and it was 4.4±0.7 months and 2.0±0.4 months, respectively. The survival period of the treated group was significantly higher (P=0.019). Nonetheless, on the multivariate analysis of the survival period, it was not an independent prognostic factor (Table 3, 4).
Table 3.
Univariate survival analysis of gastric cancer patient with bone metastasis
No. = number; SD = standard deviation; yr = year.
Table 4.
Survival rate of gastric cancer patient after bone metastasis (multivariate analysis)
Discussion
Bone metastasis frequently occurs in patients with breast cancer, lung cancer, renal cancer, prostate cancer, bladder cancer and other primary cancers. In comparison, it has been shown that bone metastasis that originates from malignant tumors of the gastrointestinal tract is rare.(2-4) In 1983, Yoshikawa and Kitaoka(6) reported that the incidence of bone metastasis is 1~20%. In 1987, Nishidoi and Koga(7) have reported that in 246 gastric cancer patients, bone metastasis was associated in 33 patients (13.4%). In our study, among the 2,150 patients diagnosed with gastric cancer from June 1992 to August 2010, bone metastasis was associated in 19 patients for a frequency of 0.9%. In other words, depending on the research institutions and investigators, the incidence of bone metastasis varies greatly. Seto et al.(8) have reported that bone scintigraphy was performed on 60 patients, and bone metastasis was suspected in 25% of these patients. Bone metastasis may occur more frequently in cases with primary cancer in the body of stomach, poorly differentiated adenocarcinoma and in cases with abundant lymph node metastasis in the vicinity. In our study, like as shown in the study reported by Seto et al., the undifferentiated histological types (14/19, 73%) were more abundant than the differentiated types (5/19, 27%). However, the survival period of the two groups was not significantly different. Thus, the survival period was not different according to the histological types. In 1995, Choi et al.(9) performed bone scintigraphy on 234 gastric cancer patients, and based on the presence or absence of hot uptake lesions, the frequency and pattern of bone metastasis were inferred and reported. According to this, there were 106 patients who showed hot uptake lesions, the estimated incidence of bone metastasis was 45.3% and an elevated serum ALP value was associated with bone metastasis. In our study, similarly, among the 19 patients, the serum ALP value was elevated in 12 patients at the time of diagnosis. Similarly, in 2006, Kusumoto et al.(10) reported on the clinical characteristics of 9 patients with bone metastasis from gastric cancer, and it was observed that the serum ALP level was elevated in all 9 patient, and the serum ALP value was an important factor for making the diagnosis of bone metastasis. Therefore, it is thought that in patients diagnosed with gastric cancer, if the ALP value is atypically elevated, then evaluation of bone metastasis is required.
In our study, bone scintigraphy was applied most frequently for the diagnosis of bone metastasis (15/19, 78%). In addition, positron emission tomography-computed tomography and MRI were used alone or in combination. On account of the use of a gamma camera and the development of test agents such as Tc-99m MDP, the sensitivity of bone scintigraphy is particularly high, and thus it is known to be the most useful screening test. Bone scintigraphy could detect the change of abnormal blood flow within the bone that developed metastasis, and so it could detect bone metastasis at the time approximately 3 months earlier than that with using plain X-rays.(11,12) Nevertheless, hot uptake lesions may be detected in Paget's disease and other metabolic bone diseases, degenerative arthritis, fractures, infectious bone diseases and other benign bone diseases or primary bone tumors, and so it has limitations of low specificity. In our study, among the 19 patients, there were only 5 patients with a solitary lesion. Several studies have examined the rate of detecting bone metastasis with a solitary lesion as detected by bone scintigraphy, and only approximately 50~55% of the cases were diagnosed as having bone metastasis.(13-16) In other words, bone scintigraphy has shortcomings that it is difficult to diagnose bone metastasis from malignant tumors in the cases with a hot uptake lesion. Therefore, it is considered that for cases with a solitary hot uptake lesion detected by bone scintigraphy, together with accurate history taking for fracture and other past medical problems, the rate of a false diagnosis of bone metastasis from gastric cancer could be decreased by making an accurate differential diagnosis from infectious bone diseases, metabolic bone diseases and other benign diseases. In our study, for the cases that a solitary lesion was detected by bone scintigraphy, efforts were made to increase the accuracy of the diagnosis by additionally performing positron emission tomography-computed tomography or magnetic resonance imaging. The accuracy of the diagnosis may be increased more by performing additional bone marrow tapping or bone marrow histological tests in parallel. Choi et al.(9) have reported that the area where bone metastasis was frequently diagnosed by bone scintigraphy was in the order of the vertebrae (66%), the costa (59%), the pelvic bone (43%), the femur (30%) and the scapula and clavicle (17%). In our study, among the 19 patients, metastasis in the vertebrae was detected in 17 cases (89%), and so similar results were obtained. Other metastases associated with bone metastasis were also examined, and particularly, 3 of the 19 patients (15%) were associated with liver cancer. In general, for gastric cancer and including early gastric cancer, the most frequently metastasized area by hematogenous metastasis is the liver.(10) In our gastric cancer patients, the rate of liver metastasis was lower than the general rate of liver metastasis. This could be explained by the difference of metastasis routes. In the cases with well differentiated tumors, metastasis is developed through the portal vein. On the other hand, in the cases with poorly differentiated cancer, bone metastasis primarily occurs through the vertebral vein system.(8,17) Several studies have reported that the vertebrae are the site where bone metastasis occurs most frequently, which supports that the vertebral vein system is the major route of bone metastasis.(9,12,18) Kusumoto et al.(10) have reported that in patients with gastric cancer, bone metastasis is more prevalent in the young age groups, but in our study, age was not found to be correlated with bone metastasis. In bone metastasis from gastric cancer, the cancer cells diffusely proliferate in the bone marrow and this can cause disseminated carcinomatosis; they also proliferate rapidly and thus induce bone destruction as well as hematological complications. Yet the developmental mechanism of bone destruction has not yet been elucidated. More studies on this are required.(10)
Yoshikawa and Kitaoka(6) and Nishidoi and Koga(7) have reported that bone metastasis from gastric cancer is associated with Borrmann's types 3 and 4, which arereferred to as scirrhous carcinoma. In our study, similarly, Borrmann's types 3 and type 4 were found in 17 cases of the entire 19 cases (89%), and these results are similar to those of the above studies. In regard to the association with the disease stage of primary gastric cancer, the 11 patients who underwent surgery (61%) were stage IV. In other words, in the Borrmann type 3 and 4 patients, bone metastasis occurs frequently in the cases with primary cancer of a high disease stage. Nonetheless, the macroscopic type and disease stage were not significantly correlated with the survival period after the diagnosis of bone metastasis. Yet the number of cases in each disease stage was too small, and a comparative analysis of the survival period of each disease stage could not be performed. Analysis of a larger number of cases is required for a clearer interpretation of the results of the analysis of our study(that the survival period according to the disease stages was not different. Among the 19 patients diagnosed with bone metastasis, 5 cases could not be treated because of the deterioration of their general condition due to the progression of gastric cancer, and the remaining 14 patients were treated. On the univariate analysis of the survival period of the treatment group and the non-treatment group, the survival period of the treatment group was 4.4±0.7 months and it was significantly longer than the 2.0±0.4 survival period of the untreated group. The most common clinical symptoms and complications of bone metastasis are bone pain, pathologic fracture and spinal cord compression. In our study, similarly, in the 19 subject patients, the majority or 16 patients (84%) were positive for bone pain. Clinically, the cause of the worst suffering of patients with bone metastasis is bone pain. Therefore, pain management for patients with bone pain is very important. In regard to radiation therapy for such bone pain, Murai et al.(19) have reported that 68 patients with bone metastasis were classified according to the type of primary tumors, and radiation therapy was effective in 73% of the lung cancer patients, 100% of the breast cancer patients and 75% of the gastric cancer patients. In addition, Yoshikawa and Kitaoka(20) have reported that in 23 gastric cancer patients associated with bone metastasis, radiation therapy was effective for the amelioration of bone pain, yet chemotherapy was not effective for this. McQuay et al.(21) have reported that in regard to the effectiveness of radiation therapy for the amelioration of bone pain, 1 month after the treatment, the bone pain was resolved completely in 1/4 of the patients, and at least 50% pain amelioration could be obtained in 1/3 of the patients. The survival period of was recently improved by treatment with MTX+5FU, S1+paclitaxel, S1+cisplatin and other chemotherapy,(22,23) and in addition it was effective on the amelioration of pain.(24) Bisphosphonate(25) has recently been used for the treatment of clinical symptoms caused by bone metastasis and the complications. It suppressed the production of proliferative factors by bones through the suppression of the reabsorption of bone, and thus it suppressed the proliferation of cancer cells.(26,27) Therefore, in patients with bone metastasis, it reduces pain and it also suppresses or delays complications caused by bone metastasis. Nonetheless, large scale studies on bone metastasis patients have not been conducted, and studies are needed on the indications for the use of Bisphosphonate and to prove its treatment effectiveness. Chung et al.(28) reported on 4 patients with bone metastasis caused by gastric cancer, and all 4 patients died within 4 months after the diagnosis of bone metastasis. In our study, similarly, the period from the diagnosis of bone metastasis to death was on average 3.8±2.6 months, and it was found that the prognosis was very poor.
In conclusion, the prognosis of bone metastasis caused by gastric cancer is very poor and the prognosis of patients may be worsened due to a delayed diagnosis. Hence, tests to assess for bone metastasis are required for gastric cancer patients at the time of the initial diagnosis and the postsurgical follow-up observation, and bone scintigraphy with its high sensitivity appears to be most useful. In such a manner, it is anticipated that the survival period as well as the quality of life may be improved with making a rapid diagnosis and by administering radiation therapy and other appropriate treatments. Studies with a large number of larger subjects are required to assess the effect ofimproving the survival period according to different treatment methods.
References
- 1.Suh CI, Suh KA, Park SH, Chang HJ, Ko JW, Ahn DH. Annual report of the central cancer registry in Korea - 1998 (Based on Registered Data from 124 Hospitals) J Korean Cancer Assoc. 2000;32:827–834. [Google Scholar]
- 2.Crivellari D, Carbone A, Sigon R, Buonadonna A, Cannizzaro R, Sorio R, et al. Gastric cancer with bone marrow invasion at presentation: case-report and review of the literature. Tumori. 1995;81:74–76. doi: 10.1177/030089169508100117. [DOI] [PubMed] [Google Scholar]
- 3.Noda N, Sano T, Shirao K, Ono H, Katai H, Sasako M, et al. A case of bone marrow recurrence from gastric carcinoma after a nine-year disease-free interval. Jpn J Clin Oncol. 1996;26:472–475. doi: 10.1093/oxfordjournals.jjco.a023267. [DOI] [PubMed] [Google Scholar]
- 4.Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer. 1950;3:74–85. doi: 10.1002/1097-0142(1950)3:1<74::aid-cncr2820030111>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Pasquini E, Gianni L, Aitini E, Nicolini M, Fattori PP, Cavazzini G, et al. Acute disseminated intravascular coagulation syndrome in cancer patients. Oncology. 1995;52:505–508. doi: 10.1159/000227520. [DOI] [PubMed] [Google Scholar]
- 6.Yoshikawa K, Kitaoka H. Bone metastasis of gastric cancer. Jpn J Surg. 1983;13:173–176. doi: 10.1007/BF02469472. [DOI] [PubMed] [Google Scholar]
- 7.Nishidoi H, Koga S. Clinicopathological study of gastric cancer with bone metastasis. Gan To Kagaku Ryoho. 1987;14(5 Pt 2):1717–1722. [PubMed] [Google Scholar]
- 8.Seto M, Tonami N, Koizumi K, Sui O, Hisada K. Bone metastasis in gastric cancer--clinical evaluation of bone scintigrams. Kaku Igaku. 1983;20:795–801. [PubMed] [Google Scholar]
- 9.Choi CW, Lee DS, Chung JK, Lee MC, Kim NK, Choi KW, et al. Evaluation of bone metastases by Tc-99m MDP imaging in patients with stomach cancer. Clin Nucl Med. 1995;20:310–314. doi: 10.1097/00003072-199504000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Kusumoto H, Haraguchi M, Nozuka Y, Oda Y, Tsuneyoshi M, Iguchi H. Characteristic features of disseminated carcinomatosis of the bone marrow due to gastric cancer: the pathogenesis of bone destruction. Oncol Rep. 2006;16:735–740. [PubMed] [Google Scholar]
- 11.Wilner D. Cancer metastasis to bone. In: Wilner D, editor. Radiology of Bone Tumors and Allied Disorders. Vol 1. Philadelphia: WB Saunders; 1982. pp. 3641–3908. [Google Scholar]
- 12.Gold RI, Seeger LL, Bassett LW, Steckel RJ. An integrated approach to the evaluation of metastatic bone disease. Radiol Clin North Am. 1990;28:471–483. [PubMed] [Google Scholar]
- 13.Corcoran RJ, Thrall JH, Kyle RW, Kaminski R, Johnson MC. Solitary abnormalities in bone scans of patients with extraosseous malignancies. Radiology. 1976;121:663–667. doi: 10.1148/121.3.663. [DOI] [PubMed] [Google Scholar]
- 14.Shirazi PH, Rayudu GV, Fordham EW. Review of solitary 18F bone scan lesions. Radiology. 1974;112:369–372. doi: 10.1148/112.2.369. [DOI] [PubMed] [Google Scholar]
- 15.Brown ML. Significance of the solitary lesion in pediatric bone scanning: concise communication. J Nucl Med. 1983;24:114–115. [PubMed] [Google Scholar]
- 16.Rappaport AH, Hoffer PB, Genant HK. Unifocal bone findings by scintigraphy. Clinical significance in patients with known primary cancer. West J Med. 1978;129:188–192. [PMC free article] [PubMed] [Google Scholar]
- 17.Diel IJ, Kaufman M, Bastert G. Metastatic bone disease: fundamental and clinical aspects. 1st ed. Santa Clara: Springer-Verlag Telos; 1994. pp. 22–23. [Google Scholar]
- 18.McNeil BJ. Value of bone scanning in neoplastic disease. Semin Nucl Med. 1984;14:277–286. doi: 10.1016/s0001-2998(84)80003-3. [DOI] [PubMed] [Google Scholar]
- 19.Murai N, Koga K, Nagamachi S, Nishikawa K, Matsuki K, Kusumoto S, et al. Radiotherapy in bone metastases--with special reference to its effect on relieving pain. Gan No Rinsho. 1989;35:1149–1152. [PubMed] [Google Scholar]
- 20.Yoshikawa K, Kitaoka H. Bone metastasis of gastric cancer. Jpn J Surg. 1983;13:173–176. doi: 10.1007/BF02469472. [DOI] [PubMed] [Google Scholar]
- 21.McQuay HJ, Collins SL, Carroll D, Moore RA. Radiotherapy for the palliation of painful bone metastases. Cochrane Database Syst Rev. 2000;(2):CD001793. doi: 10.1002/14651858.CD001793. [DOI] [PubMed] [Google Scholar]
- 22.Yasuda K, Kimura T, Seita M, Takahata T, Akazai Y. A case of gastric cancer accompanied by disseminated carcinomatosis of bone marrow with DIC recovered by sequential therapy consisting of MTX and 5-FU. Gan To Kagaku Ryoho. 2008;35:1941–1943. [PubMed] [Google Scholar]
- 23.Migita K, Watanabe A, Sakamoto C, Nakamura T, Ohyama T, Ishikawa H, et al. A case of multiple bone metastases from gastric cancer treated with combination chemotherapy of S-1 and CDDP. Gan To Kagaku Ryoho. 2007;34:929–931. [PubMed] [Google Scholar]
- 24.Fujishima Y, Yoneda R, Iwai M, Fukunaga H, Miura M, Koide M, et al. A case of advanced gastric cancer with disseminated carcinomatosis of bone marrow treated by S-1 and CDDP. Gan To Kagaku Ryoho. 2009;36:2653–2655. [PubMed] [Google Scholar]
- 25.Berenson JR, Rosen LS, Howell A, Porter L, Coleman RE, Morley W, et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer. 2001;91:1191–1200. doi: 10.1002/1097-0142(20010401)91:7<1191::aid-cncr1119>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 26.Carano A, Teitelbaum SL, Konsek JD, Schlesinger PH, Blair HC. Bisphosphonates directly inhibit the bone resorption activity of isolated avian osteoclasts in vitro. J Clin Invest. 1990;85:456–461. doi: 10.1172/JCI114459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes DE, Wright KR, Uy HL, Sasaki A, Yoneda T, Roodman GD, et al. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J Bone Miner Res. 1995;10:1478–1487. doi: 10.1002/jbmr.5650101008. [DOI] [PubMed] [Google Scholar]
- 28.Chung YS, Choi TY, Ha CY, Kim HM, Lee KJ, Park CH, et al. An unusual case of osteoblastic metastasis from gastric carcinoma. Yonsei Med J. 2002;43:377–380. doi: 10.3349/ymj.2002.43.3.377. [DOI] [PubMed] [Google Scholar]