Abstract
We report a rare case of the coexistence of a gastric small cell neuroendocrine carcinoma with a gastric adenocarcinoma. A 62-year-old man presented with epigastric soreness for 1 month. Esophagogastroduodenoscopy revealed a Borrmann type I tumor at the lesser curvature of the lower body of the stomach. The patient underwent a distal gastrectomy with D2 lymph node dissection and the resected specimen exhibited a 3.5×3.5 cm sized, fungating lesion. Two separated, not intermingling, lesions with non-adenocarcinoma components encircled by well differentiated adenocarcinoma components were identified microscopically. The non-adenocarcinoma component showed neuroendocrine features, such as a solid and trabecular pattern, and the tumor cells showed a high nuclear grade with minimal cytoplasm, indistinct nucleoli, and positive response for synaptophysin, CD56. The final pathological diagnosis was a gastric mixed exocrine-endocrine carcinoma (MEEC) composed of an adenocarcinoma and small cell neuroendocrine carcinoma of the collision type.
Keywords: Stomach neoplasms, Neuroendocrine tumor, Adenocarcinoma
Introduction
The term "mixed exocrine-endocrine carcinoma" (MEEC), which is proposed by the World Health Organization (WHO) classification of endocrine tumors, refers to the neoplasm with divergent exocrine and endocrine differentiation, especially originated in the pancreas, appendix or stomach.(1) Theses tumors were called by different names according to both the extent and the structural shape of two components: composite glandular-endocrine carcinoma, collision tumor, neuroendocrine differentiated adenocarcinoma, amphicrine tumor, goblet cell carcinoid and so on.
Nearly all neoplasms that develop in the stomach are adenocarcinomas, whereas gastric neuroendocrine tumors (NETs) represent less than 1% of all gastric neoplasms. In addition, they account for only 2~4% of all gastrointestinal NETs.(2) Most commonly, NETs are located at the appendix, ileum, or rectum, where NETs occasionally coexist with adenocarcinoma. However, in the stomach, the coexistence of NETSs and adenocarcinoma is rare, and most tumors that do coexist are composed of carcinoid or low-grade B-cell lymphoma and adenocarcinoma.(3,4) We report here a case of MEEC with adenocarcinoma and small cell neuroendocrine carcinoma.
Case Report
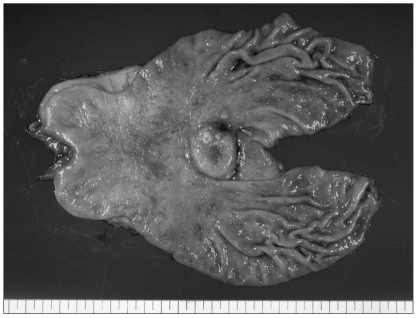
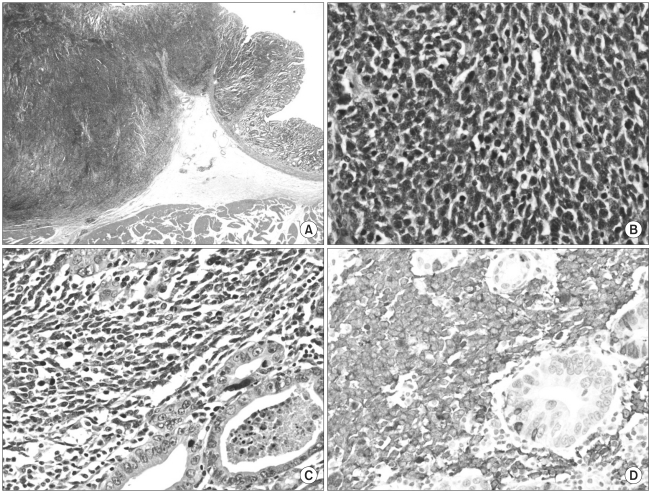
A 62-year-old man presented with epigastric soreness for 1 month. Physical examination was unremarkable and laboratory findings showed no abnormalities. Esophagogastroduodenoscopy revealed a Borrmann type I tumor at the lesser curvature of the lower body of the stomach. Contrast-enhanced computed tomography (CT) showed a 3 cm sized, elevated mass with regional enlarged lymph nodes. The diagnosis, proven from the biopsy specimens taken from the gastric tumor lesion, was poorly differentiated carcinoma. The patient underwent distal gastrectomy with Billroth II anastomosis and D2 lymph node dissection. The resected specimen exhibited a 3.5×3.5 cm sized, Borrmann type I lesion at the body of the stomach macroscopically (Fig. 1). Microscopically, routine hematoxylin-eosin-stained section showed two separated, not intermingling, lesions with different histologic features under low-power magnification (Fig. 2A). These lesions had a characteristic feature with non-adenocarcinoma components encircled by well differentiated adenocarcinoma components (Fig. 2B). The non-adenocarcinoma component showed a solid, organoid, and trabecular pattern, and the tumor cell have a small round hyperchromatic nuclei with a high nuclear grade, indistinct nucleoli, nuclear molding, and scanty cytoplasm. There was clear evidence of necrosis and increased mitotic activity (33/10 HPF) in the non-adenocarcinoma component (Fig. 2C). On immunohistochemical staining, the tumor cell showed a positive response for synaptophysin (Fig. 2D), CD56 and a focally positive response for cytokeratin AE1/3. These histopathologic and immunohistochemical findings of non-adenocarcinoma components were compatible with small cell neuroendocrine carcinoma. The small cell neuroendocrine carcinoma components and adenocarcinoma components accounted for about 65 and 35 percent of tumor lesion, respectively. Consequently, this tumor was diagnosed as gastric mixed exocrine-endocrine carcinoma composed of adenocarcinoma and small cell neuroendocrine carcinoma, of collision type.
Fig. 1.
The resected specimen shows a 3.5×3.5 cm sized, Borrmann type I lesion at the gastric body.
Fig. 2.
(A) The tumor lesion is composed of two separated and different features (hematoxylin-eosin (H&E) stain, original magnification, ×10). (B) A borderline area of small cell neuroendocrine carcinoma and well differentiated adenocarcinoma shows abrupt transition (H&E stain, original magnification, ×400). (C) The tumor cells of neuroendocrine carcinoma show a high nuclear grade, indistinct nucleoli, and nuclear molding with scanty cytoplasm, and there is increased mitotic activity (H&E stain, original magnification, ×400). (D) The tumor cells of neuroendocrine carcinoma show positive staining for synaptophysin (synaptophysin, original magnification, ×400).
The final pathologic results revealed poorly differentiated carcinoma invading the proper muscle with no lymph node metastasis (0/40), but each one positive on lymphatic and vascular invasion. The patient was diagnosed as stage IB (T2N0M0) according to the 7th edition of the International Union Against Cancer TNM classification. Postoperative recovery was uneventful, and the patient was discharged on postoperative day 7.
Discussion
In the WHO classification, Gastric NETs are categorized into 3 groups according to size, rate of proliferation and malignancy: well-differentiated tumor (carcinoid) with benign or uncertain behavior, well-differentiated neuroendocrine carcinoma (malignant carcinoid) with a low grade malignant behavior, and poorly differentiated neuroendocrine carcinoma (small cell carcinoma) with high grade malignant behavior.(1) In addition to these classifications, the most recently defined entity of non-small cell NETs with high grade malignant behavior tumor is the large cell neuroendocrine carcinoma (LCNEC), which is well established in pulmonary NETs, but very rarely reported in gastric NETs.(5) In 1993, Rindi et al.(6) classified gastric NETs into 3 types base on clinical characteristics. Type 1 is the most frequent (70~80% of all cases) and associated with chronic atrophic gastritis. This type of NETs appear as small multifocal tumors in most cases. Type 2 is rare and occurs in association with Zollinger-Ellison syndrome in multiple endocrine neoplasia type 1 (MEN-1). Type 3 is the second most common and occurs in a sporadic and solitary large form. These tumors have a metastasis rate of 10~20% at diagnosis and histologically show well to low differentiated carcinomas or even MEECs.
The spectrum of neuroendocrine and exocrine differentiation in the mixed tumor displays variable extent of two components, potentially ranging from 1 to 99%, and variable morphological patterns, ranging from a single scattered neuroendocrine cell to a well-identifiable neuroendocrine tumor cell population. MEECs are distinguished from adenocarcinoma with focal neuroendocrine differentiation by two major parameters: (1) the extension of each component (at least 30%) and (2) the structural feature of the neuroendocrine components as well differentiated organoid or poorly differentiated solid/diffuse growth patterns.(7) Under the condition of "the rule of 30%", MEECs have two separate features: intermingled(composite)-type with haphazard admixture of exocrine and endocrine elements within the same tumor mass, and the collision type with juxtaposition of both components without admixture within the same tumor mass.(8)
Contrary to an adenocarcinoma with focal neuroendocrine differentiation, which shows a similar clinical prognosis compared to the usual adenocarcinoma,(9) the clinical outcome of MEECs follows that of a more aggressive cell type. Therefore, regarding recurrence or metastasis of MEECs, the target of treatment is set similarly to the more aggressive differentiated tumors. In case of MEECs with well-differentiated neuroendocrine components of benign or low grade malignant behavior, chemotherapy would be focused on exocrine component. In contrast, in those with small cell neuroendocrine carcinoma or LCNEC, endocrine component wound be the main target of the therapy.
The most common primary sites of extrapulmonary small cell carcinoma (EPSCC) are the gastrointestinal (GI) tract, the genitourinary system, and the head and neck region.(10,11) GI EPSCC are usually diagnosed at an advanced stage and at least half are diagnosed with distant metastasis. Even when disease is localized, however, nearly all patients recur with distant metastasis. The most frequent metastatic site is the liver. Consequently, most patients with GI EPSCC have a dismal prognosis with a five-year survival of less than 15%.(12,13) Rothenstein et al.(14) studied a group of patients with GI EPSCC who underwent curative resection with a good prognosis, whereas several studies have reported that surgery with curative intent should be considered only as part of a multimodality approach for selected patients with limited-stage GI EPSCC. The multimodality treatment include adjuvant radiotherapy for incompletely resected disease and systemic chemotherapy.(12,15) The most commonly used chemotherapeutic combination regimens for GI EPSCC are cisplatin/etoposide, carboplatin/etoposide, and cisplatin/irinotecan.
In the present case, although the patient underwent curative surgery and was diagnosed with limited disease (TNM stage IB), the tumor consisted of poorly differentiated (small cell) neuroendocrine carcinoma and well differentiated tubular adenocarcinoma, as a collision type. Therefore, we are planning a close follow up strategy keeping in mind the poor prognosis of small cell carcinoma, and the possibility of potential hepatic metastasis. However, after discussing with medical oncologists, we decided to defer adjuvant chemotherapy considering the early stage of the tumor.
References
- 1.Solcia E, Klöppel G, Sobin LH, Capella C, DeLellis RA, Heitz PU, et al., editors. Histological Typing of Endocrine Tumours. WHO. World Health Organization. International Histological Classification of Tumours. 2nd ed. New York: Springer Verlag; 2000. pp. 56–70. [Google Scholar]
- 2.Klöppel G, Anlauf M. Epidemiology, tumour biology and histopathological classification of neuroendocrine tumours of the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2005;19:507–517. doi: 10.1016/j.bpg.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Kim EY, Park KC, Kwon JG. A case of double primary cancer: early gastric adenocarcinoma associated with adenocarcinoma and carcinoid. Korean J Gastroenterol. 2003;42:533–538. [PubMed] [Google Scholar]
- 4.Goteri G, Ranaldi R, Rezai B, Baccarini MG, Bearzi I. Synchronous mucosa-associated lymphoid tissue lymphoma and adenocarcinoma of the stomach. Am J Surg Pathol. 1997;21:505–509. doi: 10.1097/00000478-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Jiang SX, Mikami T, Umezawa A, Saegusa M, Kameya T, Okayasu I. Gastric large cell neuroendocrine carcinomas: a distinct clinicopathologic entity. Am J Surg Pathol. 2006;30:945–953. doi: 10.1097/00000478-200608000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Rindi G, Luinetti O, Cornaggia M, Capella C, Solcia E. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology. 1993;104:994–1006. doi: 10.1016/0016-5085(93)90266-f. [DOI] [PubMed] [Google Scholar]
- 7.Volante M, Rindi G, Papotti M. The grey zone between pure (neuro)endocrine and non-(neuro)endocrine tumours: a comment on concepts and classification of mixed exocrine-endocrine neoplasms. Virchows Arch. 2006;449:499–506. doi: 10.1007/s00428-006-0306-2. [DOI] [PubMed] [Google Scholar]
- 8.Jain D, Eslami-Varzaneh F, Takano AM, Ayer U, Umashankar R, Muller R, et al. Composite glandular and endocrine tumors of the stomach with pancreatic acinar differentiation. Am J Surg Pathol. 2005;29:1524–1529. doi: 10.1097/01.pas.0000169498.89035.f9. [DOI] [PubMed] [Google Scholar]
- 9.Capella C, La Rosa S, Uccella S, Billo P, Cornaggia M. Mixed endocrine-exocrine tumors of the gastrointestinal tract. Semin Diagn Pathol. 2000;17:91–103. [PubMed] [Google Scholar]
- 10.Haider K, Shahid RK, Finch D, Sami A, Ahmad I, Yadav S, et al. Extrapulmonary small cell cancer: a Canadian province's experience. Cancer. 2006;107:2262–2269. doi: 10.1002/cncr.22235. [DOI] [PubMed] [Google Scholar]
- 11.Wong YN, Jack RH, Mak V, Henrik M, Davies EA. The epidemiology and survival of extrapulmonary small cell carcinoma in South East England, 1970-2004. BMC Cancer. 2009;9:209. doi: 10.1186/1471-2407-9-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brenner B, Tang LH, Shia J, Klimstra DS, Kelsen DP. Small cell carcinomas of the gastrointestinal tract: clinicopathological features and treatment approach. Semin Oncol. 2007;34:43–50. doi: 10.1053/j.seminoncol.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 13.Walenkamp AM, Sonke GS, Sleijfer DT. Clinical and therapeutic aspects of extrapulmonary small cell carcinoma. Cancer Treat Rev. 2009;35:228–236. doi: 10.1016/j.ctrv.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Rothenstein J, Cleary SP, Pond GR, Dale D, Gallinger S, Moore MJ, et al. Neuroendocrine tumors of the gastrointestinal tract: a decade of experience at the Princess Margaret Hospital. Am J Clin Oncol. 2008;31:64–70. doi: 10.1097/COC.0b013e31807a2f49. [DOI] [PubMed] [Google Scholar]
- 15.Brennan SM, Gregory DL, Stillie A, Herschtal A, Mac Manus M, Ball DL. Should extrapulmonary small cell cancer be managed like small cell lung cancer? Cancer. 2010;116:888–895. doi: 10.1002/cncr.24858. [DOI] [PubMed] [Google Scholar]