Abstract
The simultaneous occurrence of a gastrointestinal stromal tumor (GIST) and a gastric adenocarcinoma is uncommon, and has rarely been reported in the literature. The present report describes the case of a 74-year-old male patient who initially presented with an adenocarcinoma that had invaded the antral mucosa. Computed tomography then revealed the presence of a suspected GIST, in the form of a 2×2 cm mass at the hilum of the spleen. In view of the advanced age of the patient, a surgical approach that would minimize risk and maximize quality of life was preferred. The patient therefore underwent simultaneous laparoscopy-assisted distal gastrectomy for the adenocarcinoma and wedge resection for the GIST. This approach was only chosen after confirming that it would be possible to preserve three or more of the short gastric arteries that supply the area below the wedge resection site. This may be considered a feasible approach to the management of the simultaneous occurrence of a mid-to-low gastric body adenocarcinoma and a high gastric body GIST.
Keywords: Gastrointestinal stromal tumors, Stomach neoplasms, Laparoscopy
Introduction
Around 95% of all malignant gastric neoplasms are adenocarcinomas. By contrast, gastric sarcomas, which arise from the mesenchymal components of the gastric wall, account for only 3% of all gastric malignancies. Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumor of the gastrointestinal tract, and typically develop in the stomach (60~70%).(1) The simultaneous occurrence of a GIST and a gastric adenocarcinoma is uncommon, and has rarely been reported in the literature. The report by Uchiyama et al.(2) is only the second to have described the treatment of this presentation with simultaneous laparoscopy-assisted distal gastrectomy and laparoscopic wedge resection. In this case, however, use of this approach was facilitated by the fact that the GIST lesions were located high in the posterior wall of the gastric body, which meant that the wedge resection would not affect the blood supply of the remnant stomach. In the present case, the GIST was located in the hilum of the spleen, whereas the adenocarcinoma was located in the antral mucosa. It was therefore difficult to decide whether a total gastrectomy was indicated, or whether a subtotal gastrectomy for the adenocarcinoma with simultaneous wedge resection of the GIST would be preferable. Having confirmed that it would be possible to preserve three or more of the short gastric arteries that supply the area below the wedge resection site, the second approach was selected. The present report demonstrates the feasibility of this approach.
Case Report
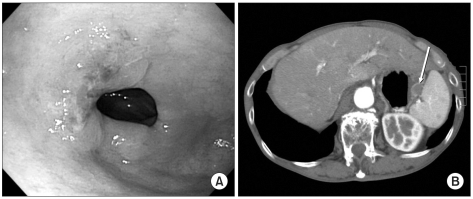
A 74-year-old man was diagnosed with early gastric cancer via endoscopy at a regional hospital. He was then transferred to our Hospital for tertiary management. Endoscopic ultrasonography was performed to determine the depth of the tumor. This showed that the lesion was confined to the mucosa (Fig. 1A). Computed tomography then revealed the presence of a suspected GIST, in the form of a 2×2 cm mass at the hilum of the spleen (Fig. 1B).
Fig. 1.
(A) Endoscopic image of the antral lesion. (B) Computed tomography revealed the presence of a suspected GIST, in the form of an approximately 2×2 cm mass (Arrow) at the hilum of the spleen. GIST = gastrointestinal stromal tumor.
The two management options were either a total gastrectomy or a subtotal gastrectomy with local resection of the hilar mass. In view of the small size of the suspected GIST and the advanced age of the patient, the latter approach was selected to minimize risk and maximize quality of life. The patient experienced no complications, and was discharged home 10 days postoperatively.
1. Operative procedure
A pneumoperitoneum was created using a carbon dioxide (CO2) pressure of up to 12 mmHg. Five trocars were inserted, including one for the telescope. The patient was then placed in the supine position and the head was elevated. After confirming the absence of systemic disease, the great omentum and lesser omentum were divided using electrocautery and ultrasonic shears (LCS; Ethicon Endo-Surgery, Cincinnati, OH, USA). A 2×2 cm mass was observed at the hilum of the spleen. Intraoperative endoscopy revealed that there was no mass in the stomach, and it was therefore concluded that the mass had extruded into the peritoneal cavity only. Careful inspection confirmed that it would be possible to preserve two or more of the short gastric arteries below the GIST (Fig. 2A, B). Wedge resection of the GIST was then performed using an Endo-GIA 60 device (Fig. 2C). The left gastroepiploic vessels and the right gastroepiploic artery and vein were then ligated and divided. The duodenum was divided using an EndoGIA 60 device, and the lymphatic tissues of stations 8, 9, 7, and 11 were dissected. After confirming the adequacy of the lymphatic dissection, a 5 cm right-subcostal minilaparotomy incision was made. The anvil of the circular stapler was inserted into the duodenum, and the minilaparotomy incision was used for the insertion of a purse-string suture. An intracorporeal Billroth I stapled anastomosis was created using a hand access device (Gel Port; Applied Medical, Rancho Santa Margarita, CA, USA).(3) Surgery was concluded after confirming that there was no bleeding in the operative field or tension at the anastomosis site.
Fig. 2.
(A) The presence of the mass at the hilum of the spleen was confirmed intraoperatively (the short arrow indicates the SMT, and the long arrow indicates the short gastric artery, which could supply the remnant stomach). (B) The SMT was dissected laparoscopically. (C) Wedge resection of the GIST was performed. SMT = submucosal tumour; GIST = gastrointestinal stromal tumor.
2. Pathology results
On visual inspection, the adenocarcinoma appeared grossly similar to early gastric cancer type IIc. The tumor was 3.2×2.2 cm in size, and the proximal and distal resection margins were 11.3 cm and 1 cm, respectively. The GIST was 2.2 cm in size, and a clear resection margin was achieved.
Histopathological examination revealed that the adenocarcinoma was moderately differentiated and confined to the mucosa (T1aN0M0, stage 1A). Its type according to the Lauren classification was intestinal. No metastasis was detected in any of the 12 retrieved lymph nodes. The GIST was benign, and consisted of spindle cells with no atypia. Immunohistochemical analyses revealed that the GIST was positive for C-kit and CD34, and negative for smooth muscle actin.
Discussion
The term GIST was introduced by Mazur and Clark in 1983.(4) This designates a heterogeneous group of mesenchymal neoplasms that consist of spindle or epithelioid cells with varying degrees of differentiation. In the earlier literature, these tumors were classified as leiomyomas, leiomyosarcomas, leiomyoblastomas, or schwannomas. However, the introduction of immunohistochemical staining and electron microscopy has revealed that GISTs are a distinct pathological entity.(1)
Although GIST is rare, with an annual incidence of approximately 10~15 cases per 1 million individuals, it is the most common mesenchymal tumor of the gastrointestinal tract.(5) The majority of GISTs are located in the stomach. However, they are also found in descending order of frequency in the small bowel, colon, and esophagus. Immunohistochemical analysis of the expression of CD117 (a marker of the c-kit gene product) and CD34 (a human progenitor cell antigen) has established that GISTs originate from the interstitial cells of Cajal.(6) Loss-of-function mutations of the c-kit gene induce the depletion of the interstitial cells of Cajal, whereas gain-of-function mutations cause their proliferation. A novel therapy for locally advanced or metastatic GIST is inhibition of the growth factor receptor c-kit tyrosine kinase.(7) Apart from the above mentioned theories, simple coincidence could also be the case, especially in geographical regions that have high incidence rates of gastric surgery. Although Helicobacter pylori infection has been implicated in the development of gastric cancer, there is no evidence that it is linked with the development of GISTs.(8)
Laparoscopic wedge resection is now considered the standard treatment for a GIST.(9) Wide negative margins are not required, since GISTs tend to grow out of, rather than diffusely infiltrate the primary organ. In general, wedge resection of a GIST on the anterior gastric wall is considered to be a straightforward procedure when the mass is extraluminal, as in the present case. However, if the tumor is large or located near the cardia or the pylorus, the eversion method or endoscopic cooperative surgery is the usual treatment of choice.(10)
The stomach is resistant to postoperative ischemia due to its rich vascular supply and extensive submucosal vascular plexus.(11) Rutter(12) was the first to report a case of ischemic necrosis of the gastric remnant following subtotal gastrectomy. The blood supply of the proximal gastric remnant is thought to arise from three main sources: the left gastric artery, the left inferior phrenic artery, and the splenic artery, together with its short gastric arteries. An experimental study by Kilgore et al.(13) demonstrated that to avoid gastric remnant infarction, it is essential to completely preserve either the left gastric artery or the splenic artery, together with it's short gastric arteries. In all of the cases reported by Kilgore et al., gastric perforation occurred along the great curvature of the stomach, which is supplied by the left gastric artery and the short gastric arteries. In 83% of cases in which the short gastric arteries had been ligated, perforation and an abscess in the peritoneal cavity occurred. By contrast, these complications occurred in only 23% of cases in which the left gastric artery had been ligated. Portal hypertension(14) and splenic venous thrombosis(15) (one cause of gastric necrosis) have also been found to impede gastric venous drainage. However, one report found that a single short gastric artery can supply the entire stomach adequately via its intramural vessel connections.(11)
In view of the advanced age of the patient in the present report, subtotal gastrectomy and wedge resection were performed to minimize risk and maximize quality of life. However, in cases where the stomach is not long enough, it is preferable to perform a subtotal gastrectomy with subsequent Billroth II reconstruction. When the number of short gastric arteries supplying the remnant stomach is insufficient, it is preferable to perform a total gastrectomy followed by esophago-jejunostomy. In the present case, the stomach was long enough for a Billroth I reconstruction, and it was possible to preserve more than two of the short gastric arteries that supply the area below the wedge resection site. As a further precaution, blood vessels were not grasped directly during surgery. We removed the upper part of short gastric artery as possible as to preserve the distal short gastric artery in order to preserve the circulation of distal stomach.
In conclusion, the simultaneous occurrence of a mid-to-low gastric body adenocarcinoma and a high gastric body GIST can be treated by the combination of a subtotal gastrectomy for the adenocarcinoma and a wedge resection for the GIST, providing that three or more of the short gastric arteries that supply the area below the wedge resection site can be preserved.
References
- 1.Heinrich MC, Corless CL. Gastric GI stromal tumors (GISTs): the role of surgery in the era of targeted therapy. J Surg Oncol. 2005;90:195–207. doi: 10.1002/jso.20230. discussion 207. [DOI] [PubMed] [Google Scholar]
- 2.Uchiyama S, Nagano M, Takahashi N, Hidaka H, Matsuda H, Nagaike K, et al. Synchronous adenocarcinoma and gastrointestinal stromal tumors of the stomach treated laparoscopically. Int J Clin Oncol. 2007;12:478–481. doi: 10.1007/s10147-007-0684-8. [DOI] [PubMed] [Google Scholar]
- 3.Joo YT, Moon HG, Lee SH, Jeong CY, Jung EJ, Hong SC, et al. Laparoscopy-assisted distal gastrectomy with intracorporeal Billroth I stapled anastomosis using a hand access device for patients with gastric cancer. Surg Endosc. 2007;21:859–862. doi: 10.1007/s00464-006-9060-4. [DOI] [PubMed] [Google Scholar]
- 4.Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol. 1983;7:507–519. doi: 10.1097/00000478-198309000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Perez EA, Livingstone AS, Franceschi D, Rocha-Lima C, Lee DJ, Hodgson N, et al. Current incidence and outcomes of gastrointestinal mesenchymal tumors including gastrointestinal stromal tumors. J Am Coll Surg. 2006;202:623–629. doi: 10.1016/j.jamcollsurg.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Miettinen M, Sarlomo-Rikala M, Lasota J. Gastrointestinal stromal tumors: recent advances in understanding of their biology. Hum Pathol. 1999;30:1213–1220. doi: 10.1016/s0046-8177(99)90040-0. [DOI] [PubMed] [Google Scholar]
- 7.Sexton JA, Pierce RA, Halpin VJ, Eagon JC, Hawkins WG, Linehan DC, et al. Laparoscopic gastric resection for gastrointestinal stromal tumors. Surg Endosc. 2008;22:2583–2587. doi: 10.1007/s00464-008-9807-1. [DOI] [PubMed] [Google Scholar]
- 8.Al-Brahim N, Radhi J, Gately J. Synchronous epithelioid stromal tumour and lipoma in the stomach. Can J Gastroenterol. 2003;17:374–375. doi: 10.1155/2003/627090. [DOI] [PubMed] [Google Scholar]
- 9.Kim MH, Song KY, Shim JH, Jung CK, Kim SN, Park CH. Laparoscopic wedge resection of GISTs involving the second portion of the duodenum. J Korean Surg Soc. 2008;74:228–232. [Google Scholar]
- 10.Hyung WJ, Lim JS, Cheong JH, Kim J, Choi SH, Noh SH. Laparoscopic resection of a huge intraluminal gastric submucosal tumor located in the anterior wall: eversion method. J Surg Oncol. 2005;89:95–98. doi: 10.1002/jso.20195. [DOI] [PubMed] [Google Scholar]
- 11.Schein M, Saadia R. Postoperative gastric ischaemia. Br J Surg. 1989;76:844–848. doi: 10.1002/bjs.1800760828. [DOI] [PubMed] [Google Scholar]
- 12.Rutter AG. Ischaemic necrosis of the stomach following subtotal gastrectomy. Lancet. 1953;265:1021–1022. doi: 10.1016/s0140-6736(53)91310-5. [DOI] [PubMed] [Google Scholar]
- 13.Kilgore TL, Jr, Turner MD, Hardy JD. Clinical and experimental ischemia of the gastric remnant. Surg Gynecol Obstet. 1964;118:1312–1316. [PubMed] [Google Scholar]
- 14.Merrill JR. Gastric necrosis and perforation aft er fundoplication. South Med J. 1981;74:1543–1545. doi: 10.1097/00007611-198112000-00034. [DOI] [PubMed] [Google Scholar]
- 15.Kanetaka K, Azuma T, Ito S, Yamaguchi S, Matsuo S, Kanematsu T. Gastric necrosis after an infarction of the spleen: report of a case. Surg Today. 2003;33:867–869. doi: 10.1007/s00595-003-2598-z. [DOI] [PubMed] [Google Scholar]